Abstract
Background
In 2014–2015, 242 individuals aged 2–89 were newly HIV-1 diagnosed in Roka, a rural commune in Cambodia. A case-control study attributed the outbreak to unsafe injections. We aimed to reconstruct the likely transmission history of the outbreak.
Methods
We assessed in 209 (86.4%) HIV-infected cases the presence of hepatitis C and B viruses (HCV, HBV). We identified recent infections using antibody (Ab) avidity testing for HIV and HCV, and HBcIgM Ab for HBV. We performed evolutionary phylogenetic analyses of viral strains. Geographical coordinates and parenteral exposure through medical services provided by an unlicensed health care practitioner were obtained from 193 cases and 1499 controls during interviews.
Results
Cases were co-infected with HCV (78.5%) and HBV (12.9%). We identified 79 (37.8%) recent (<130 days) HIV infections. Phylogeny of 202 HIV env C2V3 sequences showed a 198-sample CRF01_AE strains cluster, with time to most recent common ancestor (tMRCA) in September 2013 (95% highest posterior density, August 2012–July 2014), and a peak of 15 infections/day in September 2014. Three geospatial HIV hotspots were discernible in Roka and correlated with high exposure to the practitioner (P=0.04). Fifty-nine (38.6%) of 153 tested cases showed recent (<180 days) HCV infections. Ninety HCV NS5B sequences formed three main clades, one containing 34 subtypes 1b with tMRCA in 2012, and two with 51 subtypes 6e and tMRCAs in 2002–2003.
Conclusions
Unsafe injections in Cambodia most likely led to an explosive iatrogenic spreading of HIV, associated with a long-standing and more genetically-diverse HCV propagation.
Keywords: Cambodia, HBV, HCV, HIV, iatrogenic outbreak
INTRODUCTION
The World Health Organization (WHO) Global Health Sector Strategy 2016–2021 is paving the road to elimination of HIV and viral hepatitis as major public health threats by 2030, but the burden of new HIV, hepatitis C virus (HCV) and hepatitis B virus (HBV) infections, notably transmitted through unsafe and unnecessary medical injections, is still high [1].
In 2014–2015, one of the largest injection-related outbreak of HIV occurred in rural Cambodia among residents from the Roka commune (7985 inhabitants) (Figure 1). The details of the outbreak have been reported previously [2]. The Ministry of Health of Cambodia and the National Center for HIV/AIDS, Dermatology and Sexual Transmitted Diseases (NCHADS) conducted a case-control study determining that cases were five times more likely to have received therapeutic injections and ruled out associations with commercial sex work, injection drug use, or blood transfusion [3].
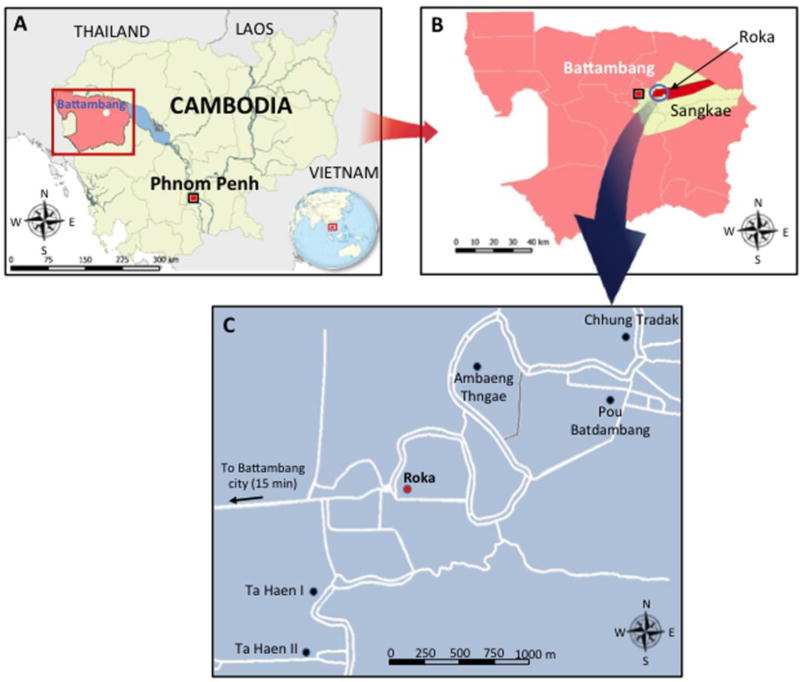
Figure 1. Geographical Location of the Iatrogenic HIV Outbreak in the Roka Commune, Cambodia, 2014 – 2015.
The map designated (A) shows Cambodia (in yellow) and the Battambang province (in pink). The map designated (B) shows the Roka commune (in red) which is located in the Sangkae district (in yellow). The map (C) shows the location of the 6 villages in the Roka commune (7985 inhabitants [inh.]). Roka village (2338 inh.) is indicated by a red circle while the other 5 villages (Ambaeng Thngae [1050 inh.], Ta Haen I [1468 inh.], Ta Haen II [1050 inh.], Pou Batdambang [731 inh.], and Chhung Tradak [1348 inh.]) are indicated by black circles. Roads are indicated by white lines.
