Abstract
Purpose
Early phase clinical trials (EPTs) have led to new, more effective treatment options for children with cancer. Despite the extensive use of EPTs in pediatric oncology, little is known about parent and child experiences during EPT participation. The purposes of this pilot study were to assess the feasibility and preliminary results of having children with cancer and their parents complete measures of treatment burden and quality of life (QOL) concurrent with EPT participation.
Methods
In this descriptive, longitudinal, pilot study, parents and children were followed for the first 60 days of an EPT. Feasibility was assessed by participant enrollment and retention, and completion of measures. Measures completed included: Demographic form (completed at baseline); Diary of Trial Experiences to capture treatment burden (completed ongoing); and PedsQL™ Quality of Life Inventories, Cancer Modules, and Family Impact Module (completed at baseline, post-first disease evaluation, and off-study). Data were analyzed using descriptive statistics.
Results
Feasibility goals of enrollment, retention, and measure completion were partially met. Preliminary treatment burden and QOL results are provided.
Conclusions
While QOL assessments may provide insight into EPT experiences, future studies need to be conducted at multiple sites and enrollment goals must account for participant attrition.
Keywords: pediatric oncology, phase I clinical trial, early phase clinical trial, treatment burden, quality of life
Early phase clinical trials (EPTs) are the first steps to test novel medical therapies in humans (Kim et al., 2008; Lee, Skolnik, & Adamson, 2005). The process of developing therapies involves a series of clinical trials in humans; after preclinical testing, therapies are tested in phase I, phase II and/or pilot, and phase III clinical trials in order to obtain sufficient evidence of the therapy’s safety and efficacy (National Institutes of Health, n.d.). For the purposes of this study, EPTs include phase I, phase II, and pilot trials of investigational therapies that are still under development and not yet approved by the United States Food and Drug Administration. The challenge with pediatric EPTs is that, due to the investigational nature of the therapies being tested, children can only participate in an EPT when standard therapies are considered ineffective. The median life expectancy of children with relapsed cancer enrolled in a phase I clinical trial is between just 3.6 – 6.4 months (Bautista et al., 2015; Kim et al., 2008; Morgenstern et al., 2014).
Treatment burden is defined as the physical, financial, time, psychosocial, and procedural demands that a treatment places on a patient and their family, as well as its impact on patient and family functioning (Eton et al., 2012; Sav, Kendall, et al., 2013; Sav, King, et al., 2013). Treatment burden is a dynamic, multidimensional concept that fluctuates over time due to severity of the patient’s condition, development of toxicities, and adjustment to the treatment. Treatment burden is different from burden caused by other factors such as symptoms or disease, because it is based on treatment for the disease, and not on either the natural history or natural symptoms of the disease (Sav, King, et al., 2013). In adult patients, treatment burden encompasses time lost from work and other activities (Henry et al., 2008). Although children may not work, their time lost is equally important and burdensome; they would also benefit from spending the time required for treatments with family and friends or carrying out their usual activities. Although research has yet to confirm this, experts hypothesize that for children with chronic illness, treatment demands such as injections, blood samples, and dietary restrictions may be particularly burdensome and negatively impact children’s quality of life (QOL) (Henry et al., 2008; Ziaian et al., 2006).
A better understanding of treatment burden in the context of EPTs may help healthcare professionals, patients, and parents to make more informed treatment decisions (Sav, Kendall, et al., 2013). Although adults’ participation in phase I clinical trials provides hope and a sense of purpose, there are also significant associated physical, emotional, and practical burdens (Cohen et al., 2007; Cox, 1999; Moore, 2001; Wootten, Abbott, Siddons, Rosenthal, & Costello, 2011). The research with adults is not generalizable to pediatric EPTs because children are reliant on parents as providers, caregivers, teachers, moral compasses, disciplinarians, and proxy decision-makers. Some experts suggest that EPTs burden children with additional medical procedures and toxicities, negatively impact QOL, limit palliation opportunities, and disrupt dying and bereavement processes (Beardsmore & Fitzmaurice, 2002; Oberman & Frader, 2003). Recent evidence, however, has demonstrated that an active palliative care program can ensure that measures of end-of-life care (e.g., presence or timing of do not attempt resuscitation orders, hospice use or length of stay) are not impacted by enrollment in a phase I clinical trial.(Levine et al., 2015) Although research on experiences of communication and decision-making during EPT consent processes has been conducted, knowledge is lacking regarding parent and child experiences of treatment burden and QOL while participating in an EPT. Therefore, the purposes of this pilot study were to assess the feasibility and preliminary results of having children with cancer and their parents complete measures of treatment burden and QOL concurrent with EPT participation.
Methods
This was a descriptive, longitudinal, pilot study with data collected from parents and children. Institutional Research Board approval was obtained for this study prior to enrolling participants. All parents and children aged ≥18 provided written documentation of informed consent; children 7 – 17 years of age provided verbal assent for participation. Recruitment occurred between June 2011 and May 2013.
All recruitment occurred at a large, Midwestern pediatric medical center. Parents and children were approached to participate after confirmation of their eligibility with the attending oncologist. The recruitment goal was 20 parent and child dyads. As this was a pilot study, the sample size was based on participants available (i.e. annual EPT enrollment projections at the pediatric medical center), rather than on statistical power (Leon, Davis, & Kraemer, 2011; Thabane et al., 2010). A 24-month maximum length was set on recruitment.
