Abstract
INTRODUCTION
The neuropsychological battery of the Uniform Data Set (UDSNB) was implemented in 2005 by the National Institute on Aging (NIA) Alzheimer Disease Centers (ADC) program to measure cognitive performance in dementia and mild cognitive impairment due to AD. This paper describes a revision, the UDSNB 3.0.
METHODS
The Neuropsychology Work Group of the NIA Clinical Task Force recommended revisions through a process of due diligence to address shortcomings of the original battery. The UDSNB 3.0 covers episodic memory, processing speed, executive function, language and constructional ability. Data from 3,602 cognitively normal participants in the National Alzheimer Coordinating Center database were analyzed.
RESULTS
Descriptive statistics are presented. Multivariable linear regression analyses demonstrated score differences by age, sex and education and were also used to create a normative calculator available online.
DISCUSSION
The UDSNB 3.0 neuropsychological battery provides a valuable non proprietary resource for conducting research on cognitive aging and dementia.
INTRODUCTION
Since 2005, the University of Washington’s National Alzheimer’s Coordinating Center (NACC) has collected the Uniform Data Set (UDS) on participants from over 30 past and present US Alzheimer’s Disease Centers (ADC). The UDS consists of data collection protocols employed systematically on participants enrolled into the Clinical Cores of each ADC1,2. Participants with clinical diagnoses of normal cognition (NC), mild cognitive impairment (MCI) and dementia of various etiologies including Alzheimer’s disease (AD) are recruited, enrolled and followed annually. Consent is obtained at the individual ADCs, as approved by individual Institutional Review Boards (IRBs), and the University of Washington’s IRB has approved the sharing of de-identified UDS data. The UDS data, include demographics, medical history, medication use, clinical and neurological exam findings, measures of function and behavior, clinical ratings of dementia severity (e.g., Clinical Dementia Rating, CDR3), and neuropsychological test scores. Systematic guidelines for clinical diagnosis are based on the most up to date published diagnostic research criteria.1,4
All UDS data collection instruments were constructed with the guidance and approval of the Clinical Task Force (CTF), a group originally constituted by the National Institute on Aging (NIA) to develop standardized methods for collecting longitudinal data that would encourage and support collaboration across the ADCs1,5. As of December 1, 2016, the NACC database contained data on 34,748 UDS participants from past and present ADCs.
ADCs used the first version of the UDSNB starting in September 2005, and in February 2008, a second version, UDSNB 2.0, was implemented with slight revisions to instructions and data collection forms. Tests in the original version of the battery, used until March 2015, were chosen to capture the continuum of cognitive decline from normal cognition through AD dementia, incorporating relevant domains described in detail previously2. An online calculator was developed to aid in scoring6.
In 2010, encouraged by recognition of the growing importance of diagnostic biomarkers7 and the identification of preclinical stages of AD8, the Neuropsychology Work Group, a committee to review the UDSNB 2.0 and make recommendations for future data collection, was convened. This paper describes the rationale and procedures for the development of UDSNB 3.0 and provides normative test scores for a cohort of cognitively normal individuals from the NACC database.
METHODS
Rationale and Procedures for Battery Design and Test Selection
The Clinical Task Force and Neuropsychology Work Group outlined the rationale for change. First, in longitudinal follow up, healthy controls showed practice effects, especially for the memory task, even reciting the story prior to administration on visits subsequent to baseline. UDSNB 2.0 measures were published tests, increasing the potential for multiple exposures either through clinical practice or in ancillary research conducted at the ADCs. Licensing costs and restrictions on sharing these instruments with intramural, extramural and international researchers also created challenges for collaboration. Furthermore, the importance of early detection required instruments that would be sensitive to earlier stages of cognitive decline or even “preclinical” states. Finally, UDSNB 2.0 lacked tests of visuospatial functions and nonverbal memory, both of which can constitute areas of early decline, particularly in those with Lewy Body disease9,10 or those with the posterior cortical atrophy variant of Alzheimer’s disease11–13. Therefore, we created novel tests to address some of the shortcomings of the existing battery, while at the same time having a mechanism for preserving longitudinal continuity with previous data.
The Work Group included members from several ADCs to ensure multicenter representation, and ex-officio members from the NACC and the NIA. The group conducted weekly or monthly conference calls, as needed, and in-person meetings 1–2 times per year to outline a strategy to assess options for change. This included considering different platforms for testing (paper-and pencil vs computer), considering whether or not to change the types of constructs tested with the UDSNB 3.0, and evaluating existing instruments for inclusion into the new battery. Criteria were developed to aid in decision-making. Early on, after reviewing a library of potential tests, the group decided to adopt nonproprietary measures to allow the ADCs to freely share the battery with collaborators. Also early on, it was decided to postpone computerized testing, as the field was rapidly evolving with increasingly sophisticated technology.
The UDSNB 2.0 faced the serious issue of a new battery disrupting the longitudinal follow up of participants tested with the initial battery since 2008. Thus, the decision was made after careful review by the Work Group and the CTF and presentations to the centers, to model the new battery on the old one and to drop or replace existing measures. Digit Symbol from the WAIS-R was dropped while the Trail Making tests and category list generation tests (animals, fruits and vegetables) were retained. In addition, four measures were replaced with similar measures developed previously by several of the centers and tested in published research studies. The section below describes the instruments.
Materials, data recording forms, and a manual for administration and scoring were created and revised with feedback from the centers. After a brief period of pilot data collection with the new instruments to refine the instructions and address any questions about administration and scoring we made additional revisions and conducted a larger pilot study (N=935) that compared the UDSNB 2.0 and 3.0 versions in individuals divided into four groups based on their MMSE scores (26–30, 21–25, 16–20, 10–15) in a “crosswalk” study14. The pairs of scores for the original and corresponding replacement tests were compared using equipercentile equating, and the analyses provided a crosswalk of equivalent test scores between the original and replacement tests (e.g., a score of 15 on the MoCA is equivalent to a score of 21 on the MMSE). The results of the crosswalk study provided good evidence for relatively reliable equivalence across both measures and that the chosen tests were reasonable replacements for the older tests. Crosswalk scores could also assist in making longitudinal comparisons.
