Abstract
Background and Purpose
The purpose of this study was to explore the relationship between cognitive impairment and brain metabolism in older subjects with HIV infection. It was hypothesized that MRS measurements related to neuronal health and function (particularly N-acetylaspartate (NAA) and glutamate (Glu)) would be lower in HIV+ subjects with worse cognitive performance.
Materials and Methods
45 HIV+ patients [58.9 ± 5.3 years; 33 male] underwent detailed neuropsychological (NP) testing and brain MRS at 7 Tesla. 24 subjects were classified as having asymptomatic cognitive impairment and 21 were classified as symptomatic cognitive impairment. Single voxel proton MR spectra were acquired from 5 brain regions and quantified using ‘LCModel’ software. Brain metabolites and NP test results were compared using non-parametric statistics and Pearson correlation coefficients.
Results
Significant differences in brain metabolites were found between symptomatic and asymptomatic subjects, with the main findings being lower measures of N-acetyl aspartate (NAA) in the frontal white matter (FWM), posterior cingulate cortex (PCC), and precuneus (PC). In the PC, glutamate (Glu) was also lower in the symptomatic group. In the FWM, PC and PCC, NAA and Glu measurements showed significant positive correlation with better performance on NP tests.
Conclusion
Compared to asymptomatic, symptomatic HIV+ subjects had lower levels of NAA and Glu, most notably in frontal white matter, which also correlated with performance on neuropsychological tests. High field MRS offers insights into the pathophysiology associated with cognitive impairment in HIV, and may be useful as a quantitative outcome measure in future treatment trials.
Introduction
Human immunodeficiency virus (HIV) associated neurocognitive disorder (HAND) is characterized by cognitive, behavioral, and motor dysfunction which impacts daily functioning1–3 and is predictive of poor survival in patients4. Combination antiretroviral therapy (cART) can improve cognitive performance and brain metabolism in some patients with HAND2, 5, 6. However, many antiretroviral drugs do not penetrate well into the central nervous system7, and neurocognitive deficits are reversed in many but not all patients8, 9. The prevalence of HAND in HIV positive (HIV+) individuals with advanced infection remains around 45-50%, and patients are now surviving for years with HIV infection and HAND as chronic conditions10, 11. Advanced age is an important risk factor for HAND; for instance, in the Hawaii Aging with HIV cohort, HIV+ individuals greater than age 50 were twice as likely to have dementia compared to HIV+ individuals who were 20-39 years of age12, 13. As HIV+ individuals are now living longer as a result of cART, the proportion of HIV+ individuals older than 50 years of age has increased. There is some evidence that HIV+ subjects may develop cognitive decline earlier than the HIV− population (i.e. more rapid aging). For the quantitative assessment of neurological involvement in older HIV+ individuals, it is important to develop and validate non-invasive imaging tools that are sensitive to changes in cognitive and behavioral status, which, for instance, may be of use as quantitative measures in future treatment trials. Measurements of regional brain metabolism may also shed some light on the neurobiology of brain aging and cognitive decline in the older HIV+ population.
Proton magnetic resonance spectroscopy (MRS) is a non-invasive technique that gives information on brain pathophysiology through measurement of brain metabolite levels14. The most common observations in HIV+ subjects are increased levels of choline (Cho)15 and myo-inositol (mI)16, thought to reflect inflammation and microglial proliferation, and decreased levels of N-acetyl aspartate (NAA) and glutamate17–19, believed to be due to neuronal injury or dysfunction15, 20–22. Metabolic abnormalities may be observed in regions of the brain with normal appearance on conventional MRI, even in subjects who are neurologically asymptomatic, and increase with increasing degrees of neurological involvement23. For these reasons, MRS has been proposed to be a suitable tool of monitoring the degree of HIV involvement in the brain, and the effects of therapy24, 25.
MRS at field strengths of 1.5, 3.0 and 4.0 Tesla (T)6, 18, 26–28 has been extensively used to investigate neurological involvement in HIV+ subjects. Higher magnetic field strengths (such as 7.0T) allow increased sensitivity and chemical shift dispersion and more reliable determination of brain metabolites, particularly for some of the smaller and overlapping signals in the spectrum, such as glutamate and glutamine29, 30.
The current study was undertaken to investigate the utility of 7.0T MRS in evaluating a cohort of older HIV+ patients with varying degrees of neurocognitive impairment. It was hypothesized that differences in brain metabolites reflecting neuronal and glial cell populations will be observed between older HIV+ individuals with and without cognitive impairment. For analysis, patients were classified either as ‘asymptomatic’ (i.e. either cognitively normal, or asymptomatic neurocognitive impairment (ANI) or ‘symptomatic’ (HAND, including those with either mild neurocognitive disorder (MND) or HIV associated dementia (HAD).
Materials and Methods
Participants and clinical testing
The study was approved the local institutional review board (IRB), and all subjects gave written informed consent. HIV+ individuals were recruited at the Institute for Clinical and Translational Research at Johns Hopkins Hospital in Baltimore, Maryland from 2013 to 2016. Inclusion criteria included adults who were HIV positive, older than 50 years, who had the ability to provide written informed consent and to ambulate at first clinic visit. Patients were excluded if they had a history of or current opportunistic central nervous system infection, schizophrenia, affective disorder or psychiatric diseases which could be a confounder for cognitive impairment, or chronic neurological disorders such as brain infarction, hemorrhage, epilepsy, and multiple sclerosis. Active substance abusers or opiate users were excluded, defined as any history of illicit drug use within 3 months preceding the baseline visit, established by subject history and urine toxicology screens. Any contraindication for 7T MRI (metal in body, claustrophobia, inner ear disorder) was also an exclusion criterion.