By February 28, 2015, the identification of new cases had winded down [2]. Among 2045 Roka residents who underwent a free and voluntary HIV screening, 242 HIV-infected cases were newly diagnosed [2], leading to a prevalence of 11.8% which is ~20 times higher than the national average (0.6%) [4]. The sources/origins of the Roka outbreak were unknown. However, it was suspected that the outbreak was rather iatrogenic, resulting from unsafe injection practices performed by a local unlicensed health-care practitioner. That lay individual, who did not hold a medical degree, provided medical services to the nearby residents in his home and also made home call visits to clients. By the end of 2014, legal authorities arrested that individual. In December 2015, he was trialed and sentenced to 25 years in prison [5].
In this study, we report the likely transmission history of this iatrogenic outbreak by providing unprecedented spatiotemporal insights, a strong association between cases and past injections, as well as a history of HCV and HBV co-infections and the evolutionary phylogenetic analyses of the viral strains.
MATERIALS AND METHODS
Sampling
We conducted our study on 209 HIV-confirmed cases who had sufficient plasma specimens for further investigation. At the time of sampling, all subjects were ART-naïve. For each case, we retrieved from the anonymously generated NCHADS database the following variables: sex, age, place of residence, history of migration to neighboring countries (such as Thailand, Laos, Malaysia, Myanmar, Vietnam), and HIV-1 RNA viral load (VL) levels (Abbott RealTime HIV-1 assay, Abbott Molecular Inc., Des Plaines, IL, USA).
Diagnostic and Recency Assays
To estimate the recency of HIV infections, all samples were tested with the Sedia™ Limiting Antigen (LAg)-Avidity enzyme-linked immunosorbent assay (ELISA) (Sedia Biosciences Portland, OR, USA), using a threshold of 1.5 for normalized optical density (ODn) values, as recommended to differentiate recent (<130 days) versus non-recent HIV infections [6]. All samples that were classified as recent by Ab avidity testing were further assessed with a HIV-1 Western-blot (WB) assay (HIV Blot 2.2, MP Diagnostics, Singapore) to define Fiebig stages of HIV-1 infection [7].
All specimens were tested for the presence of HCV Ab and RNA with a rapid diagnostic test (RDT) (SD Bioline HCV, Standard Diagnostics, Inc., Kyonggi-do, Korea) and an in-house PCR assay targeting the HCV 5’ untranslated region, respectively [8]. Samples that were positive for both HCV Ab and HCV RNA were assessed with an in-house HCV avidity ELISA [9]. We used an avidity index threshold of 43%, as recommended to distinguish recent (<6 months) from non-recent (>6 months) HCV infections in HCV-viremic patients.
All samples were tested for HBsAg with the Alere Determine HBsAg RDT (Alere Medical Co., Ltd, Chiba, Japan). In case of positive HBsAg results, specimens were further tested with ELISA for HBcIgM Ab (Bio-Rad, Marnes-la-Coquette, France). A specimen/cut-off ratio >5 was used to identify recent (<6 months) HBV infections, as recently reported [10]. In case of HBsAg negativity, subjects were assessed for HBsAb titers with ELISA (Bio-Rad, Marnes-la-Coquette, France) to identify those who were protected against HBV. Subjects were considered as negative for HBsAb when titer was <10 international units per liter (IU/L).
Viral Amplification and Sequencing
HIV RNA amplifications were done according to nested RT-PCR procedures established by the Agence Nationale de Recherches sur le SIDA et les hépatites virales (ANRS) (see http://www.hivfrenchresistance.org/) (version January 2015) (see Supplementary Table 1). A total of 202 specimens were amplified in the C2V3 region of the HIV env gene (PCR fragment length of 374 base pairs [bp]). Seven missing sequences failed PCR amplification or had low-quality chromatograms. Additionally, 192 samples were amplified in the reverse-transcriptase (RT) region (798 bp) and the protease (PR) region (507 bp) of the HIV pol gene. The HCV non-structural 5B (NS5B) genomic region (371 bp) was amplified from 90 HCV RNA positive specimens, with a semi-nested RT-PCR, as described previously [11]. Ten HCV sequences were missing due to failed PCR amplification. A total of 16 samples (11 missing samples due to failed PCR amplification or insufficient volume of plasma) were subjected to HBV DNA amplification in the S-gene (738 bp), as reported elsewhere [12]. All purified PCR products were sequenced using the Big Dye® Terminator v3•1 Cycle Sequencing kit (Applied Biosystems).
Phylogenetic Analysis
Maximum likelihood phylogenies for env C2V3 and PR-RT (following concatenation) HIV, NS5B HCV and S-gene HBV were inferred with the use of Molecular Evolutionary Genetics Analysis version 6 (MEGA6) software [13]. For reference sequences, we used sequences that were downloaded from the GenBank or Los Alamos database, as well as sequences that were amplified from subjects living in different geographical provinces in Cambodia. Phylogenetic grouping was assessed by bootstrap supports and genetic distances [14]. We also inferred Bayesian temporally resolved phylogenetic trees for HIV and HCV with the use of Bayesian Evolutionary Analysis Sampling Trees (BEAST) software, version 1.8.3 [15]. Reference sequences were amplified from Cambodian subjects infected with HIV or HCV and having a known sampling date (d/m/y), ranging from 1997 to 2014 for HIV, and between 2002 and 2015 for HCV. For each outbreak-associated cluster, we estimated the time to the most recent common ancestor (tMRCA). With HIV env C2V3 sequences, we also investigated the growth rate and lineage-through-time (LTT) of the HIV outbreak (see Supplementary Material).