Selection of Tests for the UDS Neuropsychological Battery 3.0
The Work Group recommended replacing the MMSE with the Montreal Cognitive Assessment (MoCA),15,16 Logical Memory Immediate and Delayed with the Craft Story 21 Immediate and Delayed Recall17; Digit Span Forward and Backward with the Number Span Forward and Backward Test; and the Boston Naming Test (BNT) with the Multilingual Naming Test (MINT)18. Each decision was based on the rationale outlined below.
General Cognitive Measure
The Montreal Cognitive Assessment (MoCA)15,16 was selected to replace the MMSE as a measure of overall cognitive impairment. Factors influencing this decision included the fact that the MOCA is more difficult than the MMSE as demonstrated in studies showing lower MoCA than MMSE scores in the same samples19 and hence more likely to detect subtle cognitive deficits. Furthermore, floor and ceiling effects are less common with the MoCA, which also allows for a broader range of scores in MCI samples than does the MMSE.20 Thus, the MoCA is more appropriate than the MMSE for detecting early cognitive decline. The MoCA has been validated in white21 and African American22 groups. A disadvantage of the MoCA is that it can yield lower scores in diverse healthy population-based samples23. However, an abbreviated version reportedly demonstrated predictive ability with respect to diagnosis of MCI in a low-education, illiterate sample24. In another study, MoCA was more sensitive to mild cognitive impairment and discriminated MCI from other samples better than the MMSE25,26. MoCA scores have also been shown to correlate with the Activities of Daily Living Questionnaire27 a measure of functional integrity in dementia28. The MoCA has the further advantage of yielding not only a total score (overall measure) but also index scores based on individual items tapping domains of attention, retentive memory, orientation, language, and executive function29.
The MoCA requires about 10 minutes to administer and yields a total score of 30 and the above-mentioned domain index scores. The index scores (not included in the present report) offer the potential to identify early dementia profiles of clinical dementia subtypes such as behavioral variant frontotemporal dementia and primary progressive aphasia. The Memory Index score has been shown to be especially predictive of decline from amnestic MCI to AD dementia.29 The paper-and-pencil version of the MoCA has been translated into multiple languages and dialects within languages30 and is freely available (http://www.mocatest.org/). The NACC was given permission to use it for 25 years without royalties or restrictions on sharing the test with collaborators.
Development of Domain-Specific Neuropsychological Tests
1. Episodic Memory Tests
Memory loss is the hallmark symptom of the most common clinical dementia syndrome associated with Alzheimer’s disease.31 Early studies of AD dementia emphasized the importance of measures of episodic memory, such as word list learning and story recall, in the evaluation for dementia. The group decided on a story memory test since most ADCs were already using Logical Memory, immediate and delayed recall conditions.
Craft and colleagues had designed multiple forms of a story recall test similar to Logical Memory in a study of the impact of insulin on cognition in mild AD dementia17,32. The complete set of 22 stories had previously been tested for equivalence in a diverse sample of college age adults who were administered all of the stories in counterbalanced order in the laboratory of Andrew Saykin (personal communication) and provided to the Work Group for consideration. Additional data on alternate sets of stories were included in published studies of patients undergoing systemic chemotherapy for treatment of breast cancer as well as individuals with traumatic brain injury and healthy controls33–36. In a pilot study to determine the equivalence of twenty-two stories in middle-aged and older adults the Work Group determined that three stories offered the greatest relationship to Logical Memory and to one another. These three were reviewed by the work group and one was chosen for its content relevance to a diverse population, “Craft Story 21”.
Scoring of Logical Memory allows several acceptable responses for each item recalled. Following the protocol from Craft and colleagues32, items were scored in a similar manner to Logical Memory (“paraphrase score”) but another score was also calculated (“verbatim score”), allocating a point for each item recalled exactly as delivered in the story. The verbatim score (not included in the present report) was intended to serve as potentially more sensitive than the paraphrase score in detecting very early memory decline.
Finally, we introduced a novel measure of nonverbal memory, a function not previously included in the UDSNB 2.0. Following the copy of the Benson Complex37 figure (see below under Visuospatial Test) delayed figure reproduction was tested.
2. Language Tests
The 32-item Multilingual Naming Test (MINT)18,38 was selected to replace the short BNT. The MINT was originally developed to test naming in four languages, English, Spanish, Hebrew, and Mandarin Chinese, taking care to equate the level of difficulty of items across languages. The BNT was developed in New England and designed for American English speakers and contains items that either have no equivalent word or different frequencies of usage in other languages. The MINT is sensitive to naming impairment in Alzheimer’s disease18.
Word fluency is measured with semantic and letter word list generation tests. The former were part of UDSNB 2.0, while two letter generation tasks were added (“F” and “L”) for UDSNB 3.0. Each task requires 60 seconds and correct items are totaled. Note is made of errors and rule violations.
