Abstract
Objectives
Fibroblast-like synoviocytes (FLS) produce key synovial fluid and tissue components to ensure joint integrity under healthy conditions, whereas they become cancer-like and aggressively contribute to joint degeneration in inflammatory arthritis. Here we asked whether the SOXC transcription factors SOX4 and SOX11, whose functions are critical in joint development and many cancer types, contribute to FLS activities under normal and inflammatory conditions.
Methods
We inactivated the SOXC genes in FLS of adult mice and studied consequences on joint homeostasis and tumor necrosis factor-alpha (TNFα)-induced arthritis. We used primary cells and synovial biopsies from arthritis patients to analyze the interactions between inflammatory signals and SOXC proteins.
Results
Postnatal inactivation of the SOXC genes had no major consequence on joint integrity in otherwise healthy mice. However, it hampered synovial hyperplasia and joint degeneration in transgenic mice expressing human TNFα. These effects were explained by the ability of SOX4/11 to amplify the pathogenic impact of TNFα on FLS aggressiveness, including increased cell survival and migration. SOXC RNA levels were unchanged by TNFα and other pro-inflammatory cytokines, but their proteins were strongly stabilized and able to potentiate the TNFα-induced upregulation of genes involved in FLS transformation. Substantiating the human disease relevance of these findings, the SOXC protein, but not RNA levels, were significantly higher in inflamed than non-inflamed synovium from arthritic patients.
Conclusion
SOXC proteins are targets and pivotal mediators of pro-inflammatory cytokines during FLS transformation in arthritic diseases. Their targeting could thus improve current strategies to treat these and possibly other inflammatory diseases.
The internal compartment of joint capsules, known as the synovium, is made up of synovial lining, rich in fibroblasts, and synovial intima, a loose connective tissue with interspersed fibroblasts and endothelial cells. The synovial lining and intima fibroblasts are collectively referred to as fibroblast-like synoviocytes (FLS). In healthy joints, FLS produce major constituents of the synovial fluid and synovial tissue extracellular matrix, including lubricin (encoded by Prg4), hyaluronan and heparan sulfate proteoglycans (1, 2). Apart from ensuring joint health, FLS actively contribute to the pathogenesis of chronic inflammatory joint diseases, including but not limited to rheumatoid arthritis, psoriatic arthritis and gout (3). They also contribute to the synovitis that worsens cartilage degeneration in osteoarthritis (4). In all these diseases, pro-inflammatory cytokines transform FLS into aggressive, joint-destroying cells, which increase in numbers, invade joint spaces and tissues, and produce enzymes that directly participate in the degradation of articular cartilage and subchondral bone. These cells also secrete cytokines and chemo-attractants that recruit immune cells into the hypertrophied, granulation synovial tissue, then referred to as synovial pannus. In this transformed state, FLS strikingly resemble metastatic cancer cells (3–6). To date, therapies directly aimed at preventing or reverting FLS transformation are yet to be developed. The lack of such therapies is largely due to insufficient understanding of the molecular mechanisms underlying FLS behavior in both healthy and transformed conditions.
SOX4, SOX11, and SOX12 form the group C of transcription factors containing an SRY-related high-mobility-group box (SOX) DNA-binding domain (7, 8). SOXC proteins share more identity with one another than with other SOX proteins, and they possess a unique type of transactivation domain. This domain is more potent in SOX4 and SOX11 than in SOX12 (9). Sox4 and Sox11 are primarily co-expressed in various types of multi-potent progenitor cells, and they were shown in mice to act largely in redundancy to determine the behavior and survival of these cells. Sox4-null, Sox11-null and Sox4/11 double conditional null mutant mice therefore exhibit major defects in the development of organs such as the skeleton, heart, brain and eyes (10–15). Although it is often co-expressed with Sox4 and Sox11, Sox12 only provides a minor contribution to these functions, and Sox12-null mice appear normal throughout life (10). SOX4 and SOX11 have been shown to be highly expressed in prostate, breast, leukemia, colorectal and other forms of cancer. SOX4 has been recognized as a master regulator of cell proliferation and metastasis in several cancer types, and SOX11 as a poor prognosis marker in lymphoma and breast cancer subtypes (16–20). To date, however, the importance of SOXC proteins in other adult processes, whether physiological or pathological, remains largely unknown.
We previously showed that conditional inactivation of the SOXC genes in mouse embryos drastically affected the survival and fate determination of skeletogenic cells, and resulted in a cartilaginous skeleton that failed to grow and ossify and that also completely lacked joints (12). We therefore speculated that the SOXC genes could also have critical roles in the adult skeleton. Focusing on FLS and using mouse and in vitro models as well as arthritic patient biopsies, we here demonstrate that SOX4 and SOX11 are direct targets and mediators of pro-inflammatory cytokines and are thereby required for the cancer-like transformation of FLS that plagues arthritic joints.
MATERIALS AND METHODS
Mice
Mice were used as approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Mice carrying Sox4 and Sox11 conditional-null alleles and Sox12 null alleles were as described (11, 21). The Sox4 and Sox11 conditional null alleles contain loxP sites that flank the entire coding region of the genes. Thus, upon recombination by CRE recombinase, no protein is produced from these alleles. Mice producing human TNFα carried two transgenes (22). One transgene expressed the rtTA2S Tet-ON advanced transactivator upon doxycycline treatment, and the other expressed TNFα in an rtTA2S-dependent manner. These mice received drinking water supplemented with 0.5 mg doxycycline/ml (Sigma) and 5% sucrose for 2 weeks. Prg4CreERt2 mice were previously described (23). CRE recombinase was activated by intraperitoneal injection of 1 mg tamoxifen (Sigma-Aldrich) dissolved in olive oil per 10 g body weight.
Mouse FLS cultures
FLS were isolated by digesting interphalangeal joint synovium with 1 mg/ml collagenase IV (Sigma-Aldrich) for 2 h at 37°C (24). Cells were cultured in DMEM with 10% FCS (Invitrogen) and utilized at passage 4. They were immunophenotyped by flow cytometry (Becton-Dickinson LSRII instrument) using FITC-labeled CD90.2 and APC-conjugated CD14 antibodies (Table S1).
Histology and RNA in situ hybridization
Paraffin sections of mouse limbs were generated after tissue fixation in 4% paraformaldehyde for 48 h, followed by demineralization in 0.5 M EDTA (pH 7.5) for 2 weeks. They were deparaffinized with Histo-Clear (National Diagnostics) and rehydrated in graded ethanol solutions. Staining with Lerner-3 Hematoxylin (Lerner Laboratories) and Eosin Y (Polysciences) was performed according to manufacturer’s instructions. Safranin-O (Sigma-Aldrich) at 0.1% in water was used to stain cartilage proteoglycans. RNA in situ hybridization was performed using DIG-labeled and BM-purple substrate (Roche) (25). Sox4, Sox11 and Prg4 anti-sense RNA probes were previously described (9, 26). Cell death was assessed using a fluorescent-TUNEL assay kit (Invitrogen). Cell proliferation was detected by EdU incorporation following intraperitoneal injection of 5 mg EdU (Invitrogen)/10 g body and detected using a Click-IT assay kit (Invitrogen). Slides were mounted using Permount (ThermoFisher Scientific) or DAPI-containing Vectashield (Vector Laboratories). Data were visualized with a microscope (DM2500; Leica) and captured with a digital camera (MicroPublisher 5.0 Real-Time Viewing; QImaging) using a 10X objective lens. All images were acquired using QCapture Pro 6.0 (QImaging) and processed with Photoshop 7.0 (Adobe) software.
