Abstract
Importance
Recently, tremendous prominence has been given to the investigation of the impact of different research processes as part of the Cancer Moonshot. More than half a century ago, the National Cancer Institute (NCI) established a network of publicly-funded cancer cooperative research groups to systematically evaluate new treatments for efficacy and safety.
Objective
To examine the extent to which positive NCI-sponsored cancer treatment trials have benefited cancer patients in the U.S. population.
Design
We used study data from SWOG, an NCI-sponsored Network cooperative research group. We identified all treatment trials over SWOG’s 60-year history (1956–2016) for which the new experimental therapy provided a statistically significant improvement in overall survival. We assumed the new, proven treatments from these trials established new standards for cancer care in the treatment community.
Setting
Twenty-three treatment trials were identified from a variety of different disease settings.
Main Outcomes and Measures
We estimated population life-years gained from the 23 treatment trials through 2015 by mapping the impact of the new treatments onto the U.S. cancer population, using an area-under-the-survival-curve approach that combined trial-specific hazard function and hazard ratio results, along with SEER and life-table data. Calculations were age-adjusted. Dollar return on investment was estimated as the ratio of total investment by the National Cancer Institute in the treatment trial program divided by the estimate of life-years gained.
Results
In total, 12,361 patients were enrolled to the 23 positive trials from 1965–2012. We estimated that 3.34 million years of life (95% confidence limits: 2.39–4.15 million) were gained from these 23 trials through 2015. Estimates were greater than 2 million life-years gained under 95% of model simulations. The dollar return on investment was about $125 per life year gained.
Conclusions and Relevance
SWOG treatment trials have had a substantial impact on population survival for cancer patients over 60 years. The National Cancer Institute’s investment in its cancer cooperative group research program has provided exceptional value and benefit to the American public through its research programs generating positive cancer treatment trials.
INTRODUCTION
Cancer is the leading cause of years of life lost in the U.S., exceeding all other diseases including heart disease.1 Cancer clinical trials represent a vital step in evaluating the efficacy and safety of new therapeutic approaches for malignancy. Treatments proven to work in clinical trials often become new standards of care for patients with cancer. More than half a century ago, the National Cancer Institute (NCI) established a network of publicly-funded cancer cooperative research groups to systematically evaluate new treatments for efficacy and safety.2 Little attempt has been made to quantify the impact of this clinical research system in terms of population survival gains.
SWOG, one of the original cooperative research groups, reached its 60-year anniversary in 2016. SWOG is a member of the NCI’s National Clinical Trials Network (NCTN) and Community Oncology Research Program (NCORP). SWOG was launched as a pediatric oncology group in 1956 under the name Southwest Cancer Chemotherapy Study Group (SWCCSG). In 1958, the NCI directed the SWCCSG to extend its mandate to adult malignancies. In 1973, the group was renamed the Southwest Oncology Group, later shortened to SWOG. Currently, SWOG conducts cancer treatment trials for a variety of different cancers in adults. SWOG currently has about 12,000 members from cancer clinics and centers at more than 650 institutions around the U.S., with more than 200,000 patients previously enrolled to SWOG trials and about 80 trials currently active.3
Recently, tremendous prominence has been given to the investigation of the impact of different research processes as part of the Cancer Moonshot.4 In this context, and given the history and durability of the cooperative group structure, we examined the extent to which positive SWOG cancer treatment trials over the decades have benefited cancer patients in the U.S. population, in terms of extending life.
METHODS
We first established the denominator of all randomized phase III treatment trials conducted by SWOG over the course of its 60 year history. The estimation of impact was based solely on new treatments derived from trials which showed a positive overall survival benefit in favor of the experimental arm according to the pre-specified protocol design. Overall survival was defined as the time from registration or the beginning of administration of chemotherapy until death due to any cause. Alive patients, including those lost to follow-up, were censored at the date of last contact if it occurred prior to 5 years. To better represent community practice, treatments with positive overall survival benefits that were too toxic for investigators to recommend as new treatment were excluded. The outcome of trials with respect to overall survival was identified either through article publication, internal reports of studies, or through analysis of patient level data available in the SWOG database. Information on ethical review and informed consent of participants for each of the trials was included in their study reports.
