Abstract
Rationale
CF lung disease is defined by large numbers of neutrophils and their potentially damaging products in the airway. Delayed neutrophil apoptosis is described in CF although whether this is a primary neutrophil defect or a response to the inflammatory environment is unknown. Increased levels of neutrophil extracellular traps (NETs) have been measured in CF and we wished to investigate if this is related to the apoptosis defect in CF neutrophils and could serve as a driver of inflammation.
Objectives
We hypothesized that the delay in apoptosis in CF is a primary defect and preferentially allows CF neutrophils to form NETs, contributing to inflammation.
Methods
Blood neutrophils were isolated from CF patients, CF pigs and appropriate controls. Neutrophils were also obtained from CF patients before and after commencing ivacaftor. Apoptosis was assessed by morphology and flow cytometry following ex-vivo culture. NET formation was determined by fluorescent microscopy and kinetic DNA release assays. NET interaction with macrophages was examined by measuring by cytokine generation by ELISA and qRT-PCR.
Measurements and Main Results
CF neutrophils displayed increased neutrophil survival, which was reversed by CFTR potentiator therapy and seen as a primary defect in CF pigs. CF neutrophils formed more NETs in culture and this was reversed by the cyclin-dependent kinase (CDK) inhibitor drug AT7519. NETs provided a pro-inflammatory stimulus to macrophages, which was enhanced in CF.
Conclusions
CF neutrophils have a pro-survival phenotype that is related to absence of CFTR function and may allow increased NET production and consequent inflammation.
Descriptor: Cell fate, inflammatory cells, apoptosis
Graphical abstract

Introduction
Cystic fibrosis (CF) is the most common fatal single gene disorder in Caucasian populations with a prevalence of 1 in 2000 live births. CF is a multi-organ disease with characteristic pathology observed in the lungs, pancreas, sweat glands, gut, liver and kidney. The inflammatory response to infection with lower respiratory pathogens in CF is more intense and longer lasting with most patients dying from destructive lung disease(1). The pathophysiology of CF lung disease is poorly understood but factors such impaired bacterial killing, decreased mucus clearance and over-exuberant inflammation have been described(2). Neutrophils are a key inflammatory cell in the CF lung acting as professional phagocytes, but their interaction with other immune cells in the lungs is less well defined.
The ability of neutrophils to undergo spontaneous apoptosis is both protective to the host and also drives resolution after an infectious or inflammatory insult(3). Several studies have demonstrated defects in apoptosis leading to increased neutrophil survival in CF(4–6). In the absence of normal apoptosis other mechanisms of neutrophil disposal may become important, such as is NETosis(7). NETosis is a mechanism by which neutrophils can kill bacteria, distinct from other forms of bacterial disposal, particularly phagosomal killing(8, 9). NET formation occurs when stimulated neutrophils (with IL-8, LPS, phorbol myristate acetate (PMA), or bacteria) undergo oxidative burst and emit a network of DNA, histones and anti-bacterial and pro-inflammatory proteins. Increased levels of NETs have been described in the CF airway(10, 11) although whether this represents increased production or decreased clearance from the CF lung is unclear. A role for NETs in inflammation has been demonstrated by their association with (non-infectious) inflammatory conditions such as arthritis, systemic lupus erythematosus (SLE) and gout(12–14). NETs have also been demonstrated to interact with macrophages and prime inflammation in vascular disease(15). The interaction of neutrophils with macrophages could be pivotal in CF, particularly as the CF macrophage has an over-exaggerated response to pro-inflammatory stimuli(16).
We hypothesized that neutrophil survival is constitutively increased in CF due to decreased apoptosis, allowing more neutrophils to engage in NETosis, with the interaction of NETs and macrophages promoting inflammation.
Methods
Collection of CF Patient Samples
Peripheral blood was collected from stable CF patients attending clinic with a previous diagnosis based on genetic testing and positive sweat test. Patients were considered stable if they had not required intravenous antibiotics in the past two weeks as treatment of an exacerbation. Lung transplant patients were excluded. All patients gave written consent and the study was approved by regional ethics committees (East of Scotland Research Ethics Committee 15/ES/0094, West of Scotland Research Ethics Committee 11/WS/0074). Anonymous matched healthy controls were recruited locally (Lothian Research Ethics Committee, 08/S1103/38).
