Abstract
Associations of prenatal exposure to perfluoroalkyl substances (PFASs), ubiquitous chemicals used in stain and water resistant products, with adverse birth outcomes may be confounded by pregnancy hemodynamics. We measured plasma concentrations of four PFASs in early pregnancy (median=9 weeks) among 1,645 women in Project Viva, a Boston-area cohort recruited 1999–2002. We fit multivariable models to estimate PFAS associations with birth weight-for-gestational age z-score and gestation length adjusting for sociodemographic confounders and two hemodynamic markers: 1) plasma albumin, a measure of plasma volume expansion, and 2) plasma creatinine, used to estimate glomerular filtration rate. Perfluorooctane sulfonate (PFOS) and perfluorononanoate (PFNA) were weakly inversely associated with birth weight-for-gestational age z-scores [adjusted β=−0.04 (95% confidence interval (CI): −0.08, 0.1) and −0.06 (95% CI: −0.11, −0.01) per interquartile increase, respectively]. PFOS and PFNA were also associated with higher odds of preterm birth [e.g., highest vs. lowest PFOS quartile adjusted odds ratio = 2.4 (95% CI: 1.3, 4.4)]. Adjusting for markers of pregnancy hemodynamics (glomerular filtration rate and plasma albumin), to the extent that they accurately reflect underlying pregnancy physiology, did not materially impact associations. These results suggest that pregnancy hemodynamics may not confound associations with birth outcomes when PFASs are measured early in pregnancy.
Keywords: perfluoroalkyl substances, pregnancy, fetal growth, gestational age, preterm birth, birth weight
Perfluoroalkyl substances (PFASs) are a family of synthetic compounds composed of a carbon-fluorine backbone. Many PFASs are resistant to oil and water and therefore useful in the manufacture of stain-resistant products (e.g., carpets and fabrics), nonstick coatings, food packaging and a broad range of other applications. These properties can also make them resistant to degradation and persistent in both the environment and in the body with half-lives in humans of approximately 3–5 years (1).
Diet and the indoor environment are common sources of human PFAS exposure, and PFASs are universally detected at varying serum concentrations in the U.S. population, as reported in the National Health and Nutrition Examination Survey (NHANES) (2, 3). PFASs can cross the placenta, and animal and human studies suggest that some PFASs may be developmental toxicants (4, 5).
A number of epidemiologic studies report associations of prenatal exposure to two PFASs, perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), with adverse birth outcomes, particularly reduced fetal growth (6–19), and a recent meta-analysis estimated a reduction in term birth weight of 18.9 g (95% CI: −29.8, −7.9) per 1-ng/mL increase in PFOA (20). However, other studies have reported null PFAS-birth weight associations (21–25).
There has been some concern that associations of PFASs with adverse birth outcomes are attributable to hemodynamic changes that occur during pregnancy (26, 27). Plasma volume expands at approximately six weeks gestation in response to decreased mean arterial pressure, increased cardiac output and systemic vasodilation (28); this results in serum/plasma dilution of PFAS concentrations. In addition, glomerular filtration rate (GFR) increases at six weeks gestation (28), which may accelerate PFAS excretion, as demonstrated by studies showing higher PFAS concentrations in individuals with reduced GFR (29, 30). As plasma volume expansion and changes in GFR may also be related to fetal development, including growth (31), these mechanisms could potentially induce a spurious, non-causal association between PFASs and birth outcomes.
To address these concerns we examined associations of early pregnancy PFAS plasma concentrations with birth weight-for-gestational length z-score (fetal growth), and gestational length, adjusting for confounders, including pregnancy hemodynamics, in a large, well-characterized longitudinal cohort of women who were pregnant during years that coincided with peak U.S. population exposures to PFOS and PFOA (1999–2002) (32).
METHODS
Study population
Project Viva is a prospective pre-birth cohort study in which mothers were recruited between 1999 and 2002 at their first prenatal visit to one of eight obstetric clinics of Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice in Eastern Massachusetts (33). Eligible mothers were fluent in English, had singleton gestations, were <22 weeks gestation, and had no plans to move away from the study area. Of 2,128 mothers with a live birth between November 1999 and February 2003, 1,668 (78%) provided an early pregnancy blood sample (median 9 weeks gestation; range 5–19), of which 1,645 had sufficient sample for quantification of PFASs. The Institutional Review Boards of participating institutions approved all study protocols and all participating mothers provided written informed consent. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
Quantification of PFASs and markers of pregnancy physiology
We obtained non-fasting blood samples from women at the recruitment visit, centrifuged samples and stored plasma in non-PFAS containing cryovial tubes in liquid nitrogen freezers (≤−130° Celsius). In 2014 we thawed, aliquoted and shipped samples to the Division of Laboratory Sciences at the CDC where vials were stored at or below −40° Celsius. Detailed analytic methods used for PFAS quantification were described previously (34, 35); briefly, CDC lab staff analyzed plasma by using on-line solid-phase extraction coupled with isotope dilution high-performance liquid chromatography-tandem mass spectrometry and reported concentrations of PFOA, PFOS, perfluorohexane sulfonate (PFHxS), and perfluorononanoate (PFNA). Reported concentrations for PFOS and PFOA included both linear and branched isomers. Low and high concentration quality control materials, prepared from a calf serum pool, were analyzed with the study samples, analytical standards, and with reagent and matrix blanks to ensure the accuracy and precision of the data. Limits of detection (LODs) were 0.2 ng/mL for PFOS and 0.1 ng/mL for the other three PFASs. CDC did not report numeric values below the LOD and we imputed these values as the LOD divided by the square root of 2 (36).
We sent aliquots of the samples used for PFAS measurements to the Clinical and Epidemiologic Research Laboratory at Children’s Hospital Boston (Boston, MA) for analysis of markers of pregnancy hemodynamics, including plasma albumin and creatinine. These markers have been associated with PFAS plasma concentrations in Project Viva (37).