Child inclusion criteria were: (1) age 2 to 25 years; (2) receipt of at least one therapy prior to the EPT (i.e., phase I, phase II, or pilot clinical trial); (3) consented to participate in an outpatient-based EPT for relapsed/refractory pediatric cancer; (4) EPT therapy did not include 131I-metaiodobenzylguanidine (131I-MIBG) or oncolytic virotherapy; (5) enrolled within 48 hours of first dose of EPT therapy; and (6) ability to communicate in English. Parent inclusion criteria were: (1) age ≥18; (2) self-identification as biological parent or legal guardian of child; and (3) fluency in English. Eligibility criteria were established to prospectively capture the full experience of participation in a classic EPT where therapy involves either oral or intravenous agent(s) administered on a regular schedule to a child with relapsed and/or refractory cancer. To capture as full a data set as possible, an extended range of child ages was included based on ages covered by the PedsQL modules. EPTs involving 131I-MIBG therapy and oncolytic virotherapy were excluded due to the unique requirements of these studies that necessitated prolonged isolation from support systems. The first 48 hours was selected to ensure that baseline data reflected experiences at the time the EPT started.
Procedures
Children were followed for either eight weeks (if length of treatment course was four weeks) or nine weeks (if length of treatment course was three weeks) during the EPT. This variance was due to a desire to standardize the time on study, while ensuring that children completed this study at the end of an EPT course. Assessments were completed at baseline, post-first disease evaluation, and end of this study. The baseline assessment was completed after the child was enrolled in the clinical trial, preferably before treatment started, but no more than 48 hours after the administration of the first dose of the investigational therapy. The post-disease evaluation assessment was completed after the first disease evaluation was performed, but no more than 7 days after the child/family were provided the results of the disease evaluation. The off-study assessment was completed at the end of a course, after the child had been on the EPT for 60 (+/− 5) days. In addition, parents were asked to complete the Diary of Trial Experiences on an ongoing basis (i.e. two to three times a week) at home. To ensure completeness, a study team member reviewed diary entries with the parent every 5 to 14 days throughout the study. Table 1 provides a list of measures completed with each assessment, and by which participants. Participants received $25 in cash upon completion of the baseline and post-disease evaluation assessments, and $50 in cash after completing the off-study assessment.
Table 1.
Instruments Completed by Participants at Each Assessment
| Instrument | Baseline Assessment | Post Disease Evaluation Assessment | Off-Study Assessment |
|---|---|---|---|
| Child and Family Demographics Form | Parent | ---------- | ---------- |
| Diary of Trial Experiences | Completed Throughout Study by Parent | ||
| PedsQL™ Quality of Life Inventories | Parent and Child | Parent and Child | Parent and Child |
| PedsQL™ Cancer Modules | Parent and Child | Parent and Child | Parent and Child |
| PedsQL™ Family Impact Module | Parent | Parent | Parent |
Measures
Family Demographics
Parents completed an investigator-designed Family and Patient Demographic form at the baseline assessment. In addition to standard demographics, data included family composition, type of central line access, whether central line access was placed specifically for the EPT, and distance from primary household to pediatric medical center in miles and minutes of travel time.
Child Performance Status
The child’s Lansky or Karnofsky scale scores were evaluated at each assessment by a member of the health care team and documented in the clinical trial record. These scales are similar with the Lansky scale applicable for children less than 16 years of age and the Karnofsky for those aged 16 years and older. Both scales (1) quantify cancer patients’ general well-being and activities of daily life, (2) have well-established reliability and validity, (3) are responsive to change, (4) are widely used, and (5) use a single score of 0 to 100 in increments of 10, where 0 is death and 100 is normal health with no complaints (Lansky, List, Lansky, Ritter-Sterr, & Miller, 1987; Schag, Heinrich, & Ganz, 1984; Vincent, Laliberte, Morris, & Wiemann, 1984).
EPT Data
Using an investigator-designed form, the study team extracted data from the EPT protocol, consent form, and the child’s EPT records. Data captured included: length of treatment course; frequency, number and duration of required and optional blood draws, physical exams, imaging, bone marrows, lumbar punctures, clinic visits and infusions; number of planned separate visits to a medical facility/laboratory; number of expected separate needle punctures; optional observations that the child/parent agreed to provide for the EPT (e.g. pharmacokinetic and pharmacogenetic samples); and outcomes of EPT disease evaluations.
Treatment Burden
Based on an adaptation of the Collection of Indirect and Non-medical direct costs (COIN) form (Sherman et al., 2001), the study team created the Diary of Trial Experiences to capture the treatment burden associated with EPT participation for parents and children. The COIN form was a feasible and practical method for assessing patient cost data in a study of 29 adult cancer patients being treated for prostate carcinoma (Sherman et al., 2001). Adaptations included reformatting and capturing time spent in different activities; financial costs associated with child care, lodging, and meals; venipunctures; and reasons why usual activities were missed. The additions were made by adding columns and rows as needed into the tables structuring the form, and by adding a separate section at the bottom of a page to capture venipunctures. The Diary of Trial Experiences was completed by parents on an ongoing basis and used to directly capture the number of appointments and activities related to the EPT, including time spent on and financial cost of those activities. See Table 2 for a listing of elements included in the diary.
Table 2.