3. Visuospatial Tests
The UDSNB 2.0 did not contain a visuospatial test. Visuospatial symptoms emerge in later stages of amnestic dementia due to AD but also may appear early in the clinical syndromes of posterior cortical atrophy and dementia associated with cortical Lewy Body disease. The Benson Complex Figure37 was added as a test of constructional ability (Copy condition). Figural elements are scored for presence and placement. Reproduction is tested after a delay to measure retentive memory (see above under Episodic Memory). Comparison between patients with clinical dementia of the Alzheimer type and frontotemporal dementia showed distinctive profiles of performance and associations with frontal and parietal cortical atrophy regions in the groups.37,39
5. Immediate Attention, Working Memory, Executive Attention Tests
Immediate attention span is commonly tested with Digit Span.40 For studies requiring multiple forms to reduce practice effects, a series of number sets was randomly generated to provide alternatives to the digit span test (Joel Kramer lab, personal communication). The number spans for the UDS task were randomly generated with the restriction that no digit would be adjacent to a digit that was one higher or one lower (e.g., a ‘7’ would not be succeeded or preceded by a 6 or 8). Every attempt was also made to exclude sequences that contained area codes. The number span is the longest list recalled. The total number of trials administered up to failure on two trials at one length is also recorded. Backward span is a measure of working memory. The Trail Making Tests were retained from the UDSNB 2.0 to measure processing speed (Part A) and executive attention (Part B).
Study Sample
This report is based on analyses UDS data submitted to NACC by the ADCs between March 15, 2015, and November 30, 2016. The sample was restricted to individuals who received the UDSNB3.0 and at that visit had a clinical diagnosis of normal cognition and a global CDR score of 0. If a participant had received UDSNB 3.0 more than once, data were included from only the first administration. Although some participants’ scores on the UDSNB 3.0 appeared to be outside the range of normal scores (e.g., MoCA score of 9), we chose not to remove any participants from the descriptive analyses because normalcy was not defined by the tests. Therefore, we describe the full range of scores in those with a clinical diagnosis of normal cognition and a global CDR=0.
Data, Analyses, Normative Calculator
First we describe the demographics of the sample (age, education, and sex). The mean, median, 25th and 75th percentiles, and ranges of scores for the overall sample are presented. Histograms are provided for each of the tests to illustrate the distribution of scores in the overall sample. The mean scores and standard deviations for each test are provided by age divided into five groups (<60 years, 60–69 years, 70–79 years, 80–89 years, ≥90 years) and education, divided into four groups (≤12 years, 13–15 years, 16 years, ≥17 years). Unadjusted linear regression analyses tested for differences by age or education group. Finally, we ran linear regression models to estimate the effect of age (continuous), sex, and education (continuous) on each neuropsychological measure. Adjusted linear regression models were first run with either age, gender, or education predicting the neuropsychological test score (data not shown), and then multivariable models were run with all three demographics included in the model.
We developed a calculator for the UDSNB 3.0 tests based on previously published methods used to produce the calculator for UDSNB 2.0 tests6. While our descriptive analyses focused on all participants meeting our eligibility criteria, for the normative calculator, we excluded a handful of participants who performed five standard deviations outside of the mean on any particular test to improve the distribution of residuals and better satisfy model assumptions. This restriction resulted in excluding the following participants from the regression analyses: five participants from the analysis of the MoCA, four participants from the analysis of the Benson Complex Figure Copy, sixteen participants from the analysis of the Trail Making Part A, and five participants from the analysis of the MINT.
RESULTS
The sample included 3,602 cognitively normal participants over age 60 receiving the UDSNB 3.0 (Table 1). The majority of the sample (65%) were women and were between 70 and 89 years of age (67%) and highly educated (69%). These analyses did not divide the sample by race since most participants in the sample were white (83%), with an additional 14% African American and 3% other race, reflecting the overall distribution of these groups within the ADCs receiving the UDS.
Table 1.
Sample Distribution by Gender, Age, and Education
| Age | Education | Male | Female | Total |
|---|---|---|---|---|
| <60 yrs | ≤12 | 13 | 23 | 36 |
| 13–15 | 23 | 48 | 71 | |
| 16 | 34 | 69 | 103 | |
| 17+ | 44 | 76 | 120 | |
| Missing | 6 | 6 | 12 | |
| 60–69 yrs | ≤12 | 32 | 65 | 97 |
| 13–15 | 47 | 142 | 189 | |
| 16 | 102 | 164 | 266 | |
| 17+ | 158 | 293 | 451 | |
| Missing | 3 | 7 | 10 | |
| 70–79 yrs | ≤12 | 45 | 130 | 175 |
| 13–15 | 61 | 159 | 220 | |
| 16 | 110 | 201 | 311 | |
| 17+ | 274 | 407 | 681 | |
| Missing | 1 | 7 | 8 | |
| 80–89 yrs | ≤12 | 27 | 77 | 104 |
| 13–15 | 26 | 103 | 129 | |
| 16 | 59 | 112 | 171 | |
| 17+ | 151 | 177 | 328 | |
| Missing | 1 | 1 | 2 | |
| ≥90 yrs | ≤12 | 4 | 27 | 31 |
| 13–15 | 6 | 11 | 17 | |
| 16 | 10 | 15 | 25 | |
| 17+ | 19 | 26 | 45 | |
| Missing | 0 | 0 | 0 | |
| Grand total | 1256 | 2346 | 3602 | |
As of December 2016
Means, 25th, 50th and 75th percentile, and score ranges for each test in the overall sample are reported in Table 2. Histograms demonstrate whether the distribution of test scores were approximately normal (Supplemental File 1). Tests with an approximately normal distribution of scores included Craft Story Immediate and Delayed (paraphrase and verbatim), Number Span Forward and Backward (total correct trials and longest span), the letter list generation task (F&L words), and the Benson Complex Figure Recall. Scores on the MoCA, MINT, and copy condition of the Benson Complex Figure Copy were highly skewed due to ceiling effects. However, the MoCA appears to be less affected by ceiling effects than the MMSE.2
Table 2.
