Abstract
Insulin secretion plays a central role in glucose homeostasis under normal physiological conditions as well as in disease. Current approaches to study insulin granule exocytosis either use electrophysiology or microscopy coupled to the expression of fluorescent reporters. However most of these techniques have been optimized for clonal cell lines or require dissociating pancreatic islets. In contrast, the method presented here allows for real time visualization of insulin granule exocytosis in intact pancreatic islets. In this protocol, we first describe the viral infection of isolated pancreatic islets with adenovirus that encodes a pH-sensitive green fluorescent protein (GFP), pHluorin, coupled to neuropeptide Y (NPY). Second, we describe the confocal imaging of islets five days after viral infection and how to monitor the insulin granule secretion. Briefly, the infected islets are placed on a coverslip on an imaging chamber and imaged under an upright laser-scanning confocal microscope while being continuously perfused with extracellular solution containing various stimuli. Confocal images spanning 50 µm of the islet are acquired as time-lapse recordings using a fast-resonant scanner. The fusion of insulin granules with the plasma membrane can be followed over time. This procedure also allows for testing a battery of stimuli in a single experiment, is compatible with both mouse and human islets, and can be combined with various dyes for functional imaging (e.g., membrane potential or cytosolic calcium dyes).
Keywords: Cellular Biology, Issue 127, Insulin granule, exocytosis, neuropeptide Y, pHluorin, pulsatile secretion, pancreatic islets, adenovirus
Introduction
Insulin is produced by the beta cells of the pancreatic islet and it is a key regulator of glucose metabolism1. Death or dysfunction of beta cells disturbs glucose homeostasis and leads to diabetes2. Insulin is packed in dense-core granules that are released in a Ca2+-dependent manner3. Elucidating how insulin granule exocytosis is regulated is essential to fully understand what determines insulin secretion and opens new avenues for the identification of novel therapeutic targets for the treatment of diabetes.
Insulin exocytosis has been extensively studied using electrophysiological approaches, such as membrane capacitance measurements, and microscopic approaches in combination with fluorescent molecules. Membrane capacitance measurements have good temporal resolution and allow single cell recordings. However, changes in the capacitance reflect the net surface change of the cell and do not capture individual fusion events or distinguish insulin granule fusion from other non-insulin secretory vesicles3. Microscopic approaches, such as two-photon or total internal reflection fluorescence (TIRF) microscopy in combination with fluorescent probes and vesicle cargo proteins, provide additional detail. These techniques capture single exocytotic events and also the pre- and post-exocytotic stages and can be used for studying exocytotic patterns in populations of cells3.
Fluorescent reporters can be of three types: 1) extracellular, 2) cytoplasmic, or 3) vesicular. 1) Extracellular reporters are polar tracers (e.g., dextrans, sulforhodamine B (SRB), lucifer yellow, pyranine) that can be introduced through the extracellular milieu4. The use of polar tracers allows for the investigation of the fusion pore in a population of cells and captures various intercellular structures such as blood vessels. However, they do not report on vesicle cargo behavior. 2) Cytoplasmic reporters are fluorescent probes coupled to membrane-associated proteins that face the cytoplasm and are involved in docking and exocytosis. Examples include members of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) family that have been successfully used in neuroscience for studying neurotransmitter release5. Such proteins have multiple binding partners and are not insulin-granule specific. 3) Vesicular reporters are fluorescent probes fused to vesicular cargo proteins that allow for the investigation of cargo-specific vesicle behavior. Insulin-granule specific cargo proteins include insulin, c-peptide, islet amyloid polypeptide, and NPY among others6,7. NPY is only present in insulin containing granules, and is co-released with insulin, making it an excellent partner for a fluorescent reporter8.
The fusion of different fluorescent proteins to NPY has been previously employed to study various aspects of exocytosis in neuroendocrine cells, such as the requirement of specific synaptotagmin isoforms9,10 and how the time-course of release depends on the actin cytoskeleton and on myosin II11,12. In this study, we chose pHluorin as the fluorescent reporter, which is a modified GFP that is non-fluorescent at the acidic pH inside dense core granules but becomes brightly fluorescent upon exposure to the neutral extracellular pH13. Mature insulin granules have an acidic pH below 5.5. Once the granule fuses with the plasma membrane and opens, its cargo is exposed to the neutral extracellular pH of 7.4, allowing the use of the pH-sensitive proteins pHluorin as a reporter7,14.
In view of the pH sensitive nature of pHluorin and the selective expression of NPY in insulin granules, the NPY-pHluorin fusion construct can be used to study various properties of insulin granule exocytosis. The viral delivery of the fusion construct ensures high transfection efficiency and works on primary beta cells or cell lines as well as on isolated islets. This method can also be used as a guideline for studying exocytosis in any other cell type with NPY-containing vesicles. It can also be combined with any transgenic mouse model to study effects of certain conditions (knockdowns, overexpression, etc.) on exocytosis. This technique has been previously used to characterize spatial and temporal patterns of insulin granule secretion in beta cell populations in human islets15.
Protocol
The animal ethics committee of the University of Miami has approved all the experiments.
1. Viral Infection of Intact Isolated Human or Mouse Pancreatic Islets
- Islet culture: prepare the islet culture media: Connaught Medical Research Laboratories (CMRL) 1066, 10% (v/v) FBS and 2 mM L-Glutamine.
- Human pancreatic islets are obtained from the Integrated Islet Distribution Program (NIDDK, NIH). Upon arrival, transfer the islets (~ 500 islet equivalents) to 35-mm non-tissue culture treated Petri dishes with 2 mL CMRL culture media at 37 °C, 5%/95% CO2/O2 for 24 h before viral infection.