Isolation of Human Neutrophils and Peripheral Blood Mononuclear Cells
Human peripheral blood was collected into 3.8% sodium citrate. Serum was aspirated following centrifugation of whole blood at 350×g for 20 minutes. Polymorphonuclear (PMN) cells and peripheral blood mononuclear cells (PBMC) were isolated by 6% dextran sedimentation and separated by discontinuous (72.9, 63.0 and 49.5%) Percoll (GE Healthcare, Buckinghamshire, UK), gradient as described(17). Isolated cells were washed twice in cation free Dulbecco's phosphate buffered saline (DPBS-/-) and then resuspended in appropriate culture media. In some experiments (referred to in the results section) neutrophils were isolated using Ficoll-Paque (GE Healthcare, Buckinghamshire, UK), dextran sedimentation and hypotonic lysis of residual erythrocytes as described(18)
Cell Viability and Apoptosis Measurement
Isolated neutrophils (5×106/ml) were cultured in 24 or 96 well plates in IMDM (in some experiments RPMI was substituted) supplemented with 5% autologus serum (in some experiments 10% FCS or no serum was substituted), 1% penicillin and streptomycin and 1% L-glutamine alone or in the presence of AT7519 (Astex Pharmaceuticals, Cambridge, UK), Granulocyte Macrophage-Colony Stimulating Factor (R&D Abingdon, UK) or E. coli Lipopolysacharide (LPS), (Sigma, Dorset, UK). for 24 hours at 37°C, 5% CO2. At given time points neutrophils were resuspended (1:5) in DPBS-/- supplemented with 25 mM calcium chloride and labelled with Annexin V-FLUOS (Sigma) at 1:500 and 1.0 mg/ml propidium iodide (1:300) before analysis on a BD FACS Scan, FACS Calibur, or BD Accuri cell analysers as described(19). Cytocentrifuge preparations were stained with Diff-Quick (Gamidor, Didcot, UK), to assess for morphological changes of apoptosis.
Western Blotting
Western blotting was carried out with the following antibodies Mcl-1 (1:1,000; Santa Cruz, Dallas, TX, USA), Bax (1:1,000; Santa Cruz), B-actin (1:50,000; Sigma), and HRP-conjugated secondary antibodies (1:2500; Dako Cambridgeshire, UK). See online supplement for full methods.
Microscopic Detection of Neutrophil Extracellular Traps
Neutrophils were seeded (5×104/well) into 24 well plates in RPMI1640 medium (Gibco, ThermoFisher, UK) with 5% FCS, allowed to adhere for 30 minutes and then stimulated with 10 nM PMA and incubated for 4 hours at 37°c, 5% CO2. In some experiments they were allowed to adhere for 6 hours in the presence of media alone or media with 1 μM AT and/or 2.5 ng/ml GM-CSF prior to stimulation and further incubation for 4 hours. After the incubation time, 0.15 μM SYTOX green (Invitrogen, Thermo Scientific, UK) was added before bright field and fluorescent (470/22 nm LED excitation) images were captured on an EVOS FL cell imaging system. NET formation was quantified as percentage of SYTOX positive NETs per 10× field (NETs % of total cell count on bright field), as described(17, 20). All samples were plated in duplicate and multiple fields were counted per well.
Neutrophil Extracellular Trap DNA Release Kinetic Assay
This was a modification of a published assay (17, 21). Isolated neutrophils were seeded (5×104/well) in RPMI 1640 media supplemented with 5% FCS into a flat-bottom 96 well plate and allowed to adhere for 30 minutes at 37°C, 5% CO2. Neutrophil extracellular traps were induced by addition of 10nM PMA and detected at 30 minute intervals in a Synergy HT Biotek plate reader by addition of 0.15 μM SYTOX green, a cell permeable nucleic acid stain with an excitation/emission spectra of 504/523 nm. In some experiments 1 μM AT7519 and/or 25 ng/ml GM-CSF were added to the culture media for 6 hours prior to PMA stimulation.
Human Macrophage Culture
Isolated PBMC were seeded in Iscove's modified Dulbecco's medium (IMDM) into 6 well plates for 60 minutes (Nunc Upcell™, ThermoFisher). Media was then removed, cells were washed twice in IMDM and remaining adherent monocyte cells were cultured in IMDM supplemented with 10% autologous serum, 1% penicillin and streptomycin and 1% L-glutamine for 5 days at 37°C, 5% CO2. On day 5, cells were washed twice in DPBS-/- before detachment. MDMs were detatched by resting UpCell plates at room temparature for 1hr. MDMs were then seeded into 48 well tissue culture plates at 2.5×105/well into IMDM supplemented with 10% autologous serum, 1% penicillin and streptomycin and 1% L-glutamine for a further 1-2 days at 37°C, 5% CO2.
Human NETs and Macrophage Co-culture
Isolated neutrophils were stimulated with 100nM PMA or DMSO vehicle control in HBSS-/- for 15 minutes in rolling suspension at room temperature (to stop clumping). Neutrophils were then washed three times in HBSS-/- to remove any residual PMAbefore seeding 5×105/well onto MDMs (2:1 neutrophil to MDM ratio) in 500 μl IMDM supplemented with 10% macrophage-donor serum, 1% penicillin and streptomycin and 1% L-glutamine for 24 hours at 37°C, 5% CO2. PMA treated neutrophils were considered NETting neutrophils and non-PMA treated cells as control neutrophils. Supernatants were collected at 24hrs, centrifuged at 300×g for 5 minutes to remove cell debris and frozen at -80°C. RNA was extracted from remaining adherent MDMs using the Direct-Zol™ RNA extraction kit per manufacturer's instructions (Zymo Research, Irvine, CA).