As described above, subjects were stratified by neurocognitive disorder status using the revised American Academy of Neurology (AAN) ‘Frascati’ criteria31. All subjects underwent detailed neurological, neuropsychological, laboratory and functional assessments. Clinical assessments included standardized questionnaires which assessed demographic information including primary language, reading abilities, medical, psychiatric, and neurological history, as well as a neurological examination. Serum CD4 T-cell counts and HIV RNA levels via quantitative polymerase chain reaction (PCR) in the plasma and cerebrospinal fluid were obtained. Depression symptomatology was rated using the Center for epidemiological studies depression score (CES-D)32. Hepatitis C viral status was obtained via clinical history and evaluation of laboratory findings for hepatitis C virus antibodies.
Measures of functional performance included Karnofsky Performance Scale, a questionnaire for instrumental activities of daily living (IADLs), and a questionnaire for role and physical quality of life (QOL) measures1–3, 33–35. Neuropsychological (NP) testing include the Trail Making Test, Color Trail, Grooved Pegboard Test, Digit Symbol, Stroop Test, Rey Complex Figure Test, and Hopkins Verbal Learning Test36–38. Raw scores on each neuropsychological test were converted to z-scores using published normative data31.
MR Imaging and Spectroscopy
All studies were performed on a 7T scanner (Philips ‘Achieva’, Best, The Netherlands) equipped with a 32-channel receive head coil and quadrature transmit coil (Nova Medical Inc., Wilmington, MA, USA). Brain MRI consisted of localizer images and a 3D T1-weighted MPRAGE scan with 1.2 mm isotropic voxel size. Single voxel STEAM spectra (TR/TE/TM=3000/14/25 msec) were acquired from the left frontal white matter (FWM), left basal ganglia (BG), mesial precuneus (PC), mesial posterior cingulate cortex (PCC) and left hippocampus (hippo) with and without VAPOR water suppression. These regions were chosen based on prior studies which have indicated that they are involved in HIV infection and aging10, 39–41. The voxel sizes ranged from 8 to 15 cc (Figure 1). The hippocampal voxel was carefully angulated parallel to the long axis of the hippocampus and had dimensions were 1.5×1.5×3.5cm to minimize partial volume contributions from surrounding tissues. 80 acquisitions were acquired with water suppression, and 2 unsuppressed, to give a total scan time per region of 4 min 6 sec. Prior to the acquisition of each region, field inhomogeneity was corrected up to 2nd order using the ‘FASTMAP’ localized shimming technique42 and localized power optimization43 was also performed (transmit B1 = 15 μT).
Figure 1.
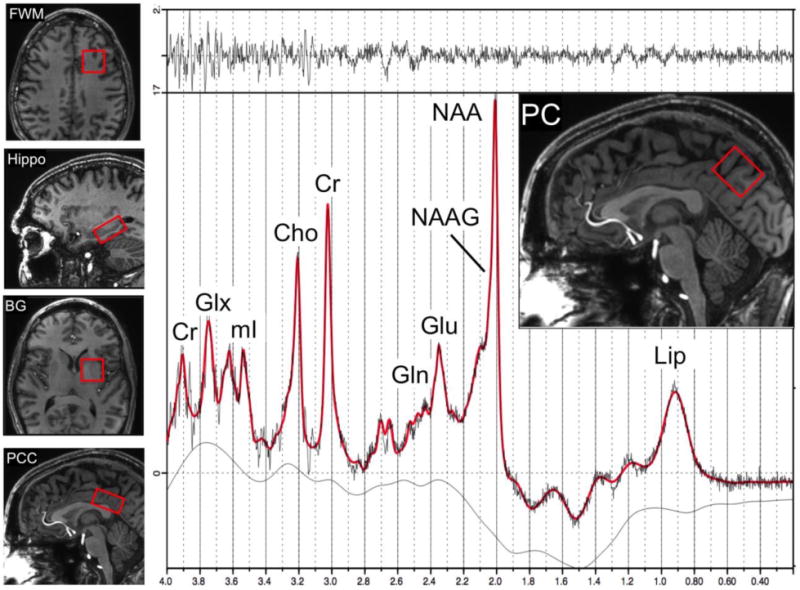
Examples of the 5 voxel locations used for brain MRS in the left frontal white matter (FWM), left hippocampus (Hippo), left basal ganglia (BG), mesial posterior cingulate cortex (PCC) and mesial precuneus (PC). An example of a spectrum from the PC in a 55 year old HIV+ subject is shown, including results from the LCModel analysis (red curve). Signals are assigned to lipids (Lip), N-acetyl aspartate (NAA), N-acetyl aspartate glutamate (NAAG), glutamate (Glu), glutamine (Gln), creatine (Cr), choline (Cho) myo-inositol (mI), and Glx (the sum of Glu and Gln). The top trace is the difference between the original data and the results of the curve-fit.
Spectra were analyzed using a basis set designed for 7T STEAM sequence incorporated in the ‘LCModel’ software package44 and quantified in approximately millimolar concentrations (referred to here as ‘institutional units’ (i.u.), since relaxation time corrections were not performed) relative to the unsuppressed water signal. Ratios relative to creatine (Cr) were also calculated. We did not do any volumetric morphometry as we reported metabolite ratios besides metabolite concentrations. The LCModel basis set contained 20 different metabolites, and also the standard LCModel macromolecule peaks. Concentration and ratio values were only included for statistical analysis if their Cramér-Rao lower bounds (CRLB) were less than or equal to 20%44. A representative PC spectrum is shown from one subject in Figure 1.