Human synovium and FLS
Synovium was collected from osteoarthritis (OA) patients undergoing total knee arthroplasty as approved by the Cleveland Clinic Institutional Review Board (Table S3). Inflamed and non-inflamed synovium were distinguished based on gross appearance and increased expression of interleukin-6 (IL6). FLS were isolated by digesting tissues for 3 h with 1 mg/ml Collagenase IV (Sigma-Aldrich) solution ([24]). They were cultured in DMEM containing 10% FCS and utilized at passage 4. RA-FLS were generated by digesting synovial biopsies of patients diagnosed with rheumatoid arthritis (RA) and were obtained by arthroscopic biopsy at the Academic Medical Centre Amsterdam, Netherlands (27). They were utilized in experiments at passage 6 or 7.
In vitro assays
Replication-deficient adenoviral particles encoding 3FLAG-SOX11, E. coli β-galactosidase (lacZ product), or CRE recombinase were generated by The University of Iowa Gene Transfer Vector Core (12). Cells were infected with 100 plaque-forming units/cell. For transient transfection of plasmids encoding FLAG-SOX4 and FLAG-SOX11, 3x105 SW982 cells (human synovial fibroblasts, ATCC) were exposed to mixtures containing 1 μg plasmid and 3 μl FuGENE6 (Roche) for up to 24 h[9]. Cells were treated with recombinant human TNFα, IL1α, IL6 or IL10 (R&D Biosystems), MG132 and cycloheximide (Sigma-Aldrich) as described in Results. Cell migration was assessed using a scratch assay. FLS were grown to confluence in 12-well dishes in DMEM containing 10% FCS, followed by overnight culture in medium containing 0.5% FCS. Wounding was performed with a 200-μL pipet tip. Cells were treated with recombinant proteins for 4 h, fixed in 4% formaldehyde, and stained with Trypan blue. SDS-PAGE and semi-dry western blotting on nitrocellulose membranes (LI-COR Biosciences) were performed under standard conditions (Table S1). Signals were detected using ECL Prime Western Blotting Reagent (Life Technologies).
RT-qPCR
Total RNA was prepared using TRIzol (Life Technologies) and RNeasy Mini Kit (QIAGEN). cDNA was synthesized using Superscript IV First-Strand Synthesis System (Life Technologies) and amplified with primers (Table S2) using SYBR Green PCR Master Mix (Applied Biosystems). PCR conditions were 1 cycle at 95°C for 10 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. Relative mRNA levels were calculated using the delta Ct method.
Statistical analysis
All data presented in this study are representative of two or more independent experiments. Quantitative data are presented as mean with standard deviation of at least triplicate biological samples. The two-tailed Student’s t-test was used to calculate the significance of sample differences in mouse and in vitro assays. Two-tailed paired t-test was used to calculate significance of differences between inflamed and non-inflamed human synovial tissues. p <0.05 was considered significant.
RESULTS
Sox4 and Sox11 are expressed in adult mouse FLS
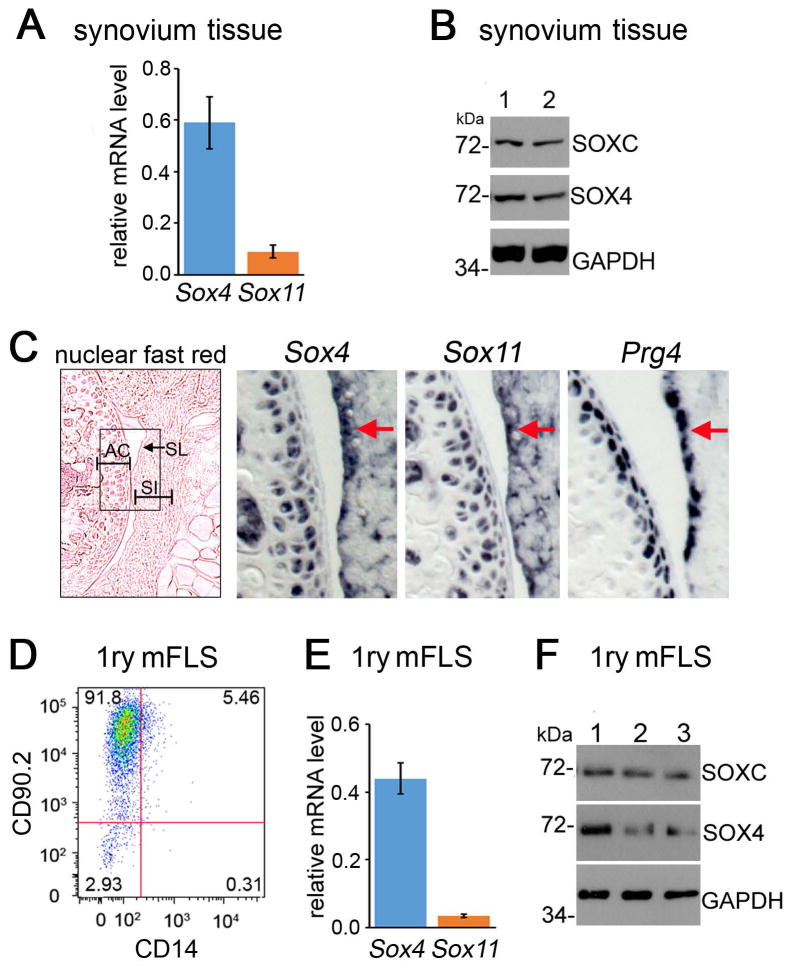
We started the study by analyzing the expression of Sox4 and Sox11 in the joints of 6-week-old mice. RT-qPCR assays revealed that both genes were expressed in synovium (Figure 1A). Their proteins were readily detected in western blot using a SOX4-specific antibody and a pan-SOXC antibody, which binds SOX11 more efficiently than SOX4 (Figures 1C and S1). This pan-SOXC antibody did not allow distinguishing SOX11 from SOX4 because both proteins exhibit an apparent molecular weight of 72 kDa in SDS-PAGE. RNA in situ hybridization showed that the expression domains of Sox4 and Sox11 overlapped with that of Prg4 (encoding lubricin) in the synovial lining, and also included the synovial intima and articular cartilage (Figure 1C). FLS account for the majority of cells present in healthy synovium[2]. They thus likely generated most, if not all, of the signals detected for SOX4 and SOX11 in whole-synovium samples. To support this conclusion, we established primary cultures of adherent synovial cells at passage4. Flow cytometry analysis indicated that most cells were FLS, as 92% expressed the fibroblastic cell surface marker CD90.2 and only 4% expressed the CD14 macrophage marker (Figure 1D). Sox4 and Sox11 mRNAs were detected in these cells (Figure 1D to F). We concluded that Sox4 and Sox11 are expressed in FLS and therefore could have important roles in FLS physiology in adult mice.
Figure 1.
SOX4 and SOX11 are expressed in mouse FLS. A, Levels of Sox4 and Sox11 RNAs measured by RT-qPCR and normalized for Gapdh RNA levels. B, Assay of SOXC proteins present in whole-synovium lysates from two wild-type mice (1 and 2). Western blots were hybridized with SOX4-specific and pan-SOXC antibodies. GAPDH detection is shown as loading control. The molecular weight of protein standards running close to the proteins of interest is indicated. C, In situ hybridization of adjacent knee sections from 6-week-old mice with Sox4, Sox11 and Prg4 RNA probes. Left panel, section stained with nuclear fast red. The box corresponds to the areas magnified in the other panels. Arrows and SL, synovial lining; SI, synovial intima; AC, articular cartilage. D, Flow cytometric phenotyping of mouse primary synovial cells at passage 4 with FITC-conjugated CD90.2 and APC-conjugated CD14 antibodies. E, Relative levels of Sox4 and Sox11 RNAs measured by RT-qPCR in similar cells as in panel D. F, SOX4 and SOX11 proteins detected in western blots of whole lysates from cells immunophenotyped in panel D.