Summary Statistics for Clinically Cognitively Normal Uniform Data Set Participants
| UDS Version 3 Neuropsychological Testa | Domain | Max. score | Sample’s scores
|
|||
|---|---|---|---|---|---|---|
| N | Mean (SD) | Q25, Q50, Q75 | Range | |||
| Montreal Cognitive Assessment (MoCA) – total score | Dementia severity | 30 | 3581 | 26.3 (2.8) | 25, 27, 28 | 9–30 |
| Craft Story 21 Recall Immed. Verbatim – total units | Memory | 44 | 3552 | 21.9 (6.6) | 17, 22, 27 | 0–38 |
| Craft Story 21 Recall Immed. Paraphrase – total units | Memory | 25 | 3552 | 16.1 (4.1) | 14, 17, 19 | 0–25 |
| Craft Story 21 Recall Delay. Verbatim – total units | Memory | 44 | 3550 | 19.1 (6.7) | 14, 19, 24 | 0–40 |
| Craft Story 21 Recall Delay. Paraphrase – total units | Memory | 25 | 3550 | 15.1 (4.3) | 12, 16, 18 | 0–25 |
| Benson Complex Figure Copy – total score | VIsuospatial | 17 | 3584 | 15.6 (1.4) | 15, 16, 17 | 0–17 |
| Benson Complex Figure Recall – total score | VIsuospatial | 17 | 3576 | 11.2 (3.1) | 9, 12, 13 | 0–17 |
| Number Span Test Forward – total correct trials | Attention | 14 | 3591 | 8.3 (2.3) | 7, 8, 10 | 2–14 |
| Number Span Test Forward – longest span | Attention | 9 | 3591 | 6.7 (1.3) | 6, 7, 8 | 3–9 |
| Number Span Test Backward – total correct trials | Attention | 14 | 3590 | 7.2 (2.2) | 6, 7, 9 | 0–14 |
| Number Span Test Backward – longest span | Attention | 8 | 3590 | 5.1 (1.3) | 4, 5, 6 | 0–8 |
| Multilingual Naming Test (MINT) – total score | Lang.-Naming | 32 | 3564 | 30.0 (2.4) | 29, 31, 32 | 0–32 |
| Phonemic Test – F-words total in 60 seconds | Lang.-Verbal fluency | 40 | 3589 | 15.1 (4.7) | 12, 15, 18 | 1–35 |
| Phonemic Test – L-words total in 60 seconds | Lang.-Verbal fluency | 40 | 3574 | 14.2 (4.5) | 11, 14, 17 | 0–35 |
| Phonemic Test – total F- and L-words | Lang.-Verbal fluency | 80 | 3572 | 29.2 (8.6) | 23, 29, 35 | 1–64 |
| Animals list generation– total in 60 seconds | Lang.-Category fluency | 77 | 3596 | 21.4 (5.7) | 17, 21, 25 | 0–49 |
| Vegetables list generation– total in 60 seconds | Lang.-Category fluency | 77 | 3590 | 15.1 (4.3) | 12, 15, 18 | 1–35 |
| Trail Making Test Part A – time in seconds | Processing speed | 150 | 3583 | 30.9 (13.4) | 22, 28, 36 | 9–150 |
| Trail Making Test Part B – time in seconds | Executive function | 300 | 3561 | 82.2 (46.3) | 54, 70, 95 | 13–300 |
Abbreviations: UDS = Uniform Data Set; Max = maximum; Lang = Language; Immed = Immediate; Delay = Delayed As of December 2016
Higher scores indicate better scores except for the Trail Making Test Parts A and B
Table 3 shows the means and standard deviations (SD) by each measure across the five age groups, and Table 4 shows means and SD for the four education groups. In the multivariable regression analyses (Table 5), women performed statistically significantly (p<0.01) better than men on the Craft Story Immediate and Delayed, Verbal Fluency Phonemic Test, and Vegetables List Generation, but worse on the Benson Copy Figure Recall, Number Span Forward, and MINT (Table 5). Women and men performed similarly, without statistically significant differences, on the Benson Complex Figure Copy, Number Span Backward, Animal List Generation, and Trail Making Parts A and B. Increasing age was associated with worse scores and increasing years of education was associated with better scores on all of the tests (p<0.01).
Table 3.
Mean neuropsychological test scores by age group
| UDS Version 3 Neuropsychological Testa | Mean (SD)
|
||||
|---|---|---|---|---|---|
| <60 years | 60–69 years | 70–79 years | 80–89 years | ≥90 years | |
| Montreal Cognitive Assessment – total score | 27.5 (2.1) | 26.9 (2.4) | 26.3 (2.7) | 25.3 (3.0) | 23.8 (3.5) |
| Craft Story 21 Recall Immed. Verbat. – total units | 23.1 (6.8) | 23.2 (6.4) | 22.0 (6.5) | 20.0 (6.6) | 17.8 (6.4) |
| Craft Story 21 Recall Immed. Paraph. – total units | 16.9 (4.1) | 16.9 (3.9) | 16.2 (4.0) | 15.0 (4.2) | 13.3 (4.4) |
| Craft Story 21 Recall Delay. Verbat. – total units | 20.9 (7.0) | 20.4 (6.4) | 19.2 (6.6) | 16.9 (6.6) | 15.1 (5.8) |
| Craft Story 21 Recall Delay. Paraph. – total units | 16.3 (4.4) | 15.9 (4.0) | 15.2 (4.1) | 13.6 (4.5) | 12.1 (4.3) |