Co-culture Supernatant ELISA
IL-8 and TNF were measured in culture supernatant using commercially available ELISAs (R&D Systems, Abingdon, UK) as per the manufacturer's instructions.
qRT-PCR of Macrophage Gene
qRT-PCR was performed using commercially available TaqMan gene expression assays (Thermo Fisher, Waltham, MA) for IL8, TNF, CXCL9, CCL17 and 18s RNA as per manufacturer's instructions. Data were expressed as fold change from control.
Scanning Electron Microscopy of Bronchial Tissue
Bronchial tissue was obtained from an explanted CF lung (under institutional board approval, University of Iowa) and fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer and processed for electron microscopy. For full methods see online supplement.
Statistical Analysis
All data are expressed as mean +/- standard error of the mean. Flow cytometry data was analysed using FlowJo software (Tree Star, Ashland, Oregon), or BD Accurri platform specific software. Data were analysed with GraphPad Prism (La Jolla, CA), by t test or ANOVA with appropriate post-test as noted in figures.
Results
CF Neutrophils Have Increased Survival Due to Less Apoptosis
First we investigated whether there was a difference in survival between CF and healthy control neutrophils by culturing overnight in the presence of 5% autologous serum. CF neutrophils displayed a pro-survival phenotype when assessed by morphology on cytocentrifuge preparations (1A-C). We confirmed this using flow cytometry by Annexin V and PI staining. Control neutrophils displayed more completed apoptosis at 24 hours than CF neutrophils (1D,E), and at 24 hours more neutrophils were viable in the CF samples suggesting a pro-survival phenotype secondary to a decrease in apoptosis (1F). Pan-caspase inhibition effectively inhibited all cell death in CF samples suggesting that those CF neutrophils dying in culture were doing so by apoptosis, thus confirming that apoptosis is occurring in CF but is delayed compared to healthy controls.
Delayed Neutrophil Apoptosis in CF is Related to a Loss of Functional CFTR
Next we assessed whether the delayed apoptosis in CF was related to decreased CFTR function in neutrophils. We obtained neutrophils (using Ficoll-Paque and red cell lysis) from 12 patients with at least 1 G551D mutation prior to and 2 days after commencing ivacaftor therapy (150 mg twice daily), a CFTR potentiator drug which increases conductance of abnormal CFTR and has a proven clinical effect (22). The samples were obtained contemporaneously as part of a previously published observational study(23, 24), but prepared and analyzed separately of the other work. Neutrophils were cultured in serum free conditions to avoid any confounding hangover effect of drug in serum. Neutrophil survival in ex-vivo culture at 24 hours was significantly decreased after ivacaftor therapy suggesting that CFTR modulation may have effects on neutrophil lifespan (2A-C), this effect was maintained when patients continued treatment for 7 days (data not shown).
Next we definitively investigated whether the increase in neutrophil survival was related to the absence of CFTR in neutrophils by utilizing CFTR null neutrophils from CF piglets. We harvested neutrophils (using Ficoll-Paque and red cell lysis) from 2 week old cftr-/- piglets (CF piglets) and WT controls. Neutrophils were cultured ex-vivo in the presence of 10% Fetal Calf Serum (FCS) to avoid any confounding CF serum effect. CF piglet neutrophils demonstrated prolonged survival in culture suggesting that increased neutrophil survival (due to decreased apoptosis) is a primary defect related to absence of CFTR from neutrophils (2C-D).
Prolonged CF Neutrophil Lifespan is not Dependent on the Inflammatory Environment
Inflammation has been suggested to prolong neutrophil lifespan by inducing changes in the expression of Bcl-2 family members, in particular Mcl-1(25) and Bax(6). Using CF patient samples we demonstrated no difference in the expression of Mcl-1 and Bax between CF neutrophils and healthy controls (3A-C), suggesting that inflammation-induced dysregulation of intrinsic apoptosis regulating proteins was not the cause of the prolonged survival of CF neutrophils. Furthermore, the addition of pro-survival stimuli GM-CSF and LPS (3D) to CF neutrophil culture served to augment the pro-survival phenotype suggesting that environmental factors could further enhance rather than cause the increased neutrophil survival in CF. To confirm that a circulating factor related to the inflammatory environment did not cause the apoptosis delay we cultured health control neutrophils in the presence of CF serum. The addition of 5% pooled CF serum to healthy neutrophil cultures did not increase neutrophil survival (3E). Next we asked whether we could therapeutically target the apoptosis delay in CF neutrophils. Culture of CF neutrophils in the presence of AT5719, a CDK inhibitor known to induce neutrophil apoptosis(26), reversed the prolonged survival phenotype of CF neutrophils (3F).