SOXC expression in the Prg4+ cell lineage may be dispensable for joint integrity in young adult mice
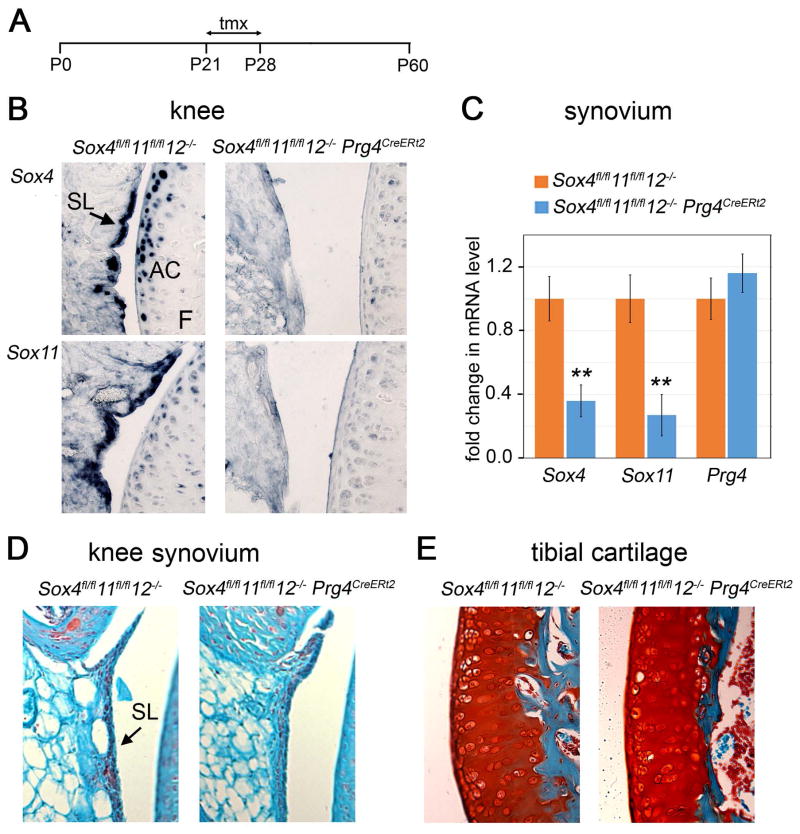
To test whether SOXC genes have a role in Prg4+ cells in juvenile and young adult joints, we generated mice harboring Sox4 and Sox11 conditional-null alleles, Sox12 null alleles, and a Prg4 allele that features a GFPCreERt2 cassette inserted into its translation initiation codon. This Prg4CreERt2 allele was previously shown to generate tamoxifen-dependent CRE recombinase activity in the synovium lining, superficial articular chondrocytes, and tendons of adult mice (23). SOXC mutant mice and age/gender-matched control mice were injected with tamoxifen at 3 to 4 weeks of age and analyzed for joint defects at 2 months of age (Figure 2A). RNA in situ hybridization showed that, as expected, the expression of Sox4 and Sox11 was downregulated in the synovium and articular chondrocytes of Sox4fl/fl11fl/fl12−/−Prg4CreERt2 mouse knees (Figure 2B). RT-qPCR assays evaluated the efficiency of Sox4 and Sox11 inactivation in the synovium at 65–75%, whereas Prg4 expression was not changed (Figure 2C). Moreover, no obvious histological changes were seen in the synovial lining and articular cartilage (Figure 2D and E). Together, these data suggested that the SOXC genes are dispensable in synovium- and cartilage-lining cells for joint integrity in young adult mice.
Figure 2.
Inactivation of SOXC genes in the Prg4+ cell lineage does not affect joint integrity in young mice. A, Schematic of experimental timeline. Tmx, tamoxifen injections. P0 to P60, postnatal days 0 to 60. B, In situ hybridization of adjacent knee sections with Sox4, and Sox11 RNA probes in mice at P60. C, Fold changes in relative levels of Sox4 and Sox11 RNAs in pools of knee and hip synovium tissue, as assessed by RT-qPCR. 3 mice were used for each genotype. *, p<0.05; **, p<0.01. D and E, Safranin-O staining (cartilage matrix) and fast green counterstaining of knee synovium and tibial plateau articular cartilage, respectively.
SOXC inactivation hinders TNFα-induced arthritis in mice
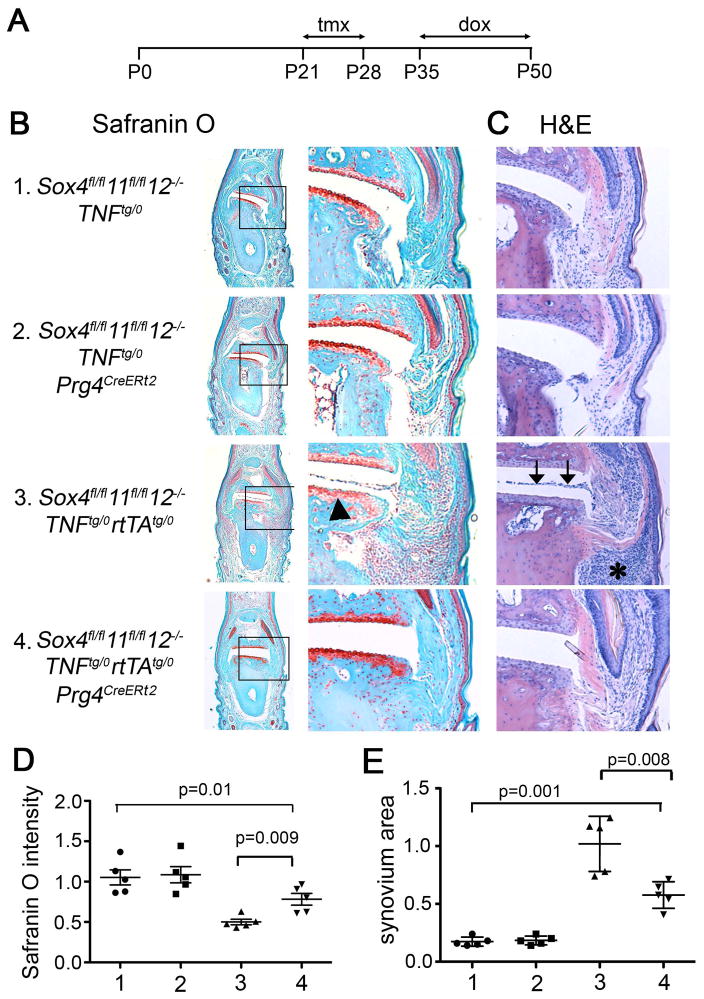
To investigate the roles of the SOXC genes in joints under the stress of inflammation, we generated Sox4fl/f11fl/fl12−/−Prg4CreERt2Tnftg/0rtTAtg/0 mice and compared them to Sox4fl/fl11fl/fl12−/−Tnftg/0 control mice (Figure S2A). Mice containing Tnftg and rtTAtg were previously shown to widely express human TNFα upon doxycycline administration and to develop inflammatory arthritis in interphalangeal joints and psoriasis-like inflammation in nail beds (22). We injected our mice with tamoxifen at 3 to 4 weeks of age, treated them with doxycycline from 5 weeks of age, and analyzed them at 7 weeks of age (Figure 3A). External analysis of the forepaws did not indicate any abnormality in SOXC-deficient (Sox4fl/fl11fl/fl12−/−Tnftg/0Prg4CreERt2) and SOXC-control mice (Sox4fl/fl11fl/fl12−/−Tnftg/0) (Figure 3B). Histological analysis of interphalangeal joints upon Safranin-O staining revealed no significant loss of proteoglycan in articular cartilage of Sox4fl/fl11fl/fl12−/−Tnftg/0Prg4CreERt2 mice (Figure 3C and D). H& E staining also revealed no differences in the synovial lining cellularity. Thus, these data, obtained for interphalangeal joints, corroborated the conclusion reached upon analyzing knee joints (Figure 2) that the SOXC genes are not critically needed for joint health in young adult mice. As expected, the interphalangeal joints of Sox4fl/fl11fl/fl12−/−Tnftg/0rtTAtg/0 mice exhibited articular cartilage damage, increased synovium cellularity, and invasion of the synovial cells into the joint cavity (Figure 3C to E). In contrast, Sox4fl/fl11fl/fl12−/−Tnftg/0rtTAtg/0Prg4CreERt2 mice had significantly reduced cartilage erosion and synovial hyperplasia, compared to Sox4fl/fl11fl/fl12−/−Tnftg/0rtTAtg/0 mice. These differences in synovium and cartilage disease status between SOXC-control and SOXC-deficient mice under the condition of TNFα expression could not be attributed to differences in the serum levels of TNFα levels of in mice of both genotypes (Figure S2B). Accordingly, the digits of TNFα-expressing SOXC-deficient mice (Sox4fl/fl11fl/fl12−/−Tnftg/0rtTAtg/0 Prg4CreERt2) were as swollen as those of TNFα-expressing SOXC-control mice (Sox4fl/fl11fl/fl12−/−Tnftg/0rtTAtg/0). Moreover, the extent of hyperplasia under the forepaw nail beds, where Prg4CreERt2 is not active, was similar in both mouse types (Figure S2C). Together, these data demonstrated that SOXC gene deletion in the Prg4 cell lineage in young adult mice hampered the ability of TNFα to exert pathogenic actions in the joints.
