Abstract
A key objective of the emerging field of personality neuroscience is to link the great variety of the enduring dispositions of human behaviour with reliable markers of brain function. This can be achieved by analyzing large sets of data with methods that model whole-brain connectivity patterns. To meet these expectations, we exploited a large repository of personality and neuroimaging measures made publicly available via the Human Connectome Project.
Using connectomic analyses based on graph theory, we computed global and local indices of functional connectivity (e.g., nodal strength, efficiency, clustering, betweenness centrality) and related these metrics to the five-factor-model (FFM) personality traits (i.e., neuroticism, extraversion, openness, agreeableness, and conscientiousness). The maximal information coefficient was used to assess for linear and non-linear statistical dependencies across the graph ‘nodes’, which were defined as distinct brain circuits identified via independent component analysis. Multi-variate regression models and ‘train/test’ machine-learning approaches were also used to examine the associations between FFM traits and connectomic indices as well as to test for the generalizability of the main findings, whilst accounting for age and sex differences.
Conscientiousness was the sole FFM trait linked to measures of higher functional connectivity in the fronto-parietal and default mode networks. This might provide a mechanistic explanation of the behavioural observation that conscientious people are reliable and efficient in goal-setting or planning.
Our study provides new inputs to understanding the neurological basis of personality and contributes to the development of more realistic models of the brain dynamics that mediate personality differences.
Keywords: Big-five, individual differences, resting-state fMRI, connectome, graph analysis
Introduction
Personality neuroscience is a rapidly growing research field that aims at understanding the neural underpinnings of variability in cognitive and emotional functions as well as the brain basis of individual differences in behaviour (Corr, 2006; Colin G. DeYoung, 2010). Extensive research in personality has shown that the complexity of human behaviour can be described by an aggregate taxonomy termed the five-factor model (FFM)(Costa & McCrae, 1992; Digman, 1990; Robert R. McCrae & Terracciano, 2005), although other models of personality have also been developed to explain the high variability in a wide range of behaviours, including clinical disorders, occupational/educational performance, and economic behaviour (Ashton et al., 2004; Cloninger, 1999; Cloninger, Przybeck, & Svrakic, 1991; Cloninger, Svrakic, & Przybeck, 1993; Corr, 2006; H. Eysenck, 1983; H. J. Eysenck, 2012; J. A. Gray, 1970; J.A. Gray & McNaughton, 2003). The FFM posits that neuroticism, extraversion, openness, agreeableness, and conscientiousness are the universal descriptors of human enduring behavioural dispositions (R. R. McCrae, 1991; R. R. McCrae & Costa, 1987; R. R. McCrae & John, 1992; Robert R. McCrae & Terracciano, 2005).
However, how and why individuals differ in these traits remain an important open question. Recently, sophisticated brain imaging techniques and new analytical methods have become available to help formulating novel theories and models regarding the neurological origin of human personality, although it must be acknowledged that neuroimaging intrinsically remains an indirect and correlational measure of brain anatomy and function. Past research has linked the FFM traits to different indices of brain structure and function, although the presence of mixed and often conflicting results in the literature limits the conclusions that can be drawn from these studies (Canli, 2004; Canli, Sivers, Whitfield, Gotlib, & Gabrieli, 2002; H. Cremers et al., 2011; H. R. Cremers et al., 2010; C. G. DeYoung et al., 2010; Dima, Friston, Stephan, & Frangou, 2015; Fischer, Wik, & Fredrikson, 1997; Hu et al., 2011; Indovina, Riccelli, Staab, Lacquaniti, & Passamonti, 2014; Kapogiannis, Sutin, Davatzikos, Costa, & Resnick, 2012; Krebs, Schott, & Duzel, 2009; Liu et al., 2013; Lu et al., 2014; Passamonti et al., 2015; Riccelli, Indovina, et al., 2017; Rodrigo et al., 2016; Servaas et al., 2013; Wright, Feczko, Dickerson, & Williams, 2007; Wright et al., 2006). Several factors may explain the inconsistences across previous findings, including the use of different analytic approaches and the fact that most of the earlier studies, with some notable exceptions (Bjornebekk et al., 2013; Holmes et al., 2012; Nostro, Muller, Reid, & Eickhoff, 2016; Riccelli, Toschi, Nigro, Terracciano, & Passamonti, 2017), have been conducted in small samples of participants.
Another important issue is the necessity to progress from accounts that describe personality differences in terms of anatomical and functional heterogeneity in isolated brain regions, to predictive frameworks that formally model the complexity of the connectivity patterns at the whole-brain level. Within this context, mathematical approaches based on graph theory have been applied to measure the architecture (‘topology’) of the brain structural and functional connectivity patterns (i.e., ‘connectomic’ approaches) (Fornito & Bullmore, 2015). The graph theoretical approach provides a series of key indices to quantify different aspects of the brain ‘connectome’ (Fornito & Bullmore, 2015). For instance, the network’s capacity to ‘route’ information across its distinct elements (‘nodes’) can be estimated by computing the efficiency of the paths (‘edges’) linking these nodes (Boccaletti, Latora, Moreno, Chavez, & Hwang, 2006). In other words, the network’s efficiency is a quantitative representation of ‘how easy’ it is for an input to ‘travel’ across the graph’s nodes. Consequently, increased efficiency reflects heightened capacity of a network to process and route relevant information across its nodes. Graph analyses also enable to quantify the degree of segregation of a network (modularity) and its capacity to integrate the information at a global or local level (i.e., global or local clustering coefficient) (Rubinov & Sporns, 2010).
Studying how ‘communications’ across large-scale brain circuits relate to each of the FFM traits has thus the potential to significantly improve our understanding of the neurological roots of human personality. The rationale behind this study was to reliably associate each of the FFM traits with functional connectivity patterns across large-scale brain networks. Although the relationship between the blood-oxygen-level dependant (BOLD) activity in single regions and the whole-brain network measures is highly complex, there is compelling evidence that ‘holistic’ neuroimaging approaches are able to predict individual variability in multiple behavioural, demographic, and life-style measures (S. M. Smith et al., 2015). However, it remains to be determined whether graph-based metrics can be reliably associated with individual differences in the FFM personality traits. To take a step in this direction, we studied the brain functional connectome in relation to the FFM in a large sample of young and healthy individuals drawn from the Human Connectome Project (HCP) (n=818, age-range: 22–37 years). The HCP is an international project that has granted open access to an unprecedented large set of demographics, personality, and neuroimaging data with high spatial and temporal resolution (McNab et al., 2013).
