Abstract
Autoimmune retinopathy (AIR) is a rare immune-mediated retinopathy associated with circulating antiretinal antibodies (ARAs). Other prominent features of AIR include visual field deficits and photoreceptor dysfunction in the setting of progressive unexplained vision loss. The role of inflammation is poorly understood in AIR. Since cytokines play a central role in the initiation and development of inflammation, we evaluated the presence of proinflammatory cytokines and chemokines in AIR patient sera. We demonstrate that IL-6 and CXCL9 are both elevated in AIR patient sera. Moreover, the presence and concentration of these 2 molecules appear to correlate with AIR patient disease severity. This cytokine profile, IL-6 and CXCL9, has been described to participate in a variety of autoimmune and inflammatory diseases. Our study provides support for an activated inflammatory process in AIR and identifies possible mechanisms that can drive autoimmunity in this disease.
Keywords: Autoimmune Retinopathy, IL-6, CXCL9, Inflammation, Retina
1. Introduction
Autoimmune retinopathy (AIR) is an inflammatory-mediated retinopathy characterized by otherwise unexplained progressive vision loss, abnormal photoreceptor function and presence of serum antiretinal antibodies. In most cases, the ophthalmic exam, including retinal exam is unremarkable making the diagnosis difficult (Comlekoglu et al., 2013). Autoimmune retinopathies can be classified into paraneoplastic (pAIR; cancer associated retinopathy and melanoma associated retinopathy) and non-paraneoplastic AIR (npAIR) (Grange et al., 2014). For the purposes of this study we will focus on the latter.
Since the first reports more than two decades ago, npAIR remains an ill-defined disorder. Despite an unremarkable exam early in the disease course, patients have abnormal electroretinography and visual fields (Fox et al., 2016). Typically, patients have no chorioretinal lesions; some may have low-grade intraocular inflammation. As the disease progresses retinal atrophy, retinal vascular attenuation and optic disc pallor may ensue along with further vision loss. Interestingly, antiretinal antibodies can also be found in healthy controls, patients with degenerative retinal disorders and patients with systemic autoimmune diseases with no ocular disease (Heckenlively et al., 1999; Shimazaki et al., 2008). Thus, unlike paraneoplastic AIR, the specificity and the role of antiretinal antibodies in npAIR are poorly understood. Clinical response to immunomodulatory agents has also been variable, casting doubt on the inflammatory nature of this disease. Nevertheless, antiretinal antibodies and visual dysfunction are the hallmarks of npAIR and most patients are typically treated with immunomodulatory agents with the hope of slowing down the progression of disease (Forooghian et al., 2008; Davoudi et al., 2017; Adamus, 2017).
In general, a critical component of autoimmune diseases and inflammatory disorders is a dysregulation of the immune response leading to inflammation. Over the years, several key cytokines have been increasingly identified as mediators of inflammation (Hunter and Jones, 2015). Newer technologies, including multiplex cytokine platforms, have made cytokine testing more accessible for clinical samples.
IL-6 is a multifunctional cytokine that displays a broad and diverse range of biologic activities including control of cell growth and cell survival (Hunter and Jones, 2015). In addition, IL-6 has been shown to mediate and regulate the immune system most notably through proliferation and activation of cytotoxic T cells, and growth and differentiation of memory B cells and plasma cells. Furthermore, IL-6 is especially well-known for its ability to trigger inflammation by inducung the acute phase response and in this way IL-6 impacts acute and perhaps chronic inflammation. Although IL-6 is most often considered to be a potent inflammatory cytokine, it has also been demonstrated to have anti-inflammatory properties. IL-6 has a complex signaling system consisting of two distinct signaling pathways and depending on the cell type, a different pathway is initiated. Recently, IL-6 antagonists (anti-IL-6R antibody) have been developed to block IL-6 from binding to its receptor, IL-6R, and ameliorate inflammation. A variety of cells produce IL-6 and several stimuli trigger IL-6 production, such as, other cytokines, (i.e. TNF-α or IL-1β) and TLR ligation (Hunter and Jones, 2015).
Over the years data have been generated that support the association of IL-6 in autoimmune diseases (Hirano, 2010). For example, the IL-6 pathway has been implicated in the pathogenesis of rheumatoid arthritis (RA), psoriasis, autoimmune liver disease and Crohn’s disease (Tanaka and Kishimoto, 2012). Studies have revealed that a number of serum (and intraocular) cytokines and chemokines, such as IL-6, IL-17, IL-23, TNF-α and VEGF have been reported as elevated in ocular inflammatory diseases (Jawad et al., 2013).