Analytical Framework
We used life-years gained as the primary measure. We estimated life-years gained from positive SWOG treatment trials using a counterfactual modeling approach. We assumed that any new, trial-proven treatment would become the new standard of care in the cancer treatment community beginning at the time of initial publication of the trial results. To establish a common impact across a panel of diverse cancers types, we assumed that the treatment effect from the new trial-proven treatment endured for 5 years, based on visual inspection of the survival functions by arm for the trials included in the analysis (data not shown). Also, we assumed that the beneficial impact of the new treatment would endure for all future patients with the given cancer diagnosis.
Estimation of Life-Years Gained
To estimate life-years gained, we mapped the treatment impact of new treatments onto the U.S. cancer population, using data from the SEER 9 registry areas.5 For each positive trial, we matched the major histology, stage, prior cancer, surgery, sex (where appropriate), age (i.e. ≥18 years), and tumor characteristic eligibility criteria from the SWOG trial to corresponding data in SEER to estimate the number of patients in the U.S. to whom the new treatment would apply. Calculations were conducted separately by 5-year age intervals, since the number of years of life an individual may live varies by age, and were further calculated for each yearly cohort of patients with a given cancer type beginning the year of trial publication until the end of the estimation period. Given the SEER 9 registry areas have consistently represented 9.5% of the U.S. population over time, we inflated each age-specific and year-specific estimate of the number of patients with a given cancer by approximately 10.5 (1/0.095).
We used the area under the Kaplan-Meier survival curve (AUSC) to estimate life-years lived for an average individual.6,7 Within the first five years after diagnosis (the “treatment benefit period”), the hazard rate for those receiving standard treatment was estimated from trial data (see below). The hazard rate for patients with the new treatment was generated using the hazard ratio for overall survival from the trial. To estimate residual AUSC in the post-treatment benefit period (i.e. after 5 years), we assumed, conservatively, that maximum survival extended only until average (age-specific) half-life, as indicated by life-table data.8 The estimation of residual AUSC was based on an exponential linear regression model (eta=exp(a+bx)) with points placed annually past the end of the treatment effect until median half-life. The convergence to approximately zero difference between the standard treatment and new treatment curves was parameterized by a point weight parameter at the final time point at median expected life. Higher weight parameters generated more rapidly converging curves and consequently fewer residual life-years gained. We chose a weight parameter of 100, at which overall estimates of life-years gained were found to achieve asymptotic stability; that is, further increases in the weight parameter had little impact on the area between the curves. The model is fit by non-linear least squares “nls” in R.9,10
The difference in the areas under the curve between those with standard treatment (STD) compared to those with the new, trial-proven treatment (EXP) represents the average life-years gained (LYG) for an average single individual due to the new treatment.
eFigure 1 gives a representative depiction for patients from SWOG trial S9008, showing how more LYG accrue to younger patients (1.58 for a 25-year-old patient from S9008) than for older patients (0.85 years for a 75-year-old patient). In particular, since more patients will be alive at the end of the treatment period for the experimental arm, life-years on average continue to accrue after the treatment effect has ended, until median half-life is attained, or until the end of the estimation period (i.e. 2015).
We partitioned age into 14 intervals (20–25, 26–30, 31–35, 36–40, 41–45, 46–50, 51–55, 56–60, 61–65, 66–70, 71–75, 76–80, 81–85, 85+). Within each age interval, there are ai individuals, and life-years gained for the individuals within the age interval i will be given by:
To obtain LYG within all 14 age intervals within a given year gives:
Separate calculations were performed for each successive year starting at initial trial publication. If Z is the number of years from trial publication to the end of the estimation period, then total life-years gained is represented by:
Forward projections for 2020, 2025, and 2030 were also calculated, but were based on positive trials identified through 2015 only.
Finally, we repeatedly conducted random sampling of the coefficient for the treatment effect from each trial, drawing from distributions based on the observed point estimate and its variation under a normal distribution. The upper and lower 2.5% quantiles from 500 iterations defined the 95% confidence limits (CLs).
Derivation of hazard rates and hazard ratios
To estimate the hazard rate for the standard arm, we obtained yearly point estimates for overall survival from patient-level trial data (if available) or from trial publications. In all instances, these point estimates were adjusted downward to better reflect survival patterns anticipated for patients in the cancer treatment population, given that trial-treated patients have been observed to have better survival than non-trial treated patients, predominantly in the first year (Supplementary Material).11 We used the hazard ratio if it was explicitly reported in trial publications; otherwise, the hazard ratio was estimated from patient-level trial data (if available), or based on overall survival point estimates reported in the main trial publication.