By using robust and highly validated methods to analyze resting-state functional magnetic resonance imaging (rsfMRI) data, we tested how individual differences in neuroticism, extraversion, openness, agreeableness, and conscientiousness are associated to global and local indices of brain functional connectivity (e.g., nodal strength, efficiency, clustering). A machine-learning validation approach based on a “training” and “testing” split of the total dataset was also employed to assess for the replicability of our main findings. We hypothesized that the FFM traits linked to less favorable outcomes (e.g., risk of developing psychiatric disorders) like neuroticism are associated to reduced brain functional connectivity (e.g., low nodal strength, low clustering, and low efficiency). Conversely, FFM traits like openness, extraversion, agreeableness, and conscientiousness (which have been linked to curiosity, social skills, and life success) were expected to be associated to measures of heightened functional connectivity (e.g., high nodal strength, high clustering, and high efficiency).
These predictions are based upon a recent study which found that functional connectomic metrics strongly relate to a ‘single-axis’ co-variation (ranging from ‘positive’ to ‘negative’ measures) in behavioural traits (S. M. Smith et al., 2015). In other words, those individuals scoring high on the ‘positive’ end of the behavioural axis linking lifestyle, demographic, and other psychometric measures (e.g., fluid intelligence) displayed stronger functional connectivity patterns than low-scoring subjects (S. M. Smith et al., 2015). Interestingly, the brain regions that most contributed to these increased functional connectivity patterns included those areas that belong to the default mode network (DMN) (e.g., the medial prefrontal and parietal cortices, temporo-parietal junction, and anterior insula). Although the precise role of each region within the DMN is still matter of debate (Leech, Kamourieh, Beckmann, & Sharp, 2011), there is robust evidence that the DMN as a whole is involved in several aspects of human cognition and behaviour, including episodic and semantic memory, imagination, decision-making, and theory of mind (R. P. Roberts et al., 2017; Schacter, 2012; Schacter et al., 2012; Schacter, Benoit, De Brigard, & Szpunar, 2015). It is thus reasonable to expect that enhanced functional connectivity patterns within and across the DMN is linked with FFM personality traits that predict ‘positive’ and favorable behavioural outcomes, although caution is always warranted when making reverse inferences in interpreting neuroimaging findings (Poldrack, 2006).
Participants & Methods
Participants
The demographic and personality variables of the HCP sample are summarized in Table 1.
Table 1.
Demographic and personality variables in the HCP sample (n=818 healthy volunteers).
| Demographic variables | ||
|
| ||
| Gender (males / females) | 367 / 451 | |
|
| ||
| Age (years) | 28.7 ± 3.7 [22–37] | |
|
| ||
| Handedness (Right / Left / Both) | 743 / 73 / 2 | |
|
| ||
| Education (years) | 14.9 ± 1.8 [11–17] | |
|
| ||
| Ethnicity (%) | Hispanic / Latino | 8.6 % |
| Not Hispanic / Latino | 90.5 % | |
| Unknown / Not Reported | 0.9 % | |
|
| ||
| Personality scores (NEO-FFI) | ||
|
| ||
| Neuroticism | 16.3 ± 7.2 [0–43] | |
|
| ||
| Extraversion | 30.7 ± 5.9 [11–47] | |
|
| ||
| Openness | 28.3 ± 6.1 [12–45] | |
|
| ||
| Agreeableness | 32.0 ± 5.0 [13–45] | |
|
| ||
| Conscientiousness | 34.5 ± 5.9 [12–48] | |
Key to table. Age, education, and personality data are expressed as mean ± standard deviation while the range in parentheses [] is expressed as minimum-maximum. NEO five-factors inventory questionnaire, NEO-FFI.
In brief, all participants were young and healthy adults with no obesity, hypertension, alcohol or tobacco misuse, anxiety, depressive or other psychiatric and neurologic disorders, or history of behavioural problems. Most participants were right-handed white Americans with a non-Hispanic or Latinos background.
Personality assessment
The FFM personality traits were assessed via the NEO five-factors inventory (NEO-FFI) (Costa & McCrae, 1992; Terracciano, 2003). The NEO-FFI is composed by 60 items, 12 for each of the five factors. For each item, participants reported their level of agreement on a 5-points Likert scale, from strongly disagree to strongly agree. The NEO instruments have been previously validated in USA and several other countries (Robert R. McCrae & Terracciano, 2005).
MRI scanning protocol and pre-processing
Resting-state fMRI (rs-fMRI) data were acquired using a 3T scanner (Siemens AG, Erlangen, Germany) (Van Essen et al., 2012). Four runs of 15 minutes each were acquired. Subjects lied within the scanner with open eyes and while fixating a bright central cross projected on a dark background. Oblique axial acquisitions were alternated between phase encoding in a right-to-left direction in one run and phase encoding in a left-to-right direction in the other run. Gradient-echo echo-planar imaging used the following parameters: TR=720 ms, TE=33.1 ms, flip angle=52°, FOV=208×180 mm, Matrix 104×90, Slice thickness=2.0 mm; 72 slices; 2.0 mm isotropic voxels, Multiband factor=8, Echo spacing=0.58 ms, BW=2290 Hz/Px. This resulted in 4800 rs-fMRI volumes in total per subject, subdivided in 4 runs of 1200 volumes each. Structural (T1-weighted) images and field maps were also acquired to aid data pre-processing.
Each 15-minute (1200 volume) run of each subject’s rs-fMRI data was pre-processed using FSL and it was minimally pre-processed according to the latest version (3.1) of the HCP pipeline (Glasser et al., 2013). Each dataset was then temporally de-meaned and had variance normalization applied according to Beckmann and colleagues (Beckmann & Smith, 2004). Group-PCA output was generated by MIGP (MELODIC’s Incremental Group-PCA), a technique that approximates full temporal concatenation of all subjects' data, from all 820 participants. This comprises the top 4500 weighted spatial eigenvectors from a group-averaged PCA (Stephen M Smith, Hyvärinen, Varoquaux, Miller, & Beckmann, 2014). The MIGP output was then fed into group-ICA using FSL's MELODIC tool (Beckmann & Smith, 2004), applying spatial-ICA at dimensionality of 15. Successively, the ICA maps were dual-regressed into each subject's 4D dataset to give a set of 15 time-courses of 4800 time points per subject. Further details regarding data acquisition and processing can be found in the HCP S900 Release reference manual available at https://www.humanconnectome.org/.