More recently, CXCL9 has been identified as a contributor to the inflammatory process. CXCL9, an IFN-triggered chemokine, is a member of the chemokine superfamily of small proteins that participate in immune and inflammatory reactions, specifically, in the induction of T cell and NK cell migration. The IFN molecules and the upregulation of the IFN signature genes are frequently seen as a major factor in autoimmune diseases, such as systemic lupus erythematous (SLE) (Hooks et al., 1979; Niewold, 2016). In addition, CXCL9 has been described to play a role in the pathogenesis of certain autoimmune diseases, including RA, giant cell arteritis and SLE (Simpson et al., 2000; Loos et al., 2006).
In order to evaluate possible inflammatory components in AIR, we screened for the presence of IL-1 β, IL-6, IL-10, GM-CSF, IFN-γ and CXCL9 with a multiplex immunoassay. In this report we demonstrate by EIA that IL-6 is upregulated in AIR patient sera. Moreover, we show that CXCL9, which attracts T cells to specific target organs, is also elevated in this disease. The presence and concentration of this cytokine signature appear to correlate with AIR patient disease severity. This data supports the concept that these two bio-regulatory molecules may contribute to the pathologic processes observed in AIR patients.
2. Materials and methods
2.1. Patient population
AIR patients who were seen at the uveitis clinic at the National Eye Institute were included and all patients provided written informed consent to participate in research studies. Patients were seen under clinical research protocols, that were approved by the Institutional Review Board at the National Institutes of Health (NIH), adhered to the tenets of the Declaration of Helsinki, and complied with the Health Insurance Portability and Accountability Act (HIPAA) and EX1996NT.
All patients met the criteria for AIR as determined by the consensus of several uveitis experts, including a negative malignancy workup and confirmed positive antiretinal antibodies. AIR was defined as the absence of fundus abnormalities with abnormal electroretinogram (ERG) and Goldman peripheral visual fields (GVF) in the presence of circulating antiretinal antibodies (Fox et al., 2016). Our cohort included patients at different clinical stages of disease (early and late stage) and severity. All patients had at least one antiretinal antibody detected on Western blot or had positive immunohistochemical staining with 7 patients testing positive for multiple antibodies on Western blot. Anti-retinal antibody tests were done in different laboratories though the majority of the patients had their testing in one commercial laboratory in the USA (Supplemental Table). Seven patients were not on treatment at the time of first sample collection, whereas 11 patients were on systemic immunosuppressive treatment or corticosteroids. Five of the “currently untreated” patients were treatment naïve, meaning they never received any systemic treatment. Four of the currently treated patients were receiving rituximab infusions during the study period (Supplemental Table).
There is currently no evidence-based scale to assess for disease severity in AIR. Measurable outcomes of the severity of disease involve gradual changes in ERG and GVF, which could be used as surrogates for disease progression and, hence, markers of disease severity. ERG can vary considerably between visits (intersession variability) for the same subject and have been reported to be up to 25–40% even among normal eyes, with possibly more intersession variability in eyes with advanced retinal disease (Fishman et al., 2005; Bach et al., 2013).
Taking into account the intersession variability, for the purposes of this study, we defined AIR disease severity as mild if there were 30–50% loss of function and moderate/severe if there were > 50% loss of photoreceptor function on ERG below the defined lower limits of normalcy (ISCEV). The Supplemental Table shows the raw ERG data for each eye of the enrolled participants at their first visit. This table also shows changes in ERG for the 5 patients that had more than one sample. Four of the 5 participants had stable poor ERG and 1 showed modest decline despite addition of another immunomodulatory agent.
2.2. Serum source and sample preparation
2.2.1. Serum source
A total of 24 frozen serum samples from 18 AIR patients were received at the Johns Hopkins Hospital from the NEI, NIH. The 18 AIR patients had an age range of 35–78 years with a gender distribution of 9 females and 9 males. In addition, blood was obtained from normal individuals at the Johns Hopkins Hospital and these serum samples served as controls in this study. Eighteen normal individuals in an age range of 25–72 years and a gender distribution of 14 females and 4 males were used in the IL-6 assay. Similarly, eighteen normal individuals in an age range of 25–82 years with a gender distribution of 11 females and 7 males were used for the CXCL9 assay.