Elements from the Diary of Treatment Experiences
| Easily Answeredq | Difficult to Answer |
|---|---|
|
|
QOL
The standardized and widely used PedsQL™ modules were used to assess QOL, including: the PedsQL™ Quality of Life Inventories (Standard Version), Cancer Modules, and Family Impact Module. The 21-to 23-item Quality of Life Inventories measure health-related QOL in children and adolescents, with subscales for physical, emotional, social, and school functioning. The 25- to 27-item Cancer Modules measure elements of health-related QOL specific for children and adolescents with cancer, with subscales for pain and hurt, nausea, procedural anxiety, treatment anxiety, worry, cognitive problems, perceived physical appearance, and communication. The 36-item Family Impact Module measures parent physical, emotional, social, and cognitive functioning; communication; worry; family daily activities; and family relationships. For all modules, 5-point response options range from ‘Never’ (100) to ‘Almost Always’ (0). The Likert scores were then transformed to a 0–100 scale; as the items in PedsQL™ modules relate to problems, higher scores indicate better QOL and less problems. The total score for each module was determined by averaging the sum of all the scores for the items answered. A change of between 4.4 to 4.5 in the total score is the standard for a minimal clinically important difference in the PedsQL™ Quality of Life Inventories (Varni, Burwinkle, & Seid, 2005). Advantages to the PedsQL™ modules are their ease of completion, demonstrated internal consistency and reliability, and established responsiveness to change when repeatedly administered in short intervals (Banks, Barrowman, & Klaassen, 2008; Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002; Varni et al., 2005; Varni & Limbers, 2009; Varni, Seid, Knight, Uzark, & Szer, 2002; Varni, Seid, & Kurtin, 2001; Varni, Sherman, Burwinkle, Dickinson, & Dixon, 2004). For most children in this study (based on child’s age), both parent report and child self-report versions of the PedsQL™ Quality of Life Inventories and Cancer Modules were available, allowing parent and child to separately complete these modules. The parent completed the Family Impact Module.
Statistical Analyses
All data were analyzed using descriptive statistics. Since children were enrolled in EPTs with varying course lengths, treatment burden per week was calculated for each participant as the sum of entries in the Diary of Trial Experiences for course 1 of the EPT divided by the number of weeks per course. Descriptive statistics of treatment burden per week were then calculated.
Feasibility was assessed by the following criteria: (1) ≥75% enrollment of all eligible parent and child dyads; (2) ≥ 80% retention of participants at the post-disease evaluation assessment; (3) ≥90% of questions answered by parents and children on each measure. Retention was evaluated as the rate of completion of the post-disease evaluation assessment by the 13 dyads who enrolled in the study, as completion of one course in the EPT was considered the minimum to be evaluable for this study. The same instruments were completed at the post-disease evaluation and off-study assessments, so selecting the post-disease evaluation provided a full set of data to be compared with the baseline assessment and ensured that the experiences of children who were only in the EPT for one course were captured.
Results
Family Demographics, Child Performance Status, and EPT Data
The accrued sample consisted of 13 parent and child dyads. The children were mostly female (69.2%) and white (76.9% white, 15.4% black, 7.7% other). The mean age of the children was 11.4 years old (SD=4.9, range 4–20) and the mean number of children in the household was 1.8 (SD=1.2, range 0–4). More than half of the children had some sort of central venous access in place (Port=38.5%, PICC=15.4%). Parents’ annual income levels were fairly evenly distributed across categories (<$20,000=15.4%; $20–$40,000=23.1%; $40–$60,000=23.1%; $60–100,000=23.1%; >$100,000=15.4%), and the majority of parents had attended college or had a professional degree (93.3%). Mean distance of the primary household from the medical center was 78.8 miles (SD=96.0, range 2–300) or 80.8 minutes (SD=70.6, range 15–240). The median baseline performance score of the children was 90 (SD=9.0, range 70–100). The children were participating on five different EPT protocols. See Table 3 for descriptive statistics summarizing requirements across the five EPT protocols.
Table 3.
Summary of Total EPT Course 1 Protocol Requirements*
| Item | Median | Mean | SD | Range |
|---|---|---|---|---|
| Length of Course (weeks) | 4 | 3.8 | 0.4 | 3 – 4 |
| Baseline Imaging (number of scans) | 2.5 | 2.8 | 1.2 | 2 – 5 |
| Physical Exams (number) | 4 | 3.8 | 0.4 | 3 – 4 |
| Required Blood Draws (number) | 12 | 11.3 | 4.7 | 4 – 16 |
| Optional Blood Draws (number) | 2.5 | 1.8 | 1.5 | 0 – 3 |
| End of Course Imaging (number of scans) | 2 | 1.5 | 1.2 | 0 – 3 |
| Total Required Observations | 20.5 | 19 | 5.9 | 10 – 26 |
n=5 different EPT protocols
Aim 1: Feasibility
Enrollment of Eligible Parent and Child Dyads
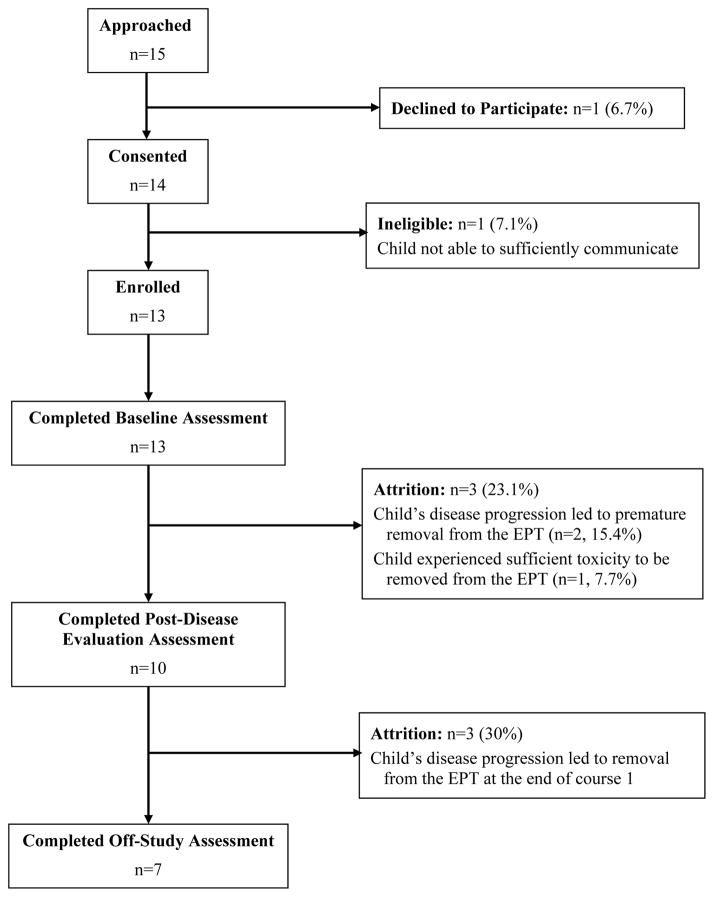
