Abstract
Borrelia mayonii is a newly described member of the Borrelia burgdorferi sensu lato complex that is vectored by the black-legged tick (Ixodes scapularis Say) and a cause of Lyme disease in Minnesota and Wisconsin. Vertebrate reservoir hosts involved in the enzootic maintenance of B. mayonii have not yet been identified. Here, we describe the first isolation of B. mayonii from naturally infected white-footed mice (Peromyscus leucopus Rafinesque) and an American red squirrel (Tamiasciurus hudsonicus Erxleben) from Minnesota, thus implicating these species as potential reservoir hosts for this newly described spirochete.
Keywords: Borrelia mayonii, Ixodes scapularis, Peromyscus leucopus, Tamiasciurus hudsonicus, Lyme disease
The recently described Lyme disease spirochete, Borrelia mayonii, causes human disease in Minnesota and Wisconsin, and it has been detected in naturally infected Ixodes scapularis Say ticks collected in both states (Pritt et al. 2016a, b). Laboratory studies have confirmed that I. scapularis is a vector for this Lyme disease spirochete (Dolan et al. 2016, Eisen et al. 2016). However, natural reservoir hosts for B. mayonii have not yet been identified. Because B. mayonii is a member of the Borrelia burgdorferi sensu lato complex (Pritt et al. 2016a, b), and because white mice are effective experimental reservoir hosts of both B. burgdorferi sensu stricto and B. mayonii (Piesman 1993, Eisen et al. 2016, Dolan et al. 2017), the same suite of rodent reservoirs implicated in the enzootic maintenance of B. burgdorferi sensu stricto are suspected to also serve as reservoirs for B. mayonii. Here, we describe the first isolation of B. mayonii from field-collected vertebrates, implicating white-footed mice (Peromyscus leucopus Rafinesque) and American red squirrels (Tamiasciurus hudsonicus Erxleben) as potential natural reservoir hosts for B. mayonii in Minnesota. We will provide a more extensive description of the study that generated these isolates in a separate paper.
Materials and Methods
Small Mammal Trapping, Animal Processing, and Ear Biopsy Culture
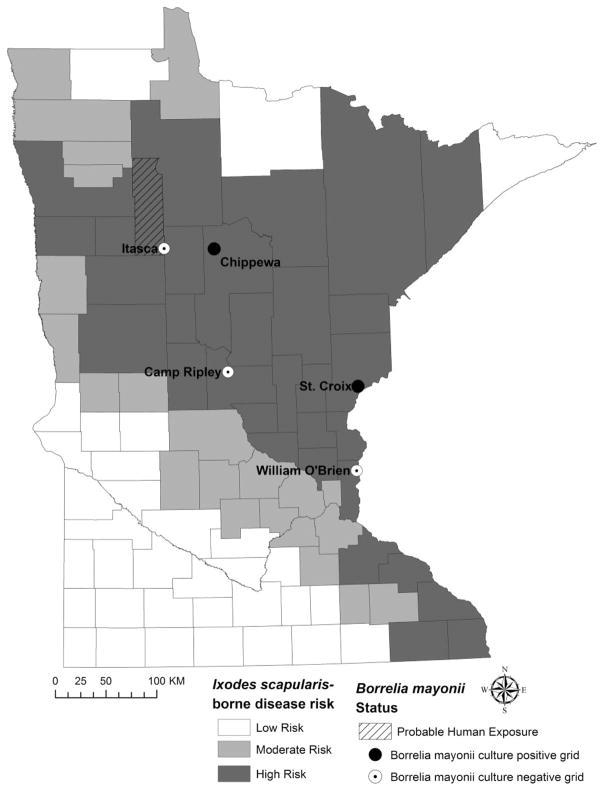
Small mammals were live-trapped using Sherman traps (H. B. Sherman Traps, Inc., Tallahassee, FL) and Tomahawk traps (Tomahawk Live Trap, Hazelhurst, WI) at five sites in Minnesota during June 2016. Trapping coincided with the peak period of nymphal I. scapularis host-seeking activity (data not shown). Study sites were located on public land in counties classified as high risk for I. scapularis-borne diseases and one site corresponded with a county of probable human exposure to B. mayonii (Pritt et al. 2016a); sites included Camp Ripley, Chippewa National Forest, Itasca State Park, Saint Croix State Park, and William O’Brien State Park (Fig. 1).