Additional and Sensitivity Analyses
The treatment effect from a SWOG trial with respect to OS may not have translated fully into the cancer treatment community. Prior evidence suggests that trial participation explains at most 5% of the variation in survival outcomes.11 Accordingly, we allowed the effectiveness of the translation to vary from as low as 75% of the hazard ratio for overall survival identified in the trial, up to 100%. We also allowed the effect of new treatment to vary from 3 to 7 years. Finally, we allowed the model weight parameter value to estimate residual AUSC to vary, with uninform probability, within the five category ranges 1:10, 11:50, 50:100, 100:200, and 200:1000.
Support of the cooperative group system represents a sizeable investment on the part of funding agencies. Therefore, we also estimated the dollar return on investment from funding agencies as the ratio of the estimated total dollars spent funding SWOG’s entire treatment trial program divided by the estimate of life-years gained over the estimation period. Federal direct funding to SWOG is publicly available by fiscal year from 1985 onward.12 Historical yearly funding amounts were inflated to constant 2015 dollars.13 In the absence of historical data prior to 1985, and given that SWOG core grants typically operate on 5-year cycles, we assumed that yearly funding prior to 1985 was equivalent to the yearly average over five years from 1985–1989, representing the earliest available funding data.
RESULTS
We identified n=205 randomized trials that were activated over the 60-year history of SWOG. Twelve of the trials are still ongoing, leaving n=193 total trials available for analysis. The trials examined a broad range of cancers and comprised 83,157 patient enrollments from January, 1962 through December, 2014. Twenty-three trials (12%) resulted in statistically significant improvement in overall survival for patients receiving the new, experimental therapy (Supplementary eFigure 2). The positive trials included 12,361 patients enrolled from 1965–2012 (eTable 1).14–36 Each of the primary experimental drugs are listed in the National Comprehensive Cancer Network (NCCN) Drugs and Biologics Compendium, except estramustine.37,38
Life-Years Gained
Figure 2 shows estimated life-years gained by study through 2015. Recently completed trials typically generated fewer life-years than older trials. Life-years gained through 2015 ranged from 313 for trial S0777 (which completed in 2015) to 748,000 for trial S7436. In contrast, by 2030, 102,000 life-years are projected to have been gained by S0777, and 1.26 million by S7436.
Figure 2.
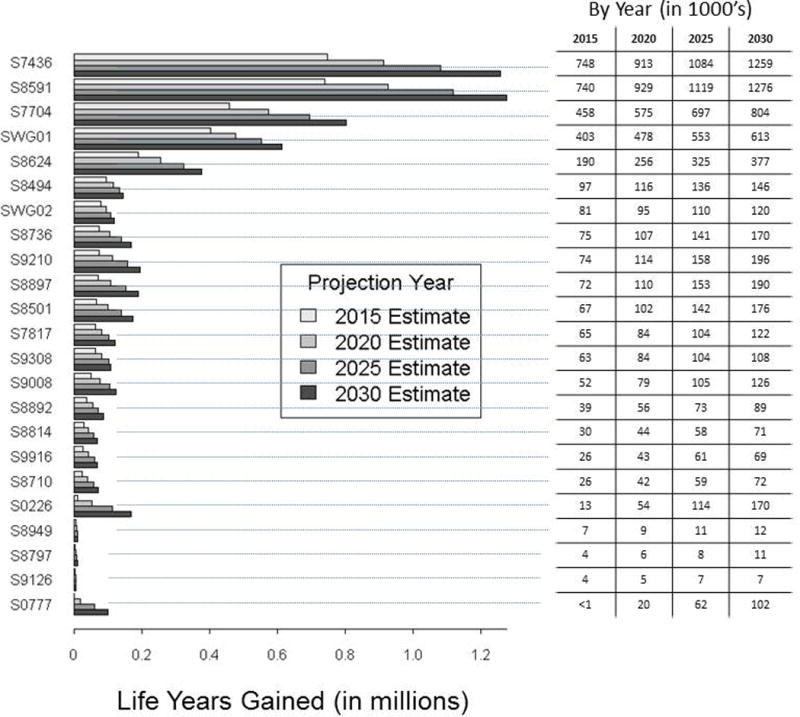
Life-years gained by study and projection year.