As shown in Figure 1, of the parents and children approached to participate (n = 15), only one parent and child dyad declined to participate (92.9% participation rate). However, one child was determined to be ineligible following consent due to communication difficulties resulting from a brain tumor, resulting in final enrollment of 13 of the 15 dyads approached to participate (86.7% enrollment rate). Both percentages were above the criteria of ≥75% enrollment, indicating that recruitment to this pilot study met feasibility criteria.
Figure 1.
Study Recruitment and Retention
Retention of Eligible Parent and Child Dyads
Per Figure 1, three children (23.1%) deteriorated due to disease progression or suffered sufficient toxicities to be withdrawn from the EPT prior to their first disease evaluation. Thus, the criteria of 80% of study participants remaining on this study and completing the post-disease evaluation assessment was not quite met (76.9% retention was achieved at this time point). Overall, only seven children (53.8% completion rate) remained on this study at the off-study assessment conducted 60 (+/− 5) days after enrollment, although no parent and child dyads were lost from this study for reasons other than the child’s removal from the associated EPT.
Completion of Measures
Each review of the Diary of Trial Experiences with the parent required between 5 – 15 minutes to complete. The reviews were either done while the parent was waiting at the medical center, or a study team member contacted the parent over the telephone. The Diary of Trial Experiences was too complicated to be completed by most participants without some assistance. Thus, rather than completing the diary at home on an ongoing basis, most parents waited and completed the form during a review with the study team member. Some questions on the diary were either too uncomfortable or too difficult for most participants to answer, and many participants elected to not provide that information. See Table 2 for a listing of elements of the diary that were observed by study team members to be easier and more difficult to answer. Overall, the feasibility criteria of 90% of the Diary of Trial Experiences being successfully completed before the review with a study team member was not met.
QOL measures were generally all completed, with only two individual child PedsQL™ modules missed during an assessment due to study team errors. The baseline assessment required between 20 – 40 minutes to complete, and was usually completed at the medical center either prior to or during an appointment (in a waiting room, clinic appointment room, or in their own room while inpatient). Two parents elected to complete their baseline PedsQL™ modules at home and return them at the next visit. The post-disease evaluation and off-study assessments required between 15 – 30 minutes to complete and all were done while the parent and child were waiting at the medical center.
There was minimal missing data from both parents and children on individual PedsQL™ modules. One parent did not respond to any of the five questions in the PedsQL™ Quality of Life Inventory related to school functioning (78.3% completion of that module) and two questions related to treatment anxiety on a PedsQL™ Cancer Module (92.6% completion). In addition, three other parents did not respond to one question in an individual PedsQL™ module (95.6 – 96.3% completion). Three different children did not answer one of the questions in one PedsQL™ module (95.6 – 96.3% completion). One child did not respond to five questions related to social functioning on a PedsQL™ Quality of Life Inventory (78.3% completion of that module). Overall, the children completed 99.4% of the questions on 52 individual PedsQL™ modules they were provided to complete, while the parents completed 99.5% of the questions on 87 modules. There were no detectable patterns to the questions not answered. However, many parents and children were observed by study team members as having difficulty answering questions related to school, particularly since many children were not attending school due to the advanced stage of their cancer and the PedsQL™ modules do not provide ‘Not Applicable’ as a response option. Thus, the school functioning subscale of the PedsQL™ Quality of Life Inventories likely resulted in inconsistent data. Overall, the feasibility criteria of 90% of the questions answered on each measure by parents and children was met.
Aim 2: Preliminary Results for Treatment Burden and QOL
Treatment Burden
Table 4 provides the descriptive statistics of per week treatment burden for parent and child dyads who completed the post disease evaluation assessment (n=10). Median data suggest that at least half of the children had an average of 3.8 appointments per week, requiring an average of 11.5 hours of time and 2.8 needle punctures per week, and resulting in an average of 9.9 hours of missed activities and $10.60 in out of pocket costs per week. Appointment hours included overnight admissions for observation experienced by 70% of the children for their first dose of EPT therapy. These overnight admissions were for monitoring and collection of timed pharmacokinetic laboratory specimens and were considered as one 24-hour long appointment. Children’s missed activities included school, attending camp, and family activities, and were almost entirely due to EPT appointments, with only three children missing activities due to not feeling well.