-
Mouse pancreatic islets can be isolated following previously established protocols16. Following isolation, culture ~ 200 islet equivalents in 35-mm non-tissue culture treated Petri dishes with 2 mL CMRL culture media at 37 °C, 5%/95% CO2/O2 for 24 h before viral infection.NOTE: Avoid using transgenic mice with GFP or YFP reporters expressed on islets to avoid fluorescence overlap with NPY-pHluorin.
-
Virus preparation
NOTE: The NPY-pHluorin fusion was cloned into the pcDNA3 vector10 and subcloned into an adenoviral vector for adenoviral production [adenovirus serotype 5 (DE1/E3)] by a recombinant adenovirus manufacturing company. The virus was aliquoted and stored at −80 °C. The viral stock is provided by the company at titers of 1012 to 1013 viral particles (~ 3×1010 – 3×1011 PFU).- For in vitro islet infection, use 106 PFU/mL, resulting in an approximate multiplicity of infection (MOI) of around 2 (see Discussion for details)
-
Viral infection of pancreatic islets
Caution: Working with adenoviruses requires Biosafety Level 2 (BSL2) procedures and certification. Check with the Institutional Biosafety Officer for guidance and training on BSL2 procedures.- Prepare human/mouse islets as described above.
-
Add 5 – 10 µL of stock virus to each 35-mm Petri dish containing human/mouse islets in 2 mL of CMRL culture media (with 10% FBS and 2 mM L-Glutamine).NOTE: Adjust the volume of the virus used according to the viral titer as provided by the company datasheet.
- Culture the islets in virus-containing media at 37 °C/5% CO2 for 24 h.
- After 24 h, aspirate the virus-containing media and replace with 2 mL CMRL culture media (with 10% FBS and 2 mM L-Glutamine).
- Culture the islets for 4 – 6 days at 37 °C/5% CO2, replacing the media every 3 days.
- After 4 – 6 days of culturing, expect around 30% of islet cells to be infected. Islets can then be used for live imaging experiments.
2. Confocal Imaging of Infected Islets
NOTE: Refer to the Table of Materials for the materials and equipment required for confocal imaging.
- Reagent preparation and experimental setup
-
Prepare extracellular solution: add 125 mM NaCl, 5.9 mM KCl, 2.56 mM CaCl2, 1 mM MgCl2, 25 mM HEPES, 0.1% BSA, pH 7.4, sterile filtered.NOTE: This buffer is normally prepared without glucose and can be stored at 4 °C for up to 1 month. Glucose is added on the day of the experiment to reach the desired final concentration.
- Prepare basal glucose (3 mM) medium: add 75 µL of 2 M glucose stock to 50 mL of extracellular solution.
- Hyperglycemic (16 mM) medium: add 400 µL of 2 M glucose stock to 50 mL of extracellular solution
- Dilute any additional stimulus (e.g., KCl or adenosine triphosphate (ATP)) in extracellular solution containing 3 mM glucose.
-
Before starting an experiment, pretreat the coverslips with poly-D-lysine by adding 30 µL of poly-D-Lysine solution (1 mg/mL) to the coverslip for 1 h and thoroughly rinsing it with H2O.NOTE: The poly-lysine coated coverslips can be stored at room temperature for up to 6 months.
-
At least 1 h before the experiment, using a 1 mL pipette, transfer the islets to a 35-mm Petri dish containing extracellular solution with 3 mM glucose. Keep the islets at 37 °C and 5% CO2.NOTE: If needed, the plasma membrane can be labeled in this step. To label the plasma membrane, add 2 µM di-8-ANEPP dye to the extracellular solution with 3 mM glucose. Incubate the islets in dye solution for 1 h at 37 °C/5% CO2. The plasma membrane dye can be excited at 488 nm and detected at 620 nm.
-
min before starting an experiment, attach the coverslip to the imaging chamber by sealing it with vacuum silicone grease. Fix the imaging chamber to the imaging platform. Using a pipette, place 20 – 30 islets on the poly-D-Lysine-treated area of the coverslip and let the islets adhere to the surface for 20 min.NOTE: It is important not to let the coverslip dry completely to avoid islet damage.
- While the islets are adhering to the coverslip, prepare the perfusion system by thoroughly rinsing it with water. Add each solution to a different channel: 3 mM glucose (channel 1), 16 mM glucose (channel 2), 16 mM glucose with 100 µM 3-isobutyl-1-methylxanthine (IBMX) and 10 µM forskolin (channel 3), 25 mM KCl in 3 mM glucose (channel 4), 10 µM ATP in 3 mM glucose (channel 5). Remove all the bubbles from the system by opening each channel separately and letting the solution flow for a few minutes, and make sure that the flow is consistent (0.5 mL/min) and the tubing is not leaking.
- Connect the single inline solution heater to the perfusion outlet tube and adjust the temperature of the outflowing buffer to 37 °C.
- Prepare the suction pump. Remove all the bubbles from the system, and make sure that the flow is consistent and the tubing is not leaking.
- Once the islets adhere to the coverslip surface, gently fill up the imaging chamber with the extracellular solution containing 3 mM glucose. Avoid washing the islets away from the surface of the coverslip.
- Place the imaging platform with the islets onto the microscope stage and connect it to the perfusion system and suction pump.
- Turn on the flow and constantly perfuse the islets with the 3G extracellular solution. The system is now ready for confocal imaging.