In total, cumulative life-years gained from all 23 positive trials is estimated to be 3.34 million (95% CLs: 2.39–4.15 million) through 2015. Figure 3 illustrates the pattern of cumulative life-years gained through 2015. Given the duration of time under examination, estimates of cumulative life-years gained were only modestly attenuated if new treatment uptake was assumed to occur after (rather than at) trial publication date (3.19 million if one year later; 3.04 million if 2 years later; 2.90 million if 3 years later). Life-years gained through 2020, 2025, and 2030 based on these 23 trials alone was projected to be 4.32 (95% CLs: 3.29–5.45 million), 5.38 (95% CLs: 3.98–6.73 million), and 6.29 million (95% CLs: 4.80–7.83 million) life-years, respectively. Thus the estimate of life-years gained will nearly double over the next 15 years.
Figure 3.
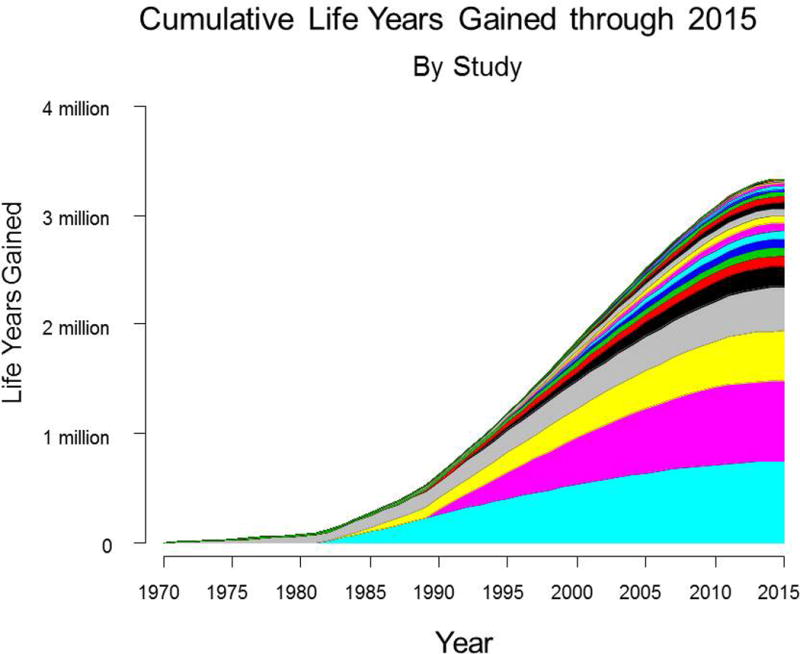
Cumulative life-years gained through 2015, by study. Each color-coded area represents cumulative life-years for a given study.
Variation in Life-Years Gained by Factors
We allowed the duration of the treatment effect to vary from 3 to 7 years by one year intervals, the effectiveness to vary from 75% to 100% of the efficacy hazard ratio, and the weight parameter to vary as specified in the Methods. The results for life-years gained through 2015 are shown in eFigure 5, indicating that life-years gained was greater than 2 million under most combinations of duration of treatment effect, effectiveness, and weight parameter assumptions.
Return on Investment
We estimated the dollar return on investment for funding agencies through support of a cooperative cancer clinical trials group. Based on the estimation method we specified, the total federal investment in SWOG treatment trials over the course of its 60-year history was $418 million. This amount includes the cost for conducting all trials during the period, including negative trials. The dollar return on investment for funding agencies is therefore the ratio of total expenditures to life-years gained, or $125 per life-year gained through 2015. Estimates for 2020, 2025, and 2030 are $113, $106, and $104 per life-year gained, respectively.
DISCUSSION
Over the course of its 60-year history, SWOG treatment trials with positive results for overall survival are estimated to have generated 3.34 million life-years gained in the U.S. The estimated dollar return on investment was $125 per life-year gained. Thus SWOG treatment trials have had a sizeable impact on population survival for cancer patients at a modest cost.