Statistical Analysis
The objective of the current study was to compare metabolite concentration and ratio values between older HIV+ patients who were either asymptomatic or symptomatic for neurocognitive impairment. Using the Shapiro-Wilk test, the data were not normally distributed; therefore, we present the data as median and interquartile range (IQR, 25th and 75th percentile). Between groups comparisons were made using the non-parametric median test. Pearson correlation coefficients were calculated between metabolite values and concentrations and neurocognitive tests.
Results
45 subjects (mean age 58.9 ± 5.3 years; 33 (73%) male) were enrolled. All patients were receiving cART. 24 subjects were classified as asymptomatic and 21 as symptomatic. Patient demographics are given in Table 1. There were no significant group differences in age, education, gender, race, CD4 count or plasma HIV RNA between groups. The estimated IQ which was significantly lower in the symptomatic group (103.7 vs. 112.9 respectively, P=0.02). Apart from some small white matter hyperintensities in 2 individuals, which were avoided during MRS voxel placement, there were no significant T2W or FLAIR lesions or other abnormalities in any of the subjects in this study.
Table 1.
Subject demographics in the two HIV+ groups: Asymptomatic neurocognitive impairment (ANI), and symptomatic HIV associated neurocognitive disorder (HAND). N = number of patients in each group. Values given are means ± standard deviation.
| ANI | HAND | P-value | |
|---|---|---|---|
| N | 24 | 21 | – |
| Age, years | 59.6 ± 5.7 | 58.2 ± 5.0 | 0.378 |
| Male, n (%) | 17 (71%) | 16 (76%) | 0.764 |
| Education, years | 14.9 ± 3.0 | 14.0 ± 2.7 | 0.263 |
| Race (% African American) | 12 (50%) | 13 (62%) | 0.655 |
| Duration of Infection (years) | 19.9±9.0 | 19.2±9.4 | 0.790 |
| CES-D | 7.7 ± 6.8 | 11.0 ± 11.7 | 0.253 |
| HART Est IQ* | 112.9 ± 12.8 | 103.7 ± 12.9 | 0.022 |
| CD4 cell count (cells/mm3) | 674 ± 281 | 676 ± 375 | 0.988 |
| Plasma HIV RNA (Log10 copies/ml) | 1.6 ± 0.1 | 2.3 ± 1.4 | 0.569 |
CES-D =Center for Epidemiological Studies Depression Score; HART Est IQ= Hopkins Adult Reading Test Estimated IQ; Plasma HIV RNA only among those with detectable viral load (n=2 ANI and n=3 HAND).
Significant P<0.05
In the frontal white matter, the median NAA/Cr ratio was lower in the symptomatic group compared to asymptomatic (1.21 vs. 1.30 respectively, P=0.005), and tNAA/Cr (1.46 vs. 1.56 respectively, p=.005) and the median myo-inositol/Cr (mI/Cr) (0.97 vs. 1.03 respectively, P=0.02) (Figure 2). There was also a trend towards lower FWM tNAA concentration in the symptomatic group compared to asymptomatic (7.46 vs. 7.81 mM respectively, P=0.06) (Figure 3). In the posterior cingulate cortex, median NAA/Cr was also significantly lower in the symptomatic group (1.16 vs. 1.21 respectively, P=0.01), as was median tNAA/Cr (1.32 vs. 1.40 respectively, P=0.02), and median mI/Cr (0.78 vs. 0.87 respectively, P=0.002) (Figure 2). In the precuneus, the median NAA/Cr was also significantly lower in the symptomatic group (1.14 vs. 1.23 respectively, P=0.02), as was median glutamate/Cr (Glu/Cr) (1.12 vs. 1.24 respectively, P=0.01) (Figure 2). There were no significant differences between groups in basal ganglia and hippocampus metabolite concentrations or ratios.
Figure 2.
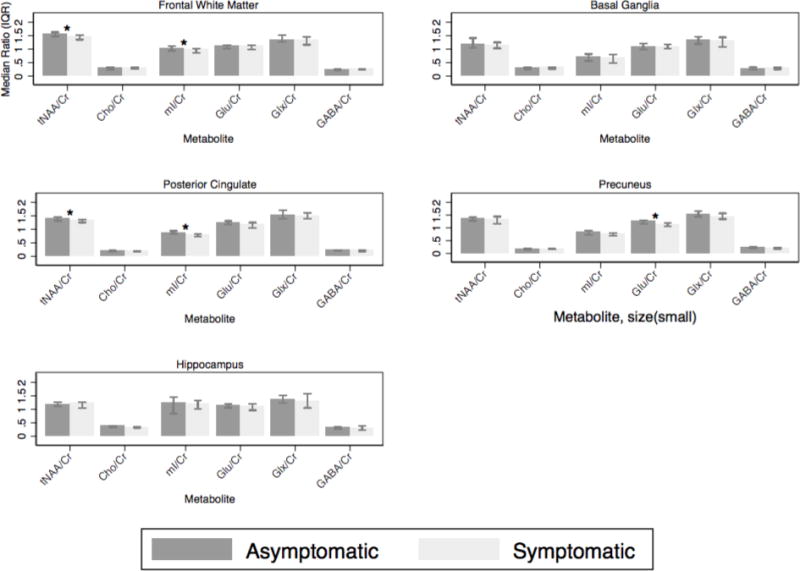
Metabolite ratios for the frontal white matter, posterior cingulate, precuneus, basal ganglia and hippocampal voxels. *= significant.
Figure 3.