-
- Confocal imaging
- Locate the islets in the microscope field with lower magnification. Once focused on the islets, switch to higher magnification objectives (e.g., 63X water immersion objective (63X/0.9 NA)).
- Open the acquisition using software (Table of Materials) and activate the resonant scanner mode.
- Select the XYZT imaging mode and configure the acquisition settings as follows:
- Turn on the Argon laser and the 488 nm laser line and adjust the laser power to 50% for pHluorin excitation.
- Collect emission at 505 – 555 nm.
- Choose a resolution of 512×512 pixels. Press the "Live" button to start imaging and adjust the gain levels (typical gain is around 600 V).
- Set the begin and end of the z-stack: focus on the top of the islet and choose "begin" and move to the last plane that can be focused and choose "end". Use a z-step size of 5 µm. The software will automatically calculate the number of confocal planes.
- Set the time interval for acquisition of each z-stack close to 1.5 – 2 s and choose the option "Acquire until stopped" for continuous imaging.
- Press the "start" button to initialize.
- Use various stimulation protocols to induce granule exocytosis by perfusing the islets with the desired stimuli. Stimulation protocols can be customized to fit the desired scientific purpose (see below).
-
Stimulation protocols
NOTE: In every stimulation protocol, start by recording at least 2 min of islet background activity during constant perfusion with extracellular solution containing 3 mM glucose. Perfuse with a stimulant for the desired period of time. The order of stimulants, duration of stimulation as well as duration of recordings can be customized to fit the desired scientific purposes. Make sure to wash islets thoroughly with extracellular solution containing 3 mM glucose before starting a new stimulation. Below find the sample stimulation protocols that have been used to demonstrate the method capabilities.-
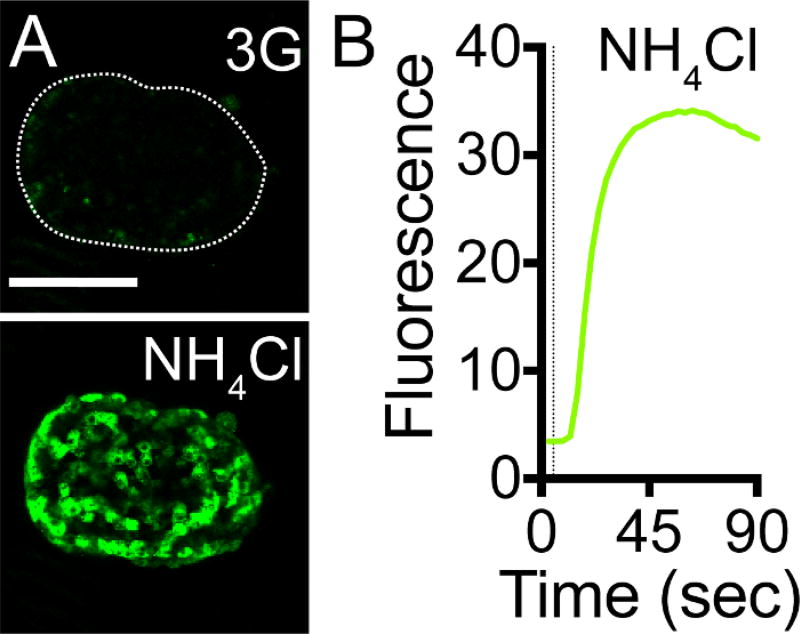
Stimulate with ammonium chloride (NH4Cl) as a positive control for pHluorin pH sensitivity and viral infection efficiency (Figure 3): 3 mM glucose (2 min) → 50 mM NH4Cl (2 min) in 3 mM glucose → 3 mM glucose (2 min)NOTE: In the NH4Cl solution, replace NaCl on an equimolar basis.
-
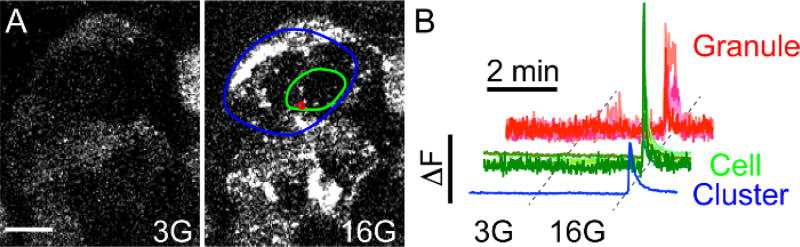
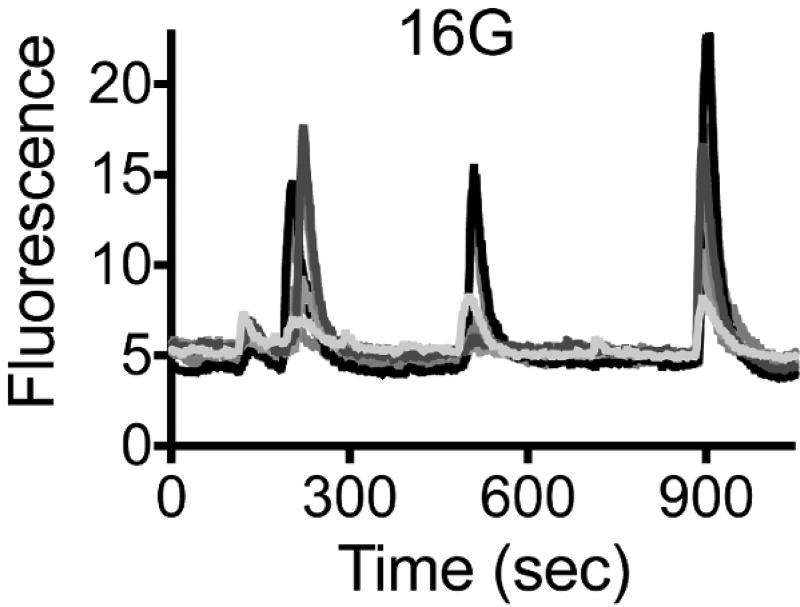
Stimulate insulin granule exocytosis by increasing the glucose concentration (Figure 5 and Figure 6): 3 mM glucose (2 min) → 16 mM glucose (15–30 min) → 3 mM glucose (2 minNOTE: In order to see several bursts of activity, perfuse the islets continuously with the 16G solution for at least 15 min.