Geographic HIV Mapping
Through a field study conducted in Roka commune, we measured with a handheld global positioning system (GPS) device the geographic coordinates of 193 HIV-infected cases and 1499 subjects who were found HIV-negative during the free and voluntary testing performed between December 2014 and February 2015. For all individuals, we also collected, through face-to-face interviews, information about whether subjects did or did not receive injections from the imprisoned practitioner. All interviewed subjects were superimposed onto a 0.004-degree latitude × 0.004-degree longitude grid. The total number of subject was summed in each cell over the entire study area. For each cell, the prevalence of HIV infection was calculated by dividing the total number of HIV-infected subjects by the total number of tested subjects, and expressed in percentage values. The resulting prevalence in each cell was mapped to the centroid position of each cell. Using the same grids, we generated one additional map depicting the interpolated frequency of history of past injections with the informal practitioner. The maps were created using inverse distance weighting, with the Quantum Geographic Information Science (QGIS™) software version 2.12.3 [16]. The correlation between the two maps was assessed using Pearson’s correlation coefficient (R2) and the t-test.
Ethical Committee Approvals
The virological investigation was approved by the Cambodia National Ethical Committee for Health Research (NECHR) (N°353/NECHR). The protocol for GPS mapping was approved by the NECHR (N°244/NECHR), and no sampling of biological material was performed during this field study. All HIV-infected cases and uninfected subjects provided written consent.
RESULTS
Patients’ Characteristics
As summarized in Table 1, median age of cases was 38.0 years (interquartile range [IQR] 18–54) (full range 2–89), and 129 (61.7%) were female. One hundred fifty nine (76.1%) were residents in the Roka village. One hundred ninety nine (95.2%) had never migrated to neighboring countries. The median HIV-1 RNA level was log10 5.16 copies HIV-1 RNA (4.5–5.6) per mL. Forty one (19.6%) cases were mono-infected with HIV single-infection, whereas most (164 [78.5%]) were co-infected with HCV. Cases showing HBV co-infection were uncommon (27 [12.9%]).
Table 1.
Main Characteristics of HIV-Infected Cases during the Iatrogenic HIV Outbreak in Roka, Cambodia, 2014 – 2015
| Variable | N=209 |
|---|---|
| Demographic characteristics | |
| Age (year) | |
| Median | 38.0 |
| Interquartile range | 19–54 |
| Full range | 2–89 |
| Age distribution | |
| <5 year | 5 (2.4) |
| 5–9 year | 22 (10.5) |
| 10–14 year | 13 (6.2) |
| 15–49 year | 89 (42.6) |
| 50–69 year | 61 (29.2) |
| ≥70 year | 19 (9.1) |
| Female sex | 129 (61.7) |
| Place of residence | |
| Roka | 159 (76.1) |
| Ambaeng Thngae | 42 (20.1) |
| Others* | 8 (3.8) |
| Migration to neighboring countries | 10 (4.8) |
| Virological characteristics | |
| Plasma HIV-1 RNA viral load (log10 copies per mL) | |
| Median | 5.16 |
| Interquartile range | 4.51–5.61 |
| Full range | 3.29–6.68 |
| Co-infection with HCV and/or HBV | |
| HCV co-infection | 141 (67.5) |
| HBV co-infection | 4 (1.9) |
| HCV and HBV co-infection | 23 (11.0) |
| Recency of HIV, HCV and HBV infections | |
| Recent HIV infection (less than 130 days) | 79 (37.8) |
| Recent HCV infection (less than 6 months); n = 153** | 59 (38.6) |
| Recent HBV infection (less than 6 months); n = 27 | 3 (11.1) |
| Protection against HBV (positive HBsAb); n = 133*** | 69 (51.9) |
Data are median (IQR and full range) or n (%) unless otherwise indicated.
Eight cases were living in Ta Haen I (n = 4), Ta Haen II (n = 3), and Pou Batdambang (n = 1)
Eleven samples were not assessed for HCV recency due to insufficient volume.
HBsAb testing was done in 133 HBsAg-negative subjects who could be further tested (sufficient volume of plasma).