2.2.2. Serum preparation
Serum was collected after centrifugation at 2000g for 15 min at 4 °C. All samples were then stored in a − 70 °C freezer until analyzed. Cytokines were evaluated using two technologies: multiplex immunoassay and enzyme-linked immunosorbent assay (EIA).
2.3. Multiplex analysis
All 24 AIR samples were analyzed on the multiplex system in a masked fashion. After analysis it was determined that 5 patients had more than one serum sample. Therefore, the serum sample obtained on the first clinic visit was used for further data analysis. Using a 6-plex, Bio-Plex® immunoassay (Bio-Rad Laboratories, Hercules, CA), samples were analyzed for IL-1 β, IL-6, GM-CSF, IFN-γ, CXCL9 and IL-10. This assay uses a bead-based flow cytometric platform designed as a capture sandwich immunoassay format, similar to EIA. Custom kits with labeled beads were purchased from Bio-Rad Laboratories. These antibody-coated magnetic beads were specific for IL-1β, IL-6, GM-CSF, IFN-γ, CXCL9 and IL-10. The median fluorescent intensity (MFI) was determined on a Luminex® 100™ instrument (Luminex Corporation, Austin, TX) using the Bio-Plex Manager™ software version 6.1 (BioRad Laboratories, Hercules, CA). Standard curves were established for the individual cytokine analyzed and the corresponding concentration value (pg/ml) was determined according to manufacturer’s instructions. All samples were run in duplicate and in a masked fashion. The mean value was used as the final concentration for that cytokine. It should be noted that the lowest concentration detected in IFN-γ system was 16 pg/ml.
Multiplex analysis revealed that the mean concentration of CXCL9 in 18 normal individuals was 129.4 pg/ml with a range of 38.4 to 383.9 pg/ml. In order to examine the predictive value of CXCL9 as a marker for AIR case status, we examined samples above versus below the 95th percentile (384 pg/ml) of the CXCL9 distribution among normal individuals. A positive value of CXCL9 was identified as the concentration greater than the 95th percentile (> 384 pg/ml).
2.4. EIA analysis
Serum IL-6 levels were confirmed using EIA (Quantikine IL-6, R&D Systems, Inc. Minneapolis, MN), which is a very sensitivity assay to quantitate low levels of serum IL-6. All serum samples were tested in duplicate and in a masked fashion, according to the manufacturer’s instructions. The plates were read immediately after application of the stop solution. The optical density of each sample was determined using VersaMax™ tunable microplate reader (Molecular Devices, Sunnyvale, CA). Results were calculated from a standard curve and reported accordingly in picograms per milliliter (pg/ml). The mean minimum detectable dose (MMD) for IL-6 was < 0.70 pg/ml and the standard curve range is 3.13 pg/ml to 300 pg/ml. The reference range for serum IL-6 was established in the Cytokine Laboratory at the Johns Hopkins University using serum samples from 18 normal individuals analyzed by the EIA R&D Systems, Inc. IL-6 assay. The mean serum value for IL-6 was 1.03 pg/ml with a range of 0.26 to 2.16 pg/ml. In order to examine the predictive value of IL-6 as a marker for AIR case status, we examined samples above versus below the 95th percentile (2.16 pg/ml) of the IL-6 distribution among normal individuals. A positive value of IL-6 was identified as the concentration greater than the 95th percentile (> 2.16 pg/ml).
2.5. Statistical analyses
The data was analyzed using the Wilcoxon rank sum test (Mann-Whitney U test). We compared the mean concentrations of CXCL9 and IL-6, respectively, among the following: 1) untreated AIR cases and normal individuals, 2) treated AIR cases and normal individuals, and 3) all 18 AIR cases (treated and untreated) and normal individuals. All statistical analyses were completed using Stata version 11 (StataCorp, LLC, College Station, TX). A p value < 0.05 was considered significant.
3. Results
3.1. Initial evaluation of serum cytokines and chemokines in AIR patient sera
Twenty-four serum samples from 18 AIR patients were initially analyzed by a multiplex immunoassay system for the presence of 5 cytokines and 1 chemokine. As is seen in Table 1, all samples tested for IL-1β were below the lower limit of quantitation (LLOQ). GM-CSF and IL-10 were detected in only 3 of 24 samples and IFN-γ was detected in 1 of 24 samples. In contrast, IL-6 was positive in 5 of 24 samples (21%) of patient sera and CXCL9 was positive in 15 of 24 samples (62.5%) of the patient sera tested. Sera from 18 individual AIR patients were further evaluated for possible correlations of treatment versus no treatment and disease severity.