-
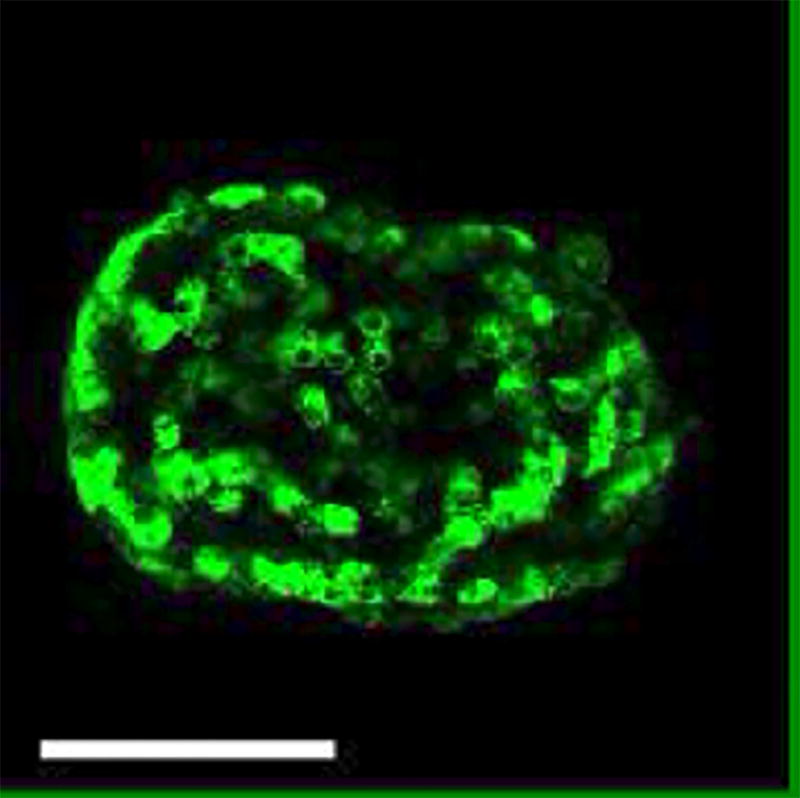
Figure 3. NPY-pHluorin is an Effective pH Sensor.
(A) Maximal projection of confocal images of a mouse islet infected with NPY-pHluorin in extracellular solution containing 3 mM glucose before (upper panel, 3G) and in the presence of 50 mM NH4Cl (lower panel). Scale bar = 100 µm. (B) Trace showing the change in mean fluorescence intensity (arbitrary units) in the whole islet induced by NH4Cl. Dashed line indicates NH4Cl application.
Figure 5. High Glucose Triggers NPY-pHluorin Fusion with the Plasma Membrane.
(A) Maximal projection of confocal images of an intact human islet infected with NPY-pHluorin in basal glucose concentration (3G – 3 mM Glucose, left panel) or in high glucose (16G – 16 mM Glucose, right panel). Extracellular solutions contained the cAMP raising agents forskolin and IBMX (see Protocol for details). Scale bar = 10 µm. (B) Traces showing changes in mean fluorescence intensities (arbitrary units) in the green channel in ROIs placed around single secretory events (red, granule), cells (green) or cell clusters (blue). High glucose was applied for 5 min after ~ 2.5 min in 3G. Fluorescence values were normalized to the initial fluorescence value (baseline).
Figure 6. Secretory Events Generate Discrete Pulses of Secretion upon High Glucose.
Traces showing changes in mean fluorescence intensity (arbitrary units) in ROIs placed around different cells within an intact human islet during sustained stimulation with 16 mM glucose. Each color represents an individual cell. Burst of secretory activity can be seen every 3 – 4 min.