Fig. 1.
Location of five small mammal trapping sites sampled during June 2016. Three trapping sites yielded no Borrelia mayonii culture-positive animals (white circles). Borrelia mayonii was cultured from animals trapped at two sites, Chippewa National Forest in north central Minnesota and Saint Croix State Park along the Minnesota border with Wisconsin. County-level designation of Ixodes scapularis-borne disease risk based on the average incidence (cases/100,000 population) of Lyme disease and human anaplasmosis cases reported to the Minnesota Department of Health, 2007–2015. Low risk is defined as <10, moderate risk is defined as 10–25, and high risk is defined as ≥25 cases/100,000 population (http://www.health.state.mn.us/divs/idepc/diseases/lyme/highrisk.html, accessed October 2016). Clearwater County is shown (hatched lines) as a location of probable human exposure to B. mayonii (Pritt et al. 2016a).
After anesthetizing an animal, we surface disinfected both ears with 70% ethanol and took a 2 by 2 mm ear biopsy from the tip of each ear. The ear biopsies were placed between the folds of a sterile alcohol pad, covered with 70% ethanol, and allowed to sit for 3–5 min. Once disinfected, we cut each ear biopsy in half and placed one half of each ear into a microcentrifuge tube with 70% ethanol and placed the other half of each ear into a 5-ml culture tube containing 4.5 ml modified Barbour-Stoenner-Kelly (BSK) media with antibiotics, as described previously (Dolan et al. 2016, Pritt et al. 2016a). Attempts to culture B. mayonii focused on ear biopsies since previous laboratory studies demonstrated that ear biopsies from infected white mice were a reliable source of live B. mayonii spirochetes (Dolan et al. 2016, 2017). Culture tubes containing ear biopsies were stored at 4 °C for 1–3 d before being shipped to the Centers for Disease Control and Prevention, Fort Collins, where they were incubated at 34 °C. At 7 d postincubation, we inverted each culture tube twice to homogenize and then removed 100 μl for DNA extraction and polymerase chain reaction (PCR) testing. Cultures testing positive for the presence of B. burgdorferi sensu lato by PCR, as described below, were monitored for motile spirochetes by dark field microscopy at 400× magnification until they reached peak spirochete density, at which point we prepared 18% glycerol stocks and stored them at −80 °C.
DNA Extraction From Culture and Ear Tissue
We heat inactivated 100 μl of culture for 30 min at 56 °C, then brought the sample volume to 200 μl with phosphate buffered saline (ThermoFisher Scientific, Waltham, MA). We extracted DNA from the 200 μl sample using the QIAcube HT automated nucleic acid isolation system and the cador Pathogen 96 QIAcube HT kit (Qiagen, Valencia, CA) per the manufacturer’s protocol for purification of nucleic acids from fluid samples with minor modifications. Briefly, the sample was lysed in a mixture of VXL buffer, carrier RNA, and proteinase K (Qiagen) without an internal control; we replaced the internal control volume specified in the manufacturer’s protocol with additional VXL buffer. A total volume of 350 μl ACB buffer (Qiagen) was added to the lysate, and the 650 μl sample was mixed and transferred to the capture plate and subjected to a 35 kPa vacuum for 3 min. All subsequent wash and dry steps were as specified in the cador Pathogen 96 QIAcube HT V3 program (Qiagen). DNA was eluted by adding 100 μl AVE buffer (Qiagen) to the column, incubating for 2 min, and applying a 55 kPa vacuum for 6 min. The eluted DNA was stored briefly at 4 °C then transferred to −80 °C pending PCR screening.
The two species of Peromyscus mice present in Minnesota cannot be reliably differentiated in the field based on morphology; therefore, to determine the species of Peromyscus that were culture positive for B. mayonii, we extracted DNA from ear biopsies for genetically based species identification. Ear biopsies were washed in 70% ethanol and placed in 100 μl PBS with 100 μg Collagenase A (Roche Applied Science, Indianapolis, IN) for 3 h at 37 °C. After the 3-h incubation, 250 μl lysis buffer (228 μl ATL, 20 μl proteinase K, 1 μg carrier RNA, and 2 μl Dx reagent (Qiagen)) was added, and samples were incubated overnight at 56 °C. DNA from 200 ml of the overnight lysis reaction was purified using a MagMax Pathogen RNA/DNA kit (Applied Biosystems) and the KingFisher Flex magnetic sample processer (ThermoFisher).