Abbreviations: Ab, antibody; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Recency, Phylogenetic Analysis and Spatial Spread of HIV
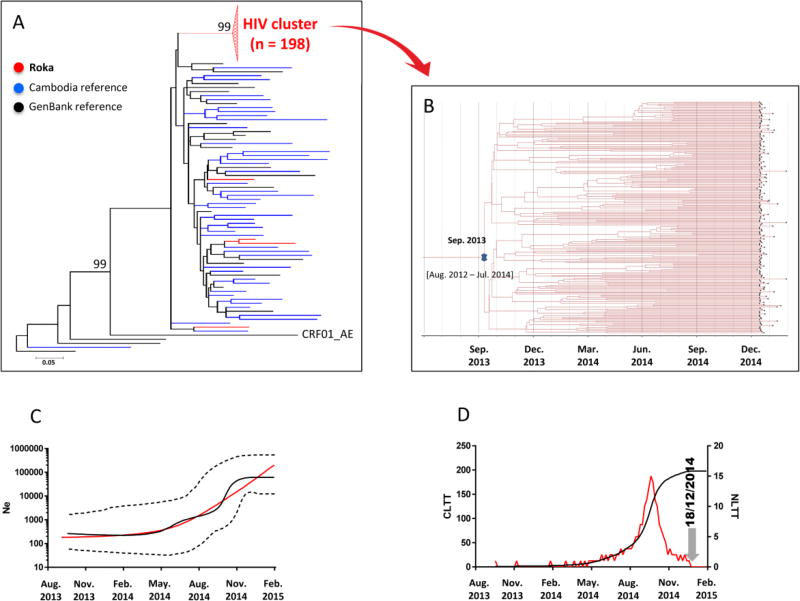
We identified recent HIV infections in 79 (37.8%) cases (Table 1). WB analysis classified subjects with recent infections at a Fiebig IV (10 [12.6%]) or V (57 [72.2%]) stage, indicating HIV transmission within the previous 30 or 100 days, respectively. Among 202 HIV C2V3 env sequences that could be analyzed, 198 (98.0%) clustered in a single well-supported monophyletic clade (bootstrap support of 99 and average intracluster nucleotide identity ± standard deviation [SD] of 99.3%±0.7) (Figure 2A and Supplementary Table 2). These sequences derived from an ancestral CRF01_AE lineage, and were not closely related to the control sequences. The remaining four C2V3 env strains, found outside the outbreak-associated cluster, were more closely related to the control sequences. Additional analysis of 192 concatenated HIV PR-RT sequences supported an identical outbreak-associated clustering pattern (see Supplementary Figure 1). We found that time to most recent common ancestor (tMRCA) of the HIV env outbreak-associated cluster was September 2013 (95% Highest Posterior Density [HDP], August 2012–July 2014) (Figure 2B). The median estimates of the effective population size inferred under the demographic expansion model indicated a pattern of constant population size followed by exponential growth (Figure 2C). The LTT-plot depicted from May 2014 a rapid rise of HIV infections with a maximum of 15 infections per a day in September 2014, followed by a decline thereafter (Figure 2D). These data were consistent with the date of arrest of the practitioner by the police (end-december 2014).
Figure 2. HIV-1 env C2V3 Sequences.
Panel A – Maximum-likelihood phylogenetic tree for HIV-1 env C2V3 sequences from 202 cases sequences (indicated in red), 45 Cambodia sequences (blue), and 24 GenBank reference sequences (black)
The Roka outbreak-associated CRF01_AE HIV cluster is represented by a red triangle. Four sequences (amplified from patients NCHADS171, NCHADS184, NCHADS185, and NCHADS116) from Roka did not group within the outbreak-associated cluster, and were isolated from individuals presenting non-recent HIV-1 infections and who were negative for HCV and HBV. The unit for the scale bar of 0.05 is the number of nucleotide substitutions per site. Genbank accession numbers for the Roka HIV env C2V3 sequences were KY570019–KY570220. Accession numbers for other Cambodia sequences were KY570221–KY570265.
Panel B – Bayesian time-scaled phylogenetic tree of 198 HIV-1 env C2V3 sequences from the Roka HIV cluster
The branh lengths represent the number of substitutions per site per year. The cross marks the mean estimate of the time to the most recent common ancestor (tMRCA) with 95% highest posterior densities (95% HPD).
Panel C – Effective viral population size over time
The Bayesian skyline plot (BSP) shows the median viral population size over time (black solid line) and 95% HPD around the estimate (black dashed lines). Expansion model is indicated by a red line.
Panel D – Lineage-through-time plot
CLTT indicates the cumulative lineage through time (black solid line) while NLTT indicates the non cumulative lineage through time (red solid line).
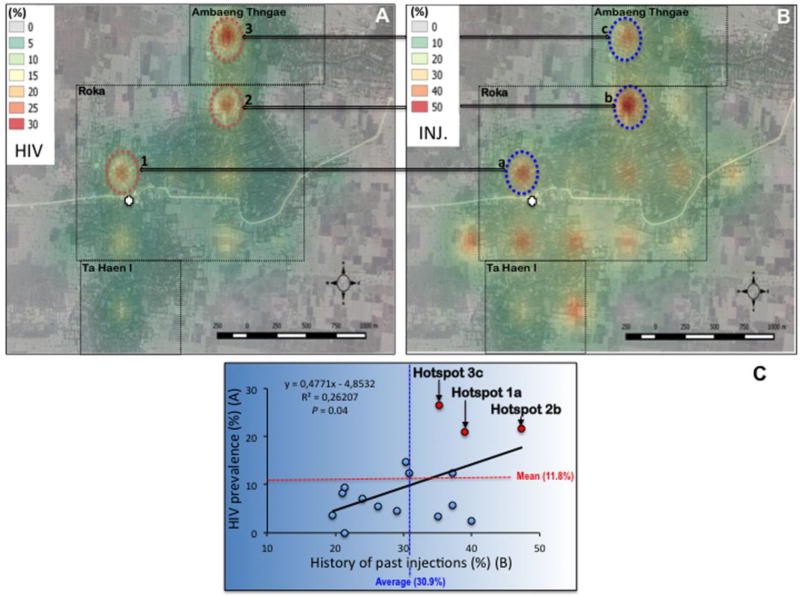
A marked heterogeneity in HIV prevalence was observed in the Roka commune. HIV cases were heavily confined in three main geographical microclusters showing HIV prevalence >20% (20.9%, 21.6%, and 26.5% for hotspots 1, 2 and 3, respectively) (Figure 3A). By contrast, some other sectors showed low (ranging between 0–5%) HIV prevalence. Regarding the three HIV hot spots, one was identified in the direct vicinity of the practitioner’s house, while the other two were located further, reflecting likely home call visits made by the informal practitioner throughout the investigated area. HIV microclusters matched hotspots of a high frequency of past injections given by the practitioner (39.0%, 47.0%, and 35.3% for hotspots a, b, and c, respectively) (Figure 3B). Overall, we found a significant correlation between HIV prevalence and past parenteral exposure to the practitioner (P = 0.04) (Figure 3C).