Representative Results
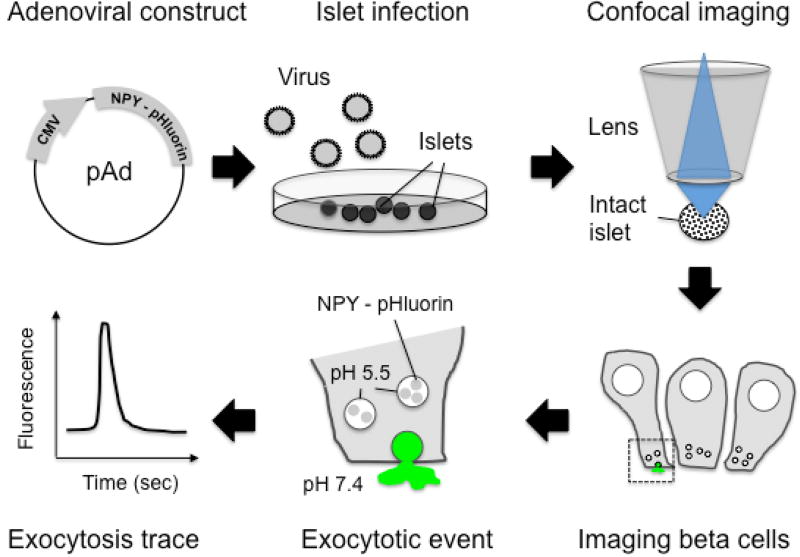
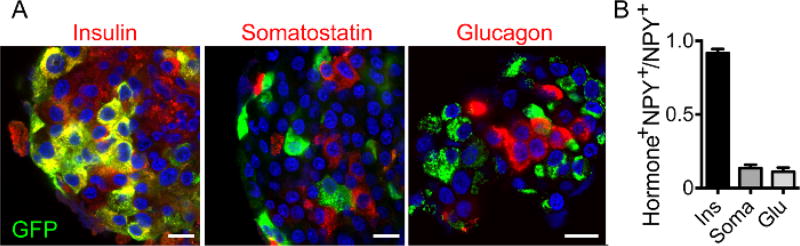
The entire workflow of the technique is shown in Figure 1. Briefly, mouse or human islets can be infected with adenovirus encoding NPY-pHluorin and imaged, after few days in culture, under a confocal microscope. As granules fuse with plasma membrane and open, an increase in fluorescence is observed and can be quantified (Figure 1). To determine if NPY-pHluorin is indeed a suitable tool to monitor insulin granule dynamics, infected islets were immunostained with antibodies against GFP and the different islet hormones insulin, somatostatin or glucagon. Most cells expressing NPY-pHluorin (GFP positive) were beta cells as they also expressed insulin (~ 90%; Figure 2A, 2B). Only a few glucagon-positive alpha cells or somatostatin-positive delta cells were GFP-labeled (Figure 2B). Importantly, in infected cells, the NPY fusion colocalized with insulin (Pearson's correlation coefficient of 0.61 ± 0.04 for GFP and insulin vs. 0.21 ± 0.05 for GFP and glucagon and 0.07 ± 0.01 for GFP and somatostatin).
Figure 1. A Scheme Illustrating the Assay Developed.
Adenovirus that encodes a pH-sensitive GFP (pHluorin) coupled to NPY were produced and used to infect mouse or human pancreatic islets. Four to six days after infection, the islets were placed on a coverslip and imaged under an upright laser-scanning confocal microscope. The NPY-pHluorin construct is expressed in insulin granules in beta cells. Its fluorescence is quenched at the acidic pH of mature insulin granules but becomes bright upon granule fusion with the plasma membrane and exposure to the neutral extracellular pH of 7.4.
Figure 2. The NPY Fusion Construct is Expressed in Insulin Granules.
(A) Confocal images of intact human islets infected with NPYpHluorin, immunostained for GFP (green) and insulin (left panel, red), somatostatin (middle, red) or glucagon (right, red). The cell nuclei are shown in blue (DAPI labeled). Scale bars = 10 µm. (B) Quantification of the fraction of GFP-positive cells that also contain either insulin (Ins), somatostatin (Soma) or glucagon (Glu). Shown are the mean ± SEMs, n = 5 islets.
We demonstrated that pHluorin is an effective pH sensor, as increasing the intracellular pH with 50 mM NH4Cl resulted in a >500% increase in the intracellular fluorescence (Figure 3A, 3B, Video 1). As the pH inside the granule increases upon fusion with the plasma membrane and the opening of the fusion pore, granules containing NPY-pHluorin become visible upon conditions that stimulate insulin exocytosis such as membrane depolarization with KCl (Figure 4, Video 2).
Video 1. NPY-pHluorin Adenovirus Efficiently Infect Islet Cells and Sense pH Changes.
Mouse islets were infected for 4 days with adenovirus encoding NPY-pHluorin. Infected islets were placed on a coverslip and mounted on the imaging chamber. To increase intracellular pH, the islets were perfused with 50 mM NH4Cl added to the extracellular solution containing 3 mM glucose. A fast and strong increase in fluorescence can be seen upon NH4Cl application. Scale bar = 100 µm. Movie speed = 5 fps. Total duration of the movie is 90 s.
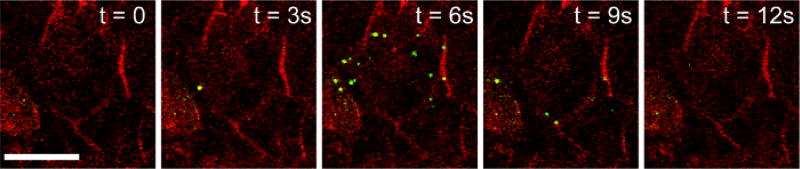
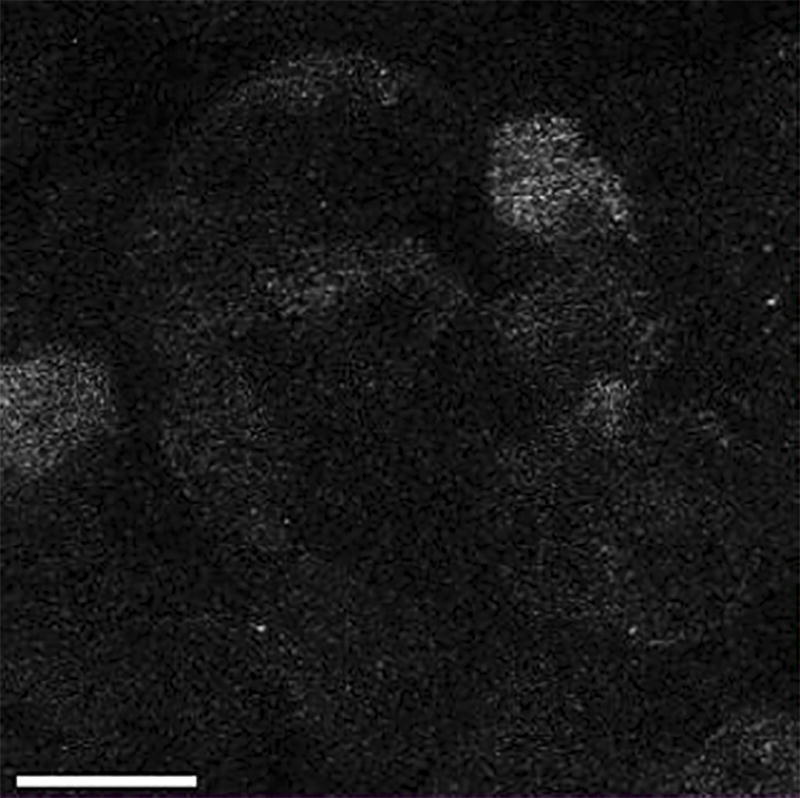
Figure 4. Granules Containing NPY-pHluorin Become Visible Once They Fuse with the Plasma Membrane and Open.
