Abstract
Purpose
Currently, in clinical practice of intensity-modulated proton therapy (IMPT), the influence of the minimum monitor unit (MU) constraint is taken into account through post-processing after the optimization is completed. This may degrade the plan quality and plan robustness. This study aims to mitigate the impact of the minimum MU constraint directly during the plan robust optimization.
Methods and Materials
Cao et al. have demonstrated a two-stage method to account for the minimum MU constraint using linear programming without the impact of uncertainties considered. In this study, we took the minimum MU constraint into consideration using quadratic optimization and simultaneously had the impact of uncertainties considered using robust optimization. We evaluated our method using 7 cancer patients with different machine settings.
Result
The new method achieved better plan quality than the conventional method. The D95% of the clinical target volume (CTV) normalized to the prescription dose was (mean [min max]): (99.4% [99.2%–99.6%]) vs. (99.2% [98.6%–99.6%]). Plan robustness derived from these 2 methods was comparable. For all 7 patients, the CTV dose-volume histogram band gap (narrower band gap means more robust plans) at D95% normalized to the prescription dose was (mean [min max]): (1.5% [0.5%–4.3%]) vs. (1.2% [0.6%–3.8%]).
Conclusion
Our new method of incorporating the minimum MU constraint directly into the plan robust optimization can produce machine-deliverable plans with better tumor coverage while maintaining high plan robustness.
Keywords: intensity-modulated proton therapy (IMPT), minimum MU constraint, L-BFGS-B, deliverable robustness, quadratic optimization
1. Introduction
Intensity-modulated proton therapy (IMPT) is receiving increased attention in cancer care for its ability to better spare normal tissue compared with intensity-modulated radiation therapy (IMRT) and the passive scattering proton therapy.1–3 In IMPT, intensities of beamlets are optimized using complicated computer algorithms to deposit adequate tumoricidal dose to targets while depositing as little dose as possible to nearby normal tissues.4
IMPT is precise but is sensitive to uncertainties caused by patient setup and range uncertainties, respiratory motion, and anatomic changes.5, 6 Several robust optimization methods have been proposed and have been shown to have great advantages by achieving robust plans while maintaining high plan quality.2, 7–25 Unfortunately in proton therapy besides uncertainties, there are machine hardware constraints, for example, field size constraint, minimum/maximum energy constraint, dose rate constraint for synchrotron-based proton therapy systems, and minimum/maximum monitor unit (MU) constraint. Among them, the minimum MU constraint is the most prominent one.26 The minimum MU constraint in proton therapy is mostly due to the minimum charge needed for the spot profile monitor to work properly. Therefore a small number of protons cannot be delivered reliably, leading to the minimum MU constraint for proton machines.
To date, however, all published robust optimization methods in IMPT ignored the effect of the minimum MU constraint during optimization. To meet the minimum MU constraint, many clinical practices still use the expedient of post-processing procedure provided by the current commercial treatment planning systems (TPS). This is a procedure that modifies the intensity map to meet the minimum MU constraint after optimization is completed. Different methods are used for post-processing. A simple one is to round up/down the spots which have intensities lower than minimum MU.27, 28 This post-processing procedure can introduce significant distortions of the dose distributions, thus degrading plan quality.26 Recently, Lin et al proposed a greedy reassignment method, which reassigned the removed spot’s intensity to its neighbor spots in order to maintain a less distorted dose distribution.29 Compared to the round up/down method, they better mitigated the impact of the minimum MU constraint.
There is a long history in photon therapy to account for the hardware constraints in treatment planning. IMRT uses multiple leaf collimators (MLCs) to form some very complicated dose distributions. Currently, most IMRT plans still need a conversion process (using a leaf-sequencing algorithm) after a fluence map optimization. This conversion process is very similar to the post-processing used in IMPT to make the IMPT plans deliverable (as discussed before). Due to the hardware constraints of MLC, plan quality degradation could happen in this conversion process for IMRT. Therefore the regularization method has been proposed in IMRT to make the optimized fluence map smoother since “smooth profiles are easier to convert into step-and-shoot segments than jagged profiles”30. In addition, non-uniform fluence map usually leads to dose distributions less robust to geometric uncertainties31 and reduces the delivery efficiency32 in IMRT. Several regularization methods have been proposed including variational methods33–36, filtering methods36–39, and iterative methods30. Alternatively, people have developed the direct machine optimization methods (DMPO) to incorporate machine constraints directly in the IMRT optimization so that the distortion to the optimized dose distribution caused by the conversion process can be avoided.40, 41, 42
The minimum MU constraint in proton therapy restricts spot intensities to be either zero or no smaller than the minimum MU, which cannot be solved by smoothing the fluence map as the regularization method does for IMRT. Some methods with the minimum MU constraint considered directly during the plan optimization have also been proposed in proton therapy. Cao et al.43 proposed one method composed of 2 optimization stages using linear programming. In the first stage, they performed an optimization to find an intermediately optimized IMPT plan without the minimum MU constraint considered. In the second stage, they performed a boundary-constrained optimization, in which the optimizer would enforce lower bound constraints for every beamlet that has positive intensity after the first stage. The limitations of this methodology are that plan robustness is not considered and the linear programming is not compatible with the existing commercial TPS. The commercial TPS for proton therapy, Raystation™ (RaySearch Laboratories), has the minimum MU constraint considered in the optimization. In the newest version of Eclipse™, the minimum MU constraint have also been considered in the new optimization algorithm (nonlinear proton optimizer (NUPO))44 via two-phase optimization strategy. In the first phase, optimal fluences are derived with respect to user-defined objectives. In the second phase, the optimal fluences are converted into deliverable spots, in which spots with smaller intensities are gradually discarded and the left spots are re-optimized. However, this method cannot be straightforwardly integrated into the most popular optimization algorithm of the Eclipse™ (proton convolution superposition optimizer (PCS)), which directly optimizes the spots’ intensities.
In this study, we integrated the minimum MU constraint into robust optimization for IMPT treatment planning based on the conventional quadratic programming. We developed a new robust optimization algorithm and used L-BFGS-B optimizer45, 46 to incorporate the minimum MU constraint into robust optimization by turning the boundary constraints on and off alternatingly during optimization, so that the algorithm will dynamically apply the boundary constraints to determine whether the intensity of one spot should be either zero or above the minimum MU on the fly. Thus, the optimization explored the full solution domain and led to a better deliverable robust plan compared to the post-processing method. We further assessed the effectiveness of our new planning method by comparing plan quality and robustness of IMPT plans for 7 patients with different spot size and different minimum MU constraint to those achieved with the use of the conventional robust optimization method.