CF Neutrophils Form More NETs Than Healthy Controls
As CF neutrophils were characterized by reduced apoptosis we then asked if they were more or less susceptible to other forms of cell fate, in particular NET formation. Freshly isolated CF neutrophils formed NETs as efficiently as healthy control neutrophils in response to 10 nm PMA (4A) measured by fluorescent microscopy after 4 hours of culture. This was confirmed by assessing NET formation by DNA release assay (4B). This also demonstrated that DNA release from CF neutrophils continued to increase towards the end of the experimental protocol, whereas control neutrophil DNA release levelled off, suggesting that as CF neutrophils aged they formed more NETs than control neutrophils (4B). We therefore assessed whether aged (viable) CF neutrophils could form more NETs than aged healthy control neutrophils. After 6 hours in culture we stimulated healthy and CF neutrophils with 10 nM PMA and measured NET production by fluorescent microscopy and DNA release. CF neutrophils formed significantly more NETs than similarly aged healthy neutrophils (4C,D). We then asked if adding pro-survival factors to control neutrophils or inducing apoptosis in CF neutrophils would affect NET formation. The addition of the GM-CSF to healthy and CF cultures for 6 hours prior to PMA stimulation increased NET formation in both groups (production being consistently higher in CF), and this was inhibited by co-treatment with AT7519 (5A, B). Cells were equally viable after 6 hours of ageing prior to the stimulation of NET formation as assessed by flow cytometry, suggesting that NET formation was not elevated in CF simply because more live neutrophils were present at the time of activation (Figure E1), but rather that cells that have a delay in apoptosis (but still viable) are more prone to NET formation on stimulation. Furthermore, promoting apoptosis in neutrophils may be a potential therapeutic intervention to stop NET formation.
NETs are Present in the CF Airway and are More Pro-Inflammatory in CF due to their Interaction with Macrophages
We used scanning EM to demonstrate the presence of NETs in the lungs of a patient with CF undergoing lung transplantation (6A) based on morphology and features similar to previous descriptions of NETs on EM (11). Next we developed a technique to enable the co-culture of NETs and monocyte derived macrophages (MDMs) at consistent ratios across experiments by pre-stimulating neutrophils with 10 nM PMA under rotating conditions then adding them to MDMs where they then form NETs demonstrating classic morphology after 4-6 hours (Figure E2) and harvesting supernatants and RNA at 24 hours (6B). The addition of NETting neutrophils to macrophages induced IL-8 and TNF production by healthy volunteer MDMs, with this effect being significantly enhanced in CF MDMs (6 C-D). This was confirmed by qRT-PCR (data not shown), which also demonstrated that CF MDMs had basal expression of the classical macrophage activation marker CXCL9(27) and that NETs induced expression CCL17 a marker of alternative activation in macrophages(28) in both CF and control MDMs, suggesting NETs promote a mixed pattern of activation in CF macrophages with both classical and alterative pathways being engaged(6 E-F).
Discussion
Our data demonstrate that prolonged neutrophil survival due to delayed apoptosis is present in CF and related to the absence of CFTR function in neutrophils, furthermore this delay in apoptosis allows CF neutrophils to form NETs more efficiently than healthy controls. Impairment of neutrophil apoptosis in CF has been reported (4–6), and previous studies have suggested that this is either a response to systemic inflammation(6) or, alternatively, a specific defect in CF neutrophils(4, 5). The reversal of the apoptosis delay in CF by treatment of patients with (the CFTR potentiator) ivacaftor suggests that modulation of CFTR function in neutrophils could have an effect on apoptosis, consistent with other studies that have demonstrated direct effects of CFTR potentiation with ivacaftor on neutrophil function following both in-vitro (29, 30) and in-vivo treatment(30). Furthermore, the observation that apoptosis is impaired in CFTR null neutrophils from CF piglets underlines a fundamental role for CFTR in neutrophil apoptosis. The expression of CFTR in neutrophils was previously contested(31), but CFTR expression a protein level has since been definitively shown in neutrophils(32, 33).
Our data suggest that the prolonged survival of CF neutrophils is not caused by the inflammatory environment in CF. The Bcl-2 family pro- and anti-apoptotic proteins Bax and Mcl-1 have been implicated in inflammation-induced neutrophil survival(6, 25). Mcl-1 excess is observed in neutrophils from patients with sepsis leading to prolonged neutrophil survival (25), we demonstrated no difference in expression between freshly isolated CF and healthy control neutrophils. Inflammation-induced Bax deficiency was suggested as a cause of delayed neutrophil apoptosis in CF and pneumonia (6) but again we demonstrated no difference in expression between CF and non-CF cells. Our data infer that the apoptosis delay in CF is an intrinsic feature of CF neutrophils and not simply related to inflammation. This finding is further underlined by the demonstration that CF serum does not induce prolonged survival in healthy control neutrophils, suggesting that there is no circulating factor influencing neutrophil survival in CF. Interestingly, treatment of CF neutrophils with pro-survival stimuli such as GM-CSF or LPS further enhances survival, which may be particularly relevant once neutrophils have reached the inflammatory environment of the CF lung (where both of these stimuli may be present), which has been shown to induce changes in neutrophil behavior and phenotype(34, 35).