Table 4.
Descriptive Statistics for Treatment Burden Per Week Documented in the Diary of Trial Experiences During EPT Course 1*
| Treatment Burden Per Week | Median | Mean | SD | Range |
|---|---|---|---|---|
| Number of Appointments | 3.8 | 3.4 | 0.8 | 2.3 – 4.3 |
| Time for Appointments (hours) | 11.5 | 11.6 | 2.7 | 7.9 – 17.8 |
| Activities Child Missed (hours) | 9.9 | 15.6 | 13.2 | 2.3 – 37.5 |
| Out of Pocket Cost ($) | 10.6 | 15.6 | 16.5 | 0 – 50 |
| Number of Needle Punctures | 2.8 | 2.7 | 1 | 0.5 – 4.3 |
Note. Children were enrolled in EPTs with varying course lengths. Per week treatment burden was calculated for each participant as the total of entries in the Diary of Trial Experiences for course 1 of the EPT, divided by the number of weeks.
n = 10 parent and child dyads completing the post-disease evaluation assessment
QOL
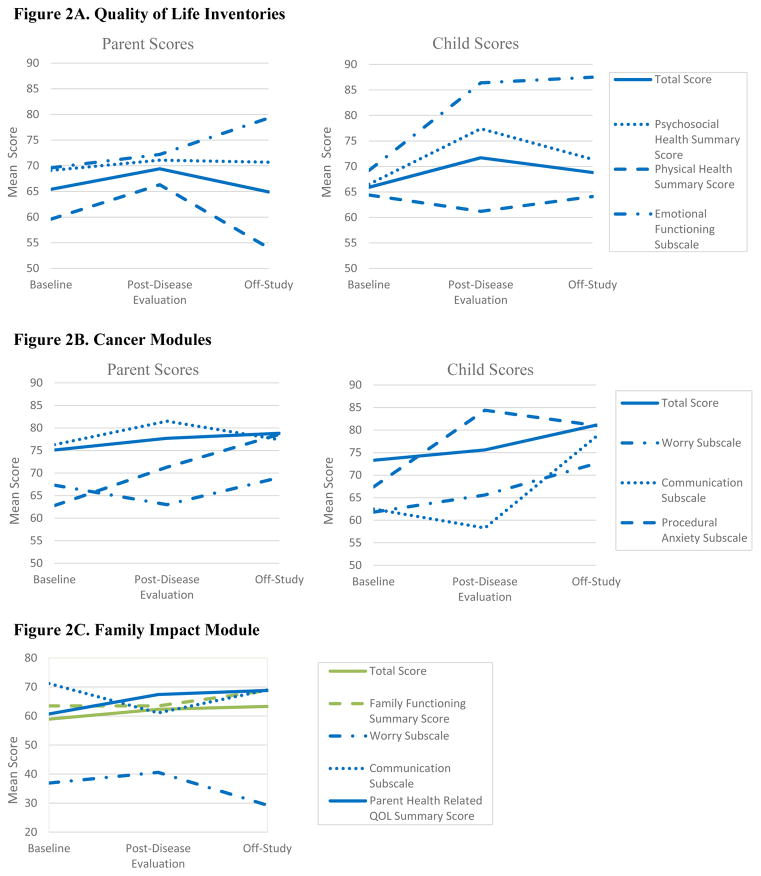
Figure 2A shows mean child and parent PedsQL™ Quality of Life Inventories scores at each assessment. While emotional health scores reported by both parents and children increased over time, other scores did not follow a continuous pattern. In general, children self-reported higher QOL scores than their parents reported on their behalf. The exception to this was the physical health summary score at post-disease evaluation and the psychosocial health summary score at baseline, which the parents generally reported as higher than their child. Statistical comparison could not be performed due to the small sample size.
Figure 2.
Mean PedsQL™ Scores at Each Assessment
Figure 2B shows mean child and parent Cancer Modules scores at each assessment. While the total scores reported by both parents and children increased over time, patterns of change for the other subscales varied over time. A wide variation between parent and child reports occurred for the communication subscale at both baseline and post-disease evaluation, with children self-reporting much lower scores than their parents on their behalf. Children generally reported higher procedural anxiety subscale scores than parents did at all time points, but particularly for the post-disease evaluation assessment; higher procedural anxiety subscale scores indicate less anxiety associated with needle sticks and other procedures performed as part of the child’s cancer care. Again, statistical analyses were not performed due to the small sample size.
Figure 2C shows the mean Family Impact Module scores as reported by parents at each assessment. Patterns of change on the worry and communication subscales varied over time, while the remaining scores continuously improved through the EPT. The overall total scores on this module were stable but low at all time points, indicating that the child’s cancer had a significant impact on the family. In particular, parents reported notably lower scores on the worry subscale, indicating that parents were very worried about their child’s cancer.
Discussion
The first major finding of this pilot study was that feasibility was not clearly established. While the goal of ≥ 75% enrollment was met, the goals for recruitment and retention were not met. In particular, the overall recruitment goal of 20 dyads was not achieved despite recruiting over a 24-month period. Challenges to recruitment included a slow accrual to non-131I-MIBG and oncolytic virotherapy EPTs and lack of sufficient study team members to approach all potential participants.