Estimation of functional connectivity
To quantify the resting-state functional connectivity among the 15 circuits (‘nodes’), the maximum information coefficient (MIC) between the time-series of each pair of circuits was computed (Reshef et al., 2011). MIC is a powerful statistic sensitive to both linear and nonlinear associations of arbitrary shape between paired variables (Reshef et al., 2011). This method was recently applied to investigate the functional connectivity patterns in patients with schizophrenia (Su, Wang, Shen, Feng, & Hu, 2013; Zhang, Sun, Yi, Wu, & Ding, 2015). The basic idea underlying MIC is that, when a relationship between two variables exists, it can be quantified via creating a grid on the scatterplot that creates a partition of the data. More formally, the MIC between two variables x and y is defined as:
where nx and ny are the number of bins of the partition of the x- and y-axis. Therefore, the MIC of two variables x and y is calculated as:
where the maximum is taken over all the possible nx by ny grids. The MIC between each pair of networks’ time-series was calculated using the MINEPY toolbox (Albanese et al., 2013) implemented in MATLAB (https://github.com/minepy/minepy). These analytical steps generated a 15 × 15 full and symmetric subject-specific matrix of functional connectivity data. These matrices were then treated as weighted networks to calculate the graph-related measures.
Local network analyses
All graph measures were computed via the Brain Connectivity Toolbox (Rubinov & Sporns, 2010) in MATLAB (https://sites.google.com/site/bctnet/). For each independent components analysis (ICA) and at the subject level, we calculated the graph measures that quantify the centrality of a node within a network (local strength and betweennes centrality) as well as its integration and segregation properties (clustering coefficient and local efficiency respectively). Local strength and betweennes centrality are two indices of centrality that measure the relative importance of a node within a network (Zuo et al., 2012). Nodes with high levels of centrality are thought to facilitate information routing in the network with a key role in the overall communication efficiency of a network. The node’s strength is the simplest measure of centrality and is defined as the sum of all the edge weights between a node and all the other nodes in the network. Regions with a high nodal strength indicate high connectivity with other nodes. Betweennees centrality of a node is defined as the fraction of all shortest paths in the network that contain a given node. If a node displays a high value of betweenness centrality, it participates in a large number of shortest paths and have an important role in the information transfer within a network. Along with centrality measures, the nodes of a network could display different levels of segregation and integration of information (Sporns, 2013). Also, the clustering coefficient is a commonly used metric to assess the segregation properties of a network. It reflects the ability of a node to communicate with other nodes with which it shares direct connections; in other words, it represents the fraction of triangles around an individual node. It is equivalent to the fraction of the node’s neighbours that are also neighbours of each other (Watts & Strogatz, 1998) and in the case of weighted networks it is calculated as the geometric mean of all triangles associated with each node (Onnela, Saramäki, Kertész, & Kaski, 2005). Finally, the ability of an efficient information transfer across distributed nodes (i.e., nodes that are not directly connected) can be quantified via local path length and local efficiency. In the case of a binary network, the local path length is the minimum number of edge that must be traversed to go from one node to another, while in a weighted network high levels of correlations are interpreted as short distances. The local efficiency is therefore the average of the inverse local path length. Local efficiency is calculated as the global efficiency of the subgraph formed by the node’s neighbours (Boccaletti et al., 2006). It measures the ability of parallel information transfer at local level.
Global network analyses
Global graph metrics describe the topology of a network in a single number which characterizes the overall organization of a network. As global measures, we computed the global strength, the global clustering coefficient and global efficiency (Boccaletti et al., 2006; Rubinov & Sporns, 2010). These measures were calculated as the average of the local strength, local clustering coefficient and the local efficiency of all nodes, respectively.
Statistical analyses
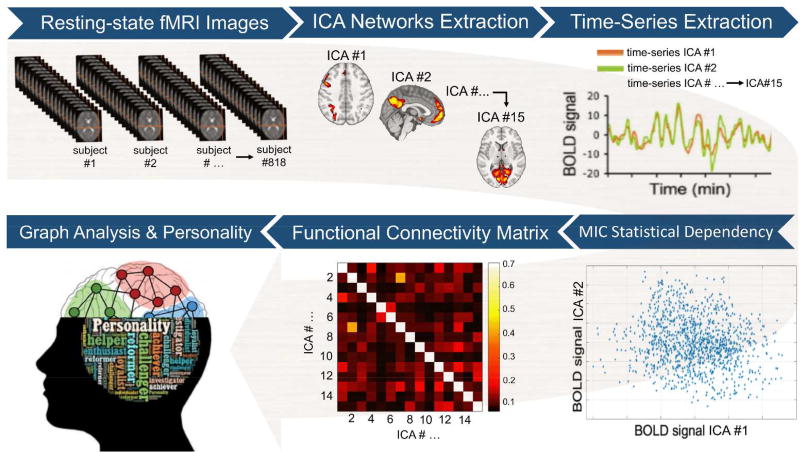
To evaluate the replicability of our inference framework, the initial sample of n=818 participants was randomly split into two sub-samples: a “training” sample (70% of participants, n=573) and a “test” sample (30% of participants, n=245). The “training” sample was used to examine the association between each of the graph measures (both global and local metrics) and the FFM personality traits. Conversely, the “test” sample was employed to assess whether the multivariate model based on the “training” sample was able to predict outcomes in the “test” sample (i.e., in an 'unseen' group of subjects to which the model was completely ‘agnostic’). More specifically, to test the associations between graph measures and personality differences, general linear models (GLMs), including each of the FFM traits as well as age and gender as nuisance covariates, were fitted using the “training” sample. The resulting P values were corrected for multiple comparisons using a false discovery rate (FDR) procedure. Associations surviving a stringent threshold of P<0.01 FDR were considered statistically significant. The GLMs fitted in the former procedure were then used to estimate the graph measures resulting in the "test" sample using the demographic variables and personality scores of the “test” sample only as inputs (in other words, the rsfMRI data of the “train” sample was not employed in this procedure). The similarity between ‘real’ graph measures (i.e., computed using rsfMRI data from the “test” sample) and ‘estimated’ graph indices (i.e., predicted using the GLM fitted on “training” data only) was assessed using the relative root mean square error (RRMSE). This approach is typically referred as external validation and tests for generalizability of the findings beyond the study population. The image analysis workflow is summarized in Figure 1.