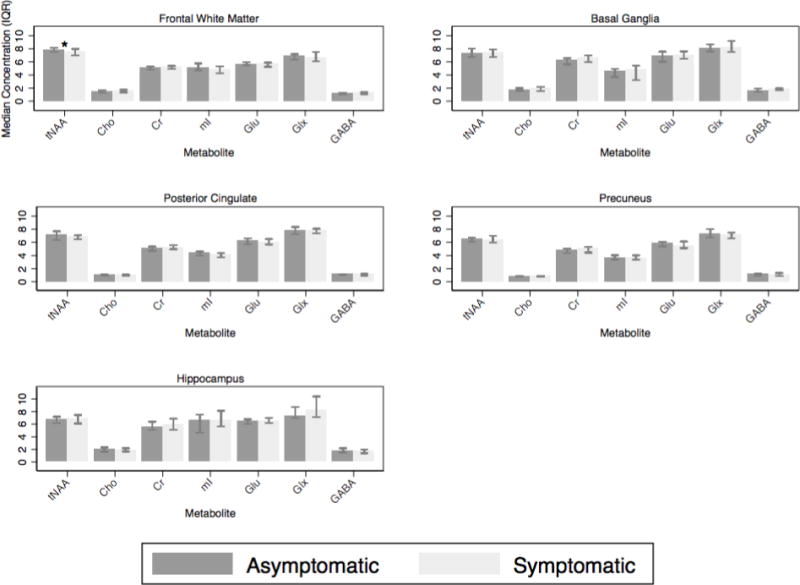
Metabolite concentrations for the frontal white matter, posterior cingulate, precuneus, basal ganglia and hippocampal voxels. *= significant.
In regards to correlations between MRS results and NP tests (Table 2, Figures 4, 5, 6), lower FWM tNAA/Cr was associated with worse performance on measures of executive function, fine motor, and psychomotor speed (Trail Making Test, part B, Grooved Pegboard Test, non-dominant hand, and Digit Symbol (P= 0.005, 0.01 and 0.001, respectively). Furthermore, lower FWM Glu/Cr was associated with lower performance on delayed recall on the Hopkins Verbal Learning Test (P = 0.02) (Figure 4). Lower tNAA/Cr in the posterior cingulate was associated with worse performance on tests of psychomotor speed (Digit Symbol and Stroop Test, P = 0.02 and 0.04 respectively) (Figure 5). Lower precuneus NAA/Cr was associated with worse performance on the Stroop Test, a test of processing speed (P=0.04); while precuneus Glu/Cr decreases were associated with worse performance in the Trail Making Test Part B, a measure of executive functioning (P=0.04) (Figure 6).
Table 2.
Pearson correlation of brain metabolites with neurocognitive test scores. Correlation coefficients (r) and significance (P values) for frontal white matter (FWM), posterior cingulate cortex (PCC) and precuneus (PC). Values in bold are significant (P<0.05)
| Trail-making test B | Grooved pegboard non-dominant hand | Digit symbol | Hopkins Verbal Learning Test Delayed recall | Stroop | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FWM | PCC | PC | FWM | PCC | PC | FWM | PCC | PC | FWM | PCC | PC | FWM | PCC | PC | |
| NAA | 0.38 (0.01) | NS | NS | 0.35 (0.01) | NS | NS | 0.46 (0.006) | 0.44 (0.008) | 0.46 (0.001) | NS | NS | NS | NS | 0.38 (0.02) | |
| NAA/Cr | 0.36 (0.01) | NS | NS | NS | NS | NS | 0.33 (0.02) | 0.35 (0.04) | 0.33 (0.04) | 0.31 (0.04) | NS | NS | NS | 0.37 (0.03) | 0.33 (0.04) |
| tNAA | 0.43 (0.003) | NS | NS | 0.45 (0.002) | NS | NS | NS | 0.48 (0.004) | NS | 0.35 (0.01) | NS | NS | NS | NS | NS |
| tNAA/Cr | 0.41 (0.005) | NS | NS | 0.35 (0.01) | NS | NS | 0.46 (0.001) | 0.39 (0.02) | NS | NS | NS | NS | NS | 0.35 (0.04) | NS |
| Glu | 0.3 (0.04) | NS | NS | 0.36 (0.01) | NS | NS | NS | 0.48 (0.003) | NS | 0.46 (0.001) | NS | NS | NS | 0.4 (0.01) | 0.46 (0.005) |
| Glu/Cr | NS | NS | 0.34 (0.04) | NS | NS | NS | NS | NS | NS | 0.32 (0.02) | NS | NS | 0.32 (0.03) | 0.38 (0.02) | NS |
| Glx | 0.29 (0.05) | NS | NS | 0.35 (0.01) | NS | NS | NS | 0.42 (0.01) | NS | 0.32 (0.03) | NS | NS | NS | NS | 0.36 (0.03) |
| Glx/Cr | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Cho | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | − 0.34 (0.04) | NS | NS | 0.4 (0.01) |
| Cho/Cr | NS | NS | NS | NS | NS | NS | 0.31 (0.04) | NS | NS | −0.37 (0.02) | NS | NS | NS | NS | NS |
| mI | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| mI/Cr | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.35 (0.04) | NS |
Figure 4.