Table 1.
Evaluation of serum cytokines in AIR patients by multiplex analysis.
| AIR patients sera | |||
|---|---|---|---|
|
| |||
| Cytokines | No. positive samplesa | Percent positive | Range (pg/ml) |
| IL-1β | 0/24 | 0 | (< 0.30) |
| IL-6 | 5/24 | 21 | (0.50 to 33) |
| IL-10 | 3/24 | 13 | (3 to 36) |
| GM-CSF | 3/24 | 13 | (0.70 to 20) |
| IFN-γ | 1/24 | 4 | (16 to 38) |
| CXCL9 | 15/24 | 62.5 | (119 to 8089) |
24 serum samples from 18 AIR patients.
3.2. Detection of CXCL9 in AIR patient sera
A comparison of CXCL9 levels detected in the sera from AIR patients and normal individuals is shown in Table 2. The mean concentration of CXCL9 in normal individuals was 129.4 pg/ml with a range of 38.4 to 383.9 pg/ml. All of the samples from the normal individuals had CXCL9 levels below the 95th percentile and were considered negative. Sixty one percent (11/18) of the AIR patient sera were positive for CXCL9 with a mean concentration of 866.9 pg/ml (Table 2).
Table 2.
Detection of CXCL9 and IL-6 in AIR patient sera and normal individuals.
| Serum sample | No. positive samplesa | Percent positive | Mean (pg/ml) | Range (pg/ml) |
|---|---|---|---|---|
| CXCL9b | ||||
| AIR patients | 11/18 | 61 | 866.9 | (119 to 4271) |
| Normal individuals | 0/18 | 0 | 129.4 | (38.4 to 383.9) |
| IL-6c | ||||
| AIR patientsb | 7/18 | 39 | 5.15 | (0.13 to 38.5) |
| Normal individuals | 0/18 | 0 | 1.03 | (0.26 to 2.16) |
Serum samples from 18 individual AIR patients.
CXCL9 level > the 95th percentile of CXCL9 among normal individuals (> 384 pg/ml) were considered positive (multiplex assay).
IL-6 level > the 95th percentile of IL-6 among normal individuals (> 2.16 pg/ml) were considered positive (EIA assay).
Of note, seven of the AIR patients in this study were not on treatment at the time the sera were collected. As seen in Fig. 1A, 5 out of these 7 patients had elevated levels of CXCL9 (mean of 1271 pg/ml). A statistically significant difference was observed when all AIR patient sera were compared to normal individual sera (p < 0.00001). Moreover, a significant difference was observed when normal individual sera were compared to untreated AIR patient sera (p < 0.0009) or compared to treated AIR patient sera (p < 0.0002). There is also a clear distinction when CXCL9 levels are compared in the sera from untreated patients (N = 7) and treated patients (N = 11). The mean concentration of CXCL9 was 1271 pg/ml in untreated patient sera and 610 pg/ml in treated patient sera (Fig. 1A).
Fig. 1.
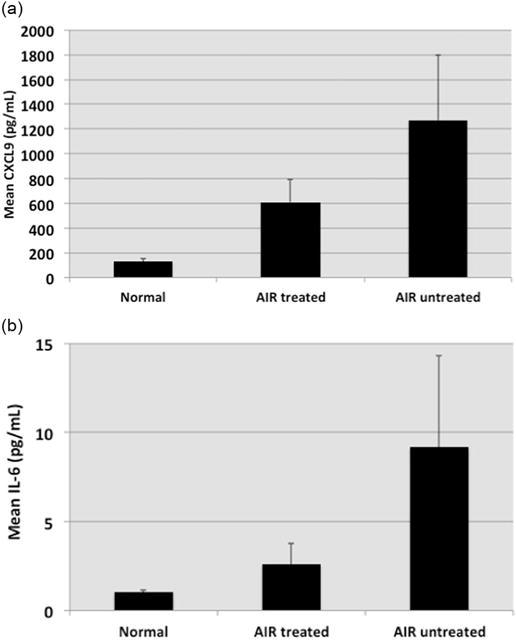
Evaluation of CXCL9 and IL-6 in sera from normal individuals (Control, N = 18), and in treated (N = 11) and untreated (N = 7) AIR Patients. Error bars represent the standard error of the mean. Top-Fig. A: Mean CXCL9 Levels. Statistical Analysis (Wilcoxon rank sum): Untreated Patients vs Normal individuals (p < 0.0009); Treated Patients vs Normal individuals (p < 0.0002); All AIR Patients vs Normal individuals (p < 0.00001). Bottom-Fig. B: Mean IL-6 levels. Statistical Analysis (Wilcoxon rank sum): Untreated Patients vs Normal Individuals (p < 0.0025); Treated Patients vs Normal individuals (p < 0.82); All AIR Patients vs Normal individuals (p < 0.082).