Figure 1. Image analysis workflow.
After initial pre-processing, the resting-state functional magnetic imaging (fMRI) data were used to extract a set of 15 separate brain circuits via independent components analysis (ICA). Next, subject-specific time-series from each ICA brain circuit were obtained. The maximal information coefficient (MIC), an index that assesses for linear and non-linear relationships in big data-sets, was used to measure statistical dependency between each pair of time-series. This led to a 15×15 functional connectivity matrix at the single-subject level. The subject-specific connectivity matrices were then used to compute local and global graph measures (i.e., strength, clustering, efficiency, and betweennees centrality). Each of these graph measures, which quantify different aspects of the brain topological organization, was finally correlated with the five-factor-model personality traits at the group level.
Results
Independent components analysis (ICA)
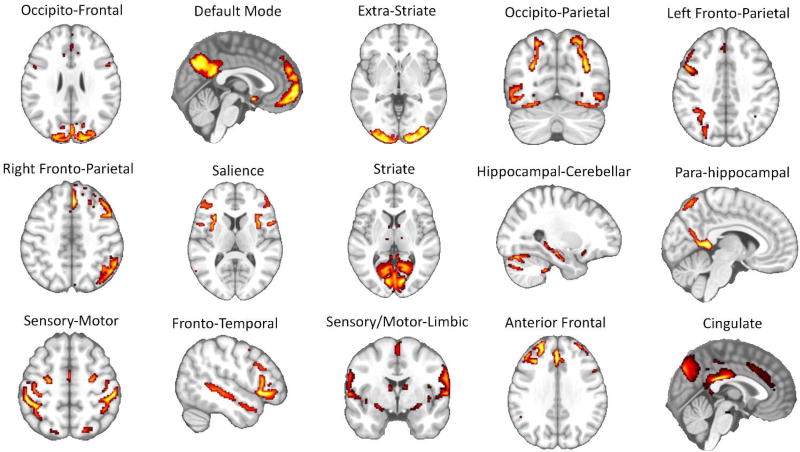
The fifteen brain networks identified via ICA were represented by a series of circuits that have been consistently reported in past rs-fMRI studies (e.g., the sensory-motor circuit, the visual circuits, the default-mode network, the left and right fronto-parietal circuits, the salience network, etc.) (Raichle, 2015; Toschi, Duggento, & Passamonti, 2017) (Figure 2 and Supplementary Table 1 for a detailed list of the anatomical regions involved in each network node).
Figure 2. Results of independent component analysis (ICA).
Fifteen separate brain functional circuits were identified during the ICA step of the image analysis pipeline (Figure 1 and methods section in the main text for further details). Each of these circuits was successively used as “node” in the graph analysis. The list of the brain areas belonging to each individual network is reported in Supplementary Table 1.
Correlations between global graph indices and FFM traits
No significant associations were found between any of the FFM personality traits and: (i) the global strength (R’s<0.084, P’s>0.14); (ii) global clustering coefficient (R’s<0.081, P’s>0.15,) and (iii) global efficiency (R’s<0.083, P’s>0.17).
Correlations between local graph indices and FFM traits
Neuroticism
No associations, either positive and negative, were found between neuroticism scores and: (i) the nodal strength (R’s<0.07, P’s>0.75); (ii) local clustering coefficient (R’s<0.06, P’s>0.88); (iii) local efficiency (R’s<0.07, P’s>0.82), and (iv) betweeness centrality (R’s<0.09, P’s>0.59)
Extraversion
As for neuroticism, no statistically significant association was found between extraversion scores and: (i) the nodal strength (R’s<0.11, P’s>0.09); (ii) local clustering coefficient (R’s<0.12, P’s>0.04); (iii) local efficiency (R’s<0.12, P’s>0.09), and (iv) betweeness centrality (R’s<0.11, P’s>0.09).
Openness
No positive or negative association was detected between openness scores and: (i) the nodal strength (R’s<0.07, P’s>0.97); (ii) local clustering coefficient (R’s<0.06, P’s>0.96); (iii) local efficiency (R’s<0.06, P’s>0.99), and (iv) betweeness centrality (R’s<0.09, P’s>0.27).
Agreeableness
No positive or negative association was detected between Agreeableness scores and: (i) the nodal strength (R’s<0.10, P’s>0.13); (ii) local clustering coefficient (R’s<0.10, P’s>0.12); (iii) local efficiency (R’s<0.10, P’s>0.15), and (iv) betweeness centrality (R’s<0.08, P’s>0.25).
Conscientiousness
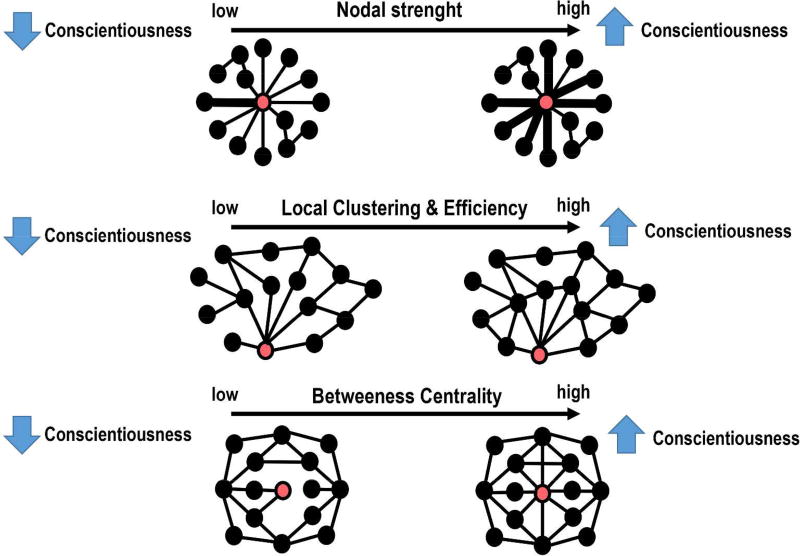
A schematic representation of the statistically significant associations between conscientiousness scores and the local graph measures is reported in Figure 3, while the statistical details are included in Table 2. In summary, significantly positive correlations were found between conscientiousness scores and the nodal strength, local clustering coefficient, and local efficiency in the left fronto-parietal network (FPN) (R’s>0.14, P’s<0.01, FDR). Furthermore, increased local clustering coefficient and betweeness centrality in the default mode network (DMN) and right FPN were associated with higher levels of conscientiousness (R’s>0.14, P’s<0.005, FDR). External validation showed good replicability, with RRMSE values of around 0.15 in the “test” sample.