Fetal growth and length of gestation
Project Viva staff abstracted birth weight (in grams) from hospital medical records. We computed birth weight-for-gestational-age and sex z-scores using a U.S. National reference (38). We computed length of gestation by subtracting the date of the last menstrual period (LMP) from the date of birth. Gestation length was also available from the ultrasound at 16–20 weeks gestation for approximately 79% of participants. For the 200 births (9%) in which gestational length derived from the LMP differed from that according to the ultrasound by > 10 days, we used the ultrasound to determine gestational duration. Because the clinical relevance of small shifts in gestational duration is unclear, we also examined PFAS-related associations with preterm birth, categorized as birth at <37 weeks of gestation.
Statistical analysis
We estimated associations of PFOS, PFOA, PFHxS and PFNA plasma concentrations with birth weight-for-gestational-age and gestation length using multivariable linear regression models. In secondary analyses, we also estimated associations of PFASs with birth weight among term births (≥37 weeks gestation). To examine associations with preterm birth we computed odds ratios (ORs) using logistic regression models. Data on covariates came from interviews and questionnaires administered during early pregnancy, mid-pregnancy and at delivery. We used a Directed Acyclic Graph (DAG) based on a priori knowledge of relationships between variables of interest, to identify potential confounders (Web Figure 1).
History of breastfeeding prior to the index pregnancy is an important variable for analyses of PFAS data (37) that was not collected in Project Viva. We therefore imputed this information using parity and breastfeeding data for the index pregnancy (collected following the birth). If the mother was multiparous (regardless of the number of previous births), and breastfed following the index pregnancy, history of breastfeeding was coded as “yes”, under the assumption that a mother who breastfed this child had a high likelihood of having breastfed an older child (39). If the mother was nulliparous or did not breastfeed the current child, history of breastfeed was coded as “no”.
All multivariable models included characteristics of the mother (age at enrollment, race/ethnicity, education, prenatal smoking, parity, history of breastfeeding prior to index pregnancy, pre-pregnancy body mass index (weight/height2), gestational age at blood collection) and the child (sex). We also included paternal education and household income.
We examined confounding by plasma albumin and GFR, both markers of pregnancy hemodynamics (26, 27). Albumin is the main binding site for PFASs as well as a marker of plasma volume expansion during pregnancy (40). GFR is a measure of the flow rate of filtered fluid through the kidney (31). We estimated GFR (eGFR) (mL/min per 1.73 m2) by plugging plasma creatinine into the Cockcroft-Gault (GFR-CG) formula [GFR-CG = (140-age) × weight (kg) × 1.04/plasma creatinine (μmol/L)]. To examine whether markers of GFR and plasma volume were biased among subgroups of women with conditions that could influence these markers, such diabetes or hypertension, we conducted a sensitivity analysis excluding women with these conditions.
We examined linearity of PFAS-outcome associations by fitting generalized additive models (GAM) with a penalized spline term and also by analyzing PFAS plasma concentrations as quartiles. We examined sex differences for associations of PFASs with fetal and infant outcomes by including an interaction term between sex and PFASs in the multivariable model. To account for missing covariate data we used chained equations to impute missing values, generating 50 imputed datasets and combining multivariable model results using PROC MI ANALYZE in SAS.
RESULTS
Participant characteristics of the 1,645 live births with prenatal maternal plasma PFAS measures are shown in Table 1. Mothers were predominantly white (69%), and at enrollment many had high educational attainment (65% with college or graduate degree), most were married or cohabitating (91%), and many had high household income (58% with >$70,000/year), and had never smoked (68%). Table 1 also shows that mothers who were older, white, had higher educational attainment, were married or cohabitating, had higher partner educational attainment, higher household income, did not smoke during pregnancy, were multiparous and had pre-pregnancy body mass index in the overweight or obese range had infants with higher birth weight-for-gestational age. These patterns were more or less consistent for length of gestation, with the exception of partner education and parity, where we did not observe meaningful differences.
Table 1.
Characteristics of Parents and Infants with PFAS Data Participating in Project Viva, Eastern Massachusetts, 1999–2002 (n=1,645) and Fetal Growth and Gestation Length by these Characteristics.
| Participant characteristic | N | % | BW for GA z-scorea (n=1,644) | Gestation length (weeks) (n=1,645) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | p-valueb | Mean (SD) | p-valueb | |||
| Maternal age at enrollment (years) | <0.0001 | 0.01 | ||||
| <20 | 55 | 3.3 | −0.43 (0.80) | 38.6 (2.1) | ||
| 20–34 | 1133 | 68.9 | 0.18 (0.95) | 39.5 (2.0) | ||
| 35+ | 457 | 27.8 | 0.30 (0.98) | 39.5 (1.9) | ||
| Maternal race/ethnicity | <0.0001 | <0.0001 | ||||
| White | 1126 | 68.5 | 0.30 (0.95) | 39.6 (1.7) | ||
| Black | 254 | 15.4 | −0.06 (0.93) | 39.1 (2.2) | ||
| Hispanic | 120 | 7.3 | −0.05 (0.98) | 39.1 (2.1) | ||
| Other | 145 | 8.8 | 0.04 (0.99) | 38.9 (2.6) | ||
| Maternal education at enrollment | <0.0001 | 0.003 | ||||
| Less than college degree | 583 | 35.5 | 0.05 (0.96) | 39.3 (2.0) | ||
| College degree | 601 | 36.5 | 0.31 (0.96) | 39.4 (2.1) | ||
| Graduate degree | 461 | 28.0 | 0.23 (0.96) | 39.7 (1.7) | ||
| Married or cohabitating at enrollment | 0.0002 | 0.03 | ||||
| Yes | 1499 | 91.1 | 0.22 (0.96) | 39.5 (1.9) | ||
| No | 146 | 8.9 | −0.10 (0.93) | 39.1 (2.5) | ||
| Partner education at enrollment | 0.001 | 0.28 | ||||
| Less than college degree | 652 | 39.6 | 0.08 (0.98) | 39.3 (2.0) | ||
| College degree | 553 | 33.6 | 0.28 (0.97) | 39.5 (1.9) | ||
| Graduate degree | 440 | 26.8 | 0.25 (0.92) | 39.5 (2.0) | ||
| Annual household income at enrollment | <0.0001 | 0.01 | ||||
| <$40K | 297 | 18.1 | −0.06 (1.02) | 39.1 (2.2) | ||
| $40K–70K | 399 | 24.2 | 0.20 (0.93) | 39.6 (1.7) | ||
| >$70K | 949 | 57.7 | 0.27 (0.95) | 39.5 (1.9) | ||
| Prenatal smoking | 0.003 | 0.01 | ||||
| Never | 1118 | 68.0 | 0.19 (0.95) | 39.3 (2.1) | ||
| Former | 310 | 18.8 | 0.32 (0.92) | 39.7 (1.6) | ||
| During pregnancy | 217 | 13.2 | 0.04 (1.07) | 39.5 (1.8) | ||
| Maternal parity | <0.0001 | 0.91 | ||||
| 0 | 800 | 48.6 | 0.02 (0.93) | 39.4 (2.1) | ||
| 1+ | 845 | 51.4 | 0.36 (0.97) | 39.4 (1.7) | ||
| Pre-pregnancy body mass indexc | <0.0001 | 0.05 | ||||
| <18.5 (underweight) | 56 | 3.4 | −0.16 (0.81) | 39.6 (1.2) | ||
| 18.5–24.9 (normal) | 954 | 58.0 | 0.11 (0.95) | 39.5 (1.9) | ||
| 25–29.9 (overweight) | 368 | 22.3 | 0.38 (0.98) | 39.5 (1.9) | ||
| 30+ (obese) | 267 | 16.2 | 0.30 (0.98) | 39.1 (2.2) | ||
PFAS= perfluoroalkyl substance; SD=standard deviation
Birth weight for gestational age z-score using a U.S. national reference standard (38).