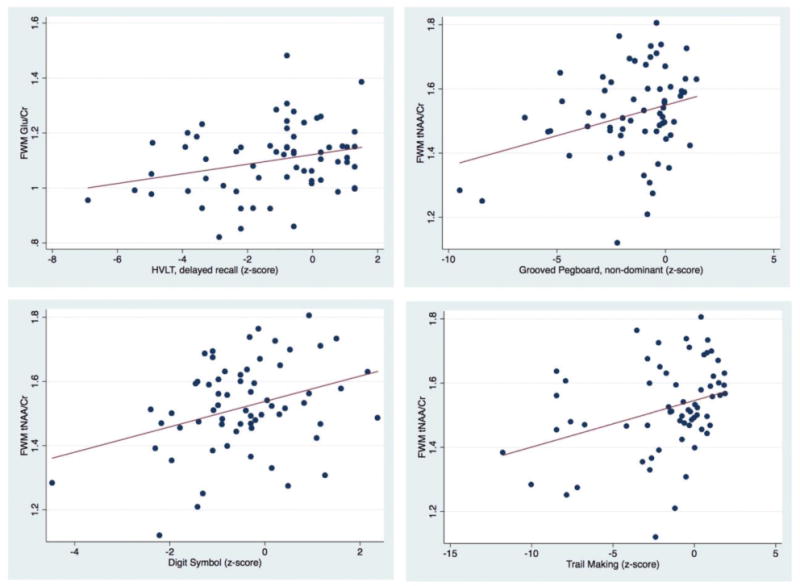
Scatter plots showing significant positive correlations of FWM Glu/Cr with HVLT delayed recall, and FWM tNAA/Cr with Grooved Pegboard non dominant, Digit Symbol and Trail-Making Test B z scores.
Figure 5.
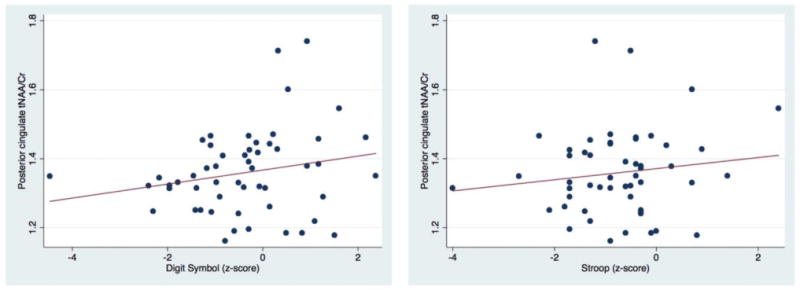
Scatter plots showing significant positive correlation of posterior cingulate tNAA/Cr with Digital Symbol and Stroop Trail z scores.
Figure 6.
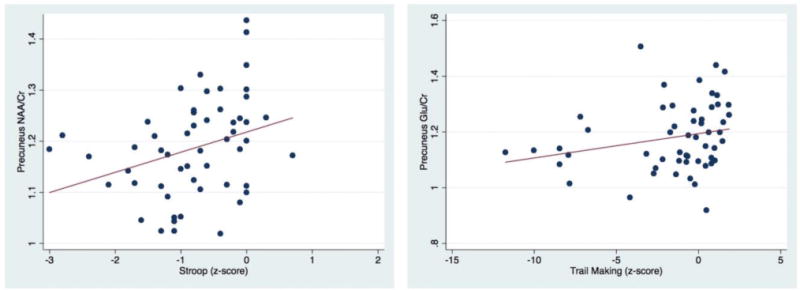
Scatter plots showing significant positive correlation of precuneus NAA/Cr and Glu/Cr with Stroop Trail and Trail Making part B z scores respectively.
Discussion
The main finding of this study is that 7T MRS was able to find significant differences in metabolite levels between symptomatic and asymptomatic HIV+ subjects, with the primary result being lower levels of (t)NAA (or the ratio (t)NAA/Cr) in frontal white matter, posterior cingulate, and precuneus. Glu/Cr was also lower in the precuneus in the symptomatic group. In addition, significant correlations were found between FWM measures of (t) NAA (and (t)NAA/Cr) and Glu (and Glu/Cr, Glx and Glx/Cr) and multiple neuropsychological test scores. Posterior cingulate cortex and precuneus measures of NAA and Glu also correlated with performance on the Digit Symbol and Stroop tests. Overall, these results suggest that MRS measurements of NAA and glutamate in these brain regions reflect neuroaxonal loss or dysfunction, which correlates with worse performance on neuropsychological tests.
Findings of decreased NAA and Glx (Glu+Gln) in HIV infection are well established from MRS studies at lower field strengths; for instance, in one study long TE performed at 1.5T found decreased levels of NAA/Cr in parietal-occipital white matter in asymptomatic HIV+ men compared to normal controls45. MR spectroscopic imaging (MRSI) studies also performed at long TE found progressive declines in NAA from HIV− controls, to asymptomatic and then symptomatic HIV+ patient groups in multiple gray and white matter regions6, 18, 46. NAA was also found to increase after initiation of cART6, 46. Previous 3T studies have also reported decreasing Glx concentrations (or Glx/Cr ratios) with increasing symptomatology in HIV infection17, 18, and decreased levels compared to HIV− controls19. Although some 3T studies have used the technique of TE-averaged PRESS19 to selectively detect Glu, generally it can be difficult to reliably separate Glu and from Gln using conventional MRS. Prior studies comparing 7T to lower field strengths have shown improved ability at 7T to quantify Glu and Gln43; the current study at 7T confirms that only significant HIV-associated correlations are found for Glu, with no significant associations found for Gln. NAA and Glu are known to be primarily located in neurons, and have been shown in previous studies of various pathologies to decrease when neuronal damage occurs. The mechanisms by which neuronal damage occur in HIV infection are complicated, but it is believed that infiltration of infected macrophages and lymphocytes result in inflammation, astrocytosis, oxidative stress and synaptodendritic injury47.