3.3. Quantitation of IL-6 in AIR patient sera
Using IL-6 EIA, additional testing of AIR patient sera was performed. As is seen in Table 2, IL-6 was detected in the sera from 18 normal individuals at levels below the 95th percentile, and therefore, considered negative. However, IL-6 was detected at positive levels in 7 sera from 18 individuals with AIR. Thus, IL-6 was identified in 39% of AIR patient samples (mean value of 5.15 pg/ml) compared to normal individuals (mean value of 1.03 pg/ml).
A comparison of IL-6 levels in treated and untreated patients is seen in Fig. 1B. Four out of 7 untreated patients had elevated levels of IL-6 (mean of 9.18 pg/ml). A significant difference for IL-6 was observed when untreated patient sera were compared to sera from normal individuals (p < 0.0025). There is also a clear distinction when IL-6 levels are compared in the sera from untreated patients (mean of 9.18 pg/ml) and treated patients (mean of 2.58 pg/ml). It is of interest to note that 7 out of these 7 untreated patients (100%) had elevated levels of either IL-6 or CXCL9.
3.4. Comparison of IL-6 and CXCL9 levels with disease severity
The 18 AIR patients were stratified into two levels of disease severity, mild (4 of 18) disease and moderate/severe (14 of 18). Of the four patients with mild disease, one had elevated levels of IL-6 and CXCL9 and another patient had elevated levels of CXCL9. The last two patients with mild disease did not have elevated levels of either cytokine. The one patient with elevated IL-6 and CXCL9 was seen at a very early time point in the disease course and was not on treatment. In the remaining 3 patients’ sera with mild disease, the mean level of IL-6 was 0.6 pg/ml, while the mean level of CXCL9 was 340 pg/ml. Of the fourteen patients, with severe disease, 50% (7 of 14) had elevated levels of IL-6 and 64% (9 of 14) had elevated levels of CXCL9. In total, 11 out of the 14 patients with severe disease had elevated levels of either IL-6 or CXCL9. In these patients’ sera, the mean level of IL-6 was 5.6 pg/ml while the mean level of CXCL9 was 735 pg/ml. These data indicate that when there is an increase in disease severity there is a trend toward elevated IL-6 or CXCL9.
3.5. Evaluation of IL-6 and CXCL9 in serially collected serum samples
Five patients had multiple serially collected serum samples tested. A comparison of IL-6 and CXCL9 in serially collected samples is shown in Table 3. The first two patients had severe disease with high levels of both IL-6 and CXCL9. The third patient also had severe disease that was associated with a high level of IL-6 (1 out of 2 samples) but normal levels of CXCL9. In contrast, the last 2 patients who had mild disease showed normal levels of IL-6 (5 out of 5 samples) and normal levels of CXCL9 (3 of 5 samples).
Table 3.
IL-6 and CXCL9 in serially collected sera samples from AIR patients.
| Patient | Date | IL-6 (pg/ml)a | CXCL9 (pg/ml)b | Disease activity |
|---|---|---|---|---|
| 3a | 1/31/2012 | 3.80 | 2077 | Severe |
| 3b | 1/29/2013 | 4.50 | 1537 | Severe |
| 4a | 1/31/2012 | 13.90 | 1472 | Severe |
| 4b | 4/16/2013 | 19.30 | 8089 | Severe |
| 14a | 11/26/2012 | 1.80 | 300 | Severe |
| 14b | 12/11/2012 | 5.50 | 363 | Severe |
| 11a | 10/11/2012 | 0.24 | 317 | Mild |
| 11b | 11/15/2012 | 0.15 | 247 | Mild |
| 13a | 11/20/2012 | 0.88 | 282 | Mild |
| 13b | 12/11/2012 | 1.20 | 386 | Mild |
| 13c | 1/29/2013 | 1.20 | 703 | Mild |
IL-6 level > the 95th percentile of IL-6 among normal individuals (> 2.16 pg/ml) were considered positive (EIA assay).