P-value for unadjusted covariate-outcome association from ANOVA.
Body mass index = kg/m2.
Median plasma concentrations of prenatal PFOS, PFOA, PFHxS and PFNA are reported in Table 2. PFASs were moderately correlated with each other, with Spearman correlation coefficients as high as 0.72 for PFOS and PFOA. Table 2 also shows that PFASs were moderately correlated with hemodynamic indicators measured in samples collected at the same time as those used to quantify PFASs, including positive correlations with plasma albumin (Spearman correlation coefficients ranged from 0.14 to 0.24), consistent with serum dilution due to blood volume expansion (higher albumin indicates less dilution, leading to higher plasma PFAS concentrations). PFASs were also negatively correlated with eGFR (−0.15 to −0.27), consistent with increased flow rate during pregnancy (higher flow rate results in more PFAS excretion and therefore lower plasma PFAS concentrations). PFOS, PFOA and PFNA were weakly inversely correlated with birth weight-for-gestational age and were not correlated with length of gestation (Table 2).
Table 2.
Summary Statistics and Correlation Coefficients for PFASs and Hemodynamic Indicators Measured in Early Pregnancy Plasma Samples Collected 1999–2002, and Birth Outcomes in Project Viva, Eastern Massachusetts (n=1,645).
| Measure | N | Median (IQR) | PFOS | PFOA | PFHxS | PFNA | eGFR-CGa | Albumin | GA at blood drawb | BW for GA z-scorec,d | Gestation lengthb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFOS (ng/mL) | 1,645 | 25.7 (16.0) | 1.00 | 0.72 | 0.50 | 0.61 | −0.19 | 0.14 | −0.13 | −0.08 | −0.05 |
| PFOA (ng/mL) | 1,645 | 5.8 (3.8) | 1.00 | 0.52 | 0.52 | −0.15 | 0.17 | −0.12 | −0.07 | 0.02 | |
| PFHxS (ng/mL) | 1,645 | 2.4 (2.2) | 1.00 | 0.42 | −0.15 | 0.14 | −0.06 | 0.01 | 0.01 | ||
| PFNA (ng/mL) | 1,645 | 0.7 (0.4) | 1.00 | −0.27 | 0.24 | −0.12 | −0.08 | −0.01 | |||
| eGFR-CGa | 1,645 | 101.4 (44.1) | 1.00 | −0.01 | 0.16 | 0.15 | 0.02 | ||||
| Albumin (g/dL) | 1,645 | 8.3 (2.4) | 1.00 | −0.06 | 0.03 | 0.02 | |||||
| GA at blood drawb | 1,645 | 9.7 (2.1) | 1.00 | 0.02 | 0.06 | ||||||
| BW for GA z-scorec,d | 1,644 | 0.2 (1.4) | 1.00 | 0.19 | |||||||
| Gestation lengthb | 1,645 | 39.7 (1.7) | 1.00 |
IQR, interquartile range; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFHxS, perfluorohexane sulfonate PFNA, perfluorononanoate; eGFR-CG, estimated glomerular filtration rate computed with the Cockcroft-Gault formula (ml/min per 1.73 m2); GA, gestational age
Glomerular filtration rate (ml/min per 1.73 m2) computed using the Cockcroft-Gault formula
Gestational age at blood draw and gestation length (weeks)
Birth weight for gestational age z-score using a U.S. national reference standard (38).
N=1,644.
Fetal growth
Table 3 shows associations of PFASs with birth weight-for-gestational-length z-scores. Adjusting for traditional sociodemographic covariates attenuated estimates to some degree, indicating the presence of positive confounding; this attenuation occurred primarily after adjusting for parity (data not shown). Adjusted models show that PFOS and PFNA were associated with small decrements in birth weight-for-gestational age z-score [β = −0.04 (95% confidence interval (CI): −0.08, 0.0 and −0.06 (95% CI: −0.11, − 1) 0.01) per interquartile (IQR) increase, respectively]. In secondary analyses (Web Table 1), we also found reductions in term birth weight per IQR increase in PFOS [β = −17.9 (95% CI: −40.9, 5.1)], PFOA [−18.5 (95% CI: −45.4, 8.3)] and PFNA [−28.2 (95% CI: −52.0, −4.4)]. While not strictly monotonic, we observed overall decrements in fetal growth across quartiles of PFOS, PFOA and PFNA (Table 3 and Web Table 1). Fetal growth associations with PFHxS were null.
Table 3.