Several studies performed at 1.5 and 3.0 T have reported reduced increased myo-inositol (mI) in HIV infection, and symptomatic HIV+ subjects compared to asymptomatic6, 19. The current study is consistent with these findings. Previous studies at lower field strengths, and usually in younger cohorts of subjects, have generally reported increased levels of mI in HIV+ subjects compared to HIV− controls, with higher mI in symptomatic HIV+ subjects compared to asymptomatic18, 48. The current study did not find strong differences in mI (or mI/Cr) between asymptomatic and symptomatic groups in most brain regions, with the exception of slightly lower mI/Cr in FWM in the symptomatic group (i.e. in the opposite direction to prior findings)18, 49. There were also few significant positive correlations between high mI and worse performance on neuropsychological tests. These findings may be due to the older nature of the HIV+ cohort studied here, with a longer disease duration, generally well-controlled viral load and less neuroinflammation than in previous studies.
Regarding correlations between brain metabolites and neuropsychological test scores, frontal white matter test NAA and Glu measures correlated positively with measures of executive function, motor speed, verbal and working memory, whereas posterior cingulate cortex and precuneus measures of NAA and Glu predominantly correlated with the Digit Symbol and Stroop tests, measures of working memory and information processing speed respectively. The precuneus and posterior cingulate cortex are both visual processing areas, and neuronal dysfunction as measured by the metabolite changes in these areas could contribute to impaired performance in neuropsychological tests which include visuo-spatial processing, such as the Digit-Symbol and Stroop tests. Specifically, decreased FWM Glu/Cr and Glx were correlated with verbal recall; while post cingulate and precuneus Glu Glu/Cr and Glx were associated with attention and working memory and information speed processing tests. These findings are generally consistent with prior studies performed at 1.5 or 3.0T; for instance, in patients receiving cART, significant correlations were found between 3T measures of Glu, Glx and NAA with performance on motor and psychomotor speed, attention and working memory6. A study performed at 1.5T25 in HIV+ subjects (the majority being on cART) assessed motor skills (fine and gross), psychomotor function, information processing speed, and verbal memory and expressed the results as a composite score (NPZ-8). Consistent with the concept of HIV+ as a predominantly subcortical dementia, NPZ-8 scores correlated positively with NAA/Cr (and NAA/Cho and NAA/mI) in the FWM, similar to the current study. Negative correlations in that study were also found between basal ganglia mI/Cr and NPZ-825. Another study at 1.5T in 45 antiretroviral naïve HIV+ subjects also found elevated frontal white matter mI and Cho compared to HIV− controls, which were correlated with slow performance in fine motor (Grooved Pegboard), psychomotor (Trail Making Test), and executive functions (Stroop tasks) tests50. The relative lack of correlations in the current study between neuropsychological test performance and measures of Cho (only PC Cho negatively correlated with Hopkins Verbal Learning Test (HVLT) Delayed recall task) or mI (PCC mI/Cr did correlate with performance on the Stroop task) most likely reflect differences in patient populations between the current and previous studies (older HIV+ subjects, all on cART).
7T MRS has been shown to offer increased signal-to-noise ratios (SNR) and spectral resolution compared to MRS at lower field strengths; however, it also presents some technical challenges, including increased chemical shift dispersion effects (CSDE), difficulty shimming, and increased radiofrequency power deposition (‘specific absorption rate’, SAR). Localized 2nd order shimming was used in this study to minimize the effects of field inhomogeneity. In addition, the STEAM pulse sequence was used for spatial localization since the 90˚ slice selective pulses in STEAM have excellent slice profiles, low CSDE and SAR51, 52. However, in the future, other sequences such as semi-LASER and/or magnetic resonance spectroscopic imaging sequences may be used which provide higher SNR, spatial resolution and coverage.
The study also has some limitations; although subjects with overt medical or neurological abnormalities were excluded, the older HIV+ subjects in this study may well have had varying degrees of risk factors commonly encountered in the general population which contribute to aging and cognitive decline, such as microvascular disease, diabetes or hypertension. All subjects were taking cART, which is known to affect brain metabolite concentrations53. Although 3D T1W images were recorded, no volumetric analysis has been performed to date in these subjects due to intensity and contrast variations due to transmit B1 inhomogeneity. However, we do not expect morphometric differences to significantly affect results, since metabolite ratios (relative to Cr) are relatively insensitive to voxel composition (particularly CSF contamination). Finally, no significant changes were observed in the hippocampus in this study, despite the well-known importance of this structure for memory and other age-related cognitive dysfunction40, 54, 55. Lack of significant findings may be in part due to the generally lower spectral quality obtained in this region due to magnetic susceptibility effects from bone/air/tissue interfaces proximal to the anterior temporal lobe, as well as partial volume with surrounding tissue due to the small size of the hippocampus. Spectral quality was also lower in the basal ganglia because of the high iron content of the globus pallidus and putamen which is known to increase line widths in MR spectra from this region56–58; this may have also contributed to the lack of significant group differences or neurocognitive correlations detectable in this region.
Conclusion
7-Tesla MRS measurements of NAA and Glu may be useful indicators of neuroaxonal loss or dysfunction and correlate with neuropsychological performance in the older subject population with HIV infection. 7T MRS may therefore be a useful adjunct technique for monitoring disease progression or response to therapy in future treatment trials. Other future studies are needed to track longitudinal changes over time, as well as comparisons to HIV− negative control subjects to determine if cognitive decline and brain metabolite changes occur at a greater rate in HIV+ subjects, and if there are specific metabolic changes only associated with HIV infection in the older population.
Acknowledgments
Funding: This work was funded by NIH RO1NS081196, P41EB015909 and P30MH075673.
DISCLOSURES: Peter Barker—RELATED: Grant: NIH*. Richard Skolasky—RELATED: Grant: NIH*; UNRELATED: Board membership: North American Spine Society, Comments: Member of Board of Directors; Grants/grants pending: NIH, PCORI; OTHER RELATIONSHIPS: Associate Editor, Quality of Life Research. Ned Sacktor—RELATED: Grant: NIH, Comments: Work supported by NIH grant NS081196*.