The primary retention challenge was that attrition was higher than expected; although the goal of ≥ 80% retention was not quite met, no participants opted to leave this study early. All attrition was due to the child’s removal from the EPT due to either toxicity or disease progression. While this was an anticipated problem, given the limited life expectancy of children with cancer enrolled in EPTs, feasibility was impacted by this attrition (Bautista et al., 2015; Kim et al., 2008; Morgenstern et al., 2014). An important implication for future research with this population is to ensure that data is captured at multiple time points, starting before the end of the first course of therapy in the EPT, to ensure that attrition does not prohibit capturing the experiences of participants who are unable to remain in the EPT.
In terms of measure completion, the goal of ≥90% of questions being answered on each measure was met for the PedsQL™ measures, but not for the Diary of Trial Experiences. This diary proved to be overly complicated to complete without the assistance of a study team member. While treatment burden data were captured using the diary, it is clear that revisions to both the format and content are needed to enhance the diary’s usability and acceptability.
To improve feasibility, the following suggestions are recommended for future studies. First, to maximize recruitment efforts and minimize bias in those approached to participate, recruitment should clearly and systematically be tasked to multiple study team members. In addition, future research should be conducted at multiple sites or within a cooperative group to enhance recruitment and generalizability of findings. Lastly, the format of the Diary of Trial Experiences should be revised to mimic EPT medication diaries (i.e. one diary per course of therapy, with one line in the diary to be completed each day of the course).
The second major finding was that while some interesting insights were provided by completion of the PedsQL™ modules, it is less clear that the Diary of Trial Experiences has sufficient value to be worth pursuing in future research. While it may be useful to obtain quantitative results regarding EPT treatment burden, in its current form this diary only measures objective elements of treatment burden (i.e. number of medications, number of appointments, and time at appointments). The subjective elements of treatment burden, including the different perceptions patients and their family have of a treatment’s burden, are not captured. These perceptions include intangible elements that significantly impact the experience of treatment burden, such as difficulty administering oral medications to a young child, the meaning attributed to side-effects of the treatment, and beliefs about a treatment’s effectiveness (Sav, King, et al., 2013). Qualitative research would be needed to identify subjective elements for inclusion. For the Diary of Trial Experiences to be valuable, it should be able to identify children or families who would benefit from further support or allow families to specify their need for further support. However, in its current format this diary does not seem to perform any better than standard psychosocial assessments already being done by social workers and other health care providers.
The third major finding was that preliminary results suggest there is value in having parents and children complete QOL measures during EPT participation. Although the PedsQL™ measures have been widely used in a variety of settings, the authors found no evidence of their use in early phase pediatric oncology clinical trials, prior to this study. In particular, having both parents and children separately complete the PedsQL™ modules, provided insight that there may be time points when parents’ and children’s perceptions of indicators of the child’s quality of life may substantively differ. An example of this is the wide variation between parent and child reports on the PedsQL™ Cancer module communication subscale at baseline and post-disease evaluation, which suggests that children may have had more difficulty communicating concerns related to their cancer at these times than their parents were aware of. This discord between child and parent reports has been acknowledged as prevalent whenever a concern is not directly observable (e.g. when asking about pain, communication, and personal experiences) (Varni, Katz, Seid, Quiggins, & Friedman-Bender, 1998; Varni, Limbers, & Burwinkle, 2007). In this population, it is unlikely that the school functioning subscale of the PedsQL™ Quality of Life Inventories will produce valid results since many children participating in EPTs do not regularly attend school and ‘Not Applicable’ is not a response option. In a study of the QOL of children with advanced cancer, Tomlinson, Hinds, Bartels, Hendershot, and Sung (2011) also reported significant missing data for the school functioning subscale. A larger study of the use of QOL measures during EPT participation is necessary to better elucidate the value they provide. In future research, to more fully understand the impact of EPT participation on physical health, it would be helpful to capture occurrence of toxicities along with completion of QOL measures. The use of PROMIS Pediatric measures (i.e. Physical Functioning - Mobility, Physical Functioning - Upper Extremity, Pain Interference, Fatigue, Depression, Anxiety, and Peer Relationships) should also be considered in future research (Hinds et al., 2013).
An additional result was that all participants in this study reported minimal financial burden directly associated with EPT participation. In particular, the reported financial burden was not grossly observed to correlate with other data, such as distance traveled. In contrast, for adults with chronic illness financial burden has emerged as the most problematic element of treatment burden (Sav, Kendall, et al., 2013). Potential explanations for this finding include: (1) Parents were unwilling to report monetary burdens; (2) Parents were unable to accurately track monetary burdens; (3) Strong levels of financial support were offered by the pediatric medical center through foundations that support families of children with cancer; and, (4) Baseline socioeconomic demographic characteristics of parents and children enrolling in a pediatric oncology EPT may differ from the general population of adults with chronic illness.
The results of this pilot study are limited by small sample size, use of a single site for recruitment, the wide inclusion age range resulting in participants aged 4 to 20 years, and attrition of study participants. In particular, only a preliminary presentation of QOL results was possible. A problem affecting all studies of this population, including ours, is that the interpretation of results is hampered by the bias created by participant attrition. Children with the most toxicities and disease progression do not remain on study to complete follow-up assessments.
Conclusions
This avenue of research is important, and likely to be feasible if conducted at multiple sites or within a cooperative group. To date, no studies have considered the impact of EPT participation, in terms of burden and QOL impact, on children with cancer and their families. Instead, current research focuses on how QOL is impacted by the child’s current health status; the impact of treatment burden on QOL has not yet been considered. While it is unclear whether the Diary of Trial Experiences, as an objective measure of treatment burden, is worthy of further research, this pilot study highlights that measures of QOL impact of EPT participation can feasibly be completed by children with cancer who are participating in an EPT and their parents, and may provide valuable insights that could guide personalized interventions.