Associations of Prenatal Plasma Concentrations of PFASs With Birth Weight-for-Gestational Age z-scorea for Participants of Project Viva, Eastern Massachusetts (n=1,644).
| PFAS | Range (ng/mL) | N | Unadjusted | Adjustedb | Adjustedb + eGFR-CG | Adjustedb + Albumin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |||
| PFOS | ||||||||||
| Continuous IQRd | 1,644 | −0.07 | −0.11, −0.02 | −0.04 | −0.08, 0.01 | −0.03 | −0.08, 0.02 | −0.04 | −0.09, 0.00 | |
| Quartile 1 | 0.1 – 18.8 | 411 | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent |
| Quartile 2 | 18.9 – 25.6 | 409 | −0.13 | −0.26, 0.01 | −0.09 | −0.22, 0.04 | −0.08 | −0.21, 0.05 | 0.09 | −0.22, 0.03 |
| Quartile 3 | 25.7 – 34.8 | 411 | −0.14 | −0.27, 0.00 | −0.09 | −0.22, 0.04 | −0.07 | −0.20, 0.06 | −0.09 | −0.22, 0.04 |
| Quartile 4 | 34.9 – 185.0 | 413 | −0.20 | −0.33, −0.07 | −0.13 | −0.26, 0.00 | −0.10 | −0.24, 0.03 | −0.14 | −0.27, 0.00 |
| PFOA | ||||||||||
| Continuous IQRc | 1,644 | −0.05 | −0.10, 0.00 | −0.02 | −0.08, 0.03 | −0.01 | −0.07, 0.04 | −0.02 | −0.08, 0.03 | |
| Quartile 1 | 0.9 – 4.1 | 412 | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent |
| Quartile 2 | 4.2 – 5.8 | 410 | −0.09 | −0.22, 0.04 | −0.04 | −0.17, 0.09 | −0.03 | −0.16, 0.10 | −0.04 | −0.17, 0.09 |
| Quartile 3 | 5.9 – 7.9 | 416 | −0.20 | −0.33, −0.07 | −0.12 | −0.25, 0.02 | −0.10 | −0.24, 0.03 | −0.12 | −0.26, 0.01 |
| Quartile 4 | 8.0 – 49.3 | 406 | −0.16 | −0.29, −0.03 | −0.07 | −0.21, 0.07 | −0.05 | −0.19, 0.09 | −0.08 | −0.22, 0.06 |
| PFHxS | ||||||||||
| Continuous IQRc | 1,644 | −0.01 | −0.04, 0.02 | 0.00 | −0.03, 0.02 | 0.00 | −0.03, 0.02 | 0.00 | −0.03, 0.02 | |
| Quartile 1 | 0.1 – 1.6 | 414 | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent |
| Quartile 2 | 1.7 – 2.4 | 409 | −0.06 | −0.20, 0.07 | −0.10 | −0.23, 0.03 | −0.08 | −0.21, 0.05 | −0.10 | −0.23, 0.03 |
| Quartile 3 | 2.5 – 3.7 | 404 | 0.06 | −0.07, 0.19 | 0.04 | −0.09, 0.17 | 0.06 | −0.07, 0.19 | 0.04 | −0.09, 0.17 |
| Quartile 4 | 3.8 – 74.5 | 417 | 0.00 | −0.13, 0.13 | 0.00 | −0.14, 0.13 | 0.02 | −0.12, 0.15 | −0.01 | −0.14, 0.12 |
| PFNA | ||||||||||
| Continuous IQRc | 1,644 | −0.08 | −0.12, −0.03 | −0.06 | −0.11, −0.01 | −0.05 | −0.10, −0.01 | −0.06 | −0.11, −0.02 | |
| Quartile 1 | 0.1 – 0.4 | 331 | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent | 0.0 | Referent |
| Quartile 2 | 0.5 – 0.6 | 462 | −0.02 | −0.16, 0.12 | −0.03 | −0.16, 0.10 | −0.02 | −0.15, 0.12 | −0.03 | −0.17, 0.10 |
| Quartile 3 | 0.7 – 0.9 | 470 | −0.19 | −0.33, −0.06 | −0.19 | −0.32, −0.06 | −0.17 | −0.31, −0.04 | −0.20 | −0.33, −0.06 |
| Quartile 4 | 1.0 – 6.0 | 381 | −0.18 | −0.32, −0.04 | −0.15 | −0.30, −0.01 | −0.13 | −0.28, 0.02 | −0.17 | −0.31, −0.02 |
PFAS= perfluoroalkyl substance; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFHxS, perfluorohexane sulfonate PFNA, perfluorononanoate; IQR, interquartile range; estimated glomerular filtration rate computed with the Cockcroft-Gault formula (ml/min per 1.73 m2)
Birth weight-for-gestational-length z-scores using a US national reference standard.
Adjusted for maternal age at enrollment, race/ethnicity, education, prenatal smoking, parity, history of breastfeeding, pre-pregnancy body mass index, pater nal education, household income, child’s sex and gestational age at blood draw.
IQR (ng/mL) for PFASs are PFOS = 16.0; PFOA = 3.8; PFHxS = 2.2; PFNA = 0.4.
Gestational length
Associations of PFASs with gestational length followed the same pattern (Table 4) as those of fetal growth, with the strongest associations for PFOS and PFNA. When we examined odds for preterm birth (dichotomized at birth <37 weeks) we estimated over two times the odds for preterm birth among women with concentrations in the highest vs. lowest quartile of PFOS [OR=2.4 (95% CI: 1.3, 4.4)]. Odds of preterm birth were weaker for PFNA and null for PFOA and PFHxS (Table 4).
Table 4.