2. Methods
In this study, IMPT plans were retrospectively generated for 7 patients with real CT images and clinical structures using both the conventional and new optimization methods with 2 different machine settings: 1) for a machine with an in-air, energy-dependent, large spot size at isocenter (σ: 5–15 mm) and spacing (1.3σ), and 2) for a small spot size (σ: 2–6 mm) and fixed spacing (5 mm). One MU is defined according to the IAEA TRS 398 during our machine commissioning. The minimum MU constraint was 0.003 MU for the small spot machine and 0.005 MU for the large spot machine. For each patient, 3 plans were generated: the conventional non-deliverable robust plan (plan A), the conventional deliverable robust plan using post-processing (plan B), and our new deliverable robust plan (plan C). They all use the same objectives and stopping criteria. To illustrate the capability of our proposed method more clearly, we also did the comparison with artificially large (10 times larger than realistic values) minimum MU setting.
2.1. Conventional robust optimization without the minimum MU constraint considered
Robust optimization methods can be implemented by different models: for example, a probabilistic robust optimization model,12, 13 an objective-wise min-max robust optimization model,8 a multiple-criteria robust optimization model,7 or a voxel-wise worst-case robust optimization model.9, 11, 47 The worst-case robust optimization model has been verified to simultaneously improve robustness and plan quality, particularly in terms of sparing normal tissues, compared with the conventional planning target volume (PTV) methods.22, 48, 49 For brevity, hereafter we will use the term robust optimization to refer to voxel-wise worst-case robust optimization.
In robust optimization, the setup uncertainty and range uncertainty are considered in the optimization process, as shown in the following formula:
Where p denotes the penalty weight of the corresponding term and D0 denotes the prescribed dose for the corresponding region of interest (ROI). The Heaviside function, H(Di − D0), is defined conventionally (ie, its value is unity if Di > D0 but zero if Di ≤ D0). The terms Di,min = minm( ) and Di,max = maxm( ) respectively indicate the minimum and maximum dose among possible doses in voxel i (m=1…9 here, is the index of the corresponding uncertainty scenario with setup and range uncertainties considered), which are calculated using in each iteration. The mth influence matrix incorporates range and setup uncertainties. ωj2 is the non-negative intensity map. Dose volume constraints (DVCs) were used following the method proposed by Wu and Mohan50. In the DVH curves, the DVCs to limit the hot spots in the DVH can be specified as: i.e., the volume receiving dose greater than should be less than Then another dose value can be derived so that in the current DVH, . Then the part in the objective function related to DVC can be written as:
Only voxels with between and are penalized. The ellipsis means that additional DVC for the target (also normal tissues) can be specified to have more complete control of the shape of the DVH curves. Similar concepts apply to the DVC to limit the cold spots in the DVH of the targets that can be used. For details of the optimization algorithm, please refer to Liu et al10, 51. The algorithm for robust optimization is shown in Figure 1 (left). In this treatment-planning process, no minimum MU constraints are considered. To make the derived robust plan deliverable, a post-processing procedure (discussed below) is needed.
Figure 1.
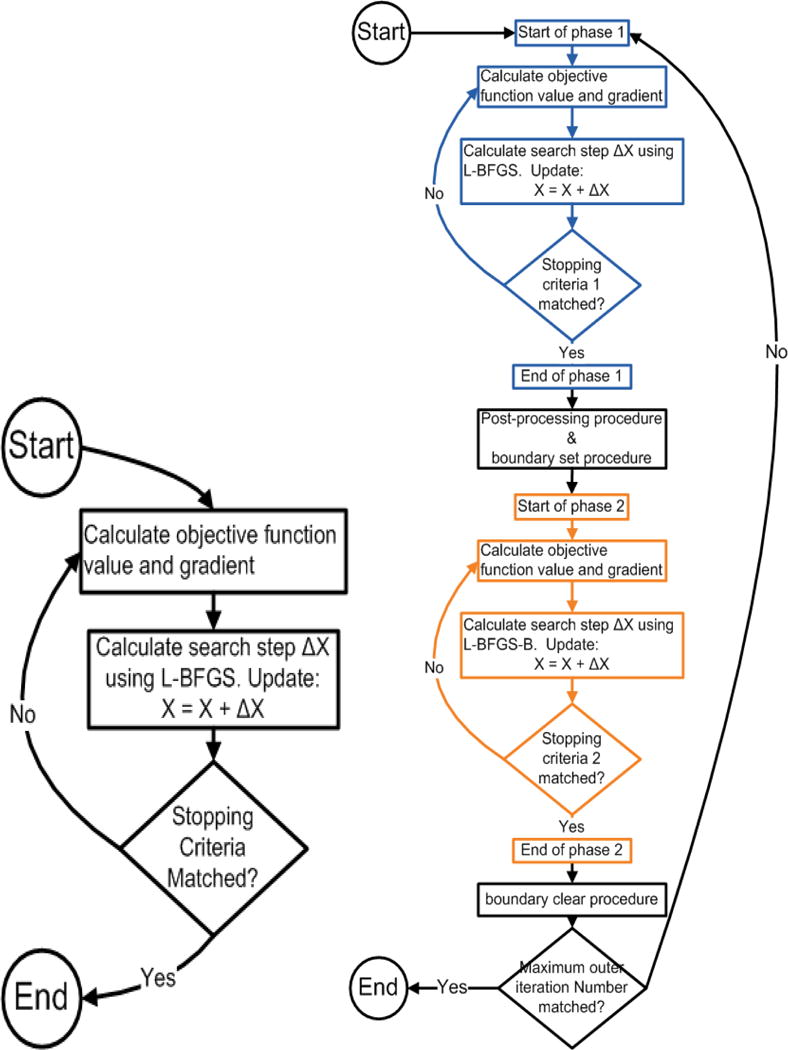
Algorithm diagrams for the conventional robust optimization (left) and the proposed deliverable robust optimization (right). L-BFGS indicates limited-memory Broyden-Fletcher-Goldfarb-Shanno algorithm.
During post-processing, the system would discard any beamlets with intensities smaller than half of the minimum MU and round the intensities of any beamlets with intensities equal to or larger than half the minimum MU up to the minimum MU. After this procedure, the solution might possibly deviate from the optimal solution. The extent of the deviation depends on the magnitude of the minimum MU constraint and the number of beamlets that are post-processed.