Figure 3. Geographical variations in interpolated HIV prevalence (panel A), frequency of history of injections with the unlicensed practitioner (panel B) across the surveillance area, and correlation (panel C).
Roka, Ambang Thngae, and Ta Haen I villages are delineated by a dotted rectangle. The practitioner’s house is depicted by a white cross. Each hotspot is indicated by a circle, associated with a number (1, 2, or 3 for HIV) or a letter (a, b, or c regarding history of past injections).
Abbreviation: INJ., history of past injections.
Recency and Phylogenetic Analysis of HCV
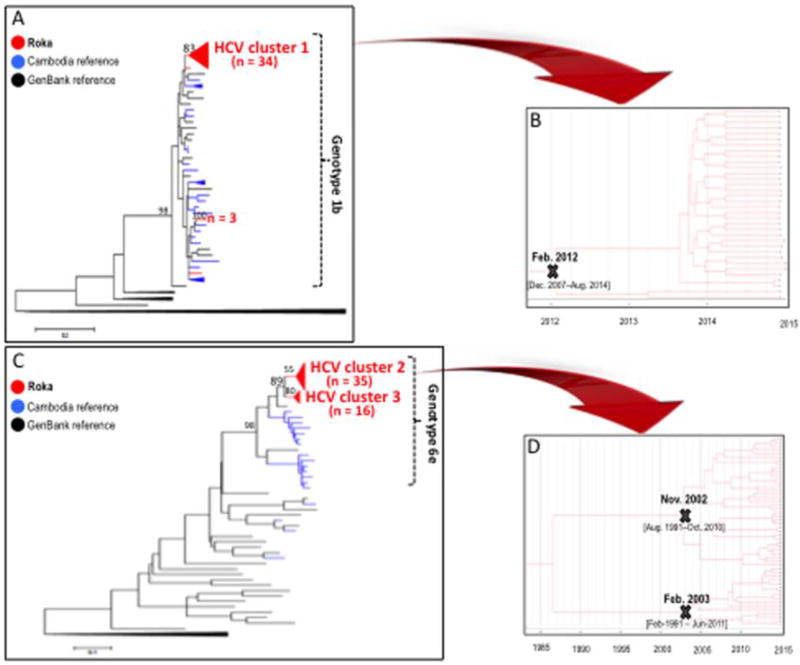
Of 153 subjects co-infected with HCV (11 missing specimens), recent HCV infections were detected in 59 (38.6%) individuals (Table 1), including 10 showing negative HCV Ab/positive HCV RNA patterns, and 49 exhibiting low-avid HCV Ab and positive HCV RNA. Non-recent HCV infections were diagnosed in 41 (26.8%) subjects with high-avid HCV Ab and positive HCV RNA. Fifty three (34.6%) persons had cleared HCV (positive HCV Ab but negative HCV RNA). Among the 100 (65.4%) individuals who were HCV viremic, we were able to amplify 90 HCV NS5B sequences. Among these, we found evidence of three main outbreak-associated clusters, including 34 HCV subtype 1b isolates in one cluster (Figure 4A and Supplementary Table 3), and 51 HCV subtype 6e strains distributed in two other clusters (Figure 4C and Supplementary Table 4). The subtype 1b cluster showed an intracluster nucleotide identity ± SD of 99.8%±0.4, and emerged in February 2012 (95% HDP, December 2007–August 2014) (Figure 4B). The 35 strains in the subtype 6e cluster 2 showed an intracluster nucleotide identity ± SD of 96.5%±1.3. The 16 isolates in the cluster 3 exhibited a nucleotide identity ± SD of 97.3%±1.6. HCV subtype 6e clusters had older tMRCAs (November 2002, 95% HDP, August 1991–October 2010; and February 2003, 95% HDP, February 1991–June 2011) (Figure 4D).
Figure 4. HCV NS5B Sequences.
Panel A – Maximum-likelihood phylogenetic tree for HCV NS5B sequences from 39 cases HCV subtype 1b sequences (red), 37 Cambodia sequences (blue), and 29 GenBank reference sequences (black)
Genbank accession numbers for the Roka HCV 1b NS5B sequences were KY569650–KY569688. Accession numbers for other Cambodia sequences were KY569689–KY569725.