The national cancer mortality rate in the U.S. has decreased by 25% since 1991, in part due to advances in treatment.39 Our findings are consistent with this observation, with the vast majority (84%) of the estimated 3.34 million life-years gained occurring since the 1990s. To provide additional context, the life-year gains from positive SWOG trials have returned approximately 1% of the estimated 360 million years of life lost due to cancer since 1969 (Supplemental Material).1,40 Alternatively, 3.34 million life-years gained would be sufficient to provide each of the approximately 600,000 individuals who died of cancer in the U.S. in 2016 with 5.6 more years of life.41 Our estimate may be conservative, since the model did not account for life-years gained from treatments showing better progression-free, recurrence-free, or disease-free survival. Such endpoints are frequently used in trials as more timely measures for the impact of treatment. Some treatments showing benefits in these outcomes in trials would also have generated survival gains in the population. Moreover, there are three other major NCI-sponsored adult cancer cooperative groups in addition to SWOG (Alliance, ECOG-ACRIN, and NRG), and one major pediatric cancer group (Children’s Oncology Group); each of these groups also have long histories of successful conduct of trials, leading to sizeable gains in population survival.42–45
The total budget of the NCI was $5.2 billion in 2016, of which $151 million was designated for the NCI’s National Clinical Trials Network.46 Only a portion of that funding is further designated towards the conduct of phase III cancer treatment trials, even as national expenditures for cancer care in the U.S. were nearly $125 billion.47 Our evidence indicates that the cost of this investment per life-year gained is very low ($125), an estimate that will continue to decrease over time given that population life-years gained from prior positive trials is growing at a faster rate than costs for trial conduct.
Investments in cooperative group clinical trials have benefited patients in ways that were not included in our model. Clinical trial discoveries have led to reductions in treatment toxicity and cancer morbidity, especially in the new era of targeted treatments. Large prevention trials conducted by the cooperative groups have identified interventions to prevent the development of new cancers, and have shown that commonly used drugs and supplements are ineffective and even harmful.48–50 Decades of NCI-sponsored clinical trial design, monitoring, and analysis has generated important insights into the science of trial conduct, which has benefited the broader clinical trial community. In an era of increased data sharing, the clinical trial databases of the cancer cooperative groups promise more important insights, which could serve as the foundations and hypotheses for future studies.51
Our model for calculating life-years gained is limited due to its simplified representation of the complex manner in which new, trial-proven treatments translate to the cancer population, and required modeling assumptions that do not fully represent real-world settings. We assumed that each of the trials changed practice, an assumption reinforced by the observation that nearly all of the trial-proven treatments were included in common medical compendia, enabling payment coverage by Medicare.52,53 However it is likely that not all of the trials changed practice alone; in some instances, trials from other research groups may also have provided positive supporting evidence for the new treatments. The model did not reflect the value of negative trials.54 As one example, many thousands of lives have likely been saved based on randomized clinical trials showing that autologous bone-marrow transplant for breast cancer – a treatment which had achieved wide dissemination in the cancer treatment community despite lack of conclusive evidence in trials – in fact did not work.55–57 Finally, the total research costs (as opposed to the phase III trial costs alone) per life year gained for any new drug is inevitably higher, since the conduct of a phase III comparative trial represents the end of the life cycle of treatment development, which also includes drug discovery, development and early stage trials. However, even accounting for these additional development costs, the investment dollar per life-year gained is likely still modest, since phase III trial costs comprise a substantial portion of total drug development costs.58–60
These findings quantify the impact of one of NCI’s large cooperate cancer clinical trial research groups on people touched by cancer. They show that the combined efforts of patient volunteers, clinical researchers, and cancer scientists have had a measurable impact on extending life for cancer patients. Moreover, the amount of the investment required to produce these gains has been relatively modest. Thus the NCI’s investment in its cancer cooperative group research program has provided exceptional value and benefit to the American public through its research programs generating positive cancer treatment trials.
Supplementary Material
Key Points.
Question
How have the NCI-sponsored Network cooperative cancer research groups benefitted patients with cancer in the general population?
Findings
In total, using data from 23 positive treatment trials from SWOG, we estimated that 3.34 million years of life were gained in the cancer population through 2015, at a cost of $125 per life year gained.
Meaning
The National Cancer Institute’s investment in its cancer cooperative group research program has provided exceptional value and benefit to the American public through its research programs generating positive cancer treatment trials.
Acknowledgments
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding/Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the following grant award numbers: 5U10CA180888-03 (CDB) and 5U10CA180819-03 (ML). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ClinicalTrials.gov registry numbers (where available): NCT00004001, NCT00075764 and NCT00644228
Author Contributions: Dr Unger had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: None reported.
References
- 1.National Cancer Institute. Cancer Trends Progress Report, Person-Years of Life Lost. Online at: http://www.progressreport.cancer.gov/end/life_lost.