2.2. Robust optimization with the minimum MU constraint considered
The minimum MU constraint is a lower boundary constraint on the intensity of every beamlet and depends on the proton scanning beam delivery machine. For example, the proton scanning beam machine in our clinic has a minimum MU constraint of 0.003 MU. Thus, the deliverable beamlet intensity is either 0 or ≥0.003 MU. The flexibility to have zero intensity for some beamlets is important because it allows optimizers to turn off a beamlet if it is not needed. However, it introduces a discontinuous feasible region to each beamlet: {0}∪[minimum MU, ∞). In quadratic programming, no optimizers can work on this feasible region directly. In order to be consistent with current practice, it is important to address this issue in the context of quadratic programming. L-BFGS is an efficient quasi-Newton method to solve an optimization problem with a quadratic objective function with low memory overhead. The L-BFGS-B version can apply boundary constraints for its solution variables. Unfortunately, it cannot handle the discontinuous feasible region problem either. To address this problem, we developed a new robust optimization algorithm to dynamically set up the boundary constraints during the optimization process by iteratively turning the boundary constraints on and off. This novel method can effectively find an optimized solution in this discontinuous feasible region composed of zero and values no smaller than the minimum MU constraint.
In our new approach, the form of the objective function remains the same as the conventional robust method. The algorithm for deliverable robust optimization is shown in Figure 1 (right). More details about the algorithm will be discussed in Sec. 2.3.
The optimization is based on the in-house developed analytical dose engine10, 51, using real CT images and clinical structures. Actually, this in-house developed TPS had been fully validated and clinically commissioned for our proton machines. We had successfully used this TPS to treat 5 complicated patients, including lung, head and neck, and central nervous system cancers (the final dose calculation was done after optimization still using a commercial TPS)52, and regularly used it as secondary MU check for our commercial TPS. The TPS is parallelized using MPI and OpenMP and running on the in-house high-performance computing cluster utilizing 48 CPU cores. Each core is Intel Xeon E5-4650 v2 CPUs at 2.40GHz.
2.3. Algorithms and procedures
2.3.1. Algorithms
As shown in Figure 1, in the conventional robust optimization, optimization is performed in one level of loop. When the maximum loop number or other optimization termination criterion is reached, the optimization will terminate. In our deliverable robust optimization, optimization is performed in 2 levels of loop: the outer loop and the inner loop. For every outer loop, 2 phases of the inner loop are performed. Phase 1 of the inner loop is performed without boundary constraints, and phase 2 of the inner loop is performed with boundary constraints. Whether to apply the minimum MU constraint for each beamlet in phase 2 is determined by the results of phase 1.
2.3.2. Procedure
2.3.2.1. Post-processing procedure
For every beamlet that has intensity ωj2 equal to or larger than half the minimum MU, its intensity is set to max (ωj2, minimum MU); otherwise, its intensity is set to zero.
2.3.2.2. Boundary set procedure
For every beamlet that has intensity ωj2 equal to or larger than half the minimum MU, a lower boundary constraint with the value of the minimum MU will be applied; otherwise, this beamlet is turned off in the following optimization.
2.3.2.3. Boundary clear procedure
During this step, the boundary constraints for all beamlets, regardless of their intensity, are cleared for the subsequent optimization. All beamlets will be available for the subsequent optimization.
2.4. Patient data and evaluation
We tested our method using 7 patients: 1 with prostate cancer (prescription dose: 76 Gy[RBE]) (RBE indicates Relative Biological Effectiveness), 3 with head-neck cancer (prescription dose: 66 Gy[RBE], 66Gy[RBE], and 60 Gy[RBE]), and 3 with lung cancer (prescription dose: 74 Gy[RBE], 66 Gy[RBE], and 66 Gy[RBE]), as shown in Table 1. For each patient, we generated 3 plans based on 2 optimization methods: plan A is generated by the conventional robust optimization method without the post-processing procedure. It contains intensities of beamlets smaller than the minimum MU. Plan B is derived from plan A using the post-processing procedure. It represents the current routine practice in the clinic. Plan C is generated by the new deliverable robust optimization method. We compared plan quality and robustness among these 3 plans. The proposed method aims to maintain the plan quality and robustness by taking into account the minimum MU constraint.
Table 1.
Patient Treatment Configurations. Energy layers are carefully selected in machine commissioning so that roughly the proton beam range difference between adjacent energy layers is 2 mm in low energy region and 5 mm in high energy region to minimize the ripples in the dose distribution in the beam direction.
| Pt | Cancer | Field | No. of Beamlets | No. of Energy Layers | Energy Range, MeV | Spot Spacing, mm | Minimum MU |
|---|---|---|---|---|---|---|---|
| 1 | Prostate | 1 | 748 | 19 | 148.85–190.55 | Energy-dependent spacing (1.3σ) | 0.005 |
| 2 | 750 | 19 | 148.85–190.55 | ||||
| 2 | Head and neck | 1 | 1,216 | 20 | 159.55–206.35 | Energy-dependent spacing (1.3σ) | 0.005 |
| 2 | 1,347 | 29 | 141.65–206.35 | ||||
| 3 | 1,326 | 25 | 146.95–203.75 | ||||
| 3 | Head and neck | 1 | 967 | 40 | 72.55–131.05 | Energy-dependent spacing (1.3σ) | 0.005 |
| 2 | 1,114 | 42 | 83.15–144.95 | ||||
| 3 | 1,056 | 46 | 72.55–141.65 | ||||
| 4 | Head and neck | 1 | 7,625 | 45 | 78.5–139.1 | 5 | 0.003 |
| 2 | 5,133 | 65 | 91.7–185.2 | ||||
| 3 | 3,243 | 37 | 78.5–129.699 | ||||
| 4 | 5,841 | 33 | 80.3–126 | ||||
| 5 | Lung | 1 | 3,075 | 49 | 72.55–122.55 | Energy-dependent spacing (1.3σ) | 0.005 |
| 2 | 3,142 | 49 | 72.55–122.55 | ||||
| 3 | 3,600 | 67 | 72.55–155.35 | ||||
| 4 | 3,657 | 53 | 72.55–129.249 | ||||
| 6 | Lung | 1 | 14,791 | 56 | 71.3–147 | 5 | 0.003 |
| 2 | 9,557 | 69 | 91.7–191.3 | ||||
| 3 | 15,061 | 58 | 80.3–156.7 | ||||
| 7 | Lung | 1 | 12,294 | 68 | 86.9–183.3 | 5 | 0.003 |
| 2 | 12,391 | 73 | 91.7–200.4 | ||||
| 3 | 16,024 | 58 | 71.3–149.2 |
Abbreviation: Pt, patient.