We restored CF neutrophil apoptosis to healthy control levels by using the CDK inhibitor AT7519, a strategy that has been demonstrated previously to decrease inflammation in sterile and infective models of inflammation(26, 36). Apoptosis is a well-described route of disposal for potentially toxic and damaging neutrophils and the phagocytosis of these apoptotic cells drives inflammation resolution(37). Targeting neutrophil apoptosis as an anti-inflammatory strategy in CF is attractive, as it would offer a universal therapy not dependent on a patient's individual CF genotype. Furthermore, engaging CF neutrophils in apoptosis more effectively may avoid more toxic forms of cell disposal such as NETosis.
We demonstrate for the first time that CF neutrophils form more NETs than healthy controls. We studied NETosis as several lines of evidence highlight the importance of NETosis in CF. Excess amounts of DNA occurs in the CF airway and has been regarded as a potential contributor to disease since the 1960s, although the presence of DNA in the form of NETs is a more contemporary finding (10, 11, 38, 39). Moreover, these studies do not definitively address whether CF neutrophils form more NETs than healthy control cells. Our study demonstrates that CF neutrophils form more NETs than controls and this is related to the reduction in CF neutrophil apoptosis. The evidence for this is several fold. Firstly, the major difference in NET formation is observed when neutrophils are aged for 6 hours in culture prior to stimulation by which time control neutrophils, although viable, will have engaged early apoptotic machinery. Secondly, if non-CF neutrophils are cultured for 6 hours in the presence of GM-CSF before stimulation (to delay apoptosis) they form equivalent numbers of NETs to aged CF neutrophils. And finally, culture for 6 hours in the presence of CDK inhibitor drug (to engage apoptosis) reduces the level of NET formation in CF to that of healthy controls. Taken together this evidence suggests that CF neutrophils form more NETs because they are less able to engage in the normal process of apoptosis. Our findings are therefore complementary to other studies that have suggested engagement of several non-apoptotic pathways are required for NET formation (40–42).
We have demonstrated that NETs are a potent pro-inflammatory signal to macrophages. The ability of NETs to kill bacteria has been described since their first description in 2004(8), but the potential inflammatory consequences of the presence of NETs in CF have to date remained unclear. We developed an assay to assess whether NETting neutrophils cultured at a consistent ratio with macrophages could promote the release of pro-inflammatory cytokines, and whether CF macrophages were more susceptible to this stimulus. NETs induced IL-8 and TNF release from MDMs, contrary to a previous publication suggesting that NET clearance is an immunologically silent process(43). However, our data are consistent with the recent finding that NETs can prime macrophages for cytokine release(15). We also demonstrated an enhanced response of CF MDMs to NETs in terms of cytokine production, in line with published data demonstrating that CF macrophages are hyper-responsive to pro-inflammatory stimuli (16), and furthermore, our data also demonstrate that the interaction of NETs with CF MDMs allows the expression of both classical and alternative markers of macrophage activation. In the clinical context we speculate that this could also contribute to non-resolving (frustrated) inflammation in CF.
In summary, we demonstrate that CF neutrophils have a prolonged survival secondary to decreased apoptosis, and that this is a primary defect in CF neutrophils. Increased NET formation by CF neutrophils is a consequence of a failure to engage in apoptosis and can be targeted by CDK inhibitor drugs. NETs are a powerful pro-inflammatory stimulus to macrophages, with CF derived macrophages being hyperresponsive. In conclusion, delayed neutrophil apoptosis enhances NET formation in CF and consequently inflammation, and therefore augmenting neutrophil apoptosis in CF may be a viable therapeutic strategy.
Supplementary Material
Figure 1. CF Neutrophils Have Increased Survival Due to Less Apoptosis.

A. Cultured CF neutrophils are more viable at 24 hours than healthy controls when assessed by morphology (n=6 CF, 5 controls). B. Freshly isolated viable neutrophils in ex-vivo culture demonstrating multilobed nuclei (×100 objective). C. Apoptotic neutrophils following prolonged ex-vivo culture demonstrating characteristic nuclear condensation (×100 objective). D. Flow plot of control neutrophils at 24 hours ex-vivo culture demonstrating small numbers of viable cells (V= annexinV –ve/PI –ve cells) with the majority of cells having entered apoptosis (A = annexinV +ve/PI –ve) or completed apoptosis (CA = annexinV +ve/PI +ve). E. Control neutrophils have higher rate of completed apoptosis (n = 19 CF and 20 controls) than CF. F. More viable neutrophils are present in CF culture at 24 hours (n =19 CF and 20 controls).G. Addition of pan-caspase inhibitor to CF neutrophil culture increases survival by inhibiting residual constitutive apoptosis. For patients details see table E1. Data presented as mean+/-SEM. Analysis with 2 way anova and Bonferoni (A, E, F), unpaired t test (G). **p<0.01 ***p<0.001
Figure 2. Delayed Neutrophil Apoptosis in CF is Related to a Loss of Functional CFTR.