*money paid to institution
Abbreviations
- cART
Combined Anti-retroviral therapy
- HIV
human immunodeficiency virus
- Cr
Creatine
- Glx
Glutamate and glutamine
- mI
myo-inositol
- Cho
Choline
- Gln
Glutamine
- Glu
Glutamate
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartyl-gluamate
- tNAA
NAA+NAAG
- GABA
γ-Aminobutyric acid
- MRS
Magnetic resonance spectroscopy
- MRSI
Magnetic resonance spectroscopic imaging
- MRI
Magnetic resonance imaging
- Lip
Lipid
- ANI
Asymptomatic neurocognitive impairment
- HAND
HIV associated neurocognitive disorder
- MND
mild neurocognitive disorder
- HAD
HIV associated dementia
- STEAM
Stimulated echo acquisition mode
- IRB
Institutional Review Board
- RNA
Ribonucleic acid
- FWM
Frontal white matter
- PCC
Posterior cingulate cortex
- PC
Precuneus
- BG
Basal Ganglia
- Hippo
Hippocampus
- HVLT
Hopkins Verbal Learning Test
- NP
Neuropsychological
- T
Tesla
- AAN
American Academy of Neurology
- CES-D
Center for epidemiological studies depression score
- PCR
Polymerase chain reaction
- IADL
Instrumental activities of daily living
- QOL
Quality of life
- VAPOR
Variable power and optimized relaxation
- LCModel
Linear Combination of Model Spectra
- CRLB
Cramér-Rao lower bounds
- PRESS
Point Resolved SpectroScopy
- i.u
Institutional units
- IQR
Interquartile range
References
- 1.Gandhi NS, Skolasky RL, Peters KB, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. Journal of neurovirology. 2011;17:159–165. doi: 10.1007/s13365-011-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakinami L, de Bruyn G, Pronyk P, et al. The impact of highly active antiretroviral therapy on activities of daily living in HIV-infected adults in South Africa. AIDS and behavior. 2011;15:823–831. doi: 10.1007/s10461-010-9776-y. [DOI] [PubMed] [Google Scholar]
- 3.Schifitto G, Kieburtz K, McDermott MP, et al. Clinical trials in HIV-associated cognitive impairment: cognitive and functional outcomes. Neurology. 2001;56:415–418. doi: 10.1212/wnl.56.3.415. [DOI] [PubMed] [Google Scholar]
- 4.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sailasuta N, Ross W, Ananworanich J, et al. Change in Brain Magnetic Resonance Spectroscopy after Treatment during Acute HIV Infection. PloS one. 2012:7. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lentz MR, Degaonkar M, Mohamed MA, et al. Exploring the relationship of macrophage colony-stimulating factor levels on neuroaxonal metabolism and cognition during chronic human immunodeficiency virus infection. Journal of neurovirology. 2010;16:368–376. doi: 10.3109/13550284.2010.513029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Archives of neurology. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford DB. HIV-associated neurocognitive disorder. Current opinion in infectious diseases. 2017;30:117–122. doi: 10.1097/QCO.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. Journal of acquired immune deficiency syndromes (1999) 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 10.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS (London, England) 2011;25:625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuenburg JK, Brodt HR, Herndier BG, et al. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999) 2002;31:171–177. doi: 10.1097/00126334-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Valcour V, Paul R, Neuhaus J, Shikuma C. The Effects of Age and HIV on Neuropsychological Performance. Journal of the International Neuropsychological Society: JINS. 2011;17:190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Zijl PC, Barker PB. Magnetic resonance spectroscopy and spectroscopic imaging for the study of brain metabolism. Annals of the New York Academy of Sciences. 1997;820:75–96. doi: 10.1111/j.1749-6632.1997.tb46190.x. [DOI] [PubMed] [Google Scholar]
- 15.Chong WK, Sweeney B, Wilkinson ID, et al. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology. 1993;188:119–124. doi: 10.1148/radiology.188.1.8099750. [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Ernst T, Leonido-Yee M, et al. Highly active antiretroviral therapy reverses brain metabolite abnormalities in mild HIV dementia. Neurology. 1999;53:782–789. doi: 10.1212/wnl.53.4.782. [DOI] [PubMed] [Google Scholar]
- 17.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. Journal of magnetic resonance imaging: JMRI. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohamed MA, Barker PB, Skolasky RL, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magnetic resonance imaging. 2010;28:1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR in biomedicine. 2009;22:326–331. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- 20.Menon DK, Ainsworth JG, Cox IJ, et al. Proton MR spectroscopy of the brain in AIDS dementia complex. Journal of computer assisted tomography. 1992;16:538–542. doi: 10.1097/00004728-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Meyerhoff DJ, MacKay S, Poole N, Dillon WP, Weiner MW, Fein G. N-acetylaspartate reductions measured by 1H MRSI in cognitively impaired HIV-seropositive individuals. Magnetic resonance imaging. 1994;12:653–659. doi: 10.1016/0730-725x(94)92460-0. [DOI] [PubMed] [Google Scholar]
- 22.Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195:58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- 23.Laubenberger J, Haussinger D, Bayer S, et al. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology. 1996;199:805–810. doi: 10.1148/radiology.199.3.8638009. [DOI] [PubMed] [Google Scholar]
- 24.Navia BA, Rostasy K. The AIDS dementia complex: clinical and basic neuroscience with implications for novel molecular therapies. Neurotoxicity research. 2005;8:3–24. doi: 10.1007/BF03033817. [DOI] [PubMed] [Google Scholar]