Figure 3.
SOXC gene inactivation protects mice from TNFα-induced joint degeneration. A, Schematic of the experimental timeline. Dox, doxycycline treatment. B and C, Staining of sections from the distal interphalangeal joint of forepaw digit 3 with Safranin-O/fast green and H&E, respectively. Arrowhead, loss of Safranin-O-stained cartilage-specific proteoglycans. Arrows, invasion the joint cavity by synovial tissue. Asterisk, synovial hyperplasia. D, Fold change in Safranin-O staining intensity in articular cartilage. E, Fold change in the size of the synovium pannus. For both panels E and F, five mice were analyzed per group. Cartilage staining intensities and synovium sizes were measured using ImageJ software in 3 sections/mouse that are 30 μm apart. *, p value in Student’s t-test.
SOXC genes are required to induce FLS aggressiveness
Increased proliferation, survival, and ability to migrate are defining pathological features of FLS (3). We used an EdU incorporation assay to test whether the SOXC genes increased the proliferation rate of synovial cells in response to TNFα. While EdU-labeled cells could be readily detected in subchondral bone, hardly any were found in interphalangeal cartilage and synovial tissue in control and TNFα-expressing mice (Figure S3A). An in vitro assay of cell cycle phases showed that primary FLS were equally proliferative when stimulated with TNFα, when forced to overexpress SOX11, and when both treatments were applied together (Fig. S3B). Thus, neither TNFα nor SOXC genes transform FLS by increasing their proliferation in our mouse model.
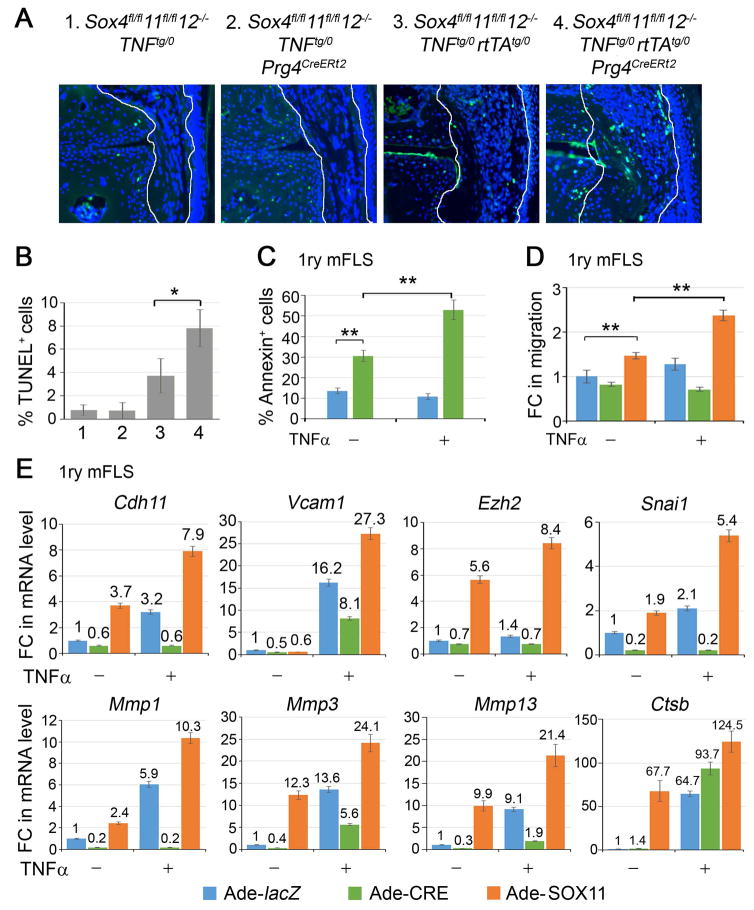
We used TUNEL staining to assess cell death. SOXC-control and SOXC-deficient mice showed 1% of dying cells in the synovium at 7 weeks of age (Figure 4A and B). Interestingly, this death rate was 3.8% in TNFα-overexpressing mice, and was twice as high (8%) in SOXC-deficient TNFα-overexpressing mice. We then tested primary FLS from Sox4fl/fl11fl/fl12−/− mice using flow cytometric quantification of annexin-V positivity (Figure 4C). These cell populations showed 3 fold more apoptotic cells when infected with a CRE-expressing adenovirus than when infected with a control, lacZ-expressing adenovirus. Upon TNFα treatment, the percentage of apoptotic cells doubled, but only in the SOXC-deficient population. Collectively, these in vivo and in vitro data suggested that the SOXC genes promote synovial hyperplasia in response to TNFα by at least in part reducing the death rate of FLS.
Figure 4.
SOXC genes promote FLS transformation. A, TUNEL staining (green) and DAPI counterstaining (blue) of cell nuclei. B, The graph shows the percentage of TUNEL-positive synovial cells from 3 mice for genotype. C, Flow cytometric quantification of Annexin V-positive cells in Sox4fl/fl11fl/fl12−/− primary FLS infected with lacZ- or CRE-expressing adenovirus for 16 h and then treated with 5 ng/ml TNFα for 8 h. D, Assay of wound repair by primary FLS. Confluent cultures of Sox4fl/fl11fl/fl12−/− FLS were infected with lacZ-, CRE- or SOX11-expressing adenovirus for 16 h, wounded with a pipette tip, and then treated or not with 5 ng/ml TNFα for 4 h. E, Fold changes in the relative levels of RNAs for the indicated genes in Sox4fl/fl11fl/fl12−/− primary FLS treated adenoviruses for 16 h and then with 5 ng/ml TNFα for 8 h, as indicated. Gapdh mRNA levels were used for normalization.*, p-value <0.05; **, p-value <0.01 in two-tailed Student’s t-test applied to triplicate cultures per condition.
Our in vivo experiments showed that SOXC inactivation precluded synovial cells from invading the joint cavity in TNFα-expressing mice. To test whether the SOXC genes directly affect FLS migration, we used an in vitro wound-healing assay, wherein a cell monolayer was locally scratched, and cells migrating in the wounded area were counted. SOXC inactivation slightly impaired cell migration, whereas SOX11 overexpression significantly increased it (Figure 4D and S3C). Interestingly, TNFα marginally increased the migration of control cells, but it was totally ineffective on SOXC-deficient cells. The effect of TNFα was potentiated by SOX11 overexpression. Since these effects were observed within 4 h of TNFα treatment, it is likely that they truly reflected increased cell migration rather than increased cell survival or proliferation.