Human isletsinfected with NPY-pHluorin adenovirus were incubated with the plasma membrane dye di-8-ANEPP (red). Islet stimulation with KCl (30 mM) triggered a sudden and transient appearance of fluorescent granules at the cell surface (shown in green). Confocal images of cells within a human islet are shown at before the exocytotic response (t = 0), during (t =3 – 9 s) and after (t = 12 s). Contrast was adjusted to remove background green fluorescence. Scale bar = 20 µm.
Video 2. Granules Containing NPY-pHluorin Become Visible Once They Fuse with the Plasma Membrane and Open.
Human islets infected with NPY-pHluorin adenovirus were incubated with the plasma membrane dye di-8-ANEPP. Islet stimulation with KCl (30 mM) triggered a sudden and transient appearance of fluorescent granules at the cell surface. A maximal projection of confocal planes is shown. Contrast was adjusted to remove background green fluorescence. Scale bar = 20 µm. Movie speed = 5 fps. Total duration of the movie is 60 s.
Single secretory events in beta cells within intact islets can be visualized with confocal time-lapse imaging of NPY-pHluorin infected islets. These occur in response to direct membrane depolarization with KCl or several other physiological stimuli (Figure 4 and Figure 7, see below). In the basal extracellular glucose concentration (3 mM), little secretory activity is observed. However, granule fusion with the plasma membrane is triggered in response to stimulation with high glucose (16 mM) (Figure 5A, Video 3). By placing the ROIs with different sizes and in different areas, the response kinetics of secretory granules can be compared with that of individual cells or groups of cells in close proximity (cluster) (Figure 5B). This type of analysis enabled determining that: 1) the individual secretory events are very synchronized and secreted within a few seconds from the average cell response, and 2) the beta cells in close proximity form functional clusters of synchronized activity.
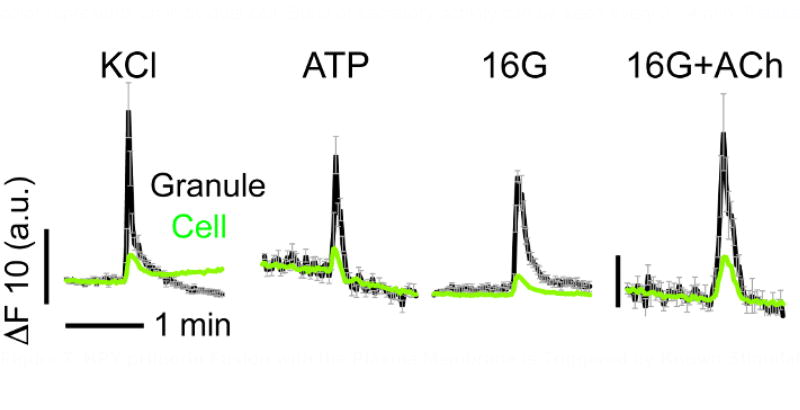
Figure 7. NPY-pHluorin Fusion with the Plasma Membrane is Triggered by Known Stimulators of Insulin Secretion.
Traces showing changes in mean fluorescence intensity (arbitrary units) in ROIs placed around individual secretory events in cells within intact human islets induced by KCl (30 mM), ATP (10 µM), high glucose (16G, 16 mM) and acetylcholine (Ach, 10 µM). KCl and ATP were applied in the basal glucose concentration (3 mM). Black lines show the average granule and gray lines reflect SEM values. Green traces show the corresponding cell response. Horizontal time scale (2 min) applies to all graphs.
Video 3. High Glucose Triggers Exocytosis of Granules Containing NPY-pHluorin.
Fusion of NPY-pHluorin containing granules is triggered in response to stimulation with high glucose (16 mM). In basal extracellular glucose concentration (3 mM), little secretory activity is observed. However, upon high glucose, insulin granules transiently appear at the plasma membrane of different cells in an islet in a synchronized fashion. High glucose was applied after ~ 2.5 min in 3G (16G label appears). A maximal projection of confocal planes is shown. Scale bar = 20 µm. Movie speed = 10 fps. Total duration of the movie is 7 min.
Insulin secretion in the body occurs in a pulsatile manner. Stimulating the NPY-pHluorin-infected islets with elevated glucose concentration for prolonged periods (15 – 20 min) gave rise to multiple pulses (or bursts) of secretion (Figure 6, Video 4). During each pulse, individual granules fuse with the plasma membrane in a synchronized fashion as shown before (Figure 5). Bursts of secretory activity can be seen every 3 – 4 min.