Panel B – Bayesian time-scaled phylogenetic tree of 34 HCV NS5B sequences from the Roka subtype 1b HCV cluster
The branch lengths represent the number of substitutions per site per year. The cross marks the mean estimate of the time to the most recent common ancestor (tMRCA) with 95% highest posterior densities (95% HPD).
Panels C – Maximum-likelihood phylogenetic tree for HCV NS5B sequences from 51 cases HCV subtype 6e sequences (red), 18 Cambodia sequences (blue), and 29 GenBank reference sequences (black)
The 51 HCV subtype 6e strains were distributed in two distinct monophyletic clusters. Genbank accession numbers for the Roka HCV 6e sequences were KY569734–KY569784. Accession numbers for other Cambodia sequences were KY569785–KY569802.
Panel D – Bayesian time-scaled phylogenetic tree of 51 HCV NS5B sequences from the Roka genotype 6e clusters (n = 51)
The branch lengths represent the number of substitutions per site per year. The mean estimate of the tMRCAs as well as 95% HPD are indicated with at key internal nodes marked with ‘x’s.
Recency and Phylogenetic Analysis of HBV
Of 27 subjects found positive for HBsAg, only three (11.1%) were reactive to HBcIgM (Table 1). From 16 HBV S-gene sequences, 10 (62.5%) were phylogenetically isolated strains whereas 6 sequences grouped together into two distinct clusters of 4 and 2 sequences within HBV-C1 genotype strains. Among 133 HBsAg-negative subjects who could be further tested, 69 (51.9%) showed HBsAb titers ≥10 IU/L (Table 1).
DISCUSSION
This study revealed a massive and spatially localized outbreak of HIV in Cambodia, associated with concomitant HCV infections, but a lack of HBV diffusion. The recency (<4–6 months) of either HIV or HCV was found in approximately one-third of infections, emphasizing the burst of the outbreak.
The monophyletic cluster of CRF01_AE HIV strains with high homology supported the iatrogenic transmission scenario which was related to a single and recent parenteral source/introduction of HIV into the Roka population. The three main HCV clusters showing older origins than the HIV cluster strengthened the iatrogenic scenario, and suggested that HCV likely originated from a long-standing spread and diverse infectious HCV strains. The geospatial correlation between HIV hotspots and high frequency of injections administered by an unlicensed practitioner highlighted his probable role behind an iatrogenic transmission [17].
This might be the first report of a massive outbreak potentially caused by an unlicensed health practitioner who performed health care practices among low-risk subjects in a rural setting. In contrast, similar outcomes have been reported in other massive (>100 cases) injection-related outbreaks among other populations at higher risk of HIV, such as: paid blood donors, as reported in Mexico (1986) [18], India (1988) [19], and China (1990–95) [20]; hospitalized children, as described in Romania (1987–92) [21, 22], Russia (1988–1989) [23], and Libya (1997–1999) [24, 25]; and persons who inject drugs, as established in Kazakhstan, Kyrgyzstan, Uzbekistan (2007–2008) [26], Pakistan (2008–09) [27], and Indiana, US (2014–2015) [28, 29]. We hypothesize that the Roka outbreak might not be an isolated incident. Other unrecognized iatrogenic outbreaks caused by similar practices could exist, especially in rural areas.
The difference we observed between HIV and HCV dynamics through time is not easy to explain. One might postulate that HIV emerged through one unidentified highly viremic HIV-infected Roka resident, who was not treated with ART (or who had interrupted his treatment), and who unfortunately received injections from the practitioner in 2013. This source was obviously rare due to the low HIV prevalence and high ART coverage in Cambodia [4]. The HIV outbreak became apparent in late 2014 due to a TB-associated comorbidity and highlights the importance of HIV testing among TB patients. In contrast, HCV emerged earlier than HIV from at least three HCV-independent introductions. However, those infections were unknown given the lack of routine diagnosis and no apparent HCV symptoms [30, 31]. Another possibility to explain this temporal difference may be related to a reduced transmissibility of HIV, with a lower ex vivo viability in needles/syringes (few hours for HIV versus at least 7 days for HCV), as documented by some researchers [32].
To explain the absence of iatrogenic HBV diffusion, two reasons could be put forward. First, even if a vast majority of Roka HBV-uninfected cases were unvaccinated (the universal vaccination in children started in 2005 in Cambodia) [33], we showed that more than half of individuals were naturally protected against HBV due to HBsAb positivity, likely resulting from past infections. Second, the proportion of inactive HBsAg carriers, with very low or undetectable serum HBV DNA levels, was likely important among HBV-infected subjects, as previously documented in Cambodia [34]. These two specific HBV patterns probably explain the low prevalence of HBV in this population.
Our study has some limitations. First, the accuracy of Ab avidity-based results for the identification of recent infections is still debatable, as we could not determine the false recent rate (FRR) of HCV infections. It is also possible that different HCV genotypes and the occurrence of HIV co-infections could have skewed our estimation of recent HCV infections in either direction [9, 35]. Moreover, the gold standard approach for measuring incidence (i.e., negative followed by positive test results through a longitudinal follow-up) cannot be used during outbreak situations. Second, only samples from HIV cases were available for hepatitis testing, as individuals diagnosed with rapid HIV tests were referred for further testing. Therefore, our survey likely missed HCV mono-infections that were part of a larger outbreak of HCV in Roka. This incomplete sampling could have generated a bias in our analysis of HCV clusters and on the timing of introduction of the most recent common ancestor. Lastly, the legal investigation by police and prosecutors, as well as interviews of cases could not clearly define the exact modus operandi of the unlicensed practitioner that led to this massive outbreak.