- 2.IOM (Institute of Medicine) A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 3.SWOG Cancer Research: Fast Facts. 2014 Online at: http://www.swog.org/Media/SWOG-Fast-Facts.pdf.
- 4.Cancer Moonshot Blue Ribbon Panel (NCI, 2016) Online at: www.cancer.gov/brp.
- 5.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2013) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016 based on the November 2015 submission.
- 6.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Amer Statist Assn. 1958;53(282):457–481. [Google Scholar]
- 7.Lee ET. Nonparametric methods of estimating survival functions. In: Lee ET, Wang JW, editors. Statistical Methods for Survival Data Analysis. 3rd. Hoboken, NJ: John Wiley & Sons; 2003. pp. 73–76. [Google Scholar]
- 8.CDC. National Center for Health Statistics – Life Tables. http://www.cdc.gov/nchs/products/life_tables.htm.
- 9.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. URL https://www.R-project.org/ [Google Scholar]
- 10.Bates DM, Chambers JM. Nonlinear models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Wadsworth & Brooks/Cole; 1992. [Google Scholar]
- 11.Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3) doi: 10.1093/jnci/dju002. dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH Research Portfolio Online Reporting Tools (RePORT) https://projectreporter.nih.gov/reporter.cfm.
- 13.United States Department of Labor, Bureau of Labor Statistics: Databases, Tables & Calculators by Subject. CPI Inflation Calculator. http://www.bls.gov/data/inflation_calculator.htm.
- 14.Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969 Jun 2;208(9):1680–5. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb JA, Rivkin SE, Spigel SC, et al. Superiority of Adriamycin over oral nitrosoureas in patients with advanced breast carcinoma. A Southwest Cancer Chemotherapy Study Group Study. Cancer. 1974 Feb;33(2):519–526. doi: 10.1002/1097-0142(197402)33:2<519::aid-cncr2820330229>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Glucksberg H, Rivkin SE, Rasmussen S, et al. Combination chemotherapy (CMFVP) versus L-phenylalanine mustard (L-PAM) for operable breast cancer with positive axillary nodes: A Southwest Oncology Group Study. Cancer. 1982 Aug 1;50(3):423–34. doi: 10.1002/1097-0142(19820801)50:3<423::aid-cncr2820500307>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Salmon SE, Haut A, Bonnet JD, et al. Alternating combination chemotherapy and levamisole improves survival in multiple myeloma: A Southwest Oncology Group Study. J Clin Oncol. 1983 Aug 1;(8):453–61. doi: 10.1200/JCO.1983.1.8.453. [DOI] [PubMed] [Google Scholar]
- 18.Samson MK, Rivkin SE, Jones SE, et al. Dose-response and dose-survival advantage for high versus low-dose cisplatin combined with vinblastine and bleomycin in disseminated testicular cancer. A Southwest Oncology Group study. Cancer. 1984 Mar 1;53(5):1029–35. doi: 10.1002/1097-0142(19840301)53:5<1029::aid-cncr2820530503>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989 Aug 17;321(7):419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 20.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996 Dec 26;335(26):1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 21.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990 Feb 8;322(6):352–8. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 22.Salmon SE, Crowley JJ, Grogan TM, Finley P, Pugh RP, Barlogie B. Combination chemotherapy, glucocorticoids, and interferon alfa in the treatment of multiple myeloma: A Southwest Oncology Group study. J Clin Oncol. 1994 Nov;12(11):2405–14. doi: 10.1200/JCO.1994.12.11.2405. [DOI] [PubMed] [Google Scholar]
- 23.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003 Aug 28;349(9):859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 24.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998 Jul 2;339(1):21–6. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 25.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000 Apr;18(8):1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 26.Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009 Dec 19;374(9707):2055–63. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998 Apr;16(4):1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 28.Hutchins LF, Green SJ, Ravdin PM, et al. Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol. 2005 Nov 20;23(33):8313–21. doi: 10.1200/JCO.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 29.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001 Dec 6;345(23):1655–9. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001 Sep 6;345(10):725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 31.List AF, Kopecky KJ, Willman CL, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: A Southwest Oncology Group study. Blood. 2001 Dec 1;98(12):3212–20. doi: 10.1182/blood.v98.12.3212. [DOI] [PubMed] [Google Scholar]
- 32.Berenson JR, Crowley JJ, Grogan TM, et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood. 2002 May 1;99(9):3163–8. doi: 10.1182/blood.v99.9.3163. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: A Southwest Oncology Group study. J Clin Oncol. 1998 Jul;16(7):2459–65. doi: 10.1200/JCO.1998.16.7.2459. [DOI] [PubMed] [Google Scholar]
- 34.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 35.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012 Aug 2;367(5):435–44. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durie BG, Hoering A, Rajkumar SV, et al. Bortezomib, Lenalidomide and Dexamethasone vs. Lenalidomide and Dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Results of the randomized phase III trial SWOG S0777. Blood (ASH Annual Meeting 177 Abstracts) 2015:653. doi: 10.1038/s41408-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network (NCCN) NCCN Drugs & Biologics Compendium (NCCN Compendium®) https://www.nccn.org/professionals/drug_compendium/content/contents.asp? Accessed February 16, 2017.