Real-Time PCR and DNA Sequencing
We screened DNA extracts from all cultures using a multiplex TaqMan real-time PCR assay. The master mix included primers and probes for the following targets: the B. burgdorferi flagellar filament cap (fliD), a pan-Borrelia 23S rDNA target, B. miyamotoi adenylosuccinate lyase (purB), and the intergenic spacer region (IGS) between 16S rDNA and 23S rDNA in B. mayonii (Table 1). The B. burgdorferi fliD target detects multiple B. burgdorferi sensu lato species, including B. burgdorferi sensu stricto and B. mayonii (Dolan et al. 2011, 2016; Hojgaard et al. 2014). Each 15-μl multiplex reaction contained 7.5 μl iQ Multiplex Powermix (Bio-Rad Laboratories, Hercules, CA), 300 nM each primer, 200 nM each probe, and 7.2 μl DNA extract. The multiplex PCR cycling conditions consisted of a 3-min, 95 °C denaturing step followed by 40 cycles of 95 °C for 10 s, 58 °C for 10 s, and 60 °C for 45 s on a C1000 Touch thermal cycler with a CFX96 real time system (Bio-Rad).
Table 1.
Primers and probes used in the multiplex TaqMan assay used to screen ear biopsy cultures for Borrelia spp. and to differentiate Peromyscus spp. for this study
| Primers and probes | Sequence 5′–3′a | Reference |
|---|---|---|
| Borrelia spp. | ||
| fliD-F | TGGTGACAGAGTGTATGATAATGGAA | Hojgaard et al. 2014 |
| fliD-R | ACTCCTCCGGAAGCCACAA | Hojgaard et al. 2014 |
| fliD-probe | FAM-TGCTAAAATGCTAGGAGATTGTCTGTCGCC-BHQ1 | Hojgaard et al. 2014 |
| 23S-F | TCGGTGAAATTGAAGTATC | This study |
| 23S-R | CARGCTATAGTAAAGGTTCA | This study |
| 23S-probe | HEX-CGTCTAACCACAAGTAATCGGCATC-BHQ1 | This study |
| purB-F | TCCTCAATGATGAAAGCTTTA | Graham et al. 2016 |
| purB-R | GGATCAACTGTCTCTTTAATAAAG | Graham et al. 2016 |
| purB-probe | CalRd610-TCGACTTGCAATGATGCAAAACCT-BHQ2 | Graham et al. 2016 |
| mIGS-F | TGTCGTTATCGGTATGTG | This study |
| mIGS-R | AAGGGCCATGATGATTTG | This study |
| mIGS–probe | Quas705-CCTTCCTACGACTTATCACCGACAG-BHQ3 | This study |
| Peromyscus spp. | ||
| Pero_F | ACAGAYGCCATCCCAGGCC | This study |
| Pero_R | AACGCTTTAAGCTTCACAGTG | This study |
HEX, hexachlorofluorescein phosphramidite; FAM, 6-carboxyfluorescein; CalRd610, CalFluor Red 610; Quas705, Quasar 705; BHQ1, BHQ2, and BHQ3 is Black Hole Quencher 1, 2, and 3, respectively.