Figure 3. Schematic representation of the main results.
Depending on the graph metric (Table 2), the red circle represents either the left or right fronto-parietal network (FPN) or default mode network (DMN), whilst the black circles represents the 14 remaining network nodes. Top row. The thicker lines in individuals with high levels of conscientiousness indicate the existence of higher strength in the ‘communications’ between the FPN and the other brain networks. Middle row. People scoring higher in conscientiousness show a higher degree of inter-connectedness between the FPN or DMN and the local networks consisting of direct neighbours of the FPN or DMN. Bottom row. The DMN has higher betweeness centrality in individuals with high levels of conscientiousness. This means that the DMN is a “hub” node in conscientious people.
Table 2.
Positive correlations between local graph metrics and conscientiousness scores.
| Local graph metric | Circuit | Mean (±SD) | T score | Pearson’s R |
P (FDR) |
RRMSE |
|---|---|---|---|---|---|---|
| Nodal strength | Left FPN | 2.37 ± 0.11 | 3.46 | 0.14 | 0.009 | 0.16 |
| Local clustering | DMN | 0.15 ± 0.008 | 3.40 | 0.14 | 0.008 | 0.15 |
| Left FPN | 0.13 ± 0.007 | 3.26 | 0.14 | 0.008 | 0.14 | |
| Local efficiency | Left FPN | 0.09 ± 0.007 | 3.53 | 0.15 | 0.006 | 0.15 |
| Betweennes-centrality | DMN | 2.17 ± 2.44 | 3.68 | 0.15 | 0.002 | 0.16 |
| Right FPN | 0.23±0.79 | 3.66 | 0.15 | 0.002 | 0.16 |
Key to Table. Standard deviation (SD); FPN, fronto-parietal network; DMN, default mode network. FDR, false discovery rate; RRMSE: Relative root mean square error.
To further explore which specific aspects of conscientiousness were linked to local graph measures, we conducted post-hoc analyses that included conscientiousness facets (i.e., C2-Order, C3-Dutifulness, C4-Achievement striving; C5-Self-Discipline) as main outcome measures. As in the previous analyses, age, sex, and the other FFM traits were also included in the GLM as nuisance covariates. We found that the betweenness centrality in the DMN was positively associated with C3 (P=0.01, FDR, RRMSE=0.17) and C4 (P=0.01, FDR, RRMSE=0.16). Finally, the betweeness centrality in the right FPN was positively associated with C3 (P=0.01, FDR, RRMSE=0.16).
Discussion
This study provides compelling new evidence that ‘local’ graph metrics based on resting state functional imaging are significantly associated with conscientiousness in a sample of 818 young and healthy adults drawn from the Human Connectome Project (HCP). More specifically, we found higher nodal strength, local clustering, and local efficiency in the left fronto-parietal network (FPN) in people scoring high in conscientiousness. Likewise, higher local clustering and betweenness centrality in the right FPN and default mode network (DMN) were positively related to conscientiousness scores. A validation approach based on a “training” and “test” split of the total dataset strongly supported the robustness, replicability, and ‘cross-validity’ of these findings.
Overall, our results demonstrate the value of applying connectomic approaches to study the brain functional connectivity in relation to the FFM of personality. The multi-variate analyses also show that the positive association between the FPN/DMN connectivity patterns and conscientiousness is not dependent on other FFM personality traits (i.e., neuroticism, extraversion, openness, and agreeableness) or potentially confounding factors like gender and age variability. Similarly, the non-significant correlations with global connectomic measures (e.g., global clustering and efficiency) suggests that individual differences in conscientiousness are mediated by specific functional dynamics across distinct cognitive nodes. In the following sections, we discuss the implication of our findings to improve the understanding of the neural underpinnings of conscientiousness as well as the main strengths and limitations of our study.
FPN and DMN connectivity patterns mediate conscientiousness
The higher nodal strength in the left fronto-parietal network (FPN) in people scoring high in conscientiousness reflects the fact that this specific circuit ‘node’ has heightened ‘communications’ with the other nodes. Highly conscientious people also show higher local clustering in the left FPN, which implies that the FPN is densely inter-connected to its neighbours and formed an elevated number of local aggregates (‘triangles’) with its most adjacent nodes. At the same time, the local efficiency in the left FPN and the betweeness centrality in the right FPN are higher in people scoring high in conscientiousness.
The FPN includes cito-architecturally complex and evolutionarily recent cortices that have been associated with inter-subject variance in several cognitive measures (Mueller et al., 2013; Zilles, Armstrong, Schleicher, & Kretschmann, 1988). Furthermore, a recent study in n=126 people from the HCP database reported that the functional connectivity patterns involving the FPNs were the most distinguishing features (‘fingerprints’) to predict variability in cognitive functioning across different individuals (Finn et al., 2015). Although the FPNs are particularly engaged during tasks that require high levels of attention and cognitive control, their connectivity patterns at rest can also predict subject-specific performance with a high degree of precision (Finn et al., 2015; Miranda-Dominguez et al., 2014). This may depend on the fact the FPN ‘nodes’ act as flexible ‘hubs’ and therefore play a critical role in coordinating the activity and functioning of several other brain regions (Finn et al., 2015; Miranda-Dominguez et al., 2014).
The enhanced connectivity patterns in FPNs in people scoring high in conscientiousness can therefore be interpreted as a ‘sign’ of increased cognitive control and flexible behaviour in these individuals, bearing in mind the shortcomings of making reverse inference in cognitive neuroscience (Poldrack, 2006). This is in keeping with several observations showing that conscientious people are efficient in pursuing their objectives and planning, which are themselves critical predictors of academic or occupational success, healthy life-styles, and ultimately longevity (Noftle & Robins, 2007; Ozer & Benet-Martinez, 2006; B. W. Roberts, Lejuez, Krueger, Richards, & Hill, 2014; Sutin et al., 2016). Our data are also consistent with past neuroimaging studies that have implicated the dorsolateral prefrontal cortex (DLPFC) and other PFC areas (e.g., the anterior cingulate cortex-ACC, which is also part of the FPN) in conscientiousness (Bunge & Zelazo, 2016; C. G. DeYoung et al., 2010; Forbes et al., 2014; Jackson, Balota, & Head, 2011; Kapogiannis et al., 2012; Matsuo et al., 2009; Whittle et al., 2009). Nevertheless, it is important to emphasize that our results show that it is the FPN connectivity patterns with the other ‘nodes’ which is linked with conscientiousness rather than the activity in the DLPFC/ACC in isolation. This is a key issue, especially when considering the necessity to progress from models of personality that consider the function of single brain regions, to more naturalistic frameworks that aim at describing individual differences in behavioural traits in terms of large-scale networks dynamics.