| Benson Complex Figure Copy – total score | 15.7 (1.2) | 15.8 (1.2) | 15.6 (1.3) | 15.4 (1.6) | 15.5 (1.5) |
| Benson Complex Figure Recall – total score | 12.6 (2.4) | 11.9 (2.8) | 11.2 (3.0) | 10.1 (3.3) | 9.4 (3.6) |
| Number Span Test Forward – total correct trials | 8.7 (2.4) | 8.4 (2.3) | 8.3 (2.3) | 8.0 (2.3) | 7.6 (2.1) |
| Number Span Test Forward – longest span | 6.9 (1.3) | 6.7 (1.3) | 6.7 (1.3) | 6.5 (1.3) | 6.3 (1.3) |
| Number Span Test Backward – total correct trials | 7.8 (2.3) | 7.4 (2.2) | 7.1 (2.2) | 6.8 (2.2) | 6.4 (2.4) |
| Number Span Test Backward – longest span | 5.4 (1.3) | 5.2 (1.3) | 5.1 (1.3) | 4.9 (1.3) | 4.7 (1.4) |
| Multilingual Naming Test (MINT) – total score | 30.1 (2.0) | 30.3 (2.0) | 30.1 (2.2) | 29.6 (2.6) | 28.4 (3.4) |
| Phonemic Test – F-words total in 60 seconds | 16.3 (4.4) | 15.5 (4.6) | 14.9 (4.7) | 14.6 (4.8) | 13.6 (5.0) |
| Phonemic Test – L-words total in 60 seconds | 15.3 (4.3) | 14.8 (4.4) | 14.0 (4.4) | 13.7 (4.5) | 12.5 (4.7) |
| Phonemic Test – total F- and L-words | 31.3 (8.3) | 30.0 (8.4) | 28.8 (8.5) | 28.2 (8.6) | 26.1 (9.1) |
| Animals list generation– total in 60 seconds | 23.6 (5.3) | 22.7 (5.5) | 21.2 (5.4) | 19.5 (5.6) | 17.0 (5.4) |
| Vegetables list generation– total in 60 seconds | 16.3 (4.1) | 16.0 (4.3) | 15.0 (4.2) | 14.0 (4.3) | 12.4 (4.1) |
| Trail Making Test Part A – time in seconds | 22.3 (8.4) | 27.3 (9.6) | 31.0 (10.8) | 36.2 (13.3) | 42.0 (14.3) |
| Trail Making Test Part B – time in seconds | 55.4 (27.2) | 70.1 (33.8) | 82.7 (41.4) | 102.0 (55.2) | 140.6 (75.3) |
Abbreviations: UDS = Uniform Data Set; SD = standard deviation; Immed. = Immediate; Delay. = Delayed; Verbat = Verbatim; Paraph = Paraphrase
Higher scores indicate better scores except for the Trail Making Test Parts A and B; higher scores indicate slower time to completion
Table 4.
Mean neuropsychological test scores by education group
| UDS Version 3 Neuropsychological Testa | Mean (SD)
|
|||
|---|---|---|---|---|
| ≤12 years | 13–15 years | 16 years | 17+ years | |
| Montreal Cognitive Assessment (MoCA) – total score | 24.1 (3.7) | 25.7 (2.9) | 26.6 (2.4) | 26.9 (2.2) |
| Craft Story 21 Recall Immed. Verbatim – total units | 19.6 (7.1) | 21.2 (6.5) | 22.0 (6.5) | 22.8 (6.4) |
| Craft Story 21 Recall Immed. Paraphrase – total units | 14.5 (4.7) | 15.6 (4.0) | 16.3 (4.1) | 16.7 (3.8) |
| Craft Story 21 Recall Delay. Verbatim – total units | 16.6 (7.1) | 18.3 (6.8) | 19.2 (6.6) | 20.0 (6.4) |
| Craft Story 21 Recall Delay. Paraphrase – total units | 13.2 (5.0) | 14.4 (4.4) | 15.3 (4.3) | 15.8 (4.0) |
| Benson Complex Figure Copy – total score | 15.2 (1.5) | 15.5 (1.4) | 15.7 (1.3) | 15.8 (1.2) |
| Benson Complex Figure Recall – total score | 10.2 (3.6) | 11.0 (3.2) | 11.4 (3.1) | 11.5 (2.9) |
| Number Span Test Forward – total correct trials | 7.4 (2.2) | 7.9 (2.2) | 8.4 (2.2) | 8.6 (2.3) |
| Number Span Test Forward – longest span | 6.2 (1.3) | 6.5 (1.3) | 6.8 (1.3) | 6.9 (1.3) |
| Number Span Test Backward – total correct trials | 6.1 (2.3) | 6.7 (2.2) | 7.3 (2.2) | 7.5 (2.2) |
| Number Span Test Backward – longest span | 4.5 (1.4) | 4.9 (1.3) | 5.2 (1.3) | 5.3 (1.3) |
| Multilingual Naming Test (MINT) – total score | 28.5 (2.9) | 29.4 (2.4) | 30.2 (2.0) | 30.5 (2.0) |
| Phonemic Test – F-words total in 60 seconds | 12.8 (4.7) | 13.9 (4.4) | 15.2 (4.6) | 16.1 (4.5) |
| Phonemic Test – L-words total in 60 seconds | 11.9 (4.5) | 13.0 (4.4) | 14.3 (4.1) | 15.2 (4.3) |
| Phonemic Test – total F- and L-words | 24.7 (8.6) | 26.7 (8.3) | 29.4 (8.1) | 31.2 (8.2) |
| Animals list generation– total in 60 seconds | 18.5 (5.4) | 19.9 (5.3) | 21.3 (5.1) | 22.8 (5.7) |
| Vegetables list generation– total in 60 seconds | 13.5 (4.0) | 14.7 (4.0) | 15.1 (4.1) | 15.7 (4.5) |
| Trail Making Test Part A – time in seconds | 35.1 (14.4) | 32.1 (12.3) | 29.7 (11.0) | 29.2 (10.9) |
| Trail Making Test Part B – time in seconds | 112.7 (69.8) | 91.4 (51.5) | 76.1 (36.4) | 73.9 (35.9) |
Abbreviations: UDS = Uniform Data Set; SD = standard deviation; Immed. = Immediate; Delay. = Delayed;
Higher scores indicate better scores except for the Trail Making Test Parts A and B
Table 5.