In conclusion, our study provided the detailed serologic, genomic and geospatial investigation of an iatrogenic outbreak that was recognized in rural Cambodia in 2014–2015. Our unfortunate experience might serve as a global warning, notably for the South-East Asia region, which is an area of major concern for the “iatrogenic risk” of blood-borne viruses. Avidity testing and genomic surveillance, in conjunction with spatial analysis, are key milestones to more forcefully advocate for reducing unsafe injection practices and further promoting oral drugs, in countries with concentrated epidemics, and also in those with generalized epidemics where outbreaks are probably undiagnosed and/or neglected due to higher background noise.
Supplementary Material
Key points.
This study provided the serologic, genomic and geospatial investigation of an iatrogenic outbreak that was recognized in rural Cambodia in 2014–2015, showing a massive and spatially localized outbreak of HIV, associated with HCV infections, but a lack of HBV diffusion.
Acknowledgments
We are indebted to all patients living in the Roka commune and to Sophon Sern for collecting information from exposed residents in Roka; to Robert Newman, M.D., Ph.D., who reviewed carefully this manuscript, and to Ahmed Saadani Hassani, M.D. for technical assistance; to Patrice Piola, M.D., Ph.D., for statistical support; and to Daiana Mir da Silva, MSc, and Gonzalo Bello Bentancor, Ph.D., for their assistance in phylogenetic analysis.
Financial support. This work was supported by grants from the 5% Initiative Expertise France, the Institut Pasteur International Network (IPIN) and the French National Agency for Research on AIDS and Viral Hepatitis (ANRS). This research has been also partially been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC), under the terms of the projects (Cooperative Agreement) number: 3U2GGH000989.
Footnotes
Author contributions. FR, JN, SP, DF, AR, and VS conceived the study. FR, JN, DZ, SP, CGG, CM, SM, CG, GL, SK, KP, AK, and CY generated and managed the data. FR, JN, BR, AB, SP, ML, LF, WK, FB, MF, CM, JCP, and TB contributed to the data analysis. FR, JN, and AR contributed to writing the first draft of the paper.
Disclaimer. The content is solely the responsibility of the authors, and the findings and conclusions in this study do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. We declare no competing interests.
References
- 1.World Health Organization. Global hepatitis Report, 2017. WHO; Geneva, Switzerland: [(accessed May 10, 2017)]. 2017. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ [Google Scholar]
- 2.Vun MC, Galang RR, Fujita M, et al. Cluster of HIV Infections Attributed to Unsafe Injection Practices--Cambodia, December 1, 2014–February 28, 2015. MMWR Morbidity and mortality weekly report. 2016;65(6):142–5. doi: 10.15585/mmwr.mm6506a2. [DOI] [PubMed] [Google Scholar]
- 3.Saphonn V, Fujita M, Samreth S, et al. Cluster of HIV Infections Associated With Unsafe Injection Practices in a Rural Village in Cambodia. Journal of acquired immune deficiency syndromes. 2017;75(3):e82–e6. doi: 10.1097/QAI.0000000000001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vun MC, Fujita M, Rathavy T, et al. Achieving universal access and moving towards elimination of new HIV infections in Cambodia. Journal of the International AIDS Society. 2014;17:18905. doi: 10.7448/IAS.17.1.18905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Phnom Penh Post. Doctor gets 25 years for HIV outbreak. [(accessed May 10, 2017)];2015 http://www.phnompenhpost.com/national/doctor-gets-25-years-hiv-outbreak.