Last but not least, we found that the default mode network (DMN) showed higher local clustering and betweenness centrality in relation to high conscientiousness scores. This finding was predicted on the basis of recent data showing that connectivity patterns involving the DMN strongly predict variability in a single ‘positive-to-negative’ behavioural axis (S. M. Smith et al., 2015). There is also evidence that the DMN significantly contributes to working memory performances via the dynamic reconfiguration of its interactions with other networks, which suggests that the DMN is actively involved during the execution of cognitive demanding tasks (Vatansever, Menon, Manktelow, Sahakian, & Stamatakis, 2015). Overall, high-level cognitive functioning is critical in human evolution and is likely to be central in the life of conscientious people. Hence, we speculate that the enhanced DMN ‘interplay’ with other nodes may help explaining, in mechanistic terms, why conscientious individuals are able to efficiently elaborate complex plans like imaging and planning future scenarios. This hypothesis is corroborated by our post-hoc analyses showing that local measures in the DMN (i.e., local clustering and betweeness centrality) are respectively linked to the C3-Dutifulness facet (i.e., reliable, dependable, careful, scrupulous and strictly adherent to rules) and C4-Achievement Striving facet (i.e., industrious, enterprising, ambitious, purposeful, and driven) of conscientiousness.
Strengths & limitations
The main strengths of our study are: (i) the large, homogeneous, and well-characterized sample of participants in terms of FFM personality traits, demographic variables, and neuroimaging data, which in itself offers greater statistical power compared to several previous studies, and (ii) the fact that we employed robust statistical approaches (i.e., machine-learning) to show specificity and replicability of our main findings. We note, however, that the effects sizes were relatively small (T’s~3.5), although in the typical range of other recent studies using similar sample sizes in healthy individuals (Mackey et al., 2016; S. M. Smith et al., 2015). There was also a relatively high number of statistical tests, although we strived to attenuate this potential problem with the use of stringent statistical procedures to correct for multiple comparisons (P<0.01, FDR).
The fact that conscientiousness was the sole personality trait related to ‘connectomic’ metrics does not necessarily imply that the other FFM traits do not have such brain correlates. Several reasons why the other FFM traits were not related to functional connectomic indices may be speculated–even if not resolved by our dataset. These include: (i) type II errors; (ii) non-linear relationships between personality traits and brain connectomic metrics; (iii) the fact that our models were multivariate rather than univariate, which means that the shared variance explained by the other FFM traits was factored out while analyzing the effect of each FFM trait; (iv) the possibility that correlations between brain functional connectomic measures and other personality traits do exist but can only be revealed by ‘meta-trait’ measures (C. G. DeYoung, Peterson, & Higgins, 2002) or traits from other models of personality, not the FFM.
Perhaps more importantly, our study suggests that different neuroimaging modalities and analytical techniques may be able to reveal the unique and exquisite nature of how the brain mediates each of the FFM traits. Consistent with this idea, we have recently found in n=509 individuals from the same HCP dataset that measures of cortical anatomy (i.e., cortical thickness, folding, and surface area) were strongly and differently associated with each of the FFM traits (Riccelli, Toschi, et al., 2017). Hence, brain structural heterogeneity is likely to be an underlying substrate of variability in all FFM traits while the same may not be true for functional measures that assess more transient ‘communication’ patterns. Different functional connectivity approaches (e.g., time-variant connectivity methods) are thus warranted to reveal in more detail the complexity of the neural dynamics mediating individual differences in personality (Riccelli, Passamonti, Duggento, Guerrisi, Indovina, Terracciano, et al., 2017; Riccelli, Passamonti, Duggento, Guerrisi, Indovina, & Toschi, 2017).
Summary & conclusions
To summarize, we found robust and specific associations between conscientiousness and graph measures of local connectivity in the FPN and DMN. These highly integrated circuits include different parts of the prefrontal and parietal cortices, a set of brain regions that have significantly evolved in human beings and have been consistently implicated in goal-setting and planning, two high-order cognitive functions in which conscientious people excel.
Supplementary Material
Acknowledgments
Grant support: Roberta Riccelli is funded by the University “Tor Vergata” of Rome, Italy, while Luca Passamonti is funded by the Medical Research Council (MRC) (MR/P01271X/1) at the University of Cambridge, UK. Antonio Terracciano is supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG053297 and R03AG051960. Iole Indovina is funded by the Italian Ministry of Health (PE-2013-02355372). Data collection and sharing for this project was provided by the MGH-USC Human Connectome Project (HCP; Principal Investigators: Bruce Rosen, M.D., Ph.D., Arthur W. Toga, Ph.D., Van J. Weeden, MD). The HCP project is supported by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Mental Health (NIMH) and the National Institute of Neurological Disorders and Stroke (NINDS) (Principal Investigators: Bruce Rosen, M.D., Ph.D., Martinos Center at Massachusetts General Hospital; Arthur W. Toga, Ph.D., University of Southern California, Van J. Weeden, MD, Martinos Center at Massachusetts General Hospital).
The authors would like to thank Dr. Gaetano Valenza (University of Pisa) for insightful discussion of connectivity estimation using MIC.