Multivariable linear regression coefficients and 95% confidence intervals for sex, age, and education
| UDS Version 3 Neuropsychological Testa | Female | Age (years) | Education (years) |
|---|---|---|---|
|
| |||
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |
| Montreal Cognitive Assessment – Total score | 0.35 (0.18, 0.52)** | −0.08 (−0.08, −0.07)** | 0.33 (0.30, 0.36)** |
| Craft Story 21 Recall Immediate - Verbatim | 0.96 (0.51, 1.40)** | −0.13 (−0.15, −0.11)** | 0.41 (0.33, 0.48)** |
| Craft Story 21 Recall Immediate - Paraphrase | 0.60 (0.33, 0.88)** | −0.08 (−0.09, −0.07)** | 0.28 (0.23, 0.33)** |
| Craft Story 21 Recall Delayed - Verbatim | 0.78 (0.33, 1.23)** | −0.15 (−0.17, −0.13)** | 0.43 (0.35, 0.51)** |
| Craft Story 21 Recall Delayed - Paraphrase | 0.52 (0.23, 0.81)** | −0.10 (−0.11, −0.09)** | 0.32 (0.27, 0.37)** |
| Benson Complex Figure Copy – Total score | 0.04 (−0.05, 0.14) | −0.01 (−0.02, −0.01)** | 0.07 (0.06, 0.09)** |
| Benson Complex Figure Recall – Total score | −0.47 (−0.67, −0.26)** | −0.09 (−0.09, −0.08)** | 0.16 (0.12, 0.19)** |
| Number Span Forward – Total correct trials | −0.29 (−0.45, −0.14)** | −0.03 (−0.03, −0.02)** | 0.15 (0.13, 0.18)** |
| Number Span Forward – Longest span | −0.16 (−0.25, −.0.07)** | −0.01 (−0.02, −0.01)** | 0.08 (0.07, 0.10)** |
| Number Span Backward – Total correct trials | −0.10 (−0.25, 0.05) | −0.03 (−0.04, −0.03)** | 0.17 (0.14, 0.20)** |
| Number Span Backward – Longest span | −0.06 (−0.15, 0.03) | −0.02 (−0.02, −0.01)** | 0.10 (0.08, 0.11)** |
| Multilingual Naming Test – Total score | −0.81 (−0.96, −0.66)** | −0.03 (−0.04, −0.02)** | 0.22 (0.19, 0.25)** |
| Verbal Fluency Phonemic Test –Total Correct F-words | 0.54 (0.22, 0.85)** | −0.05 (−0.07, −0.04)** | 0.42 (0.36, 0.47)** |
| Verbal Fluency Phonemic Test –Total correct L-words | 0.63 (0.33, 0.92)** | −0.05 (−0.07, −0.04)** | 0.45 (0.40, 0.50)** |
| Verbal Fluency Phonemic Test – Total correct F and L-words | 1.12 (0.55, 1.69)** | −0.09 (−0.12, −0.07)** | 0.86 (0.76, 0.96)** |
| Category Fluency: Animals – Total score | 0.35 (−0.02, 0.71) | −0.15 (−0.16, −0.13)** | 0.57 (0.50, 0.63)** |
| Category Fluency: Vegetables – Total score | 2.49 (2.22, 2.77)** | −0.08 (−0.10, −0.07)** | 0.31 (0.26, 0.36)** |
| Trail Making Test Part A – Time in seconds | 0.03 (−0.72, 0.78) | 0.45 (0.41, 0.48)** | −0.73 (−0.86, −0.60)** |
| Trail Making Test Part A – Correct lines/Time in seconds | −0.00 (−0.02, 0.02) | −0.01 (−0.01, −0.01)** | 0.02 (0.01, 0.02)** |
| Trail Making Test Part B – Time in seconds | 1.58 (−1.29, 4.45) | 1.64 (1.50, 1.77)** | −4.65 (−5.15, −4.15)** |
| Trail Making Test Part B – Correct lines/Time in seconds | 0.00 (−0.01, 0.01) | −0.01 (−0.01, −0.01)** | 0.01 (0.01, 0.01)** |
Abbreviations: UDS = Uniform Data Set; CI = Confidence Interval
Statistically significant at p<0.05;
statistically significant at p<0.01
Higher scores indicate better scores except for the Trail Making Test Parts A and B time in seconds
For the data to be useful in characterizing research participants, a calculator was created to indicate the level of performance on each measure. The calculator uses the intercepts, regression coefficients, and root mean square errors (RMSE) resulting from the regression analyses described above to calculate unadjusted and adjusted z-scores for individuals of a particular sex, age, and/or education level. The RMSE is the square root of the average squared differences between the observed score and the predicted score, which we substitute as an estimate for a population standard deviation. The adjusted z-scores are calculated for each test adjusting for a single demographic characteristic (i.e., sex, age, or education) and adjusting for all three of these demographics. One can enter an individual’s demographics and raw test scores, and the calculator uses the resulting z-scores to calculate percentile estimates that indicate the individual’s level of impairment on any given test (e.g., Low Average, or Severely Impaired). Two new variables were also added to this calculator to improve the precision of percentile estimates for Trail Making Part A and Part B. These two tests are terminated if the subjects cannot complete within a specified time length (150 seconds and 300 seconds for A and B, respectively), resulting in the same score regardless of how many lines are correctly connected. We added connections-per-second (correct lines connected divided by the time to completion) for both Part A and B. These two new variables provide more accurate Z-scores and percentiles for the Trail Making tests.
The normative calculator for the UDSNB 3.0 tests can be found on NACC’s website (http://www.alz.washington.edu/WEB/UDS3_NormsCalculator.xlsx).