Associations of Prenatal Plasma Concentrations of PFASs With Gestation Length (Weeks) and Preterm Birth (Birth <37 Weeks Gestation vs.≥37 weeks) for Participants of Project Viva, Eastern Massachusetts (n=1,645).
| Range (ng/mL) | N | Gestational Length (weeks) | Preterm (<37 weeks) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Unadjusted | Adjusteda | Adjusteda + eGFR-CG | Adjusteda + Albumin | N preterm | Adjusteda | ||||||||
|
|
|
||||||||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | OR | 95% CI | ||||
| PFOS | |||||||||||||
| Continuous IQRb | 1645 | −0.10 | −0.19, 0.00 | −0.08 | −0.17, 0.02 | −0.06 | −0.16, 0.03 | −0.08 | −0.17, 0.01 | 120 | 1.1 | 1.0, 1.3 | |
| Quartile 1 | 0.1 − 18.8 | 411 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 16 | 1.0 (ref) | |||||
| Quartile 2 | 18.9 − 25.6 | 410 | −0.24 | −0.51, 0.03 | −0.20 | −0.47, 0.06 | −0.17 | −0.44, 0.09 | −0.20 | −0.47, 0.06 | 31 | 2.0 | 1.1, 3.7 |
| Quartile 3 | 25.7 − 34.8 | 411 | −0.15 | −0.42, 0.11 | −0.08 | −0.35, 0.19 | −0.05 | −0.32, 0.23 | −0.09 | −0.36, 0.18 | 33 | 2.0 | 1.1, 3.7 |
| Quartile 4 | 34.9 − 185.0 | 413 | −0.43 | −0.69,−0.16 | −0.36 | −0.64, −0.09 | −0.31 | −0.59, −0.03 | −0.37 | −0.65, −0.10 | 40 | 2.4 | 1.3, 4.4 |
| PFOA | |||||||||||||
| Continuous IQRb | 1645 | −0.04 | −0.69,−0.16 | −0.05 | −0.16, 0.06 | −0.03 | −0.14, 0.08 | −0.05 | −0.16, 0.06 | 120 | 1.0 | 0.9, 1.3 | |
| Quartile 1 | 0.3 – 4.1 | 413 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 26 | 1.0 (ref) | |||||
| Quartile 2 | 4.2 – 5.8 | 410 | 0.07 | −0.20, 0.34 | 0.05 | −0.22, 0.32 | 0.07 | −0.20, 0.34 | 0.05 | −0.22, 0.32 | 30 | 1.1 | 0.6, 2.0 |
| Quartile 3 | 5.9 – 7.9 | 416 | 0.02 | −0.25, 0.28 | 0.00 | −0.28, 0.28 | 0.05 | −0.24, 0.33 | 0.00 | −0.28, 0.28 | 30 | 1.1 | 0.6, 1.9 |
| Quartile 4 | 8.0 – 49.3 | 406 | −0.03 | −0.29, 0.24 | −0.04 | −0.33, 0.24 | 0.01 | −0.28, 0.30 | −0.05 | −0.33, 0.24 | 34 | 1.2 | 0.7, 2.2 |
| PFHxS | |||||||||||||
| Continuous IQRb | 1645 | 0.03 | −0.03, 0.08 | 0.02 | −0.04, 0.07 | 0.02 | −0.03, 0.08 | 0.02 | −0.04, 0.07 | 120 | 1.0 | 0.9, 1.1 | |
| Quartile 1 | 0.1 – 1.6 | 415 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 26 | 1.0 (ref) | |||||
| Quartile 2 | 1.7 – 2.4 | 409 | 0.12 | −0.15, 0.38 | 0.08 | −0.18, 0.35 | 0.11 | −0.16, 0.38 | 0.08 | −0.19, 0.35 | 23 | 0.9 | 0.5, 1.7 |
| Quartile 3 | 2.5 – 3.7 | 404 | −0.13 | −0.39, 0.14 | −0.18 | −0.45, 0.09 | −0.15 | −0.42, 0.12 | −0.19 | −0.46, 0.08 | 41 | 1.8 | 1.1, 3.1 |
| Quartile 4 | 3.8 – 74.5 | 417 | 0.00 | −0.27, 0.26 | −0.10 | −0.37, 0.17 | −0.06 | −0.33, 0.22 | −0.10 | −0.38, 0.17 | 30 | 1.3 | 0.7, 2.2 |
| PFNA | |||||||||||||
| Continuous IQRb | 1645 | −0.08 | −0.18, 0.01 | −0.07 | −0.17, 0.02 | −0.06 | −0.15, 0.04 | −0.08 | −0.17, 0.02 | 120 | 1.2 | 1.0, 1.4 | |
| Quartile 1 | 0.1 – 0.4 | 331 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 20 | 1.0 (ref) | |||||
| Quartile 2 | 0.5 – 0.6 | 463 | 0.12 | −0.15, 0.40 | 0.10 | −0.17, 0.38 | 0.14 | −0.14, 0.42 | 0.10 | −0.18, 0.38 | 23 | 0.8 | 0.5, 1.6 |
| Quartile 3 | 0.7 – 0.9 | 470 | 0.01 | −0.27, 0.28 | −0.02 | −0.30, 0.26 | 0.03 | −0.25, 0.31 | −0.03 | −0.31, 0.25 | 39 | 1.4 | 0.8, 2.6 |
| Quartile 4 | 1.0 – 6.0 | 381 | −0.09 | −0.38, 0.20 | −0.07 | −0.37, 0.22 | −0.01 | −0.31, 0.30 | −0.08 | −0.38, 0.22 | 38 | 1.7 | 0.9, 3.0 |
PFAS= perfluoroalkyl substance; PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFHxS, perfluorohexane sulfonate PFNA, perfluorononanoate; IQR, interquartile range; estimated glomerular filtration rate computed with the Cockcroft-Gault formula (ml/min per 1.73 m2)
Adjusted for maternal age at enrollment, race/ethnicity, education, prenatal smoking, parity, history of breastfeeding, pre-pregnancy body mass index, paternal education, household income, child’s sex and gestational age at blood draw.
IQR (ng/mL) for PFASs are PFOS = 16.0; PFOA = 3.8; PFHxS = 2.2; PFNA = 0.4.
Confounding by pregnancy physiology
Adjusting for eGFR only slightly attenuated associations of PFASs with birth outcomes (e.g., PFOS-birth weight-for-gestational age associations attenuated from −0.04 to −0.03) (Tables 3 and 4). Estimates were unchanged after adjusting for plasma albumin (Tables 3 and 4). Excluding women with conditions that could bias markers of GFR and plasma volume, including hypertension or diabetes (n=39 women), did not impact effect estimates (data not shown).