Finally, we tested whether SOXC proteins impacted the effect of TNFα on the expression of genes required for efficient cell migration and FLS transformation. These genes include Cdh11 (cadherin 11) and Vcam1 (vascular cell adhesion protein 1), which encode cell surface proteins, Ezh2 (histone methyltransferase Zeste homologue 2), Snai1 (Snail family transcriptional repressor 1), Mmp1, Mmp3 and Mmp13 (matrix metalloproteinases) and Ctsb (cathepsin B). Sox4/11 loss blocked or reduced the ability of TNFα to upregulate the expression of each one of these genes, except Ctsb. SOX11 overexpression was able to upregulate the expression of Cdh11, Snai1, Mmp1, Mmp3 and Mmp13. and combined TNFα treatment and SOX11 overexpression greatly increased expression of all eight genes.
Taken together, these data indicated that SOXC genes enhance the ability of TNFα to transform FLS into aggressive, cancer-like cells.
SOX4/11 proteins are stabilized by inflammatory cytokines
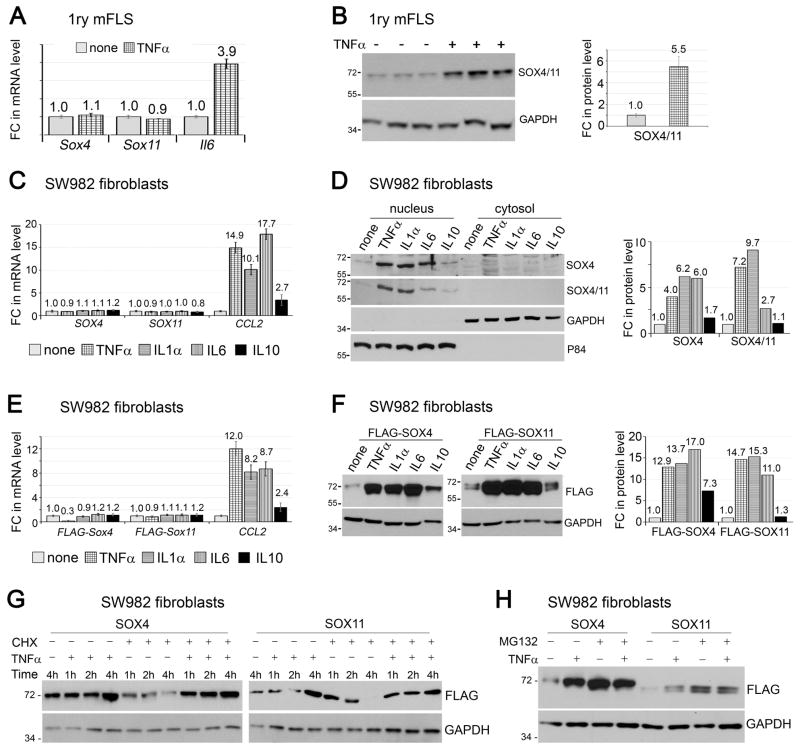
Having discovered that they potentiate TNFα signaling in FLS, we next asked whether the SOXC genes are targets of this pathway. The treatment of mouse primary FLS with TNFα did not change the levels of SOX4 and SOX11 RNAs, in spite of significantly increasing the RNA level of IL6, a TNFα signaling target (Figure 5A). Interestingly, however, the same treatment upregulated the level of SOX4/11 protein by more than 5 fold (Figure 5B). We then asked whether other cytokines abundantly present in arthritic joints had effects comparable to those of TNFα. We tested IL1α and IL6, which are also pro-inflammatory, and IL10, which is anti-inflammatory, on human synovial sarcoma-derived FLS-like SW982 cells. As in mouse primary FLS, SOX4 and SOX11 RNAs were readily detected in SW982 cells (Figure 5C). None of the cytokines changed the levels of these RNAs, even though TNFα, IL1 and IL6 increased the RNA level of CCL2 (encoding a monocyte chemo-attractant protein known as CCL2 or MCP1) by more than 10 fold, and IL10 increased it by almost 3 fold. Remarkably, TNFα, IL1, IL10 and IL6 all increased the levels of SOX4/11 protein with a robustness closely paralleling their effect on CCL2 RNA level (Figure 5D). The SOX proteins were primarily located in the cell nucleus in all conditions, indicating that the cytokines did not affect their nuclear-cytoplasmic shuttling.
Figure 5.
SOX4/11 proteins are stabilized by inflammatory cytokines. A and B, Mouse FLS at passage 4 were treated with 5 ng/ml TNFα for 8 h. Fold changes (FC) in Sox4, Sox11 and Il6 RNA levels were then measured by RT-qPCR (A) and fold changes in SOXC protein levels by western blots of whole-cell extracts from triplicate cultures (B). C and D, SW982 cells were treated with 5 ng/ml TNFα, 10 ng/ml IL1α, 10ng/ml IL6, or 10 ng/ml IL10 for 8 h. Fold changes in RNA (C) and protein (D) levels were then assayed. The P84 nuclear protein and GAPDH cytoplasmic protein were used as protein loading controls. E and F, SW982 cells were transfected with expression plasmids encoding FLAG-SOX4 and FLAG-SOX11 for 16 h and were then induced with cytokines, as in panels C and D. Fold changes in RNA (E) and protein (F) levels were then assayed in whole-cell extracts. G, SW982 cells were transfected with expression plasmids encoding FLAG-SOX4 and FLAG-SOX11 for 16 h, after which time their medium was supplemented with 0 or 5 μg/ml cycloheximide (CHX) for 15 min and then with 0 or 5 ng/ml TNFα for 1, 2, or 4 h. The levels of FLAG-tagged proteins were assayed in western blots using whole-cell extracts. H, Western blots of SW982 cells transfected with a FLAG-SOX4 or FLAG-SOX11 expression plasmid for 16 h, and then induced with 5 ng/ml TNFα for 6 h. MG132 was added at 10 μM 30 min prior to the addition of TNFα.
The ability of the cytokines to increase the SOX4/11 protein levels, but not the RNA levels, implied the involvement of a post-transcriptional mechanism. To investigate the nature of this mechanism, we first utilized expression plasmids encoding FLAG-tagged mouse SOX4 and SOX11 proteins. The plasmids featured a heterologous CMV promoter and besides carrying the SOXC coding sequences, lacked any Sox4 or Sox11 regulatory sequences. As seen for the endogenous proteins, the inflammatory cytokines increased the levels of the FLAG-SOX4 and FLAG-SOX11 proteins without affecting their transcript levels (Figure 5E and 5F). This result consolidated the notion of a post-transcriptional effect and, since the expression plasmids lacked Sox4 and Sox11 5′ and 3′ untranslated sequences, it suggested that the effect is post-translational.
To investigate whether inflammatory cytokines affected SOX4 and SOX11 mRNA translation, we expressed FLAG-SOX4 and FLAG-SOX11 in SW982 cells and treated the cells with TNFα. The protein levels robustly increased by 4 h (Figure 5G). Blockade of new protein synthesis with the translation elongation inhibitor cycloheximide caused a progressive loss of the FLAG-SOX4 and FLAG-SOX11 proteins in TNFα-untreated cells, but not in TNFα-treated cells. These results thus pointed both to a short life of the SOXC proteins in the absence, but not in the presence of TNFα. To validate this novel notion, we tested the effect of blocking the proteasome protein degradation pathway with the MG132 compound. As anticipated, both TNFα and MG132 increased the levels of FLAG-SOX4 and FLAG-SOX11 proteins, and their combined treatments did not further raise the protein levels (Figure 5H). Therefore, TNFα signaling likely increases the SOX4 and SOX11 protein levels by inhibiting the proteasome pathway.. Together, these data indicated that pro-inflammatory cytokines robustly increase SOXC protein stability in FLS.