We do the normalization by dividing the doses with the prescription dose so that different cases with different prescription doses can be compared directly. The following dosimetric parameters were used to compare plan quality and robustness: for any structure, the minimum dose that covers 95% volume of the structure with the highest dose (D95%); the minimum dose that covers 25% volume of the ROI (D25%) with the highest dose; the minimum dose that covers 5% volume of the ROI (D5%) with the highest dose; the minimum dose that covers 1% volume of the ROI (D1%) with the highest dose; and the normalized volume as a percentage of the ROI that received at least 20 Gy[RBE] or 35 Gy[RBE] (V20, V35).
2.5. Robustness quantification
To evaluate or compare the robustness of IMPT plans, we used a robustness quantification technique that displayed the envelope of all dose-volume histograms in band graphs of the 9 dose distributions associated with the corresponding range or setup uncertainties. The width of the band is used to indicate the plan robustness between the competing plans. This robust quantification technique is effective for determining an IMPT plan’s sensitivity to setup and range uncertainties.10, 51, 53
3. Results
3.1. Comparison of plan qualities
3.1.1. Comparison of nominal scenario plan qualities
Plan A is the plan without the minimum MU constraint considered. We performed the optimization with the minimum MU as 0.003 and 0.005 MU for the small and large spot machines, respectively. Consistent conclusions are derived from the results of 7 patients with different machines. Results are shown in Table 2.
Table 2.
Results for All 7 Patientsa
| Pt | Statistic | Plan A | Plan B | Plan C |
|---|---|---|---|---|
| 1 | CTV D95% | 0.996 (0.991–0.996) | 0.996 (0.990–0.996) | 0.996 (0.991–0.996) |
| CTV D5% | 1.009 (1.006–1.010) | 1.009 (1.006–1.010) | 1.009 (1.006–1.010) | |
| Bladder D25% | 0.059 (0.017–0.137) | 0.059 (0.017–0.137) | 0.059 (0.016–0.137) | |
| Rectum D25% | 0.360 (0.170–0.632) | 0.360 (0.169–0.634) | 0.356 (0.172–0.637) | |
| 2 | CTV D95% | 0.993 (0.961–0.993) | 0.986 (0.954–0.987) | 0.993 (0.960–0.993) |
| CTV D5% | 1.040 (1.036–1.046) | 1.036 (1.030–1.041) | 1.039 (1.037–1.046) | |
| Brainstem D1% | 0.755 (0.639–0.848) | 0.751 (0.634–0.842) | 0.761 (0.646–0.851) | |
| Brain D5% | 0.446 (0.397–0.506) | 0.442 (0.393–0.502) | 0.448 (0.399–0.511) | |
| 3 | CTV D95% | 0.992 (0.953–0.992) | 0.990 (0.952–0.990) | 0.992 (0.949–0.992) |
| CTV D5% | 1.059 (1.056–1.069) | 1.058 (1.054–1.067) | 1.059 (1.056–1.070) | |
| Brain D5% | 0.662 (0.599–0.702) | 0.660 (0.597–0.700) | 0.661 (0.596–0.701) | |
| Optic chiasm D1% | 0.998 (0.976–1.013) | 0.995 (0.974–1.010) | 1.001 (0.979–1.018) | |
| 4 | CTV D95% | 0.996 (0.989–0.996) | 0.992 (0.987–0.992) | 0.996 (0.989–0.996) |
| CTV D5% | 1.021 (1.016–1.023) | 1.018 (1.014–1.020) | 1.023 (1.018–1.028) | |
| Spinal cord D1% | 0.311 (0.269–0.364) | 0.309 (0.266–0.361) | 0.316 (0.271–0.368) | |
| Brain D5% | 0.093 (0.062–0.118) | 0.092 (0.061–0.116) | 0.096 (0.063–0.127) | |
| 5 | CTV D95% | 0.994 (0.974–0.994) | 0.988 (0.971–0.988) | 0.994 (0.974–0.994) |
| CTV D5% | 1.044 (1.035–1.051) | 1.041 (1.032–1.048) | 1.045 (1.036–1.051) | |
| Right lung V20 | 0.289 (0.266–0.315) | 0.288 (0.265–0.313) | 0.298 (0.274–0.324) | |
| Spinal cord D1% | 0.029 (0.023–0.065) | 0.029 (0.023–0.065) | 0.030 (0.024–0.065) | |
| 6 | CTV D95% | 0.994 (0.981–0.994) | 0.992 (0.981–0.992) | 0.994 (0.979–0.994) |
| CTV D5% | 1.050 (1.024–1.060) | 1.049 (1.023–1.058) | 1.054 (1.027–1.057) | |
| Lung V20 | 0.341 (0.321–0.362) | 0.342 (0.322–0.362) | 0.341 (0.322–0.362) | |
| Heart V35 | 0.121 (0.101–0.144) | 0.121 (0.101–0.144) | 0.121 (0.101–0.144) | |
| 7 | CTV D95% | 0.996 (0.991–0.996) | 0.996 (0.991–0.996) | 0.996 (0.989–0.996) |
| CTV D5% | 1.012 (1.005–1.012) | 1.012 (1.006–1.013) | 1.017 (1.007–1.017) | |
| Total lung V20 | 0.281 (0.270–0.290) | 0.281 (0.270–0.290) | 0.285 (0.275–0.292) | |
| Spinal cord D1% | 0.258 (0.227–0.348) | 0.259 (0.224–0.350) | 0.240 (0.219–0.351) |
Abbreviations: CTV, clinical target volume; DX%, the minimum normalized dose that covers X% of the region of interest with the highest dose; MU, monitor unit; Pt, patient; VX, the normalized volume as a percentage of the region of interest that received at least X Gy[RBE].
Values are norminal (min-max values of uncertainty scenarios).
Figure 2 shows the results for 3 patients (patient #1, #2, and #5) with two sets of minimum MU settings: realistic (0.005 MU) and artificially large (0.05 MU). In the realistic minimum MU setting, in terms of target coverage, the results from the conventional robust optimization method after post-processing (plan B) had a notable deviation from plan A, except for patient 1, for whom the effect of the minimum MU was negligible. Thus, compared with the conventional robust optimization method, our novel deliverable robust optimization method can prevent target coverage from degrading due to the impact of the minimum MU, while having organ protections comparable to that of plan A. In the artificially large minimum MU setting, it is more clear that our proposed method can preserve the target coverage while the post-processing significantly impaired the target coverage.