Sex Differences
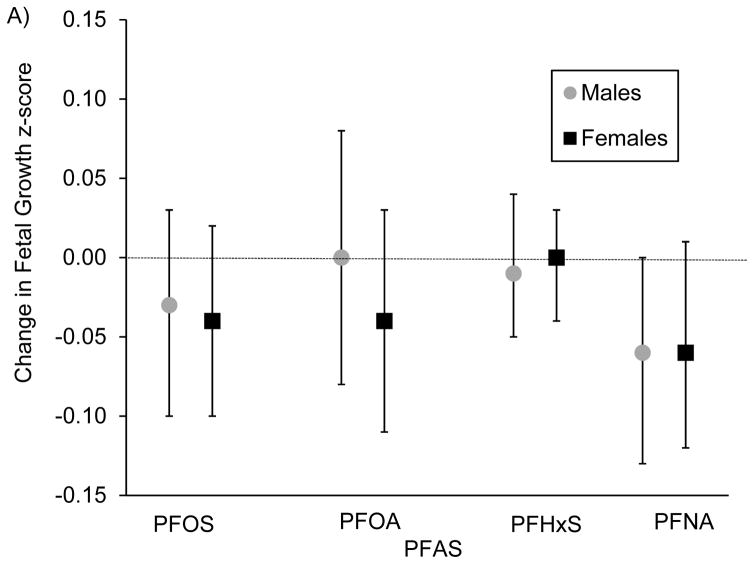
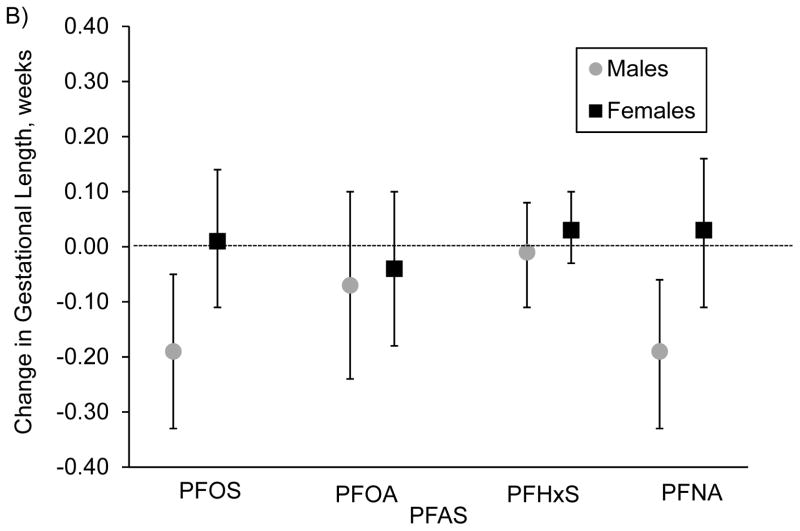
We did not observe strong evidence for sex differences in PFAS-birth weight-for-gestational age associations (Figure 1A). For gestational length, associations with PFOS were stronger among males [β per IQR increase in PFOS=−0.19 (95% CI: −0.33, −0.05)] vs. females [β=0.01 (95% CI: −0.11, 0.14)] (interaction p-value=0.09)0.14 (Figure 1B). We found similar patterns for PFNA [β=−0.19 (95% CI: −0.33, −0.06) for males vs. β=0.03 95% CI: −0.11, 0.16) for females; interaction p-value=0.01]. We did not find evidence for sex differences for any other studied associations.
Figure 1.
Associations of prenatal plasma concentrations of PFASs with A) fetal growth (birth weight for gestational age) z-score (n=1,644) and B) gestational length (weeks) (n=1,645), stratified by sex and adjusted for maternal age at enrollment, race/ethnicity, education, prenatal smoking, parity, history of breastfeeding, pre-pregnancy BMI, paternal education, household income and gestational age at blood draw, for participants of Project Viva.
DISCUSSION
This large prospective study measured of PFAS plasma concentrations in pregnant women before the U.S. phase-out of PFOS (41) and PFOA (42). As a result, plasma concentrations of these PFASs (37), and PFOS in particular, are considerably higher in this cohort relative to concentrations reported in more recent U.S. national surveys (43, 44), but similar to concentrations in a nationally representative survey with measures during the same time period (3).
In this context, we observed modest reductions in fetal growth with higher concentrations of PFOS, PFOA and PFNA. Higher PFOS and PFNA concentrations were also associated with shorter gestational length, though primarily among males. Associations of PFHxS with all birth outcomes were null. Notably, we did not observe confounding by measures of pregnancy hemodynamics, including eGFR and plasma albumin.
Gestational exposure to some PFASs has been linked with poorer fetal growth and development in animal models (45, 46). Though a direct mechanism for the impact of PFAS exposure on fetal growth has not been characterized, structural homology of PFASs with fatty acids may induce disruption of lipid metabolism (47), which could interfere with fetal growth. PFASs can also alter thyroid hormone levels (48) and activate peroxisome proliferator-activated receptors (PPAR) (49), pathways that may influence fetal growth.
Two meta-analysis, one of 9 published studies (20) and another of 7 studies (all of which were included in the 9-study meta-analysis) (50), reported an 18.9 (95% CI: −29.8, −7.9) and 14.7 g (95% CI: −21.76, −7.8) reduction in birth weight, respectively, per 1-ng/mL increase in serum or plasma PFOA. These associations were considerably stronger than those we detected in the current study: converting PFOA estimates per IQR increase (3.8 ng/mL) in Web Table 1 to per 1-ng/mL increase we found a reduction in term birth weight of 4.9 g (95% CI: −11.9, 2.2). In addition, the 7-study meta-analysis reported a 5.0 g (95% CI: −8.9, −1.1) reduction in birth weight per 1-ng/mL increase in serum or plasma PFOS, compared with only a 1.1 g (95% CI: −2.6, 0.3) reduction in term birth weight per 1-ng/mL increase in PFOS in the current study (computed using estimates in Web Table 1).