DISCUSSION
This paper reports the results from a study to develop normative data for the Version 3.0 revision of the Uniform Data Set Neuropsychological Battery. The complete UDS contains not only neuropsychological battery but also demographic, medical, family history, neurological, biomarker, psychiatric, and functional data and available post mortem diagnosis on Clinical Core participants who have been followed longitudinally. Earlier versions have been collected since 2005 and stored in the database of the National Alzheimer Coordinating Center at the University of Washington. All the data are available for sharing with researchers and therefore provide a rich source for generating hypotheses and investigating cognitive aging and dementia in a well-defined cohort.
The current revision of the neuropsychological battery provides an updated set of tests, targeting predominantly the symptoms of the most typical, amnestic, presentation of Alzheimer’s disease. The tests are nonproprietary and have the potential to increase sensitivity over former measures to very early symptoms of cognitive decline in older individuals with different levels of education. The new measures are similar to the old measures but have also enriched the standard data collection with novel scores to enhance available data using a relatively brief battery. The normative calculator provides a convenient tool to characterize the level of performance on the measures of the UDSNB 3.0 battery. The battery and the calculator are available on line (https://www.alz.washington.edu/WEB/npsych_means.html) (http://www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER3/UDS3_npsych_worksheets_C2.pdf)
There are some limitations to the study reported above. Although the ADCs encourage the participation of a diverse sample with respect to gender, education and race, there was an over-representation of individuals who were white, female, and highly educated. Thus, the findings are most relevant to research settings where these demographics are representative of the research volunteers. It will be important to expand the normative data for under represented groups and also for population-based samples. Another limitation is that the battery focuses on the spectrum from healthy cognition to dementia of the Alzheimer type and does not explicitly target symptoms associated with other forms of dementia. The Clinical Task Force has introduced additional data collection modules, however, including specialized tests of symptoms related to frontotemporal dementia. Plans are under way to further expand clinical symptom assessment in other dementia syndromes. The availability of the UDSNB 3.0 at no cost to researchers will aid in encouraging more consistent and systematic data collection in disparate studies of cognitive aging and dementia.
Figure 1.0.
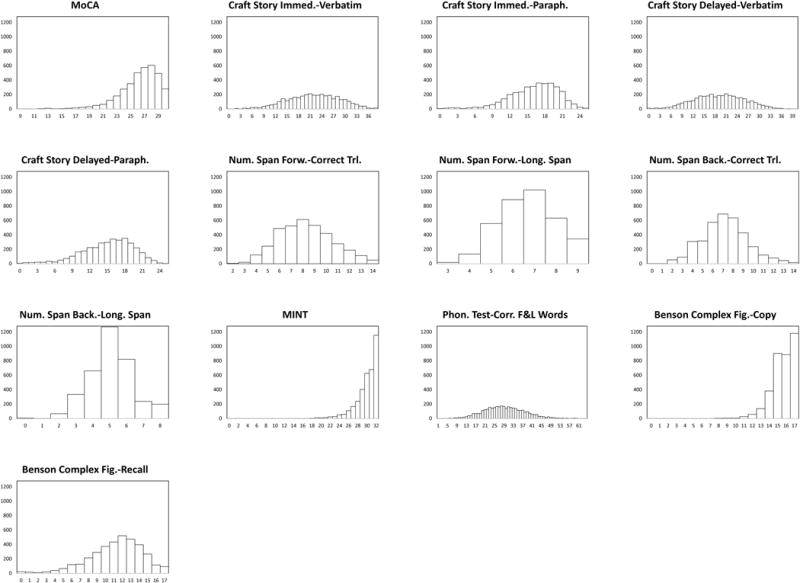
Histograms showing score distributions for each measure on the UDSNB 3.0. From these graphs, many of the measures have a normal or near normal distribution, with the exception of the MoCA total score, the score for the copy of the Benson Complex Figure, and the total score for the MINT.
Research in Context.
Systematic Review
The first version of the UDSNB was based on a review of the literature (e.g., Ovid and PubMed) for cognitive domains and specific constructs sensitive to age-related decline and Alzheimer’s disease and the review was updated with information about newer instruments for screening and constructs not previously included in the original version. Some tests incorporated into the UDSNB 3.0 were copyrighted and permission was given to the National Alzheimer Coordinating Center, University of Washington, from the authors to use the tests in the battery.
Interpretation
The UDSNB 3.0 is a valuable nonproprietary resource for researchers.
Future Directions
Data continue to be accumulated and can be reanalyzed on a larger, more demographically diverse sample. Clinical groups of cognitively normal and cognitive impaired individuals will be compared in future publications. Data are also available to researchers via requests to the National Alzheimer Coordinating Center.
Acknowledgments
The Uniform Data Set Neuropsychology Work Group dedicates this paper to the memory of Dr. Steven Ferris, who passed away on April 5, 2017. He was a cherished and highly respected member of our community who made major contributions to the creation of the UDS and to the work that went into this publication. We all mourn his loss.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
The Uniform Data Set Neuropsychology Work Group also wishes to thank the following individuals for giving their permission to include tests they created in the UDSNB 3.0: Zaid Nasreddine, M.D. (MoCA); Suzanne Craft, Ph.D. (“Craft Story” and scoring method); Andrew Saykin, PhD. for providing data on the equivalence of forms of the Craft Story to Logical Memory; Tamar Gollan, Ph.D. for the Multilingual Naming Test (MINT); Joel Kramer for providing alternate number strings for the Number Span test; Kate Possin and Joel Kramer for the Benson Complex Figure Test. We also wish to thank Nina Silverberg, PhD, for her insightful leadership throughout the conduct of this project.
Kathleen Welsh-Bohmer has received honoraria from Merck, Roche,T3D, Diffusion, and Biogen Companies. She is currently receiving funding from Takeda Pharmaceutical Company in her role as the Neuropsychology Lead for the TOMMORROW clinical trial program.
Dan Marson serves as a consultant for Janssen.
Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Dr. Morris is currently participating in clinical trials of antidementia drugs from Eli Lilly and Company, Biogen, and Janssen. Dr. Morris serves as a consultant for Lilly USA. He receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants # P50AG005681; P01AG003991; P01AG026276 and UF01AG032438.
Footnotes
DISCLOSURES
Sandra Weintraub is participating in clinical trials of antidementia drugs from Eli Lilly and Company and has no other conflicts of interest to declare.
Lilah Besser, Hiroko H. Dodge, Merilee A. Tylan, Steven Ferris, Felicia C. Goldstein, David Salmon, Joel Kramer, Bruno Giordani, Xiao-Hua Zhou, Walter A. Kukull, David Loewenstein, Dan Mungas, and Creighton Phelps have no conflicts of interest to declare.
References
- 1.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007 Jul-Sep;21(3):249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009 Apr-Jun;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules.[see comment] Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006 Oct-Dec;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 5.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004 Oct-Dec;18(4):270–277. [PubMed] [Google Scholar]
- 6.Shirk SD, Mitchell MB, Shaughnessy LW, et al. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther. 2011;3(6):32. doi: 10.1186/alzrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010 Jan;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and neuropathological findings in Lewy body dementias. Brain Cogn. 1996 Jul;31(2):166–175. doi: 10.1006/brcg.1996.0040. [DOI] [PubMed] [Google Scholar]
- 10.Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31:148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- 11.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007 Oct;130(Pt 10):2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 12.Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res. 1997;37(24):3609–3625. doi: 10.1016/S0042-6989(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 13.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- 14.Monsell SE, Dodge HH, Zhou XH, et al. Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord. 2016 Apr-Jun;30(2):134–139. doi: 10.1097/WAD.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasreddine ZS, Phillips N, Chertkow H. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2012 Mar 06;78(10):765–766. doi: 10.1212/01.wnl.0000413072.54070.a3. author reply 766. [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996 Jan-Feb;17(1):123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 18.Ivanova I, Salmon DP, Gollan TH. The multilingual naming test in Alzheimer’s disease: clues to the origin of naming impairments. J Int Neuropsychol Soc. 2013 Mar;19(3):272–283. doi: 10.1017/S1355617712001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larner AJ. Screening utility of the Montreal Cognitive Assessment (MoCA): in place of–or as well as–the MMSE? Int Psychogeriatr. 2012 Mar;24(3):391–396. doi: 10.1017/S1041610211001839. [DOI] [PubMed] [Google Scholar]
- 20.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ, Alzheimer’s Disease Neuroimaging I Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015 Sep 07;15:107. doi: 10.1186/s12877-015-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam B, Middleton LE, Masellis M, et al. Criterion and convergent validity of the Montreal cognitive assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc. 2013 Dec;61(12):2181–2185. doi: 10.1111/jgs.12541. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein FC, Ashley AV, Miller E, Alexeeva O, Zanders L, King V. Validity of the montreal cognitive assessment as a screen for mild cognitive impairment and dementia in African Americans. J Geriatr Psychiatry Neurol. 2014 Sep;27(3):199–203. doi: 10.1177/0891988714524630. [DOI] [PubMed] [Google Scholar]
- 23.Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011 Sep 27;77(13):1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- 24.Julayanont P, Tangwongchai S, Hemrungrojn S, et al. The Montreal Cognitive Assessment-Basic: A Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults. J Am Geriatr Soc. 2015 Dec;63(12):2550–2554. doi: 10.1111/jgs.13820. [DOI] [PubMed] [Google Scholar]
- 25.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009 Feb;24(2):197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- 26.Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013 Sep;9(5):529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson N, Barion A, Rademaker A, Rehkemper G, Weintraub S. The Activities of Daily Living Questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord. 2004 Oct-Dec;18(4):223–230. [PubMed] [Google Scholar]
- 28.Durant J, Leger GC, Banks SJ, Miller JB. Relationship between the Activities of Daily Living Questionnaire and the Montreal Cognitive Assessment. Alzheimer’s & dementia: diagnosis, assessment & disease monitoring. 2016;4:43–46. doi: 10.1016/j.dadm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc. 2014 Apr;62(4):679–684. doi: 10.1111/jgs.12742. [DOI] [PubMed] [Google Scholar]
- 30.Conti S, Bonazzi S, Laiacona M, Masina M, Coralli MV. Montreal Cognitive Assessment (MoCA)-Italian version: regression based norms and equivalent scores. Neurol Sci. 2015 Feb;36(2):209–214. doi: 10.1007/s10072-014-1921-3. [DOI] [PubMed] [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Craft S, Asthana S, Schellenberg G, et al. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000 Apr;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 33.Conroy SK, McDonald BC, Ahles TA, West JD, Saykin AJ. Chemotherapy-induced amenorrhea: a prospective study of brain activation changes and neurocognitive correlates. Brain Imaging Behav. 2013 Dec;7(4):491–500. doi: 10.1007/s11682-013-9240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conroy SK, McDonald BC, Smith DJ, et al. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res Treat. 2013 Jan;137(2):493–502. doi: 10.1007/s10549-012-2385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson RJ, Ahles TA, Saykin AJ, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007 Aug;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald BC, Flashman LA, Arciniegas DB, et al. Methylphenidate and Memory and Attention Adaptation Training for Persistent Cognitive Symptoms after Traumatic Brain Injury: A Randomized, Placebo-Controlled Trial. Neuropsychopharmacology. 2017 Apr 05; doi: 10.1038/npp.2016.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011 Jan;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gollan TH, Weissburger G, Runnqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Bilingualism: Language and Cognition. 2011;13:215–218. doi: 10.1017/S1366728911000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. 2010 Dec;16(6):466–487. doi: 10.1080/13554791003730600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, Texas: The Psychological Corporation; 1987. [Google Scholar]