SOX4/11 protein levels are elevated in inflamed synovium of arthritis patients
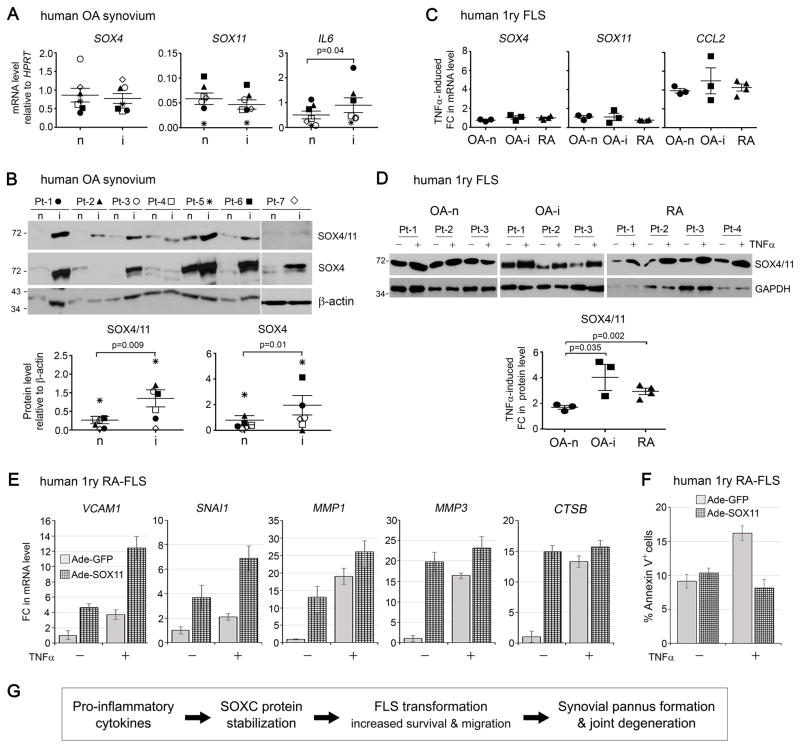
In order to establish whether SOXC protein stabilization also happens in human synovium subjected to pro-inflammatory signals, we separately collected non-inflamed and inflamed synovium biopsies from the same OA patients’ joints. Inflamed synovium was recognized based on hyperplasia and erythema and was confirmed by measuring the IL6 mRNA level (Figure 6A). Interestingly, while the levels of SOX4 and SOX11 RNAs showed no consistent pattern of inflammation-dependent change in the 7 patients that we tested (Figure 6A), the SOX4/11 protein levels were higher in inflamed synovium tissue than in non-inflamed patient-matched synovium biopsies. The increase varied between <2 fold and >9 fold (Figure 6B). Together, these data support the novel assertion that the SOXC proteins are stabilized in the inflamed synovium of OA patients.. We next compared the effect of TNFα on the SOX4/11 mRNA and protein levels in primary FLS isolated from OA and RA patients. Similar to mouse FLS, human FLS from OA and RA patients responded to TNFα treatment by increasing their CCL2 mRNA level without a significant change in SOX4 and SOX11 RNAs levels (Figure 6C). Interestingly, the level of SOX4/11 protein increased less than or equal to 2 fold in the FLS isolated from non-inflamed OA synovium, and increased significantly more, up to 3 or 4 fold in the FLS isolated from inflamed OA and RA synovium (Figure 6D). This suggests that cells isolated from inflammatory tissue have acquired increased responsiveness to TNFα with regards to SOX4/11 protein stabilization.
Figure 6.
SOX4/11 protein levels are increased in the inflamed synovium and FLS of patients with arthritis. A and B, FLS were isolated from non-inflamed synovium of OA patients (OA-n), inflamed synovium of OA patients (OA-i) and RA patients. They were treated with 5 ng/ml TNFα for 8 h. TNFα-induced fold changes in RNA (A) and protein (B) levels are presented. HPRT mRNA levels and GAPDH protein levels were used for normalization. C and D, Non-inflamed (n) and inflamed (i) synovium tissue biopsies were collected from seven osteoarthritic patients (Pt-1 to -7) and RNA (C) and protein (D) levels were directly assessed by RT-qPCR and western blotting, respectively. HPRT RNA levels and β-actin protein levels were used, respectively, for normalization. E, Fold changes in the relative levels of RNAs for the indicated genes in RA-FLS treated with adenoviruses for 16 h and then with 5 ng/ml TNFα for 8 h, as indicated. HPRT mRNA levels were used for normalization. F, Percentage of apoptotic RA-FLS treated with adenoviruses for 16 h and then with 5 ng/ml TNFα for 16 h. G, Model of the inflammation-SOX4/11 axis uncovered in this study. Pro-inflammatory signals triggered by such cytokines as TNFα, IL1, and IL6, induce the stabilization and accumulation of the SOX4/11 proteins in FLS. The SOXC transcription factors are thereby allowed to actively participate in the aggressive transformation of FLS by upregulating genes that promote cell survival and migration. Hence, SOXC proteins are targets and mediators of inflammatory pathways that cause accelerated joint degeneration in arthritic diseases.
Finally, we tested whether overexpression of SOXC proteins in human primary RA-FLS impacted the ability of TNFα to upregulate the expression of genes required for efficient cell migration and invasion. We found that, similarly to results obtained in mouse FLS, SOX11 overexpression alone was sufficient to increase the expression levels of VCAM1 and SNAI1, whose gene products are known to promote cell migration (Figure 6E). Gene encoding for the proteolytic enzymes MMP1, MMP3 and CTSB, which help the FLS become invasive, also had their expression increased. The combination of SOX11 overexpression and TNFα addition resulted in higher expression of VCAM1, SNAI1 and MMP1 than when both treatments were applied independently. Furthermore, the percentage of FLS undergoing apoptosis upon TNFα treatment was reduced by SOX11 overexpression (Figure 6F). Together, these data strongly suggest that SOXC proteins contribute towards enhancing the aggressive phenotype of RA-FLS.
DISCUSSION
This study has uncovered that the SOXC proteins are pivotal targets of a pro-inflammatory cytokine-initiated molecular axis that leads to the development of arthritic lesions in synovial joints (Figure 6G). While suggesting that the SOXC proteins are dispensable for joint integrity in juvenile and young adult mice, it demonstrated that these proteins are required in the Prg4+ cell lineage for synovial pannus formation and articular cartilage degeneration in TNFα-induced arthritic disease. It has then shown that SOXC proteins are very unstable in FLS under regular conditions, but robustly stabilized upon stimulation by TNFα and other pro-inflammatory cytokines. Pro-inflammatory pathways thereby use these transcription factors to amplify the expression of genes that promote FLS survival and migration, which are critical events in inflammation-induced FLS transformation.
Our observation that SOXC genes may be dispensable for joint integrity in juvenile and young adult mice was not predicted. Indeed, we were able to detect the proteins and their RNAs in synovium and articular cartilage of adult mice, and we previously showed the indispensability of SOXC genes for joint formation in mouse embryos (11, 13–15, 28–30). A possible explanation is that the SOXC proteins are present at very low levels or in an inactive form under adult healthy conditions and therefore cannot exert significant functions. While SOXC inactivation did not cause major changes in the joints of young adult mice, it is possible that it would cause problems in the long-term and in particular upon aging. Testing this possibility will require further investigations. The finding that SOXC genes are necessary for FLS transformation in arthritic mice is the first evidence that these genes and, in fact, any member of the SOX family, are involved in positively mediating or exacerbating inflammatory pathways. Interestingly, SOXC RNA levels were not changed in FLS treated with these cytokines, but the SOXC protein levels were quickly and dramatically increased. Thus, constitutive expression of the SOXC genes allows healthy FLS to quickly respond to pro-inflammatory signals.