References
- Albanese D, Filosi M, Visintainer R, Riccadonna S, Jurman G, Furlanello C. Minerva and minepy: a C engine for the MINE suite and its R, Python and MATLAB wrappers. Bioinformatics. 2013;29(3):407–408. doi: 10.1093/bioinformatics/bts707. [DOI] [PubMed] [Google Scholar]
- Ashton MC, Lee K, Perugini M, Szarota P, de Vries RE, Di Blas L, De Raad B. A six-factor structure of personality-descriptive adjectives: solutions from psycholexical studies in seven languages. Journal of Personality and Social Psychology. 2004;86(2):356–366. doi: 10.1037/0022-3514.86.2.356. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Fjell AM, Walhovd KB, Grydeland H, Torgersen S, Westlye LT. Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. Neuroimage. 2013;65:194–208. doi: 10.1016/j.neuroimage.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang D-U. Complex networks: Structure and dynamics. Physics reports. 2006;424(4):175–308. [Google Scholar]
- Bunge SA, Zelazo PD. A Brain-Based Account of the Development of Rule Use in Childhood. Current Directions in Psychological Science. 2016;15(3):118–121. doi: 10.1111/j.0963-7214.2006.00419.x. [DOI] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of Personality. 2004;72(6):1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JD. Amygdala response to happy faces as a function of extraversion. Science. 2002;296(5576):2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Personality and Psychopathology. American Psychiatric Press; 1999. [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. normative data. Psychological Reports. 1991;69(3 Pt 1):1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50(12):975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Corr P. Understanding biological psychology. Blackwell; 2006. [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cremers H, van Tol MJ, Roelofs K, Aleman A, Zitman FG, van Buchem MA, van der Wee NJ. Extraversion is linked to volume of the orbitofrontal cortex and amygdala. PloS one. 2011;6(12):e28421. doi: 10.1371/journal.pone.0028421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJ, Roelofs K. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49(1):963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- DeYoung CG. Personality Neuroscience and the Biology of Traits. Social and Personality Psychology Compass. 2010;4(12):1165–1180. doi: 10.1111/j.1751-9004.2010.00327.x. [DOI] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience. Brain structure and the big five. Psychological Science. 2010;21(6):820–828. doi: 10.1177/0956797610370159. 'doi':0956797610370159 [pii]10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM. Higher-order factors of the Big Five predict conformity: Are there neuroses of health? Personality and Individual Differences. 2002;33(4):533–552. 'doi':Pii S0191-8869(01)00171-4 Doi 10.1016/S0191-8869(01)00171-4. [Google Scholar]
- Digman JM. Personality structure: Emergence of the five-factor model. Annual review of psychology. 1990;41(1):417–440. [Google Scholar]
- Dima D, Friston KJ, Stephan KE, Frangou S. Neuroticism and conscientiousness respectively constrain and facilitate short-term plasticity within the working memory neural network. Human Brain Mapping. 2015;36(10):4158–4163. doi: 10.1002/hbm.22906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H. Psychophysiology and personality: Extraversion, neuroticism and psychoticism. Physiological correlates of human behavior. 1983;3:13–30. [Google Scholar]
- Eysenck HJ. A Model for Personality. Springer Berlin Heidelberg: 2012. [Google Scholar]
- Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature Neuroscience. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Wik G, Fredrikson M. Extraversion, neuroticism and brain function: A PET study of personality. Personality and Individual Differences. 1997;23(2):345–352. [Google Scholar]
- Forbes CE, Poore JC, Krueger F, Barbey AK, Solomon J, Grafman J. The role of executive function and the dorsolateral prefrontal cortex in the expression of neuroticism and conscientiousness. Social Neuroscience. 2014;9(2):139–151. doi: 10.1080/17470919.2013.871333. [DOI] [PubMed] [Google Scholar]
- Fornito A, Bullmore ET. Connectomics: a new paradigm for understanding brain disease. European Neuropsychopharmacology. 2015;25(5):733–748. doi: 10.1016/j.euroneuro.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Polimeni JR. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8(3):249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry Into the Function of the Septo-hippocampal System. OUP Oxford; 2003. [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. The Journal of Neuroscience. 2012;32(50):18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Erb M, Ackermann H, Martin JA, Grodd W, Reiterer SM. Voxel-based morphometry studies of personality: issue of statistical model specification--effect of nuisance covariates. Neuroimage. 2011;54(3):1994–2005. doi: 10.1016/j.neuroimage.2010.10.024. 'doi':S1053-8119(10)01316-9 [pii] 10.1016/j.neuroimage.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Indovina I, Riccelli R, Staab JP, Lacquaniti F, Passamonti L. Personality traits modulate subcortical and cortical vestibular and anxiety responses to sound-evoked otolithic receptor stimulation. Journal Psychosomatic Research. 2014;77(5):391–400. doi: 10.1016/j.jpsychores.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of Aging. 2011;32(12):2162–2171. doi: 10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Sutin A, Davatzikos C, Costa P, Jr, Resnick S. The five factors of personality and regional cortical variability in the baltimore longitudinal study of aging. Human Brain Mapping. 2012 doi: 10.1002/hbm.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Duzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biological Psychiatry. 2009;65(2):103–110. doi: 10.1016/j.biopsych.2008.08.019. 'doi':S0006-3223(08)01013-5 [pii] 10.1016/j.biopsych.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. The Journal of Neuroscience. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WY, Weber B, Reuter M, Markett S, Chu WC, Montag C. The Big Five of Personality and structural imaging revisited: a VBM - DARTEL study. Neuroreport. 2013;24(7):375–380. doi: 10.1097/WNR.0b013e328360dad7. [DOI] [PubMed] [Google Scholar]
- Lu F, Huo Y, Li M, Chen H, Liu F, Wang Y, Chen H. Relationship between personality and gray matter volume in healthy young adults: a voxel-based morphometric study. PloS one. 2014;9(2):e88763. doi: 10.1371/journal.pone.0088763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Chaarani B, Kan KJ, Spechler PA, Orr C, Banaschewski T Consortium, I. Brain Regions Related to Impulsivity Mediate the Effects of Early Adversity on Antisocial Behavior. Biological Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.12.027. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping. 2009;30(4):1188–1195. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR. The five-factor model and its assessment in clinical settings. Journal of Personality Assessment. 1991;57(3):399–314. doi: 10.1207/s15327752jpa5703_2. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;52(1):81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- McCrae RR, John OP. An introduction to the five-factor model and its applications. Journal of Personality. 1992;60(2):175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Terracciano A. Universal features of personality traits from the observer's perspective: data from 50 cultures. Journal of personality and social psychology. 2005;88(3):547. doi: 10.1037/0022-3514.88.3.547. [DOI] [PubMed] [Google Scholar]