We detected associations of PFNA with birth outcomes in the current study, however, given the low plasma concentrations of PFNA in Project Viva compared with other more commonly studied PFASs, such as PFOS and PFOA, these results should be interpreted with caution. Only a few other studies have examined associations of PFNA with birth outcomes, presumably because of the relatively low PFNA concentrations, with mixed findings (9, 13, 51).
A primary objective of the current study was to evaluate whether adjusting for pregnancy hemodynamics impacted PFAS-birth outcome associations. Adjusting for albumin and eGFR did not materially alter effect estimates, suggesting that there was little or no confounding by these hemodynamic markers. Only one previous study, the Norwegian Mother and Child Cohort Study, adjusted for plasma albumin when examining associations of PFOA and PFOS measured in early gestation (17 weeks) with birth outcomes (19). This analysis also showed no confounding by albumin. We are not aware of any previous studies of PFASs and birth outcomes that adjusted for GFR. However, confounding by GFR was examined in a study that used simulated data of maternal and cord plasma PFOA and PFOS concentrations (50). In contrast to the current study, this simulation study observed considerable confounding by GFR of the PFAS-term birth weight association, with strong attenuation of both PFOA and PFOS-related associations with term birth weight after adjusting for GFR. One explanation for these conflicting findings is that plasma samples in Project Viva were drawn early in pregnancy, when pregnancy hemodynamic changes are just beginning. We speculate that this confounding may therefore only be present in studies in which blood is drawn later in pregnancy. This is further supported by the previous literature, where studies with the strongest reported PFOA-birth weight associations measured PFOA in cord serum at birth (7, 52), while studies with weaker birth weight associations measured PFOA in early pregnancy (first trimester or early second trimester) (6, 8, 19, 21). The suggestion that confounding by eGFR has less influence when PFOS and PFOA concentrations are measured in early pregnancy is also supported by simulated data (50).
Although a cohort with blood samples drawn late in pregnancy (second or third trimester) might best reveal the extent of potential confounding by plasma volume expansion and GFR, our results do suggest that studies that examine associations of birth outcomes with PFASs in serum/plasma drawn early in pregnancy are unlikely to be substantially confounded by pregnancy hemodynamics.
When we compare our birth weight results (Web Table 1) to four studies that measured PFASs in early pregnancy (9–17 weeks) (6, 8, 19, 21), as these are likely to be the associations that are least confounded by pregnancy hemodynamics, we find some consistency across individual PFASs. For example, the four studies find that a 1 ng/mL increase in PFOA is associated with a reduction in birth weight of 11 to 34 grams, whereas in our study we report a 19 gram reduction in birth weight (Web Table 1). However, these previous studies observed null associations of PFOS with birth weight, while we observed associations for PFOS that were comparable to our PFOA associations. One previous study reported lower birth weight with exposure to PFHxS (8), though our study and another previous study (21) found null associations.
A limitation of our analysis examining confounding by pregnancy hemodynamics is that we used markers measured in early pregnancy plasma: creatinine for GFR and albumin for plasma volume expansion. Whether these markers adequately represent pregnancy hemodynamics is unclear. Confounder measurement error would have resulted in residual confounding when controlling for these variables in multivariable models. However, given that we observed minimal attenuation when we included these markers as covariates, we would not expect residual confounding to completely account for our observed associations.
This study had a number of strengths. We had a large sample size with participants recruited before the voluntary phase out of PFOS and PFOA, and thus during the time of likely peak exposure to these PFASs in the United States (32). We also adjusted for key confounders, including parity, sociodemographic factors and pregnancy hemodynamics.
In conclusion, concentrations of early pregnancy PFOS, PFOA and PFNA were inversely associated, albeit modestly, with fetal growth and length of gestation in Project Viva. These findings, in a population enrolled when exposures to PFOS and PFOA were likely at their peak in the United States, are consistent with other studies showing weak inverse associations of PFOS and PFOA measured early in pregnancy with fetal growth. Measurement of PFASs in early pregnancy in future studies may avoid confounding by pregnancy hemodynamics. Studies that measure PFASs in later pregnancy, however, should consider adjusting for markers of pregnancy hemodynamics.
Supplementary Material
Acknowledgments
Work was supported by the following NIH grants: R01ES021447, R01HD34568, K23ES024803. We thank Drs. Kayoko Kato, Ayesha Patel, and Tao Jia for quantifying plasma PFAS and Drs. Matthew Longnecker, Ana Maria Mora, and Maria Harris for their valuable feedback on the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the US Government, the Department of Health and Human Services (DHHS), the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or DHHS. The authors declare they have no competing financial interests.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CI
Confidence Interval
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- PFASs
perfluoroalkyl substances
- PFHxS
perfluorohexane sulfonate
- PFNA
perfluorononanoate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
Footnotes
Conflict of interest: none declared.