Acknowledgments
Funding: This research study was generously funded with a Cincinnati Children’s Hospital Medical Center Carolyn Stoll Fund Nursing Research Grant ($5,000). Ms. Crane’s doctoral studies are supported by: Grants 1F31 NR015393A and 2T32 NR007066 from the National Institute of Nursing Research of the National Institutes of Health, and a Doctoral Scholarship in Cancer Nursing numbers DSCN-13-267-01- SCN and DSCNR-15-081-03-SCN from the American Cancer Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
STACEY CRANE, MSN RN CPON:
Ms. Crane is a predoctoral nursing fellow at Indiana University School of Nursing in Indianapolis, IN with seven years of experience as a phase I pediatric oncology clinical trial research nurse.
LORI BACKUS, BA CCRP:
Ms. Backus is the supervisor of the pediatric oncology developmental therapeutics research team at Cincinnati Children’s Hospital Medical Center.
BETH STOCKMAN, BSN RN:
Ms. Stockman is a clinical trial research nurse in the pediatric oncology developmental therapeutics research team at Cincinnati Children’s Hospital Medical Center.
JANET S. CARPENTER, PhD RN FAAN:
Dr. Carpenter is a Distinguished Professor at Indiana University and the Associate Dean for Research and Scholarship at Indiana University School of Nursing in Indianapolis, IN.
LI LIN, MS:
Li Lin is a statistician with the Research in Patient Services at Cincinnati Children’s Hospital Medical Center.
JOAN HAASE, PhD RN FAAN:
Dr. Haase is the Holmquist Professor in Pediatric Oncology Nursing and Co-Director for the Research in Palliative and End-of-Life Communication and Teaching (RESPECT) Signature Center at Indiana University in Indianapolis, IN.
Contributor Information
STACEY CRANE, Predoctoral Fellow, Indiana University School of Nursing, 600 Barnhill Dr., NU345, Indianapolis, IN, 46202.
LORI BACKUS, Supervisor, Pediatric Oncology Developmental Therapeutics Research Team, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH, 45229.
BETH STOCKMAN, Developmental Therapeutics Research Nurse, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH, 45229.
JANET S. CARPENTER, Distinguished Professor, Indiana University School of Nursing, 600 Barnhill Dr., NU340G, Indianapolis, IN, 46202.
LI LIN, Statistician, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH, 45229.
JOAN HAASE, Holmquist Professor in Pediatric Oncology Nursing, Indiana University School of Nursing, 600 Barnhill Dr., NUE431, Indianapolis, IN, 46202.
References
- Banks BA, Barrowman NJ, Klaassen R. Health-related quality of life: changes in children undergoing chemotherapy. Journal of Pediatric Hematology/Oncology. 2008;30(4):292–297. doi: 10.1097/MPH.0b013e3181647bda. [DOI] [PubMed] [Google Scholar]
- Bautista F, Di Giannatale A, Dias-Gastellier N, Fahd M, Valteau-Couanet D, Couanet D, … Geoerger B. Patients in pediatric phase I and early phase II clinical oncology trials at Gustave Roussy: A 13-year center experience. Journal of Pediatric Hematology Oncology. 2015;37(2):e102–110. doi: 10.1097/mph.0000000000000237. [DOI] [PubMed] [Google Scholar]
- Beardsmore S, Fitzmaurice N. Palliative care in paediatric oncology. European Journal of Cancer. 2002;38(14):1900–1910. doi: 10.1016/s0959-8049(02)00216-2. [DOI] [PubMed] [Google Scholar]
- Cohen MZ, Slomka J, Pentz RD, Flamm AL, Gold D, Herbst RS, Abbruzzese JL. Phase I participants’ views of quality of life and trial participation burdens. Supportive Care in Cancer. 2007;15(7):885–890. doi: 10.1007/s00520-007-0216-0. [DOI] [PubMed] [Google Scholar]
- Cox K. Researching research: patients’ experiences of participation in phase I and II anti-cancer drug trials. European Journal of Oncology Nursing. 1999;3(3):143–152. [Google Scholar]
- Eton DT, de Oliveira DR, Egginton JS, Ridgeway JL, Odell L, May CR, Montori VM. Building a measurement framework of burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Related Outcome Measures. 2012;3:39–49. doi: 10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the US. Supportive Care in Cancer. 2008;16(7):791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, DeLuca H, … Gross HE. PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood & Cancer. 2013;60(3):402–408. doi: 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- Kim A, Fox E, Warren K, Blaney SM, Berg SL, Adamson PC, … Widemann BC. Characteristics and outcome of pediatric patients enrolled in phase I oncology trials. Oncologist. 2008;13(6):679–689. doi: 10.1634/theoncologist.2008-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60(7):1651–1656. doi: 10.1002/1097-0142(19871001)60:7<1651::aid-cncr2820600738>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lee DP, Skolnik JM, Adamson PC. Pediatric phase I trials in oncology: An analysis of study conduct efficiency. Journal of Clinical Oncology. 2005;23(33):8431–8441. doi: 10.1200/jco.2005.02.1568. [DOI] [PubMed] [Google Scholar]
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research. 2011;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DR, Johnson LM, Mandrell BN, Yang J, West NK, Hinds PS, Baker JN. Does phase 1 trial enrollment preclude quality end-of-life care? Phase 1 trial enrollment and end-of-life care characteristics in children with cancer. Cancer. 2015 doi: 10.1002/cncr.29230. Retrieved from: Retrieved online from http://onlinelibrary.wiley.com/doi/10.1002/cncr.29230/pdf. [DOI] [PMC free article] [PubMed]
- Moore S. A need to try everything: Patient participation in phase I trials. Journal of Advanced Nursing. 2001;33(6):738–747. doi: 10.1046/j.1365-2648.2001.01715.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Hargrave D, Marshall LV, Gatz SA, Barone G, Crowe T, … Moreno L. Toxicity and outcome of children and adolescents participating in phase I/II trials of novel anticancer drugs: The Royal Marsden experience. Journal of Pediatric Hematology/Oncology. 2014;36(3):218–223. doi: 10.1097/mph.0000000000000003. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. NIH Clinical Research Trials and You. n.d Retrieved from http://www.nih.gov/health/clinicaltrials/basics.htm.