Figure 2.
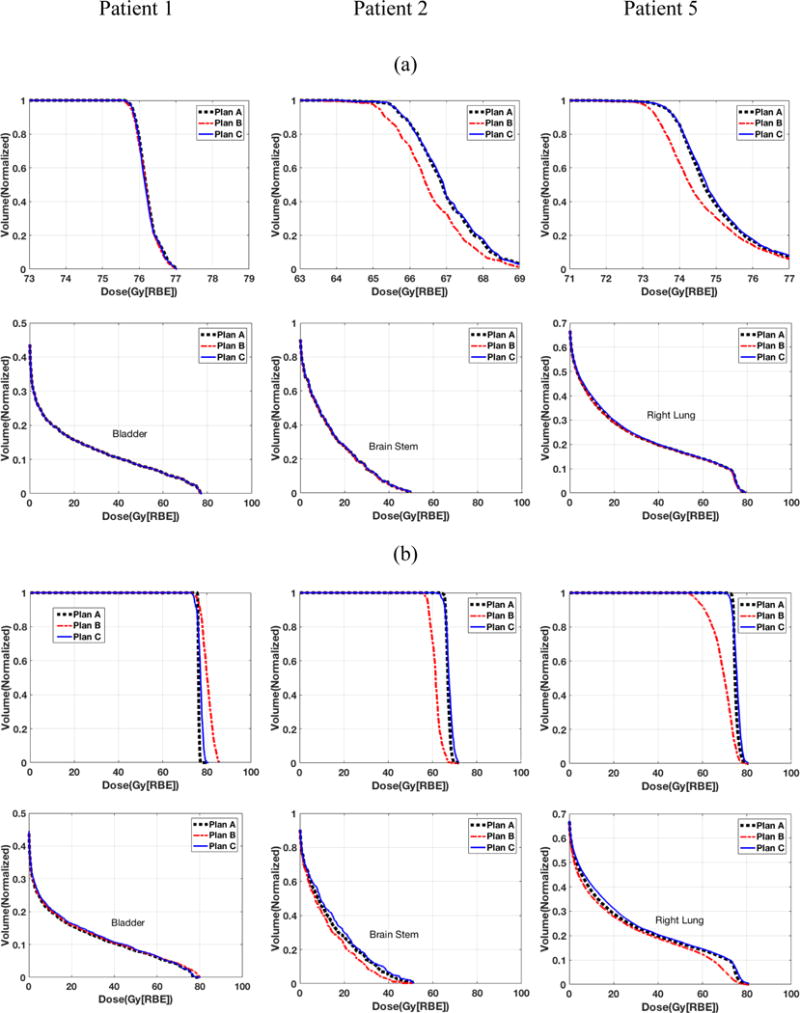
Dose-volume histograms (DVHs) for all 3 methods in patient 1 with prostate cancer (first column), patient 2 with head-neck cancer (second column), and patient 5 with lung cancer (third column). The first row indicates DVHs for CTVs; the second row indicates DVHs for organ at risk. Part (a) is the test with realistic minimum MU constraint and part (b) is with artificially large (10 times larger than that in part (a)) minimum MU constraint. Please note that figures in the top row are zoomed in.
3.1.2. Comparison of plan robustness
Our method not only minimized the influence of the minimum MU constraint on nominal plan quality but also maintained high plan robustness. A complete comparison of robustness (width of the band of CTV at D95%) for the 7 patients is shown in Figure 3. Our method prevented the nominal plan quality from degrading while also preserved the plan robustness. This trend was clearer when the method was applied to settings with larger minimum MU constraint.
Figure 3.
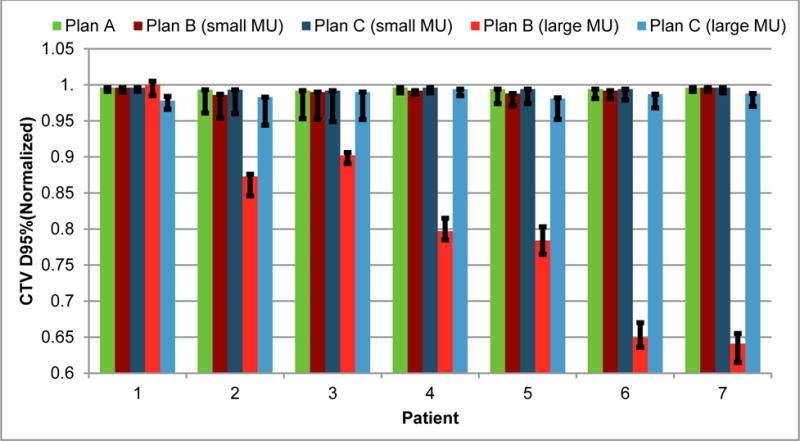
Comparison of CTV D95% (CTV, clinical target volume; D95%, the minimum normalized dose that covers 95% of the region of interest with the highest dose of plans A, B, and C with realistic (small) minimum MU constraint and artificially 10 times larger (large) minimum MU constraint, in all 7 patients. Error bars indicate the DVH family band width at D95%, which indicates plan robustness.
3.2. Performance
For a fair comparison of the performance of our new minimum robust optimization method, the time consumed is calculated so that the number of total optimization loops was normalized to 300 for both the deliverable robust optimization and the conventional robust optimization methods. Comparison of the computation time between the 2 methods showed that the deliverable robust optimization required less time for most patients (Table 3). The unexpected sweetness of faster calculation per inner iteration may be due to the newer implementation of L-BFGS-B compared to L-BFGS.
Table 3.
Total CPU Time Normalized to 300 Loops
| Patient | Conventional Robust Optimization | Deliverable obust Optimization |
|---|---|---|
| 1 | 2 min, 38 s | 2 min, 16 s |
| 2 | 8 min, 9 s | 7 min, 38 s |
| 3 | 13 min, 43 s | 13 min, 15 s |
| 4 | 500 min, 15 s | 438 min, 9 s |
| 5 | 71 min, 48 s | 71 min, 25 s |
| 6 | 40 min, 21 s | 44 min, 0 s |
| 7 | 66 min, 5 s | 62 min, 50 s |
4. Discussion
In this study, we developed a new robust optimization method using a quadratic objective function to account for the minimum MU constraint in IMPT. By dynamically turning on and off the boundary constraints of the solution variables directly in the optimization, our new method derived the optimized solution in the discontinuous feasible region and generated deliverable robust plans without the conventional post-processing procedure.