CXCL9 level > the 95th percentile of CXCL9 among normal individuals (> 384 pg/ml) were considered positive (multiplex assay).
4. Discussion
The data presented in this study demonstrate increased inflammatory molecules, IL-6 and CXCL9, in the sera of AIR patients. Moreover, an increase in AIR disease severity was found to be associated with an increase in the proportion and concentration of positive samples for both cytokines. In contrast, none of the other cytokines analyzed, IL-1 β, GM-CSF, IFN-γ or IL-10 was significantly increased in the AIR patient cohort.
Although the presence of CXCL9 and IL-6 was not always detected in the same serum sample, patients with severe disease more frequently had elevated CXCL9, IL-6 or both cytokines in their sera. Furthermore, IL-6 and CXCL9 levels were higher when only patients that were not on any systemic immunomodulatory treatment were considered, strengthening the possibility that inflammation may play a role in the pathogenesis of AIR. However, whether a lower cytokine concentration among treated patients corresponds to clinical improvement has yet to be determined. Our findings of elevated IL-6 and CXCL9 underscore the prominent role of these cytokines in inflammatory and autoimmune processes. However, our selection of cytokines herein was small and it is very possible that numerous other cytokines may be elevated or depressed and this cytokine milieu may contribute to the elevation of CXCL9 and IL-6. Additional research is needed and may reveal other cytokines that participate in this degenerative process.
IL-6 stimulates the inflammatory process while chemokines, such as CXCL9, orchestrate immune cell migration into specific tissues (Islam et al., 2016). Increasing evidence for IL-6 and CXCL9 has clearly implicated the involvement of these mediators in the development of autoimmune diseases. For example, the persistent production of IL-6 has been associated with causing the onset and development of autoimmunity (Hirano, 2010; Hunter and Jones, 2015). In these disorders IL-6 acts as a B cell stimulatory factor and activates B cells into antibody producing plasma cells. IL-6 can also mediate T cell development in autoimmunity. In combination with TGF-β, IL-6 induces the differentiation of CD4 T cells into Th17 cells, thereby, making IL-17 available within the autoimmune environment. Alternatively, IL-6 has been reported to inhibit the ability of TGF-β to induce regulatory T cells. It is this Th17/T-regulatory imbalance that is implicated in the onset and progression of autoimmune diseases (Tanaka and Kishimoto, 2012)
The presence of IFN in human autoimmune diseases was first described in SLE patients in 1979, where its detection correlated with disease activity (Hooks et al., 1979). The presence of IFNs in many of these autoimmune diseases is now recognized as an integral component of their pathologic process (Niewold, 2016). IFN-α and IFN-γ are the cytokines that trigger the expression of CXCL9, CXCL10 and CXCL11. A major function of CXCL9 is to attract T cells that contain CXCR3 on their surface to the site of inflammation (Islam et al., 2016). This chemokine plays a critical role in the migration of T cells from the blood vessel into the brain or retina (Lee et al., 2007; Morrell et al., 2011).
Recently, CXCL9 has been identified in patients with autoimmune diseases and in animal models of autoimmunity. In humans CXCL9 has been implicated in the pathogenesis of RA and multiple sclerosis (Loos et al., 2006; Simpson et al., 2000). Furthermore, the CXCL9 signature was enhanced in a murine model of a retinal degenerative disease, experimental coronavirus retinopathy (ECOR). ECOR is a disease triggered by a coronavirus in genetically susceptible animals (BALB/c mice) and is associated with antiretinal autoimmune reactivity (Detrick and Hooks, 2010). The virus also replicates in the retina of genetically resistant mice (CD-1) but these animals do not develop a retinal degeneration nor do the mice develop antiretinal antibodies (Hooks et al., 1993; Detrick and Hooks, 2010). In addition, elevated levels of IFN-γ, CXCL9 and CXCL10 were detected in the sera and enhanced gene expression for CXCL9 and CXCL10 was noted in the retina early in this disease (Detrick et al., 2008). Notably, this cytokine profile was observed one day prior to infiltration of cells into the retina. These studies implicate CXCL9 expression in the development of retinal autoimmunity.