- 6.Duong YT, Kassanjee R, Welte A, et al. Recalibration of the limiting antigen avidity EIA to determine mean duration of recent infection in divergent HIV-1 subtypes. PloS one. 2015;10(2):e0114947. doi: 10.1371/journal.pone.0114947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17(13):1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bukh J, Purcell RH, Miller RH. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(1):187–91. doi: 10.1073/pnas.89.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudy-Graffin C, Lesage G, Kousignian I, et al. Use of an anti-hepatitis C virus (HCV) IgG avidity assay to identify recent HCV infection. Journal of clinical microbiology. 2010;48(9):3281–7. doi: 10.1128/JCM.00303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JW, Kwak KM, Kim SE, et al. Differentiation of acute and chronic hepatitis B in IgM anti-HBc positive patients. World journal of gastroenterology. 2015;21(13):3953–9. doi: 10.3748/wjg.v21.i13.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budkowska A, Kakkanas A, Nerrienet E, et al. Synonymous mutations in the core gene are linked to unusual serological profile in hepatitis C virus infection. PloS one. 2011;6(1):e15871. doi: 10.1371/journal.pone.0015871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitnik R, Pinho JR, Bertolini DA, Bernardini AP, Da Silva LC, Carrilho FJ. Hepatitis B virus genotypes and precore and core mutants in Brazilian patients. Journal of clinical microbiology. 2004;42(6):2455–60. doi: 10.1128/JCM.42.6.2455-2460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular biology and evolution. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragonnet-Cronin M, Hodcroft E, Hue S, et al. Automated analysis of phylogenetic clusters. BMC bioinformatics. 2013;14:317. doi: 10.1186/1471-2105-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular biology and evolution. 2012;29(8):1969–73. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.QGIS version 2.14. http://www.qgis.org/api/2.14/
- 17.Janjua NZ, Butt ZA, Mahmood B, Altaf A. Towards safe injection practices for prevention of hepatitis C transmission in South Asia: Challenges and progress. World journal of gastroenterology. 2016;22(25):5837–52. doi: 10.3748/wjg.v22.i25.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avila C, Stetler HC, Sepulveda J, et al. The epidemiology of HIV transmission among paid plasma donors, Mexico City, Mexico. Aids. 1989;3(10):631–3. doi: 10.1097/00002030-198910000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bhimani GV, Gilada IS. HIV prevalence in people with no fixed abode: A study of blood donorship patterns and risk determinants. Presented at the 8th Int Conf AIDS; 1992; Amsterdam, the Netherlands. 1992. Abstract No. MoC00937. [Google Scholar]
- 20.Wu Z, Liu Z, Detels R. HIV-1 infection in commercial plasma donors in China. Lancet. 1995;346(8966):61–2. doi: 10.1016/s0140-6736(95)92698-4. [DOI] [PubMed] [Google Scholar]
- 21.Apetrei C, Loussert-Ajaka I, Collin G, et al. HIV type 1 subtype F sequences in Romanian children and adults. AIDS research and human retroviruses. 1997;13(4):363–5. doi: 10.1089/aid.1997.13.363. [DOI] [PubMed] [Google Scholar]
- 22.Patrascu IV, Dumitrescu O. The epidemic of human immunodeficiency virus infection in Romanian children. AIDS research and human retroviruses. 1993;9(1):99–104. doi: 10.1089/aid.1993.9.99. [DOI] [PubMed] [Google Scholar]
- 23.Bobkov A, Garaev MM, Rzhaninova A, et al. Molecular epidemiology of HIV-1 in the former Soviet Union: analysis of env V3 sequences and their correlation with epidemiologic data. Aids. 1994;8(5):619–24. [PubMed] [Google Scholar]
- 24.de Oliveira T, Pybus OG, Rambaut A, et al. Molecular epidemiology: HIV-1 and HCV sequences from Libyan outbreak. Nature. 2006;444(7121):836–7. doi: 10.1038/444836a. [DOI] [PubMed] [Google Scholar]
- 25.Yerly S, Quadri R, Negro F, et al. Nosocomial outbreak of multiple bloodborne viral infections. The Journal of infectious diseases. 2001;184(3):369–72. doi: 10.1086/322036. [DOI] [PubMed] [Google Scholar]
- 26.Thorne C, Ferencic N, Malyuta R, Mimica J, Niemiec T. Central Asia: hotspot in the worldwide HIV epidemic. The Lancet Infectious diseases. 2010;10(7):479–88. doi: 10.1016/S1473-3099(10)70118-3. [DOI] [PubMed] [Google Scholar]
- 27.Ansari JA, Salman M, Safdar RM, et al. HIV/AIDS outbreak investigation in Jalalpur Jattan (JPJ), Gujrat, Pakistan. Journal of epidemiology and global health. 2013;3(4):261–8. doi: 10.1016/j.jegh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters PJ, Pontones P, Hoover KW, et al. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. The New England journal of medicine. 2016;375(3):229–39. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- 29.Strathdee SA, Beyrer C. Threading the Needle--How to Stop the HIV Outbreak in Rural Indiana. The New England journal of medicine. 2015;373(5):397–9. doi: 10.1056/NEJMp1507252. [DOI] [PubMed] [Google Scholar]
- 30.De Weggheleire A, An S, De Baetselier I, et al. A cross-sectional study of hepatitis C among people living with HIV in Cambodia: Prevalence, risk factors, and potential for targeted screening. PloS one. 2017;12(8):e0183530. doi: 10.1371/journal.pone.0183530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westbrook RH, Dusheiko G. Natural history of hepatitis C. Journal of hepatology. 2014;61(1 Suppl):S58–68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Thompson SC, Boughton CR, Dore GJ. Blood-borne viruses and their survival in the environment: is public concern about community needlestick exposures justified? Australian and New Zealand journal of public health. 2003;27(6):602–7. doi: 10.1111/j.1467-842x.2003.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 33.Mao B, Patel MK, Hennessey K, Duncan RJ, Wannemuehler K, Soeung SC. Prevalence of chronic hepatitis B virus infection after implementation of a hepatitis B vaccination program among children in three provinces in Cambodia. Vaccine. 2013;31(40):4459–64. doi: 10.1016/j.vaccine.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada H, Fujimoto M, Svay S, et al. Seroprevalence, genotypic distribution and potential risk factors of hepatitis B and C virus infections among adults in Siem Reap, Cambodia. Hepatology research : the official journal of the Japan Society of Hepatology. 2015;45(4):480–7. doi: 10.1111/hepr.12367. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd SJ, McDonald SA, Palmateer NE, et al. HCV avidity as a tool for detection of recent HCV infection: Sensitivity depends on HCV genotype. Journal of medical virology. 2018;90(1):120–30. doi: 10.1002/jmv.24919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.