- 25.Paul RH, Yiannoutsos CT, Miller EN, et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. The Journal of neuropsychiatry and clinical neurosciences. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- 26.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magnetic resonance in medicine. 2001;45:765–769. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antiviral therapy. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 28.Young AC, Yiannoutsos CT, Hegde M, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014;83:1592–1600. doi: 10.1212/WNL.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs 7T. Magnetic resonance in medicine. 2009;62:868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephenson MC, Gunner F, Napolitano A, et al. Applications of multi-nuclear magnetic resonance spectroscopy at 7T. World journal of radiology. 2011;3:105–113. doi: 10.4329/wjr.v3.i4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of youth and adolescence. 1991;20:149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- 33.Giebel CM, Challis D, Montaldi D. The newly revised interview for deteriorations in daily living activities in dementia (R-IDDD2): distinguishing initiative from performance at assessment. International psychogeriatrics. 2017;29:497–507. doi: 10.1017/S1041610216002003. [DOI] [PubMed] [Google Scholar]
- 34.Kaur N, Belchior P, Gelinas I, Bier N. Critical appraisal of questionnaires to assess functional impairment in individuals with mild cognitive impairment. International psychogeriatrics. 2016;28:1425–1439. doi: 10.1017/S104161021600017X. [DOI] [PubMed] [Google Scholar]
- 35.Fantoni M, Izzi I, Del Borgo C, et al. Inter-rater reliability of a modified Karnofsky Scale of Performance Status for HIV-infected individuals. AIDS patient care and STDs. 1999;13:23–28. doi: 10.1089/apc.1999.13.23. [DOI] [PubMed] [Google Scholar]
- 36.Gaines JJ, Shapiro A, Alt M, Benedict RH. Semantic clustering indexes for the Hopkins Verbal Learning Test-Revised: initial exploration in elder control and dementia groups. Applied neuropsychology. 2006;13:213–222. doi: 10.1207/s15324826an1304_2. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. The Clinical neuropsychologist. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 38.Woods SP, Scott JC, Conover E, Marcotte TD, Heaton RK, Grant I. Test-retest reliability of component process variables within the Hopkins Verbal Learning Test-Revised. Assessment. 2005;12:96–100. doi: 10.1177/1073191104270342. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, Lee PL, Yiannoutsos CT, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. NeuroImage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 40.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. What is normal in normal aging? Effects of Aging, Amyloid and Alzheimer’s Disease on the Cerebral Cortex and the Hippocampus. Progress in neurobiology. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driscoll I, Troncoso JC, Rudow G, et al. Correspondence between in vivo (11)C-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta neuropathologica. 2012;124:823–831. doi: 10.1007/s00401-012-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Öz G, Tkáč I. SHORT-ECHO, SINGLE-SHOT, FULL-INTENSITY 1H MRS FOR NEUROCHEMICAL PROFILING AT 4T: VALIDATION IN THE CEREBELLUM AND BRAINSTEM. Magnetic resonance in medicine. 2011:65. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradhan S, Bonekamp S, Gillen JS, et al. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magnetic resonance imaging. 2015;33:1013–1018. doi: 10.1016/j.mri.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson ID, Miller RF, Miszkiel KA, et al. Cerebral proton magnetic resonance spectroscopy in asymptomatic HIV infection. AIDS (London, England) 1997;11:289–295. doi: 10.1097/00002030-199703110-00005. [DOI] [PubMed] [Google Scholar]
- 46.Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. Journal of neurovirology. 2011;17:220–229. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of neurology. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 48.Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. Journal of neuroimmunology. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 49.Mohamed MA, Lentz MR, Lee V, et al. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology. 2010;254:577–586. doi: 10.1148/radiol.09081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. NeuroImage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 51.Wijtenburg SA, Rowland LM, Edden RA, Barker PB. Reproducibility of brain spectroscopy at 7T using conventional localization and spectral editing techniques. Journal of magnetic resonance imaging: JMRI. 2013;38:460–467. doi: 10.1002/jmri.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mekle R, Mlynarik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magnetic resonance in medicine. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 53.Sailasuta N, Ananworanich J, Lerdlum S, et al. Neuronal-glia markers by Magnetic Resonance Spectroscopy in HIV Before and After Combination Antiretroviral Therapy. Journal of acquired immune deficiency syndromes (1999) 2016;71:24–30. doi: 10.1097/QAI.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castellano JF, Fletcher BR, Kelley-Bell B, Kim DH, Gallagher M, Rapp PR. Age-Related Memory Impairment Is Associated with Disrupted Multivariate Epigenetic Coordination in the Hippocampus. PloS one. 2012:7. doi: 10.1371/journal.pone.0033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castellano JF, Fletcher BR, Patzke H, et al. Reassessing the Effects of Histone Deacetylase Inhibitors on Hippocampal Memory and Cognitive Aging. Hippocampus. 2014;24:1006–1016. doi: 10.1002/hipo.22286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daugherty A, Raz N. Age-Related Differences in Iron Content of Subcortical Nuclei Observed in vivo: A Meta-Analysis. NeuroImage. 2013;70:113–121. doi: 10.1016/j.neuroimage.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haacke EM, Miao Y, Liu M, et al. Correlation of change in R2* and phase with putative iron content in deep gray matter of healthy adults. Journal of magnetic resonance imaging: JMRI. 2010;32:561–576. doi: 10.1002/jmri.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Persson N, Wu J, Zhang Q, et al. Age and Sex Related Differences in Subcortical Brain Iron Concentrations among Healthy Adults. NeuroImage. 2015;122:385–398. doi: 10.1016/j.neuroimage.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