References
- 1.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calafat AM, Kuklenyik Z, Reidy JA, et al. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the national health and nutrition examination survey (NHANES) Environ Sci Technol. 2007;41(7):2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Wong LY, Kuklenyik Z, et al. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115(11):1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198(2):231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol. 2009;27(3–4):212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Fei C, McLaughlin JK, Tarone RE, et al. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apelberg BJ, Witter FR, Herbstman JB, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maisonet M, Terrell ML, McGeehin MA, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120(10):1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen MH, Ha EH, Wen TW, et al. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One. 2012;7(8):e42474. doi: 10.1371/journal.pone.0042474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu K, Xu X, Peng L, et al. Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ Int. 2012;48:1–8. doi: 10.1016/j.envint.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Stein CR, Savitz DA, Dougan M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol. 2009;170(7):837–846. doi: 10.1093/aje/kwp212. [DOI] [PubMed] [Google Scholar]
- 12.Washino N, Saijo Y, Sasaki S, et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect. 2009;117(4):660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Adgent M, Su PH, et al. Prenatal Exposure to Perfluorocarboxylic Acids (PFCAs) and Fetal and Postnatal Growth in the Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2016;124(11):1794–1800. doi: 10.1289/ehp.1509998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Cock M, De Boer MR, Lamoree M, et al. Prenatal exposure to endocrine disrupting chemicals and birth weight-A prospective cohort study. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2016;51(2):178–185. doi: 10.1080/10934529.2015.1087753. [DOI] [PubMed] [Google Scholar]
- 15.Bach CC, Bech BH, Nohr EA, et al. Perfluoroalkyl Acids in Maternal Serum and Indices of Fetal Growth: The Aarhus Birth Cohort. Environ Health Perspect. 2016;124(6):848–854. doi: 10.1289/ehp.1510046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenters V, Portengen L, Rignell-Hydbom A, et al. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ Health Perspect. 2016;124(3):365–372. doi: 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishi R, Nakajima T, Goudarzi H, et al. The Association of Prenatal Exposure to Perfluorinated Chemicals with Maternal Essential and Long-Chain Polyunsaturated Fatty Acids during Pregnancy and the Birth Weight of Their Offspring: The Hokkaido Study. Environ Health Perspect. 2015;123(10):1038–1045. doi: 10.1289/ehp.1408834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect. 2013;121(10):1207–1213. doi: 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitworth KW, Haug LS, Baird DD, et al. Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2012;175(12):1209–1216. doi: 10.1093/aje/kwr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson PI, Sutton P, Atchley DS, et al. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122(10):1028–1039. doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamm MP, Cherry NM, Chan E, et al. Maternal exposure to perfluorinated acids and fetal growth. J Expo Sci Environ Epidemiol. 2010;20(7):589–597. doi: 10.1038/jes.2009.57. [DOI] [PubMed] [Google Scholar]
- 22.Savitz DA, Stein CR, Bartell SM, et al. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology. 2012;23(3):386–392. doi: 10.1097/EDE.0b013e31824cb93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savitz DA, Stein CR, Elston B, et al. Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the mid-Ohio Valley. Environ Health Perspect. 2012;120(8):1201–1207. doi: 10.1289/ehp.1104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolan LA, Nolan JM, Shofer FS, et al. The relationship between birth weight, gestational age and perfluorooctanoic acid (PFOA)-contaminated public drinking water. Reprod Toxicol. 2009;27(3–4):231–238. doi: 10.1016/j.reprotox.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee ES, Han S, Oh JE. Association between perfluorinated compound concentrations in cord serum and birth weight using multiple regression models. Reprod Toxicol. 2016;59:53–59. doi: 10.1016/j.reprotox.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Savitz DA. Guest editorial: biomarkers of perfluorinated chemicals and birth weight. Environ Health Perspect. 2007;115(11):A528–529. doi: 10.1289/ehp.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savitz DA. Invited commentary: interpreting associations between exposure biomarkers and pregnancy outcome. Am J Epidemiol. 2014;179(5):545–547. doi: 10.1093/aje/kwt314. [DOI] [PubMed] [Google Scholar]
- 28.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney international. 1998;54(6):2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 29.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol. 2011;174(8):893–900. doi: 10.1093/aje/kwr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins DJ, Josson J, Elston B, et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect. 2013;121(5):625–630. doi: 10.1289/ehp.1205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morken NH, Travlos GS, Wilson RE, et al. Maternal glomerular filtration rate in pregnancy and fetal size. PLoS One. 2014;9(7):e101897. doi: 10.1371/journal.pone.0101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Cousins IT, Scheringer M, et al. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: the remaining pieces of the puzzle. Environ Int. 2014;69:166–176. doi: 10.1016/j.envint.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Oken E, Baccarelli AA, Gold DR, et al. Cohort Profile: Project Viva. Int J Epidemiol. 2014;44(1):37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato K, Basden BJ, Needham LL, et al. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218(15):2133–2137. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 35.Kuklenyik Z, Needham LL, Calafat AM. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem. 2005;77(18):6085–6091. doi: 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- 36.Hornung RW, Reed LW. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 37.Sagiv SK, Rifas-Shiman SL, Webster TF, et al. Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ Sci Technol. 2015;49(19):11849–11858. doi: 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oken E, Kleinman KP, Rich-Edwards J, et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC pediatrics. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips G, Brett K, Mendola P. Previous breastfeeding practices and duration of exclusive breastfeeding in the United States. Maternal and child health journal. 2011;15(8):1210–1216. doi: 10.1007/s10995-010-0694-4. [DOI] [PubMed] [Google Scholar]
- 40.D’Eon JC, Simpson AJ, Kumar R, et al. Determining the molecular interactions of perfluorinated carboxylic acids with human sera and isolated human serum albumin using nuclear magnetic resonance spectroscopy. Environmental toxicology and chemistry/SETAC. 2010;29(8):1678–1688. doi: 10.1002/etc.204. [DOI] [PubMed] [Google Scholar]
- 41.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Perfluoroalkyls. (Draft for Public Comment) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2009. [Google Scholar]
- 42.US Environmental Protection Agency. [Accessed 12 September 2016];PFOA Stewardship Program. 2006 https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/and-polyfluoroalkyl-substances-pfass-under-tsca#tab-3.
- 43.Kato K, Wong LY, Jia LT, et al. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45(19):8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. http://www.cdc.gov/exposurereport/ [Google Scholar]
- 45.Lau C, Anitole K, Hodes C, et al. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 46.DeWitt JC, editor. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. New York, NY: Springer; 2015. [Google Scholar]
- 47.Vanden Heuvel JP, Thompson JT, Frame SR, et al. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92(2):476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 48.Knox SS, Jackson T, Frisbee SJ, et al. Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J Toxicol Sci. 2011;36(4):403–410. doi: 10.2131/jts.36.403. [DOI] [PubMed] [Google Scholar]
- 49.DeWitt JC, Shnyra A, Badr MZ, et al. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Critical reviews in toxicology. 2009;39(1):76–94. doi: 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- 50.Verner MA, Loccisano AE, Morken NH, et al. Associations of Perfluoroalkyl Substances (PFAS) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK) Environ Health Perspect. 2015;123(12):1317–1324. doi: 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Yang L, Li J, et al. Occurrence of perfluoroalkyl substances in cord serum and association with growth indicators in newborns from Beijing. Chemosphere. 2017;169:396–402. doi: 10.1016/j.chemosphere.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 52.Fromme H, Mosch C, Morovitz M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44(18):7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.