Video 4. Prolonged Stimulation with High Glucose Triggers Several Bursts of Exocytosis.
Prolonged stimulation with high glucose gives rise to multiple pulses of secretion islets, during which granules transiently appear. Pulses of secretory activity can be seen every ~ 3 – 4 min. Confocal plane of a human islet is shown. A pseudocolor scheme was applied for fluorescence intensity. Scale bar = 20 µm. Movie speed = 30 fps. Total duration of the movie is 20 min.
Transient increases in fluorescence in NPY-pHluorin containing granules could also be observed in response to the purinergic agonist ATP (10 µM) or the muscarinic agonist acetylcholine (ACh, 10 µM) (Figure 7), both of which are known stimulators of insulin secretion in human islets. As expected, the membrane depolarization with KCl (30 mM) also triggered insulin granule exocytosis (Figure 5). Several stimuli can be applied to the same islet preparation as long as the islets are washed well in between stimuli. Make sure to alter the order of application when repeating the experiment.
Discussion
This manuscript describes a technique that can be used to visualize exocytosis of insulin granules in beta cells within intact pancreatic islets by confocal microscopy. It uses NPY-pHluorin as the fluorescent reporter cloned into adenovirus to ensure a high transfection efficiency.
Although the method was highly efficient in our hands, it might require some modifications that primarily depend on two parameters: 1) the quality of islet preparation, and 2) the titer of viral stock with optimization of infection conditions. Prior to the viral infection of islets, allow the islet preparation to recover from isolation and transportation stress overnight at 37 °C. Inspect the islet morphology under a light microscope throughout the infection/expression period and check for signs of decreased viability such as cells protruding from the islet surface or presence of hypoxic cells (darker cells) in the islet center18. Each viral stock may vary in titer and infection efficiency. Make sure to adjust the volume of the virus used according to the viral titer and the time of culture prior to the microscopy assay. The most critical step is the optimization of the infection conditions (e.g., number of viral particles, culture time before assay) so that enough of the reporter is expressed and the islet integrity and responsiveness are maintained. When optimizing the infection protocol and with each new viral batch, collect a few islets at different time points and check whether exocytosis can be elicited with KCl (30 mM) and/or high glucose (16 mM). We found that the assay works better and islet cells remain healthy and glucose responsive when the MOI is low (around 2) and the culture time is extended (around 5 days) to increase the expression levels of the fusion construct. Our results are in line with a previous study showing that efficient expression of gene products can be better achieved at lower transfecting concentrations of the adenoviral vector while preserving islet function19.
The technique has, however, some limitations. One is that by using NPY-pHluorin as a reporter, granules cannot be visualized before they fuse with the plasma membrane and the fusion pore opens. To study insulin granule trafficking instead of exocytosis, adenovirus encoding NPY-eGFP can be used instead. Another limitation is that, to a lower extent, NPY-pHluorin can be targeted to non-insulin containing granules. However, the majority of the cells that were infected with NPY-pHluorin also expressed insulin and less than 10% of the cells expressed glucagon or somatostatin. Indeed, the glucose dependency of granule secretion, its pulsatile pattern and co-localization with insulin, indicated that most secretory events represent cargo release from insulin granules. To guarantee monitoring of insulin exocytosis is occurring, the assay should be performed under conditions that stimulate insulin secretion (e.g., high glucose). Nevertheless, to improve the specificity of beta cell expression, NPY-pHluorin could be put under the control of a more selective promoter such as the rat insulin promoter. Another limitation of the technique is that it requires expression of exogenous proteins in insulin granules, which can perturb their biogenesis and/or behavior20. This should be considered when interpreting the results of the experiments and, if possible, the conclusions should be validated using other insulin granule cargo proteins (e.g., C-peptide, islet amyloid polypeptide) as reporters.
This protocol also recommends including cAMP-raising agents (IBMX and forskolin) to potentiate the effects of high glucose on insulin granule exocytosis. This stimulation protocol mimics the activation of physiologically relevant amplifying pathways (triggered by incretins or paracrine and neural signaling molecules) that potentiate insulin secretion by increasing cAMP in the pancreatic beta cells. Nevertheless, in the absence of cAMP raising agents, high glucose (16 mM) also elicits granule exocytosis but the number of secretory events observed is lower. Granules still appear in discrete bursts, are synchronized with the average cell response, and show similar burst periods: 1.4 – 6.6 min in high glucose plus IBMX/forskolin vs. 1.5 – 10 min in high glucose alone.
The assay described here is significant with respect to existing methods as it has sufficient spatial and temporal resolution to visualize single fusion events in real time in beta cells within intact islets. For instance, it revealed the high synchronization with which insulin granules are released in human islets upon glucose stimulation. Moreover, if islets are well attached to the coverslip, they can be imaged for prolonged periods (at least 15 – 20 min). This was used to show that in human islets exocytosis occurs in distinct bursts that appear in periods similar to those of pulsatile in vivo insulin secretion. The assay also allows for testing the effect of several stimuli on insulin exocytosis using murine or human islets. Furthermore, because it does not require islet cell dissociation like other methods, it can be applied to study the behavior of beta cell populations within an islet. Indeed, we observed that neighboring beta cells form clusters of ~ 5 – 10 cells in which activity is synchronized. Beta cells from the same cluster are probably coupled by gap junction channels made of connexin 3621. Their synchronized response illustrates the beta cell connectivity that is essential for proper insulin secretion22.