If a sample tested positive for B. mayonii using the multiplex TaqMan assay, we tested it again using the real-time PCR assay with which Pritt et al. (2016a) first identified B. mayonii sp. nov. in patient and tick samples. This assay targets the oligopeptide permease A2 gene (oppA2) and uses fluorescent resonance energy transfer (FRET) and melting curve analysis to detect and differentiate B. burgdorferi sensu lato species (Babady et al. 2008, Pritt et al. 2016a). Reactions were prepared as described in Babady et al. (2008) with minor modifications. Each 20-μl reaction contained 5 μl template, 1X LightCycler FastStart DNA Master HybProbe (Roche Applied Science), 3 mM additional MgCl2 for a total MgCl2 concentration of 4 mM, 500 nM each primer (BOR 1, BOR 2, BOR 3, and BOR 4), 200 nM fluorescein-labeled probe, 200 nM LC RED 640-labeled probe 1, and 300 nM LC RED 640-labeled probe 2 (Babady et al. 2008). The Biotechnology Core Facility Branch at the Centers for Disease Control and Prevention (Atlanta, GA) synthesized all oligonucleotides. Reactions were run on a LightCycler 2.0 instrument (Roche Applied Science), and cycling conditions were as described by Babady et al. (2008). Positive controls included DNA from cultured B. burgdorferi B31 and B. mayonii MN14-1539 (Pritt et al. 2016a, b). We used primers BOR 1 and BOR 2 (Babady et al. 2008) to amplify and sequence the oppA2 target from B. burgdorferi B31, B. mayonii MN14-1539, and B. mayonii PCR positive samples. Each 50-μl amplification reaction included 25 μl Premix Ex Taq Hot Start PCR Solution (Takara Bio USA, Mountain View, CA), 500 nM each primer, and 5 μl template. Cycling conditions were identical to the amplification conditions used for the FRET assay (Babady et al. 2008) with a final 5-min extension step at 72 °C. We purified the product using the QIAquick PCR Purification Kit (Qiagen), and we sequenced 500 pg – 1 ng purified amplicon using BigDye Terminator v3.1 Ready Reaction Mix (ThermoFisher). The BigDye Xterminator Kit (ThermoFisher) was used to remove unincorporated dyes before analyzing the samples on an ABI 3130XL genetic analyzer. Using Lasergene 12 software (DNASTAR, Madison, WI), we assembled a consensus sequence for each sample from two forward and two reverse sequences. Each consensus sequence was based on at least double coverage of every nucleotide (nt).
To differentiate P. leucopus from P. maniculatus (Wagner), a 10-μl PCR reaction was performed using 5 μl 2x Sso Advanced SYBR Green supermix (BioRad, Hercules, CA), 4.9 μl DNA, and 1 μl primers Pero_F and Pero_R for a final primer concentration of 400 nM (Table 1). The PCR cycling conditions included a 2-min denaturing step at 98 °C followed by 40 cycles of 98 °C for 10 s, 61 °C for 10 s, and 72 °C for 30 s, followed by melting curve analysis on a C1000 Touch thermal cycler with a CFX96 real time system (BioRad).
Regulatory Compliance
Animal use and experimental procedures were in accordance with an approved protocol on file with the Centers for Disease Control and Prevention Division of Vector-Borne Diseases Animal Care and Use Committee (Fort Collins, CO).
Results and Discussion
We screened 226 ear cultures representing nine species of rodents captured in Minnesota during June 2016 (Table 2). Among the 226 rodent ear cultures screened with the multiplex TaqMan assay, 99 (44%) were positive for B. burgdorferi sensu lato (B. burgdorferi fliD and Borrelia 23S rDNA positive) and six (2.7%) tested positive for B. mayonii (B. burgdorferi fliD, Borrelia 23S rDNA, and B. mayonii IGS positive). We observed motile spirochetes in all cultures testing positive for B. burgdorferi sensu lato DNA. The six B. mayonii positive samples were isolated from T. hudsonicus (n = 2) and from Peromyscus spp. mice identified genetically as P. leucopus (n = 4). Of the six samples that were positive for B. mayonii by the TaqMan assay and were subjected to further testing using the oppA2 assay, four of the samples, including one from T. hudsonicus and three from P. leucopus, had melting temperatures between 60.44 °C and 60.63 °C, consistent with the melting temperature determined for the B. mayonii MN14-1539 positive control (60.46 °C). A second T. hudsonicus sample and one P. leucopus sample had melting temperatures of 62.34 °C and 62.45 °C, respectively, which was intermediate to the melting temperatures for the B. mayonii MN14-1539 and B. burgdorferi sensu stricto B31 (63.09 °C) controls. We suspect that these samples may be coinfected with B. burgdorferi sensu stricto and B. mayonii. For the more detailed publication of this study, we will undertake additional testing to more conclusively characterize these two samples. We sequenced the oppA2 target from the four samples that had melting temperatures consistent with B. mayonii. With the primer sequences removed from either end, all four samples yielded 220-nt consensus sequences identical to the amplicon sequence from B. mayonii MN14-1539 (GenBank CP015796). We conclude that at least four individual rodents from two study sites yielded ear biopsies that contained live B. mayonii spirochetes. These animals included one T. hudsonicus collected from the Chippewa National Forest in Cass County in north-central Minnesota, and three P. leucopus caught at Saint Criox State Park, Pine County, near the Wisconsin border (Fig. 1).