Neutrophils were harvested from 12 patients with at least 1 G551D mutation before and after starting the CFTR potentiator ivacaftor, and cultured ex-vivo for 24 hours. A. and B. demonstrate example flow plots (following 24 hrs ex-vivo culture) from an individual patient before (A) and 2 days after (B) starting ivacaftor, showing that neutrophil survival decreased with treatment. C. Combined data for 12 patients receiving ivacaftor, neutrophil viability decreased significantly with treatment (p<0.05). D. Neutrophils were harvested from 2 week old CF piglets and wild type (WT) controls. Flow cytometry following 24 hours ex-vivo culture demonstrated increased survival in CF (5 CF pigs and 5 WT controls). E. Representative cytocentrifuge preparations of CF and WT neutrophils at 24 hours and ×100 magnification demonstrating increased numbers of apoptotic neutrophils in WTs at 24 hours. Data presented as mean+/-SEM. Analysis with paired t test (C), unpaired t test (D). *p<0.05
Figure 3. Prolonged Neutrophil Lifespan is not Dependent on the Inflammatory Environment in CF.

CF neutrophils have normal baseline apoptotic signaling of A. Mcl-1 (anti-apoptotic) and B. Bax (pro-apoptotic) proteins when freshly isolated, (n=8 CF and 8 healthy controls, patient details in table E2). C. Mcl-1 and Bax densitometry. D. CF neutrophils have a normal response to culture with pro-survival stimuli GM-CSF (20ng/ml) and LPS (10ng/ml) for 24 hrs (n=19 CF and 20 controls, table E1). E. The primary apoptosis defect in CF is not due to a circulating CF serum factor. Healthy control neutrophils were cultured in media containing 5% pooled CF serum and this did not lead to increased neutrophil survival (n= 3 separate healthy donors). F. The survival defect in CF could be corrected by culture with AT7519 (1μM) for 24 hrs to augment neutrophil apoptosis and effectively reduce survival to healthy control levels (n=19 CF and 20 controls, table E1). Data presented as mean+/-SEM. Analysis with unpaired t test (C), 1 way anova with Newman Keulspost test (D,F). *p<0.05, **p<0.01, ***p<0.001
Figure 4. CF Neutrophils Form More NETs Than Healthy Controls.
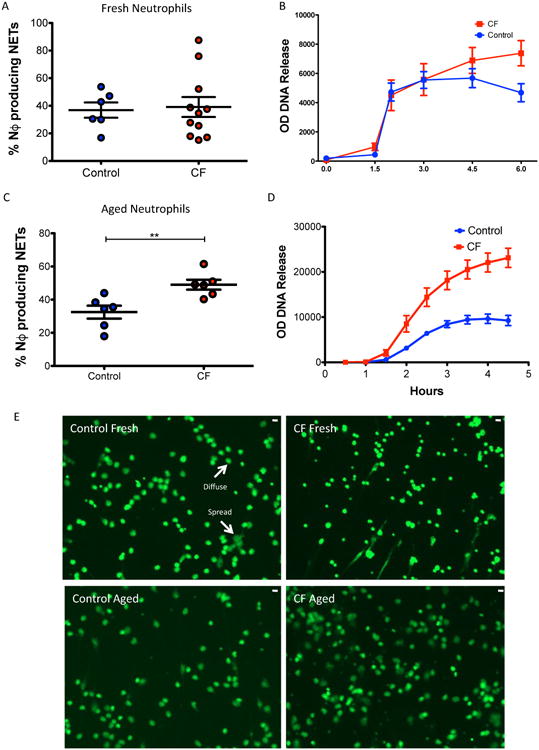
Under conditions of ageing in culture CF neutrophils form more NETs than healthy controls A. Freshly isolated neutrophils form similar amounts of NETs as healthy controls (11 CF vs. 6 controls) following stimulation with 10nM PMA. B. DNA release as surrogate marker of NET production yielded similar results from PMA treated freshly isolated neutrophils (7 CF vs. 6 controls), although we observed a non-statistically significant increased DNA release in the later stages by CF neutrophils. C. Neutrophils aged in culture for 6 hours prior to stimulation with PMA (10nM) demonstrated increased NET production by CF neutrophils vs. Controls (n=6 CF vs. 6 controls). D. Increased NET production was confirmed by DNA release assay (n=6 CF vs. 6 controls). E. Representative fluorescent microscopy following addition of Sytox green in non-fixed cells, demonstrating increased PMA-induced NETs in CF following neutrophil ageing compared to controls (and also non-aged CF). Both diffuse morphology and spread morphology NETs are seen (scale bar = 10 microns) Patient data for these experiments table E3. Data presented as mean+/-SEM. Analysis with unpaired t test (A and C), 2 way anova with Bonferoni (D). **p<0.01, ***p<0.001
Figure 5. Increased NET formation is Associated With The Apoptosis Delay in CF.