In the future, this technique can be combined with other fluorescent dyes for functional imaging to visualize in real time how changes in cytosolic calcium or membrane potential, for instance, correlate with granule exocytosis in beta cells. In particular, as new pH sensitive red fluorescent proteins are becoming available14,23 and can be fused to NPY or other insulin granule cargo proteins, green dyes for functional imaging can be used. Furthermore, this technique can be combined with a mouse model for in vivo imaging of vascularized islets transplanted into the anterior chamber of the mouse eye24. Islets can be infected with adenoviruses 48 – 72 h before transplantation into the eye. Immune deficient mice should be used if human islets are transplanted. Using this model, the subcellular spatial pattern of insulin granule secretion can be correlated with vascular arrangements25.
Acknowledgments
The authors thank Marcia Boulina from the DRI imaging core facility for help with the microscopes. This work was supported by NIH grants 1K01DK111757-01 (JA), F31668418 (MM), R01 DK111538, R33 ES025673 and R56 DK084321 (AC).
Footnotes
Video Link
The video component of this article can be found at https://www.jove.com/video/56089/
Disclosures
The authors declare they have no competing financial interests.
References
- 1.Roder PV, Wong X, Hong W, Han W. Molecular regulation of insulin granule biogenesis and exocytosis. Biochem J. 2016;473(18):2737–2756. doi: 10.1042/BCJ20160291. [DOI] [PubMed] [Google Scholar]
- 2.Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A. Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem J. 2015;466(2):203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 3.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297(5585):1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron. 2012;73(1):121–134. doi: 10.1016/j.neuron.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michael DJ, Xiong W, Geng X, Drain P, Chow RH. Human insulin vesicle dynamics during pulsatile secretion. Diabetes. 2007;56(5):1277–1288. doi: 10.2337/db06-0367. [DOI] [PubMed] [Google Scholar]
- 7.Ohara-Imaizumi M, et al. Monitoring of exocytosis and endocytosis of insulin secretory granules in the pancreatic beta-cell line MIN6 using pH-sensitive green fluorescent protein (pHluorin) and confocal laser microscopy. Biochem J. 2002;363(Pt 1):73–80. doi: 10.1042/0264-6021:3630073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whim MD. Pancreatic beta cells synthesize neuropeptide Y and can rapidly release peptide co-transmitters. PLoS One. 2011;6(4):e19478. doi: 10.1371/journal.pone.0019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuboi T, Rutter GA. Multiple forms of "kiss-and-run" exocytosis revealed by evanescent wave microscopy. Curr Biol. 2003;13(7):563–567. doi: 10.1016/s0960-9822(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhu D, et al. Synaptotagmin I and IX function redundantly in controlling fusion pore of large dense core vesicles. Biochem Biophys Res Commun. 2007;361(4):922–927. doi: 10.1016/j.bbrc.2007.07.083. [DOI] [PubMed] [Google Scholar]
- 11.Aoki R, et al. Duration of fusion pore opening and the amount of hormone released are regulated by myosin II during kiss-and-run exocytosis. Biochem J. 2010;429(3):497–504. doi: 10.1042/BJ20091839. [DOI] [PubMed] [Google Scholar]
- 12.Felmy F. Modulation of cargo release from dense core granules by size and actin network. Traffic. 2007;8(8):983–997. doi: 10.1111/j.1600-0854.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 13.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394(6689):192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 14.Gandasi NR, et al. Survey of Red Fluorescence Proteins as Markers for Secretory Granule Exocytosis. PLoS One. 2015;10(6):e0127801. doi: 10.1371/journal.pone.0127801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almaca J, et al. Spatial and temporal coordination of insulin granule exocytosis in intact human pancreatic islets. Diabetologia. 2015;58(12):2810–2818. doi: 10.1007/s00125-015-3747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do OH, Low JT, Thorn P. LepRdb) mouse model of type 2 diabetes: pancreatic islet isolation and live-cell 2-photon imaging of intact islets. J Vis Exp. 2015;(99):e52632. doi: 10.3791/52632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna ST, et al. Kiss-and-run exocytosis and fusion pores of secretory vesicles in human beta-cells. Pflugers Arch. 2009;457(6):1343–1350. doi: 10.1007/s00424-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 18.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber M, et al. Adenoviral transfection of isolated pancreatic islets: a study of programmed cell death (apoptosis) and islet function. J Surg Res. 1997;69(1):23–32. doi: 10.1006/jsre.1997.4995. [DOI] [PubMed] [Google Scholar]
- 20.Michael DJ, et al. Fluorescent cargo proteins in pancreatic beta-cells: design determines secretion kinetics at exocytosis. Biophys J. 2004;87(6):L03–05. doi: 10.1529/biophysj.104.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serre-Beinier V, et al. Cx36 makes channels coupling human pancreatic beta-cells, and correlates with insulin expression. Hum Mol Genet. 2009;18(3):428–439. doi: 10.1093/hmg/ddn370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutter GA, Hodson DJ. Beta cell connectivity in pancreatic islets: a type 2 diabetes target? Cell Mol Life Sci. 2015;72(3):453–467. doi: 10.1007/s00018-014-1755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Rosendale M, Campbell RE, Perrais D. pHuji, a pH-sensitive red fluorescent protein for imaging of exo- and endocytosis. J Cell Biol. 2014;207(3):419–432. doi: 10.1083/jcb.201404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speier S, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14(5):574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low JT, et al. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia. 2014;57(8):1655–1663. doi: 10.1007/s00125-014-3252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]