Like SOX4 and SOX11, SOX9 is also a target of pro-inflammatory cytokines, but the underlying mechanisms are distinct for the two types of SOX proteins, and their consequences are opposite. Indeed, the SOXC proteins are stabilized in response to pro-inflammatory pathways, whereas the SOX9 RNA and protein levels are quickly and sharply downregulated. While SOXC inactivation was found here to be protective for articular cartilage, SOX9 inactivation leads to chondrocyte de-differentiation and hence to cartilage degeneration (31). These opposite mechanisms and consequences are not unexpected when one considers that SOX9 belongs to the SOXE group in the SOX family and that the SOXC and SOXE proteins share only about 50% identity in their DNA-binding domain and none outside this domain, and that the two types of genes have very different patterns of expression and modes of regulation. Moreover, we previously showed that the SOXC proteins are not only very different from SOX9, but also have anti-chondrogenic properties that prevent presumptive joint and perichondrial mesenchyme in mouse embryos from undergoing ectopic chondrogenesis (12). One of these properties was to stabilize β-catenin and thereby to synergize with canonical WNT signaling to efficiently repress the levels and the activity of SOX9. By extrapolation, we surmise that SOXC inactivation in the joints of our mice may have protected articular cartilage at least in part by increasing the levels of SOX9 in chondrocytes.
Our findings suggest that SOXC genes contribute to synovial hyperplasia in arthritic disease by increasing FLS survival, but not by affecting cell proliferation. Similarly, we and others previously showed that the SOXC genes promote survival of skeletal mesenchyme, neuronal progenitors and other types of progenitor cells, without affecting cell proliferation (11, 30). It is known that FLS are not highly proliferative in adult life. Only a small proportion of the FLS is known to undergo an extremely slow rate of proliferation similar to that of stem-like cells (32, 33). Moreover, it has also been shown that cell proliferation does not play a significant role in TNFα-induced FLS transformation (3). Hence, our findings that SOXC genes do not affect cell proliferation in the synovium are in line with these observations. Transformed FLS are known to resist TNFα-induced apoptosis in spite of expressing functional FAS (cell surface death receptors), TRAILR1 and 2 (TNF-related apoptosis-inducing ligand receptors), and TNFR1 and 2 (TNF receptors) (34). Here, we showed that the absence of the SOXC genes makes TNFα-exposed FLS lose their apoptosis resistance.
We also showed that besides promoting cell survival the SOXC genes allowed FLS invasion of joint cavities and migration over plastic surfaces. Studies in epithelial cancers showed that SOX4 and SOX11 promote metastasis. The epithelial mesenchymal transition transcription factor SNAI1 and histone methyltransferase EZH2 are direct transcriptional targets of SOX4 in cancer cells (19). Both genes are also TNFα signaling targets with key roles in promoting FLS invasiveness (35, 36). CDH11 is an FLS surface marker and a target of TNFα (37). Its inactivation protects mice from developing TNFα-induced joint degeneration. We have shown here that Ezh2, Cdh11 and Snai1 are targets of the SOXC/TNFα molecular axis. It is thus likely that SOXC proteins directly upregulate the expression of TNFα target genes that support FLS transformation in arthritic disease.
In spite of years of research in many processes, mechanisms underlying SOXC regulation remain largely unknown. SMAD proteins activated by TGFβ were shown to directly upregulate SOX4 transcription in cancer cells and T-cells (4, 38). Our study sheds new light on SOXC regulation by showing that protein stabilization is central downstream of inflammatory signals. We speculate that a similar mechanism is likely at play in specific developmental and physiological processes. However, the signals that then stabilize the SOXC proteins are yet-to-be identified.
Direct targeting of pro-inflammatory cytokines with biological compounds is a prevalent strategy in current-day clinical practice to reduce or delay permanent joint degeneration. This strategy, however, is neither ideal nor sufficient because global interference with the immune system results in such unwanted effects as development of drug resistance, infections and cancers (39–42). We identified that downregulating the expression of SOXC genes in the synovial lining and articular chondrocytes significantly reduced synovitis and articular cartilage erosion. We therefore propose the SOXC genes or proteins as alternative or complementary drug targets for treating inflammation-induced joint pathology. Targeting SOXC genes/proteins is especially tempting considering that these factors have been found so far to be critical in disease development, but not in adult homeostatic processes. However, more studies are needed to validate their candidacy as drug targets. SOXC transcription factors are expressed in a broad range of tissues and cell types, including immune cells that accumulate in the synovial pannus (43). Further understanding of the mechanistic regulation of the interactions between SOXC proteins and inflammation in additional models of inflammation-induced arthritis will be necessary. Although transcription factors have long been considered undruggable, a number of compounds directed towards preventing their interactions with DNA or protein partners have recently been successfully developed. They include small molecule inhibitors that target STAT3 dimerization, and p53-Mdm2, p300-HIF1α, and SOX18-RBPJ interactions (44–47). Therefore, targeting the SOXC proteins or their stabilization mechanism using systemic delivery of small molecule inhibitors is an option to be explored as it could soon become a safe and effective therapy for arthritic diseases and other types of inflammatory diseases.
Supplementary Material
Acknowledgments
Supported by a grant from the Arthritis National Research Foundation to PB (ANRF1405PB), grants from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases to VL (AR046249 and AR068308), to PB (AR070736) and to GM (AR063733), and a grant from the Rheumatology Research Foundation to VL.
We thank T. Haqqi and all members of the Lefebvre, Muschler and Haqqi laboratories for help during the study. We thank M. Longworth and O. Wessely for advice during manuscript preparation.