- McNab JA, Edlow BL, Witzel T, Huang SY, Bhat H, Heberlein K, Wald LL. The Human Connectome Project and beyond: initial applications of 300 mT/m gradients. Neuroimage. 2013;80:234–245. doi: 10.1016/j.neuroimage.2013.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Carpenter SD, Grant KA, Kroenke CD, Nigg JT, Fair DA. Connectotyping: model based fingerprinting of the functional connectome. PloS one. 2014;9(11):e111048. doi: 10.1371/journal.pone.0111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, Liu H. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77(3):586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noftle EE, Robins RW. Personality predictors of academic outcomes: big five correlates of GPA and SAT scores. Journal of Personality and Social Psychology. 2007;93(1):116–130. doi: 10.1037/0022-3514.93.1.116. [DOI] [PubMed] [Google Scholar]
- Nostro AD, Muller VI, Reid AT, Eickhoff SB. Correlations Between Personality and Brain Structure: A Crucial Role of Gender. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnela J-P, Saramäki J, Kertész J, Kaski K. Intensity and coherence of motifs in weighted complex networks. Physical Review E. 2005;71(6):065103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- Ozer DJ, Benet-Martinez V. Personality and the prediction of consequential outcomes. Annual Review of Psychology. 2006;57:401–421. doi: 10.1146/annurev.psych.57.102904.190127. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Terracciano A, Riccelli R, Donzuso G, Cerasa A, Vaccaro M, Quattrone A. Increased functional connectivity within mesocortical networks in open people. Neuroimage. 2015;104:301–309. doi: 10.1016/j.neuroimage.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitve Sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philosophical Transactions of the Royal Society of London. Series B. 2015;370(1668) doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef DN, Reshef YA, Finucane HK, Grossman SR, McVean G, Turnbaugh PJ, Sabeti PC. Detecting novel associations in large data sets. Science. 2011;334(6062):1518–1524. doi: 10.1126/science.1205438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccelli R, Indovina I, Staab JP, Nigro S, Augimeri A, Lacquaniti F, Passamonti L. Neuroticism modulates brain visuo-vestibular and anxiety systems during a virtual rollercoaster task. Human Brain Mapping. 2017;38(2):715–726. doi: 10.1002/hbm.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccelli R, Passamonti L, Duggento A, Guerrisi M, Indovina I, Terracciano A, Toschi N. Dynamical brain connectivity estimation using GARCH models: An application to personality neuroscience. 2017; 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2017. pp. 3305–3308. [DOI] [PubMed] [Google Scholar]
- Riccelli R, Passamonti L, Duggento A, Guerrisi M, Indovina I, Toschi N. Dynamic inter-network connectivity in the human brain. 2017; 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2017. pp. 3313–3316. [DOI] [PubMed] [Google Scholar]
- Riccelli R, Toschi N, Nigro S, Terracciano A, Passamonti L. Surface-based morphometry reveals the neuroanatomical basis of the five-factor model of personality. Social Cognitive Affective Neurosciences. 2017 doi: 10.1093/scan/nsw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Lejuez C, Krueger RF, Richards JM, Hill PL. What is conscientiousness and how can it be assessed? Developmental Psychology. 2014;50(5):1315–1330. doi: 10.1037/a0031109. [DOI] [PubMed] [Google Scholar]
- Roberts RP, Wiebels K, Sumner RL, van Mulukom V, Grady CL, Schacter DL, Addis DR. An fMRI investigation of the relationship between future imagination and cognitive flexibility. Neuropsychologia. 2017;95:156–172. doi: 10.1016/j.neuropsychologia.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo AH, Di Domenico SI, Graves B, Lam J, Ayaz H, Bagby RM, Ruocco AC. Linking trait-based phenotypes to prefrontal cortex activation during inhibitory control. Social Cognitive Affective Neurosciences. 2016;11(1):55–65. doi: 10.1093/scan/nsv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. American Psychologist. 2012;67(8):603–613. doi: 10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76(4):677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, De Brigard F, Szpunar KK. Episodic future thinking and episodic counterfactual thinking: intersections between memory and decisions. Neurobiology of Learning and Memory. 2015;117:14–21. doi: 10.1016/j.nlm.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas MN, van der Velde J, Costafreda SG, Horton P, Ormel J, Riese H, Aleman A. Neuroticism and the brain: a quantitative meta-analysis of neuroimaging studies investigating emotion processing. Neuroscience & Biobehavioral Reviews. 2013;37(8):1518–1529. doi: 10.1016/j.neubiorev.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Smith SM, Hyvärinen A, Varoquaux G, Miller KL, Beckmann CF. Group-PCA for very large fMRI datasets. Neuroimage. 2014;101:738–749. doi: 10.1016/j.neuroimage.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, Miller KL. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nature Neurosciences. 2015;18(11):1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Current opinion in neurobiology. 2013;23(2):162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Su L, Wang L, Shen H, Feng G, Hu D. Discriminative analysis of non-linear brain connectivity in schizophrenia: an fMRI Study. Frontiers in human neuroscience. 2013;7:702. doi: 10.3389/fnhum.2013.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Luchetti M, Artese A, Oshio A, Terracciano A. The five-factor model of personality and physical inactivity: A meta-analysis of 16 samples. Journal of Research in Personality. 2016;63:22–28. doi: 10.1016/j.jrp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A. The Italian version of the NEO PI-R: conceptual and empirical support for the use of targeted rotation. Personality and individual differences. 2003;35(8):1859–1872. doi: 10.1016/S0191-8869(03)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi N, Duggento A, Passamonti L. Functional connectivity in amygdalar-sensory/(pre)motor networks at rest: new evidence from the Human Connectome Project. European Journal of Neurosciences. 2017;45(9):1224–1229. doi: 10.1111/ejn.13544. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens T, Bucholz R, Curtiss SW. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA. Default Mode Dynamics for Global Functional Integration. The Journal of Neuroscience. 2015;35(46):15254–15262. doi: 10.1523/JNEUROSCI.2135-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’networks. nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Whittle S, Allen NB, Fornito A, Lubman DI, Simmons JG, Pantelis C, Yucel M. Variations in cortical folding patterns are related to individual differences in temperament. Psychiatry Research. 2009;172(1):68–74. doi: 10.1016/j.pscychresns.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D. Neuroanatomical correlates of personality in the elderly. Neuroimage. 2007;35(1):263–272. doi: 10.1016/j.neuroimage.2006.11.039. 'doi':S1053-8119(06)01126-8 [pii] 10.1016/j.neuroimage.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM. Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex. 2006;16(12):1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun S, Yi M, Wu X, Ding Y. MIC as an appropriate method to construct the brain functional network. BioMedical research international, 2015. 2015 doi: 10.1155/2015/825136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anatomy and Embryology. 1988;179(2):173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- Zuo X-N, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP. Network centrality in the human functional connectome. Cerebral cortex. 2012;22(8):1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.