It is important for the developed methods to be compatible with the current commercial TPS for rapid translation to clinical practice. All the commercial TPS are using nonlinear programming in optimization. Nonlinear programming based on quadratic objective functions (i.e., quadratic optimization) has been used in radiation therapy treatment planning for decades. Compared with linear programming, quadratic optimization usually generates plans with smoother dose-volume histogram curves54. Also, because of its simplistic formulations and the capability to use quasi-Newton optimization methods, it can achieve clinically acceptable plans much more quickly than linear programming54. Therefore we believe that it is still valuable to develop a method which can integrate the minimum MU constraint into robust optimization for IMPT treatment planning using an optimization algorithm similar to PCS. Therefore, it can be more readily incorporated into the current IMPT treatment planning workflow (such as the most popular version of the optimization algorithm, PCS, in Eclipse™).
Proton dose distribution is highly sensitive to patient setup and range uncertainties. Therefore, it is important to consider plan robustness in IMPT treatment planning. Recently, many groups have proposed different robust optimization methods that have achieved robust plans while preserving the nominal plan quality compared with the margin-based method. In this study, we based on the voxel-wise worst-case robust optimization which operates on a given set of uncertainty scenarios. Fredriksson et al, have introduced a new robust optimization method which maximizes the possibility of the clinical acceptance with the variable extent of uncertainties that can be incorporated55. This method explored more possibilities in terms of plan quality and demonstrated the relationship between the extent of uncertainties considered and the nominal plan quality. This provides users more control in the tradeoff between nominal plan quality and plan robustness. In our future work, we plan to provide the same control with a different approach.
It is worth noting that, in spite of the fact that our deliverable robust optimization was currently implemented on the basis of the voxel-wise worst-case robust optimization method, it is essentially independent of the choice of the optimization model. Therefore, it can be applied to a broad range of optimization models: either robust or non-robust, and either proton or photon therapy. It can also be used in beam angle optimization, optimization in the reduction of energy layers, and spot position optimization since these optimizations all potentially could lead to a substantial number of spots with small intensities. Therefore, integration of the minimum MU constraint into plan optimization could be important for these problems.
Preservation of target coverage while accounting for the minimum MU constraint is important in clinic practice. Degradation of the target coverage, although small, could substantially increase local recurrence. Local recurrence is one of the major causes of patient mortality in many disease sites. In the current clinical practice, the treatment planner sometimes has to adjust the spot spacing and re-optimize the plan by trial-and-error if the impact of the minimum MU constraint turns out to be significant (decreasing the target coverage a lot). Our method would relieve the treatment planner from the burden of selecting appropriate spot spacing by trial-and-error to minimize the impact of the minimum MU constraint. More importantly, since the minimum MU constraint are directly incorporated into the treatment planning, a post-processing is not needed as in the current clinical practice and no dose distribution degradation would take place due to the minimum MU constraint.
The parameters of our proton machines such as spot spacing and discrete energy layers are tuned with great efforts so that the impact of the minimum MU is minimized during the machine commissioning. Therefore, the benefit of deliverable robust optimization is diluted here. We also evaluated our method with larger minimum MU constraint (up to ten times larger). Results showed that our method can still mitigate the effect of the much larger minimum MU constraint (Figure 3). It suggests that our method is valuable for systems with larger minimum MU constraint and for proton centers, in which the technical support of the careful adjustment of the related configurations to minimize the influence of the minimum MU is not available.
Our method has some limitations. Because of practical considerations, only 9 uncertainty scenarios, composed of rigid iso-center shifts due to patient setup uncertainty and minimum/maximum range uncertainty, were considered in the optimization. This could either underestimate or overestimate the effect of uncertainties.56 Also a study including a larger patient cohort is needed to get statistically significant results.
In summary, compared with the conventional post-processing methods, our new method of incorporating the minimum MU constraint directly into robust optimization can produce machine-deliverable plans with better tumor coverage while maintaining high plan robustness.
Acknowledgments
This research was supported by the National Cancer Institute (NCI) Career Developmental Award K25CA168984, Arizona Biomedical Research Commission Investigator Award, the Fraternal Order of Eagles Cancer Research Fund Career Development Award, the Lawrence W. and Marilyn W. Matteson Fund for Cancer Research, Mayo Arizona State University Seed Grant, and the Kemper Marley Foundation.
Footnotes
Disclosure of Conflicts of Interest
The authors have no relevant conflicts of interest to disclose.