References
- 1.Iwanaga T, Shikichi M, Kitamura H, Yanase H, Nozawa-Inoue K. Morphology and functional roles of synoviocytes in the joint. Arch Histol Cytol. 2000;63:17–31. doi: 10.1679/aohc.63.17. [DOI] [PubMed] [Google Scholar]
- 2.Smith MD. The normal synovium. Open Rheumatol J. 2011;5:100–106. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA. passive responders and imprinted aggress. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattaram P, Chandrasekharan U. The joint synovium: A critical determinant of articular cartilage fate in inflammatory joint diseases. Semin Cell Dev Biol. 2017;62:86–93. doi: 10.1016/j.semcdb.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 5.De Bari C. Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res Ther. 2015;17:113. doi: 10.1186/s13075-015-0634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140:4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dy P, Penzo-Mendez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins--Sox4, Sox11 and Sox12--exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36:3101–3117. doi: 10.1093/nar/gkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoser M, Potzner MR, Koch JM, Bosl MR, Wegner M, Sock E. Sox12 deletion in the mouse reveals nonreciprocal redundancy with the related Sox4 and Sox11 transcription factors. Mol Cell Biol. 2008;28:4675–4687. doi: 10.1128/MCB.00338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattaram P, Penzo-Mendez A, Sock E, Colmenares C, Kaneko KJ, Vassilev A, Depamphilis ML, Wegner M, Lefebvre V. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun. 2010;1:9. doi: 10.1038/ncomms1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattaram P, Penzo-Mendez A, Kato K, Bandyopadhyay K, Gadi A, Taketo MM, Lefebvre V. SOXC proteins amplify canonical WNT signaling to secure nonchondrocytic fates in skeletogenesis. J Cell Biol. 2014;207:657–671. doi: 10.1083/jcb.201405098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang KC, Hertz J, Zhang X, Jin XL, Shaw P, Derosa BA, Li JY, Venugopalan P, Valenzuela DA, Patel RD, et al. Novel Regulatory Mechanisms for the SoxC Transcriptional Network Required for Visual Pathway Development. J Neurosci. 2017;37:4967–4981. doi: 10.1523/JNEUROSCI.3430-13.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul MH, Harvey RP, Wegner M, Sock E. Cardiac outflow tract development relies on the complex function of Sox4 and Sox11 in multiple cell types. Cell Mol Life Sci. 2014;71:2931–2945. doi: 10.1007/s00018-013-1523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potzner MR, Tsarovina K, Binder E, Penzo-Mendez A, Lefebvre V, Rohrer H, Wegner M, Sock E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;137:775–784. doi: 10.1242/dev.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilir B, Osunkoya AO, Wiles WGt, Sannigrahi S, Lefebvre V, Metzger D, Spyropoulos DD, Martin WD, Moreno CS. SOX4 Is Essential for Prostate Tumorigenesis Initiated by PTEN Ablation. Cancer Res. 2016;76:1112–1121. doi: 10.1158/0008-5472.CAN-15-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roisman A, Stanganelli C, Nagore VP, Richardson GV, Scassa ME, Bezares RF, Cabrejo M, Slavutsky I. SOX11 expression in chronic lymphocytic leukemia correlates with adverse prognostic markers. Tumour Biol. 2015;36:4433–4440. doi: 10.1007/s13277-015-3083-1. [DOI] [PubMed] [Google Scholar]
- 18.Vervoort SJ, van Boxtel R, Coffer PJ. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: friend or foe? Oncogene. 2013;32:3397–3409. doi: 10.1038/onc.2012.506. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schubeler D, van Nimwegen E, Christofori G. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–783. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd JH, Uray IP, Mazumdar A, Tsimelzon A, Savage M, Hilsenbeck SG, Brown PH. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget. 2016;7:13106–13121. doi: 10.18632/oncotarget.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penzo-Mendez A, Dy P, Pallavi B, Lefebvre V. Generation of mice harboring a Sox4 conditional null allele. Genesis. 2007;45:776–780. doi: 10.1002/dvg.20358. [DOI] [PubMed] [Google Scholar]
- 22.Retser E, Schied T, Skryabin BV, Vogl T, Kanczler JM, Hamann N, Niehoff A, Hermann S, Eisenblatter M, Wachsmuth L, et al. Doxycycline-induced expression of transgenic human tumor necrosis factor alpha in adult mice results in psoriasis-like arthritis. Arthritis Rheum. 2013;65:2290–2300. doi: 10.1002/art.38026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozhemyakina E, Zhang M, Ionescu A, Ayturk UM, Ono N, Kobayashi A, Kronenberg H, Warman ML, Lassar AB. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015;67:1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ntougkos E, Chouvardas P, Roumelioti F, Ospelt C, Frank-Bertoncelj M, Filer A, Buckley CD, Gay S, Nikolaou C, Kollias G. Genomic responses of mouse synovial fibroblasts during TNF-driven arthritogenesis greatly mimic those of human rheumatoid arthritis. Arthritis Rheumatol. 2017;69:1588–1600. doi: 10.1002/art.40128. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wixler V, Cromme C, Retser E, Meyer LH, Smyth N, Muhlenberg K, Korb-Pap A, Koers-Wunrau C, Sotsios Y, Bassel-Duby R, et al. FHL2 regulates the resolution of tissue damage in chronic inflammatory arthritis. Ann Rheum Dis. 2015;74:2216–2223. doi: 10.1136/annrheumdis-2013-205061. [DOI] [PubMed] [Google Scholar]
- 28.Gnedeva K, Hudspeth AJ. SoxC transcription factors are essential for the development of the inner ear. Proc Natl Acad Sci U S A. 2015;112:14066–14071. doi: 10.1073/pnas.1517371112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schilham MW, Clevers H. HMG box containing transcription factors in lymphocyte differentiation. Semin Immunol. 1998;10:127–132. doi: 10.1006/smim.1998.0114. [DOI] [PubMed] [Google Scholar]
- 30.Thein DC, Thalhammer JM, Hartwig AC, Crenshaw EB, 3rd, Lefebvre V, Wegner M, Sock E. The closely related transcription factors Sox4 and Sox11 function as survival factors during spinal cord development. J Neurochem. 2010;115:131–141. doi: 10.1111/j.1471-4159.2010.06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami S, Lefebvre V, de Crombrugghe B. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-alpha. J Biol Chem. 2000;275:3687–3692. doi: 10.1074/jbc.275.5.3687. [DOI] [PubMed] [Google Scholar]
- 32.Roelofs AJ, Zupan J, Riemen AHK, Kania K, Ansboro S, White N, Clark SM, De Bari C. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun. 2017;8:15040. doi: 10.1038/ncomms15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 34.Orosa B, Gonzalez A, Mera A, Gomez-Reino JJ, Conde C. Lysophosphatidic acid receptor 1 suppression sensitizes rheumatoid fibroblast-like synoviocytes to tumor necrosis factor-induced apoptosis. Arthritis Rheum. 2012;64:2460–2470. doi: 10.1002/art.34443. [DOI] [PubMed] [Google Scholar]
- 35.Lauzier A, Lavoie RR, Charbonneau M, Gouin-Boisvert B, Harper K, Dubois CM. Snail Is a Critical Mediator of Invadosome Formation and Joint Degradation in Arthritis. Am J Pathol. 2016;186:359–374. doi: 10.1016/j.ajpath.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Trenkmann M, Brock M, Gay RE, Kolling C, Speich R, Michel BA, Gay S, Huber LC. Expression and function of EZH2 in synovial fibroblasts: epigenetic repression of the Wnt inhibitor SFRP1 in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1482–1488. doi: 10.1136/ard.2010.143040. [DOI] [PubMed] [Google Scholar]
- 37.Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 38.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes D, Iwamura C, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-beta and suppresses T(H)2 differentiation. Nat Immunol. 2012;13:778–786. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaeverbeke T, Truchetet ME, Kostine M, Barnetche T, Bannwarth B, Richez C. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology (Oxford) 2016;55:210–220. doi: 10.1093/rheumatology/kev277. [DOI] [PubMed] [Google Scholar]
- 40.Tragiannidis A, Kyriakidis I, Zundorf I, Groll AH. Invasive fungal infections in pediatric patients treated with tumor necrosis alpha (TNF-alpha) inhibitors. Mycoses. 2017;60:222–229. doi: 10.1111/myc.12576. [DOI] [PubMed] [Google Scholar]
- 41.Atzeni F, Gianturco L, Talotta R, Varisco V, Ditto MC, Turiel M, Sarzi-Puttini P. Investigating the potential side effects of anti-TNF therapy for rheumatoid arthritis: cause for concern? Immunotherapy. 2015;7:353–361. doi: 10.2217/imt.15.4. [DOI] [PubMed] [Google Scholar]
- 42.Ehrenstein MR, Wing C. The BAFFling effects of rituximab in lupus: danger ahead? Nat Rev Rheumatol. 2016;12:367–372. doi: 10.1038/nrrheum.2016.18. [DOI] [PubMed] [Google Scholar]
- 43.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 44.Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, Iiyama T, Asao N, DiGiovanni J, Sano S. Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a Stat3 inhibitor. J Invest Dermatol. 2011;131:108–117. doi: 10.1038/jid.2010.255. [DOI] [PubMed] [Google Scholar]
- 45.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Chene P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3:102–109. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- 47.Fontaine F, Overman J, Moustaqil M, Mamidyala S, Salim A, Narasimhan K, Prokoph N, Robertson AA, Lua L, Alexandrov K, et al. Small-Molecule Inhibitors of the SOX18 Transcription Factor. Cell Chem Biol. 2017;24:346–359. doi: 10.1016/j.chembiol.2017.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.