- 38.Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J. 2010 Dec;4(6):380–4. doi: 10.5489/cuaj.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 Jan;67(1):7–30. doi: 10.3322/caac.21387. Epub 2017 Jan 5. [DOI] [PubMed] [Google Scholar]
- 40.Horm JW, Sondik EJ. Person-years of life lost due to cancer in the United States, 1970 and 1984. American Journal of Public Health. 1989 Nov;79(11):1490–1493. doi: 10.2105/ajph.79.11.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 42.Alliance for Clinical Trials in Oncology. https://www.allianceforclinicaltrialsinoncology.org/main/
- 43.ECOG-ACRIN Research Group. http://ecog-acrin.org/
- 44.NRG Oncology. https://www.nrgoncology.org/
- 45.Children’s Oncology Group. https://www.childrensoncologygroup.org/
- 46.National Cancer Institute budget and appropriations. https://www.cancer.gov/about-nci/budget.
- 47.National Cancer Institute cancer prevalence and cost of care projections. https://costprojections.cancer.gov/expenditures.html.
- 48.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009 Jan 7;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 50.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 51.Warren E. Strengthening Research through Data Sharing. N Engl J Med. 2016 Aug 4;375(5):401–3. doi: 10.1056/NEJMp1607282. [DOI] [PubMed] [Google Scholar]
- 52.Omnibus Budget Reconciliation Act of 1993. www.gpo.gov/fdsys/pkg/BILLS-103hr2264enr/pdf/BILLS-103hr2264enr.pdf. Accessed February 10, 2017.
- 53.Center for Medicare and Medicaid Services regulations and guidance transmittal. Subject: “Compendia and Authoritative Sources for use in the Determination of a ‘Medically Accepted Indication’ of Drugs and Biologicals Used Off-Label in an AntiCancer Chemotherapeutic Regimen”. 2008 Oct 24; http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r96bp.pdf. Accessed February 10, 2017.
- 54.Unger JM, Barlow WE, Ramsey SD, LeBlanc M, Blanke CD, Hershman DL. The scientific impact of positive and negative phase 3 cancer clinical trials. JAMA Oncol. 2016 Jul 1;2(7):875–81. doi: 10.1001/jamaoncol.2015.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stadtmauer EA, O’Neill A, Goldstein LJ, et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. Philadelphia Bone Marrow Transplant Group. N Engl J Med. 2000 Apr 13;342(15):1069–76. doi: 10.1056/NEJM200004133421501. [DOI] [PubMed] [Google Scholar]
- 56.Farquhar C, Marjoribanks J, Lethaby A, Azhar M. High-dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database Syst Rev. 2016 May;20(5):CD003139. doi: 10.1002/14651858.CD003139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rettig RA, Jacobson PD, Farquhar CM, Wade MA. False hope: bone marrow transplantation for breast cancer. New York: Oxford University Press; 2007. [Google Scholar]
- 58.DiMasi JA, Hansen RW, Grabowski HG. The Price of Innovation: New Estimates of Drug Development Costs. Journal of Health Economics. 2003;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 59.DiMasi JA, Grabowski HG. The Cost of Biopharmaceutical R&D: Is Biotech Different? Managerial and Decision Economics. 2007;28(4–5):469–479. [Google Scholar]
- 60.Pharmaceutical Research and Manufacturers of America. Annual Report 2011. 2011 http://www.phrma.org/sites/default/files/159/phrma_2011_annual_report.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