References
- 1.Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. International Journal of Radiation Oncology*Biology*Physics. 2011;80:1015–1022. doi: 10.1016/j.ijrobp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuschke M, Kaiser A, Pottgen C, Lubcke W, Farr J. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012;104:45–51. doi: 10.1016/j.radonc.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Sejpal S, Komaki R, Tsao A, Chang JY, Liao Z, Wei X, Allen PK, Lu C, Gillin M, Cox JD. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004–3013. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 4.Lomax A. Intensity modulation methods for proton radiotherapy. Physics in Medicine and Biology. 1999;44:185. doi: 10.1088/0031-9155/44/1/014. [DOI] [PubMed] [Google Scholar]
- 5.Lomax AJ. Habilitation Thesis. ETH; 2004. [Google Scholar]
- 6.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Physics in Medicine and Biology. 2008;53:1043–1056. doi: 10.1088/0031-9155/53/4/015. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Unkelbach J, Trofimov A, Madden T, Kooy H, Bortfeld T, Craft D. Including robustness in multi-criteria optimization for intensity-modulated proton therapy. Physics in Medicine and Biology. 2012;57:591–608. doi: 10.1088/0031-9155/57/3/591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredriksson A, Forsgren A, Hardemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Medical Physics. 2011;38:1672–1684. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Li Y, Li X, Cao W, Zhang X. Influence of robust optimization in intensity-modulated proton therapy with different dose delivery techniques. Med Phys. 2012;39:3089–4001. doi: 10.1118/1.4711909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Zhang X, Li Y, Mohan R. Robust optimization in intensity-modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Physics in Medicine and Biology. 2008;53:1689–1700. doi: 10.1088/0031-9155/53/6/013. [DOI] [PubMed] [Google Scholar]
- 12.Unkelbach J, Bortfeld T, Martin BC, Soukup M. Reducing the sensitivity of IMPT treatment plans to setup errors and range uncertainties via probabilistic treatment planning. Medical Physics. 2009;36:149–163. doi: 10.1118/1.3021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unkelbach J, Chan TCY, Bortfeld T. Accounting for range uncertainties in the optimization of intensity modulated proton therapy. Physics in Medicine and Biology. 2007;52:2755–2773. doi: 10.1088/0031-9155/52/10/009. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Liao Z, Schild SE, Liu Z, Li H, Li Y, Park PC, Li X, Stoker J, Shen J, Keole S, Anand A, Fatyga M, Dong L, Sahoo N, Vora S, Wong W, Zhu XR, Bues M, Mohan R. Impact of respiratory motion on worst-case scenario optimized intensity-modulated proton therapy for lung cancers. Practical Radiation Oncology. 2015;5:e77–e86. doi: 10.1016/j.prro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopf A-C, Boye D, Lomax A, Mori S. Adequate margin definition for scanned particle therapy in the incidence of intrafractional motion. Physics in Medicine and Biology. 2013;58:6079–6094. doi: 10.1088/0031-9155/58/17/6079. [DOI] [PubMed] [Google Scholar]
- 16.Rietzel E, Bert C. Respiratory motion management in particle therapy. Medical Physics. 2010;37:449–460. doi: 10.1118/1.3250856. [DOI] [PubMed] [Google Scholar]
- 17.Graeff C, Durante M, Bert C. Motion mitigation in intensity modulated particle therapy by internal target volumes covering range changes. Medical Physics. 2012;39:6004–6013. doi: 10.1118/1.4749964. [DOI] [PubMed] [Google Scholar]
- 18.Graeff C. Motion mitigation in scanned ion beam therapy through 4D-optimization. Phys Medica. 2014;30:570–577. doi: 10.1016/j.ejmp.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Graeff C, Luechtenborg R, Eley JG, Durante M, Bert C. A 4D-optimization concept for scanned ion beam therapy. Radiotherapy and Oncology. 2013;109:419–424. doi: 10.1016/j.radonc.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Eley JG, Newhauser WD, Richter D, Luechtenborg R, Saito N, Bert C. Robustness of target dose coverage to motion uncertainties for scanned carbon ion beam tracking therapy of moving tumors. Physics in Medicine and Biology. 2015;60:1717–1740. doi: 10.1088/0031-9155/60/4/1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graeff C, Constantinescu A, Luechtenborg R, Durante M, Bert C. Multigating, a 4D Optimized Beam Tracking in Scanned Ion Beam Therapy. Technology in Cancer Research & Treatment. 2014;13:497–504. doi: 10.7785/tcrtexpress.2013.600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Schild SE, Chang JY, Liao Z, Chang Y-H, Wen Z, Shen J, Stoker J, Ding X, Hu Y, Sahoo N, Herman MG, Vargas C, Keole S, Wong W, Bues M. Exploratory Study of 4D Versus 3D Robust Optimization in Intensity-Modulated Proton Therapy for Lung Cancer. International Journal of Radiation Oncology • Biology • Physics. 2015 doi: 10.1016/j.ijrobp.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Frank SJ, Li X, Li Y, Zhu RX, Mohan R. PTV-based IMPT optimization incorporating planning risk volumes vs robust optimization. Medical Physics. 2013;40:021709–021708. doi: 10.1118/1.4774363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Liao Z, Schild SE, Liu Z, Li H, Li Y, Park PC, Li X, Stoker J, Shen J, Keole S, Anand A, Fatyga M, Dong L, Sahoo N, Vora S, Wong W, Zhu XR, Bues M, Mohan R. Impact of respiratory motion on worst-case scenario optimized intensity modulated proton therapy for lung cancers. Practical Radiation Oncology. 2015;5:e77–86. doi: 10.1016/j.prro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Zhang X, Park P, Liu W, Chang J, Liao Z, Frank S, Li Y, Poenisch F, Mohan R, Gillin M, Zhu R. Robust optimization in intensity-modulated proton therapy to account for anatomy changes in lung cancer patients. Radiotherapy and Oncology. 2015;114:367–372. doi: 10.1016/j.radonc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Sahoo N, Zhang X, Robertson D, Li H, Choi S, Lee A, Gillin M. Intensity modulated proton therapy treatment planning using single-field optimization: the impact of monitor unit constraints on plan quality. Medical physics. 2010;37:1210–1219. doi: 10.1118/1.3314073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Sahoo N, Zhang X, Robertson D, Li H, Choi S, Lee A, Gillin M. Intensity modulated proton therapy treatment planning using single-field optimization: The impact of monitor unit constraints on plan quality. Medical physics. 2010;37:1210–1219. doi: 10.1118/1.3314073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doolan PJ, Alshaikhi J, Rosenberg I, Ainsley CG, Gibson A, D’Souza D, Bentefour EH, Royle G. A comparison of the dose distributions from three proton treatment planning systems in the planning of meningioma patients with single-field uniform dose pencil beam scanning. Journal of Applied Clinical Medical Physics. 2015;16 doi: 10.1120/jacmp.v16i1.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Kooy H, Craft D, Depauw N, Flanz J, Clasie B. TH-CD-209-01: A Greedy Reassignment Algorithm for the PBS Minimum Monitor Unit Constraint. Medical Physics. 2016;43:3885–3886. doi: 10.1088/0031-9155/61/12/4665. [DOI] [PubMed] [Google Scholar]