Table 2.
Small mammals captured at five sites in Minnesota—June 2016
| Camp Ripley | Chippewa National Forest | Itasca State Park | St. Croix State Park | William O’Brien State Park | Total | |
|---|---|---|---|---|---|---|
| Clethrionomys gapperi | 6 | 16 | 5 | 7 | 4 | 38 |
| Glaucomys volans | 0 | 0 | 0 | 3 | 0 | 3 |
| Microtus pennsylvanicus | 0 | 0 | 1 | 0 | 0 | 1 |
| Peromyscus spp. | 21 | 33 | 19 | 49 | 29 | 151a |
| Sciurus carolinensis | 0 | 0 | 0 | 0 | 1 | 1 |
| Tamias striatus | 19 | 4 | 1 | 2 | 0 | 26 |
| Tamiasciurus hudsonicus | 0 | 4 | 0 | 1 | 0 | 5 |
| Zapus hudsonicus | 4 | 0 | 0 | 0 | 0 | 4 |
Ear biopsy cultures were not started for three juvenile Peromyscus spp., one each from Camp Ripley, Chippewa National Forest, and St. Croix State Park.
Field studies, such as the one described here, are a first step toward understanding the enzootic maintenance and underlying ecology of the novel pathogen, B. mayonii, in the Upper Midwest. Further field studies are needed to more definitively identify reservoir hosts and to elucidate their roles in B. mayonii maintenance. Here, we report the first isolation of B. mayonii spirochetes from a naturally infected American pine squirrel and from white-footed mice, thus implicating these species as potential reservoirs. Molecular detection of pathogens from ear biopsies and blood samples will provide a better estimate of infection prevalence in hosts by species and will be reported in a follow-up study. In addition, experimental reservoir competence studies are required to confirm that white-footed mice and American red squirrels are capable of infecting feeding ticks. Laboratory experiments with white mice (Dolan et al. 2017), however, strongly suggest that rodents yielding B. mayonii culture-positive ear biopsies are able to both maintain an infection for many months and serve as a source of infection for feeding larval ticks. Notably, at least one I. scapularis larva or nymph was found on each of the B. mayonii-positive animals. The two American red squirrels were infested with a total of 4 nymphs. Among the four white-footed mice, a total of 20 larvae and 3 nymphs were recovered.
The white-footed mouse is a primary enzootic reservoir for B. burgdorferi throughout its range (Anderson et al. 1987, Anderson 1988, Magnarelli et al. 1988), which extends from southeastern Canada southward through the eastern half of the United States including the Great Plains (Lackey et al. 1985). American red squirrels are also distributed across the range, and have been found infected with or seropositive for B. burgdorferi in New York (LoGiudice et al. 2003, Hersh et al. 2014), Michigan (Hamer et al. 2010), and southeastern Canada (Bouchard et al. 2011, Mechai et al. 2016). Presently, it appears that the enzootic reservoir hosts important for the persistence of B. burgdorferi in the eastern United States likely serve as important hosts for B. mayonii in the Upper Midwest. Moreover, our study indicates that at least one potential reservoir host species for B. mayonii occurs commonly across the Northeast. Although B. mayonii has yet to be detected from the Northeast, this study and previous vector competence studies for I. scapularis together indicate that both vector ticks and rodent reservoirs occur commonly across the Northeast and B. mayonii will be able to establish there should it be introduced via infected ticks or vertebrates.
Acknowledgments
We thank Dave Neitzel, Jenna Bjork, Franny Dorr, Elizabeth Schiffman, and Robert Prose (Minnesota Department of Health) for assistance in the field and Marc Dolan (CDC) for advice on field protocols. We also thank Christine Brown (Chippewa National Forest), Rick Dunkley (Saint Croix State Park), Adam Thompson (Camp Ripley), Wayne Boerner (William O’Brien State Park), Bob Chance and David Biesboer (Itasca State Park), for assistance with field logistics. We thank Bob Fischer (Rocky Mountain Laboratory/NIH) for providing Peromyscus maniculatus DNA.