A. Healthy control and CF neutrophils were cultured for 6 hours in the presence of GM-CSF(2.5ng/ml) +/- AT7519 (1μM) and then stimulated with PMA (10nM). The addition of GM-CSF to control neutrophils increased NET formation to that of CF levels. This effect was reversed by AT7519 suggesting that inducing the apoptosis process stopped NET formation (n=6 CF and 6 healthy controls). B. Representative fluorescent microscopic images showing sytox positive diffuse NETs in culture, enhanced by GM-CSF and inhibited by AT7519 in a CF patient (scale bar = 10 microns). Data presented as mean+/-SEM. Analysis with 1 way anova with Newman-Keulspost test (A). **p<0.01, ***p<0.001
Figure 6. NETs are Present in the CF Airway and are More Pro-Inflammatory in CF due to Their Interactions with Macrophages.

A. Scanning EM of bronchus from explanted CF patient lung showing characteristic strands of NET-like material associated with neutrophils and bacteria. B. Outline of novel NET/MDM co-culture system. C. NETs are pro-inflammatory to MDMs, causing an increase in IL-8 production after 24 hrs of co-culture and this effect is more pronounced on CF MDMs (n = 8 CF and 7 Healthy MDM donors) D. NETs also induced TNF production from MDMs. E. NETs induce a non-significant increase in CXCL-9 expression in healthy controls, whereas CXCL-9 is over-expressed at baseline in CF. F. NETs also induce CCL17 expression in MDMs. CF MDM donor characteristics are displayed in table E4. Data presented as mean+/-SEM. Analysis by 1 way anova with Newman-Keulspost test (C-F) **p<0.01, ***p<0.001
Acknowledgments
We would like to thank the nursing staff of the Scottish National CF service for help with patient sample collection. We would also like to acknowledge use of the University of Iowa Central Microscopy Research Facility and assistance with the electron microscopy.
Sources of Support: RDG is a Wellcome Trust Fellow (WT093767). DJD is an MRC Senior Research Fellow (G1002046). Also supported by the UK Medical Research Council (MR/K013386/1 [AGR, RD, and CH]) and the Wellcome Trust (WT094415 [CDL] and WT096497 [DAD]). AT7519 was a kind gift from Astex Pharmaceuticals (Cambridge, UK). Specimen collection from the patients receiving ivacaftor was supported by an unrestricted investigator-initiated grant from Vertex Pharmaceuticals (Boston, MA, USA).
Footnotes
This article has an online data supplement, which is accessible from this issue's table of content online at www.atsjournals.org
Author Contributions: RDG, GH, KHR, MS, RHD, AM, JMF, LP, BMM, CDL, DAD performed research. RDG, AGR, DJD, CH, MKBW, PKS, SCD, PBM, DAS, EFM designed the research. RDG, MKBW, AGR, and DJD wrote the manuscript. All authors approved final manuscript.
References
- 1.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, Schidlow DV, Stokes DC, Wohl ME, Kaplowitz H, Wyatt MM, Stryker S. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28:231–41. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–63. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 3.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–145. doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Moriceau S, Kantari C, Mocek J, Davezac N, Gabillet J, Guerrera IC, Brouillard F, Tondelier D, Sermet-Gaudelus I, Danel C, Lenoir G, Daniel S, Edelman A, Witko-Sarsat V. Coronin-1 Is Associated with Neutrophil Survival and Is Cleaved during Apoptosis: Potential Implication in Neutrophils from Cystic Fibrosis Patients. J Immunol. 2009;182:7254–7263. doi: 10.4049/jimmunol.0803312. [DOI] [PubMed] [Google Scholar]
- 5.Moriceau S, Lenoir G, Witko-Sarsat V. In Cystic Fibrosis Homozygotes and Heterozygotes, Neutrophil Apoptosis Is Delayed and Modulated by Diamide or Roscovitine: Evidence for an Innate Neutrophil Disturbance. J Innate Immun. 2010;2:260–266. doi: 10.1159/000295791. [DOI] [PubMed] [Google Scholar]
- 6.Dibbert B, Weber M, Nikolaizik WH, Vogt P, Schöni MH, Blaser K, Simon HU. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: A general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci U S A. 1999;96:13330–13335. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer M, Shan Q, D'Ortona S, Maurer R, Mitchell R, Olesen H, Thiel S, Huebner J, Gadjeva M. Cystic Fibrosis Sputum DNA Has NETosis Characteristics and Neutrophil Extracellular Trap Release Is Regulated by Macrophage Migration-Inhibitory Factor. J Innate Immun. 2014;6:765–79. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, Huml M, Stoiber W, Hector A, Griese M, Hannig M, Studnicka M, Vitkov L, Hartl D. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros. 2011;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang PX, Cheng J, Zou S, D'Souza AD, Koff JL, Lu J, Lee PJ, Krause DS, Egan ME, Bruscia EM. Pharmacological modulation of the AKT/microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun. 2015;6:6221. doi: 10.1038/ncomms7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray RD, Lucas CD, Mackellar A, Li F, Hiersemenzel K, Haslett C, Davidson DJ, Rossi AG. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm Lond Engl. 2013;10:12. doi: 10.1186/1476-9255-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol Clifton NJ. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- 19.Lucas CD, Allen KC, Dorward DA, Hoodless LJ, Melrose LA, Marwick JA, Tucker CS, Haslett C, Duffin R, Rossi AG. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J. doi: 10.1096/fj.12-218990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–9. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffery U, Kimura K, Gray R, Lueth P, Bellaire B, LeVine D. Dogs cast NETs too: Canine neutrophil extracellular traps in health and immune-mediated hemolytic anemia. Vet Immunol Immunopathol. 2015;168:262–268. doi: 10.1016/j.vetimm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordoñez C, Elborn JS VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hisert KB, Schoenfelt KQ, Cooke G, Grogan B, Launspach JL, Gallagher CG, Donnelly SC, Welsh MJ, Singh PK, McKone EF, Becker L. Ivacaftor-Induced Proteomic Changes Suggest Monocyte Defects May Contribute to the Pathogenesis of Cystic Fibrosis. Am J Respir Cell Mol Biol. 2016;54:594–597. doi: 10.1165/rcmb.2015-0322LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam RJ, Hisert KB, Dodd JD, Grogan B, Launspach JL, Barnes JK, Gallagher CG, Sieren JP, Gross TJ, Fischer AJ, Cavanaugh JE, Hoffman EA, Singh PK, Welsh MJ, McKone EF, Stoltz DA. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight. 2016;1:e86183. doi: 10.1172/jci.insight.86183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fotouhi-Ardakani N, Kebir DE, Pierre-Charles N, Wang L, Ahern SP, Filep JG, Milot E. Role for myeloid nuclear differentiation antigen in the regulation of neutrophil apoptosis during sepsis. Am J Respir Crit Care Med. 2010;182:341–350. doi: 10.1164/rccm.201001-0075OC. [DOI] [PubMed] [Google Scholar]
- 26.Lucas CD, Dorward DA, Tait MA, Fox S, Marwick JA, Allen KC, Robb CT, Hirani N, Haslett C, Duffin R, Rossi AG. Downregulation of Mcl-1 has anti-inflammatory pro-resolution effects and enhances bacterial clearance from the lung. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci J Virtual Libr. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 28.Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur J Immunol. 2006;36:1882–1891. doi: 10.1002/eji.200635972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pohl K, Hayes E, Keenan J, Henry M, Meleady P, Molloy K, Jundi B, Bergin DA, McCarthy C, McElvaney OJ, White MM, Clynes M, Reeves EP, McElvaney NG. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999–1009. doi: 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratcher PE, Rowe SM, Reeves G, Roberts T, Szul T, Harris WT, Tirouvanziam R, Gaggar A. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2016;15:67–73. doi: 10.1016/j.jcf.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeon DJ, Condliffe AM, Cowburn AS, Cadwallader KC, Farahi N, Bilton D, Chilvers ER. Prolonged survival of neutrophils from patients with ΔF508 CFTR mutations. Thorax. 2008;63:660–661. doi: 10.1136/thx.2008.096834. [DOI] [PubMed] [Google Scholar]
- 32.Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, Nauseef WM, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol. 2008;83:1345–53. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weldon S, McNally P, McAuley DF, Oglesby IK, Wohlford-Lenane CL, Bartlett JA, Scott CJ, McElvaney NG, Greene CM, McCray PB, Taggart CC. miR-31 dysregulation in cystic fibrosis airways contributes to increased pulmonary cathepsin S production. Am J Respir Crit Care Med. 2014;190:165–174. doi: 10.1164/rccm.201311-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, Davies ZA, Herzenberg LA, Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci. 2008;105:4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makam M, Diaz D, Laval J, Gernez Y, Conrad CK, Dunn CE, Davies ZA, Moss RB, Herzenberg LA, Herzenberg LA, Tirouvanziam R. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc Natl Acad Sci. 2009;106:5779–5783. doi: 10.1073/pnas.0813410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–64. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 37.Haslett C, Savill JS, Whyte MK, Stern M, Dransfield I, Meagher LC. Granulocyte apoptosis and the control of inflammation. Philos Trans R Soc Lond B Biol Sci. 1994;345:327–333. doi: 10.1098/rstb.1994.0113. [DOI] [PubMed] [Google Scholar]
- 38.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011;6:e28526. doi: 10.1371/journal.pone.0028526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18:581–8. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itakura A, McCarty OJT. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am J Physiol - Cell Physiol. 2013;305:C348–C354. doi: 10.1152/ajpcell.00108.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol Baltim Md. 2013;1950191:2647–2656. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