- Oberman M, Frader J. Dying children and medical research: Access to clinical trials as benefit and burden. American Journal of Law and Medicine. 2003;29(2–3):301–317. [PubMed] [Google Scholar]
- Sav A, Kendall E, McMillan SS, Kelly F, Whitty JA, King MA, Wheeler AJ. ‘You say treatment, I say hard work’: treatment burden among people with chronic illness and their carers in Australia. Health and Social Care in the Community. 2013;21(6):665–674. doi: 10.1111/hsc.12052. [DOI] [PubMed] [Google Scholar]
- Sav A, King MA, Whitty JA, Kendall E, McMillan SS, Kelly F, … Wheeler AJ. Burden of treatment for chronic illness: A concept analysis and review of the literature. Health Expectations. 2013 doi: 10.1111/hex.12046. Retrieved from: http://onlinelibrary.wiley.com/doi/10.1111/hex.12046/pdf. [DOI] [PMC free article] [PubMed]
- Schag CC, Heinrich RL, Ganz P. Karnofsky performance status revisited: Reliability, validity, and guidelines. Journal of Clinical Oncology. 1984;2(3):187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- Sherman EJ, Pfister DG, Ruchlin HS, Rubin DM, Radzyner MH, Kelleher GH, … Scher HI. The Collection of Indirect and Nonmedical Direct Costs (COIN) form: a new tool for collecting the invisible costs of androgen independent prostate carcinoma. Cancer. 2001;91(4):841–853. [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, … Goldsmith CH. A tutorial on pilot studies: The what, why and how. BMC Medical Research Methodology. 2010;10(1) doi: 10.1186/1471-2288-10-1. Retrieved from: http://www.biomedcentral.com/1471-2288/10/1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D, Hinds PS, Bartels U, Hendershot E, Sung L. Parent reports of quality of life for pediatric patients with cancer with no realistic chance of cure. Journal of Clinical Oncology. 2011;29(6):639–645. doi: 10.1200/JCO.2010.31.4047. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Seid M. The PedsQL(TM) as a pediatric patient-reported outcome: reliability and validity of the PedsQL(TM) Measurement Model in 25,000 children. Expert Review of Pharmacoeconomics & Outcomes Research. 2005;5(6):705–719. doi: 10.1586/14737167.5.6.705. [DOI] [PubMed] [Google Scholar]
- Varni JW, Katz ER, Seid M, Quiggins DJ, Friedman-Bender A. The pediatric cancer quality of life inventory-32 (PCQL-32): I. Reliability and validity. Cancer. 1998;82(6):1184–1196. doi: 10.1002/(sici)1097-0142(19980315)82:6<1184::aid-cncr25>3.0.co;2-1. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=ohst1&AN=9506367. [DOI] [PubMed] [Google Scholar]
- Varni JW, Limbers C, Burwinkle TM. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. Journal of Pediatric Psychology. 2007;32(9):1151–1163. doi: 10.1093/jpepsy/jsm008. [DOI] [PubMed] [Google Scholar]
- Varni JW, Limbers CA. The PedsQL™ 4.0 generic core scales young adult version: Feasibility, reliability and validity in a university student population. Journal of Health Psychology. 2009;14(4):611–622. doi: 10.1177/1359105309103580. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQLTM 4.0 Generic Core Scales: Sensitivity, responsiveness, and impact on clinical decision-making. Journal of Behavioral Medicine. 2002;25(2):175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Medical Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL Family Impact Module: preliminary reliability and validity. Health and quality of life outcomes. 2004;2(55) doi: 10.1186/1477-7525-2-55. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=ohstr&AN=15450120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M, Laliberte L, Morris J, Wiemann M. The Karnofsky performance status scale. Cancer. 1984;53:2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wootten AC, Abbott JM, Siddons HM, Rosenthal MA, Costello AJ. A qualitative assessment of the experience of participating in a cancer-related clinical trial. Supportive Care in Cancer. 2011;19(1):49–55. doi: 10.1007/s00520-009-0787-z. [DOI] [PubMed] [Google Scholar]
- Ziaian T, Sawyer MG, Reynolds KE, Carbone JA, Clark JJ, Baghurst PA, … Staugas RE. Treatment burden and health-related quality of life of children with diabetes, cystic fibrosis and asthma. Journal of Paediatrics and Child Health. 2006;42(10):596–600. doi: 10.1111/j.1440-1754.2006.00943.x. [DOI] [PubMed] [Google Scholar]