References Cited
- Anderson JF. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:180–191. doi: 10.1111/j.1749-6632.1988.tb31852.x. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Johnson RC, Magnarelli LA. Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. J Clin Microbiol. 1987;25:1564–1566. doi: 10.1128/jcm.25.8.1564-1566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babady NE, Sloan LM, Vetter EA, Patel R, Binnicker MJ. Percent positive rate of Lyme real-time polymerase chain reaction in blood, cerebrospinal fluid, synovial fluid, and tissue. Diagn Microbiol Infect Dis. 2008;62:464–466. doi: 10.1016/j.diagmicrobio.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Beauchamp G, Nguon S, Trudel L, Milord F, Lindsay LR, Bélanger D, Ogden NH. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in southeastern Canada: implications for Borrelia burgdorferi transmission. Ticks Tick-Borne Dis. 2011;2:183–190. doi: 10.1016/j.ttbdis.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Schulze TL, Jordan RA, Dietrich G, Schulze CJ, Hojgaard A, Ullmann AJ, Sackal C, Zeidner NS, Piesman J. Elimination of Borrelia burgdorferi and Anaplasma phagocytophilum in rodent reservoirs and Ixodes scapularis ticks using a doxycycline hyclateladen bait. Am J Trop Med Hyg. 2011;85:1114–1120. doi: 10.4269/ajtmh.2011.11-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, Williams MA, Pritt BS, Schriefer ME, Eisen L. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick-Borne Dis. 2016;7:665–669. doi: 10.1016/j.ttbdis.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Breuner NE, Hojgaard A, Hoxmeier JC, Pilgard MA, Replogle AJ, Eisen L. Duration of Borrelia mayonii infectivity in an experimental mouse model for feeding Ixodes scapularis larvae. Ticks Tick-Borne Dis. 2017;8:196–200. doi: 10.1016/j.ttbdis.2016.11.002. http://dx.doi.org/10.1016/j.ttbdis.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Eisen L, Breuner NE, Hojgaard A, Hoxmeier JC, Pilgard MA, Replogle AJ, Biggerstaff BJ, Dolan MC. Comparison of vector efficiency of Ixodes scapularis (Acari: Ixodidae) from the Northeast and Upper Midwest of the United States for the Lyme disease spirochete Borrelia mayonii. J Med Entomol. 2016;53:tjw160. doi: 10.1093/jme/tjw160. http://dx.doi.org/10.1093/jme/tjw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) Ticks Tick-Borne Dis. 2016;7:1230–1235. doi: 10.1016/j.ttbdis.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Tsao JL, Walker ED, Hickling GJ. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. EcoHealth. 2010;7:47–63. doi: 10.1007/s10393-010-0287-0. [DOI] [PubMed] [Google Scholar]
- Hersh MH, Ostfeld RS, McHenry DJ, Tibbetts M, Brunner JL, Killilea ME, LoGiudice K, Schmidt KA, Keesing F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE. 2014;9:e99348. doi: 10.1371/journal.pone.0099348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick-Borne Dis. 2014;5:49–351. doi: 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Lackey JA, Huckaby DC, Ormiston BG. Peromyscus leucopus. Mamm Species Arch. 1985;247:1–10. [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA, Anderson JF, Hyland KE, Fish DU, Mcaninch JB. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J Clin Microbiol. 1988;26:1138–1141. doi: 10.1128/jcm.26.6.1138-1141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechai S, Margos G, Feil EJ, Barairo N, Lindsay LR, Michel P, Ogden NH. Evidence for host-genotype associations of Borrelia burgdorferi sensu stricto. PLoS ONE. 2016;11:e0149345. doi: 10.1371/journal.pone.0149345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Hoang Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect Dis. 2016a;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper Midwestern United States. Int J Sys Evol Microbiol. 2016b:66. doi: 10.1099/ijsem.0.001445. http://dx.doi.org/10.1099/ijsem.0.001445. [DOI] [PMC free article] [PubMed]