- 30.Carlsson F, Forsgren A. Iterative regularization in intensity-modulated radiation therapy optimization. Medical Physics. 2006;33:225–234. doi: 10.1118/1.2148918. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Y, Michalski D, Censor Y, Galvin JM. Inherent smoothness of intensity patterns for intensity modulated radiation therapy generated by simultaneous projection algorithms. Phys Med Biol. 2004;49:3227–3245. doi: 10.1088/0031-9155/49/14/015. [DOI] [PubMed] [Google Scholar]
- 32.Kim T, Zhu L, Suh T-S, Geneser S, Meng B, Xing L. Inverse planning for IMRT with nonuniform beam profiles using total-variation regularization (TVR) Medical Physics. 2011;38:57–66. doi: 10.1118/1.3521465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chvetsov AV, Calvetti D, Sohn JW, Kinsella TJ. Regularization of inverse planning for intensity-modulated radiotherapy. Med Phys. 2005;32:501–514. doi: 10.1118/1.1844111. [DOI] [PubMed] [Google Scholar]
- 34.Chvetsov AV. L-curve analysis of radiotherapy optimization problems. Med Phys. 2005;32:2598–2605. doi: 10.1118/1.1949750. [DOI] [PubMed] [Google Scholar]
- 35.Alber M, Nusslin F. Intensity modulated photon beams subject to a minimal surface smoothing constraint. Phys Med Biol. 2000;45:N49–52. doi: 10.1088/0031-9155/45/5/403. [DOI] [PubMed] [Google Scholar]
- 36.Spirou SV, Fournier-Bidoz N, Yang J, Chui CS, Ling CC. Smoothing intensity-modulated beam profiles to improve the efficiency of delivery. Med Phys. 2001;28:2105–2112. doi: 10.1118/1.1406522. [DOI] [PubMed] [Google Scholar]
- 37.Webb S, Convery DJ, Evans PM. Inverse planning with constraints to generate smoothed intensity-modulated beams. Phys Med Biol. 1998;43:2785–2794. doi: 10.1088/0031-9155/43/10/008. [DOI] [PubMed] [Google Scholar]
- 38.Llacer J, Agazaryan N, Solberg TD, Promberger C. Degeneracy, frequency response and filtering in IMRT optimization. Phys Med Biol. 2004;49:2853–2880. doi: 10.1088/0031-9155/49/13/007. [DOI] [PubMed] [Google Scholar]
- 39.Sun X, Xia P. A new smoothing procedure to reduce delivery segments for static MLC-based IMRT planning. Med Phys. 2004;31:1158–1165. doi: 10.1118/1.1713279. [DOI] [PubMed] [Google Scholar]
- 40.Dobler B, Koelbl O, Bogner L, Pohl F. Direct machine parameter optimization for intensity modulated radiation therapy (IMRT) of oropharyngeal cancer – a planning study. Journal of Applied Clinical Medical Physics. 2009;10:4–15. doi: 10.1120/jacmp.v10i4.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broderick M, Leech M, Coffey M. Direct aperture optimization as a means of reducing the complexity of intensity modulated radiation therapy plans. Radiation Oncology. 2009;4:8. doi: 10.1186/1748-717X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hårdemark B, Liander A, Rehbinder H, Löf J. RaySearch White Paper Stockholm. Sweden: RaySearch Laboratories AB; 2003. Direct machine parameter optimization with RayMachine® in Pinnacle3®. [Google Scholar]
- 43.Cao W, Lim G, Li X, Li Y, Zhu XR, Zhang X. Incorporating deliverable monitor unit constraints into spot intensity optimization in intensity-modulated proton therapy treatment planning. Physics in medicine and biology. 2013;58:5113. doi: 10.1088/0031-9155/58/15/5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard M, Beltran C, Mayo CS, Herman MG. Effects of minimum monitor unit threshold on spot scanning proton plan quality. Medical physics. 2014;41 doi: 10.1118/1.4892057. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C, Byrd RH, Nocedal J. L-BFGS-B: Algorithm 778: L-BFGS-B, FORTRAN routines for large scale bound constrained optimization. ACM Transactions on Mathematical Software. 1997;23:550–560. [Google Scholar]
- 46.Morales JL, Nocedal J. Remark on “Algorithm 778: L-BFGS-B: Fortran subroutines for large-scale bound constrained optimization”. ACM Transactions on Mathematical Software (TOMS) 2011;38:7. [Google Scholar]
- 47.Liu W, Patel S, Shen J, Hu Y, Harrington D, Ding X, Halyard M, Schild SE, Wong W, Ezzell G, Bues M. Robustness Quantification Methods Comparison in Volumetric-Modulated Arc Therapy to treat Head and Neck Cancer. Practical Radiation Oncology. 2016 doi: 10.1016/j.prro.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Frank SJ, Li X, Li Y, Park P, Dong L, Zhu XR, Mohan R. Effectiveness of Robust Optimization in Intensity-Modulated Proton Therapy Planning for Head and Neck Cancers. Med Phys. 2013;40:051711–051718. doi: 10.1118/1.4801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue T, Widder J, van Dijk LV, Takegawa H, Koizumi M, Takashina M, Usui K, Kurokawa C, Sugimoto S, Saito AI, Sasai K, van’t Veld AA, Langendijk JA, Korevaar EW. Limited impact of setup and range uncertainties, breathing motion and interplay effects in robustly optimized intensity modulated proton therapy for stage III non-small cell lung cancer. International Journal of Radiation Oncology • Biology • Physics. doi: 10.1016/j.ijrobp.2016.06.2454. [DOI] [PubMed] [Google Scholar]
- 50.Wu QW, Mohan R. Algorithms and functionality of an intensity modulated radiotherapy optimization system. Medical Physics. 2000;27:701–711. doi: 10.1118/1.598932. [DOI] [PubMed] [Google Scholar]
- 51.Liu W, Li Y, Li X, Cao W, Zhang X. Influence of robust optimization in intensity-modulated proton therapy with different dose delivery techniques. Med Phys. 2012;39 doi: 10.1118/1.4711909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu W. Robustness quantification and robust optimization in intensity-modulated proton therapy. In: Rath A, Sahoo N, editors. Particle Radiotherapy: Emerging Technology for Treatment of Cancer. Springer; 2015. [Google Scholar]
- 53.Trofimov A, Unkelbach J, DeLaney TF, Bortfeld T. Visualization of a variety of possible dosimetric outcomes in radiation therapy using dose-volume histogram bands. Practical Radiation Oncology. 2012;2:164–171. doi: 10.1016/j.prro.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P, Fan N, Shan J, Schild SE, Bues M, Liu W. Mixed integer programming with dose-volume constraints in intensity-modulated proton therapy. Journal of Applied Clinical Medical Physics. 2017;18:29–35. doi: 10.1002/acm2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fredriksson A, Forsgren A, Hardemark B. Maximizing the probability of satisfying the clinical goals in radiation therapy treatment planning under setup uncertainty. Medical Physics. 2015;42:3992–3999. doi: 10.1118/1.4921998. [DOI] [PubMed] [Google Scholar]
- 56.Quan M, Liu W, Wu R, Li Y, Frank SJ, Zhang X, Zhu XR, Mohan R. Preliminary evaluation of multi-field and single-field optimization for the treatment planning of spot-scanning proton therapy of head and neck cancer. Med Phys. 2013;40:081709. doi: 10.1118/1.4813900. [DOI] [PMC free article] [PubMed] [Google Scholar]