Another example of CXCL9 involvement in autoimmunity was demonstrated by Lang and associates in which they show that CXCL9 production in the liver was required for the development of murine autoimmune liver disease (Lang et al., 2006). In this system liver-specific cytotoxic CD8 T cells were able to migrate, infiltrate and destroy liver cells that expressed CXCL9. Together, these data underscore the important role of CXCL9 in the development of autoimmune pathology.
Since AIR is characterized by retinal pathology a potential source of IL-6 and CXCL9 may be within the retina (Hooks et al., 2008). The retinal pigment epithelial (RPE) cell and the retinal vascular endothelial cell are rich sources of IL-6 and CXCL9 (Lee et al., 2007; Nagineni et al., 2012). The RPE cell possesses 9 TLRs with a high concentration of TLR 3 which can drive cytokine production (Kumar et al., 2004).
In humans, elevated CXCL9 in serum of patients with ocular inflammatory diseases associated with sarcoidosis has also been shown to correlate with disease activity (Takeuchi et al., 2006). Interestingly, elevated serum CXCL9 along with IL-6 and other proinflammatory cytokines have also been reported in a patient with CRB1-associated retinal dystrophy. In this patient cytokine levels were decreased in response to immunomodulatory treatment. These findings suggest that there may be a significant contribution by inflammation in known genetic retinal dystrophies where activated resident retinal cells may be responsible for proinflammatory cytokine production (Verhagen et al., 2016).
Clearly, the presence of IL-6 and CXCL9 in AIR patients is not specific to this disease since these molecules can be identified in systemic inflammatory and autoimmune processes. Moreover, IL-6 and a CXCL9 related chemokine, CXCL10, have been identified in other retinal disorders (Ambati et al., 2013). A notable example is seen in Age Related Macular Degeneration (AMD), in which IL-6 is an independent prognostic marker associated with the disease progression (Seddon et al., 2005). Additional studies identified that CXCL10 is highly expressed in the retina of AMD patients (Mo et al., 2010). Whether or not these cytokines contribute to retinal pathology is still unknown. Nevertheless, the data presented here provides support to the hypothesis that AIR pathogenesis may be associated with an inflammatory processes.
Cytokine-mediated pathways are pivotal to the development of autoimmune diseases. While elevated IL-6 and CXCL9 are not unique to AIR, the data presented here indicate that the inflammatory cytokine signature of elevated IL-6 and CXCL9 may contribute to the retinal pathology observed in AIR patients. Continued studies on these pathways will be useful in the discovery of future strategies for AIR diagnosis and management.
Supplementary Material
Abbreviations
- AIR
Autoimmune retinopathy
- RPE
retinal pigment epithelium
- AMD
Age-Related-Macular Degeneration
- ECOR
experimental coronavirus retinopathy
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jneuroim.2017.12.014.
References
- Adamus G. Impact of autoantibodies against glycolytic enzymes on pathogenicity of autoimmune retinopathy and other autoimmune disorders. Front Immunol. 2017 Apr 28;8:505. doi: 10.3389/fimmu.2017.00505. https://doi.org/10.3389/f.mmu.2017.00505 (eCollection 2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati S, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Ophthalmology. 2013;126:1–7. doi: 10.1007/s10633-012-9353-y. [DOI] [PubMed] [Google Scholar]
- Comlekoglu DU, Thompson IA, Sen HN. Autoimmune retinopathy. Curr Opin Ophthalmol. 2013;24:598–605. doi: 10.1097/ICU.0b013e3283654e1e. [DOI] [PubMed] [Google Scholar]
- Davoudi S, Ebrahimiadib N, Yasa C, Sevgi DD, Roohipour R, Papavasilieou E, Comander J, Sobrin L. Outcomes in autoimmune retinopathy patients treated with rituximab. Am J Ophthalmol. 2017;180:124–132. doi: 10.1016/j.ajo.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Detrick B, Hooks JJ. Immune regulation in retina. Immunol Res. 2010;47:153–161. doi: 10.1007/s12026-009-8146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrick B, Lee MT, Chin MS, Hooper L, Chan CC, Hooks JJ. Experimental coronavirus retinopathy (ECOR): retinal degeneration susceptible mice have an augmented interferon and chemokine (CXCL9, CXCL10) response early after virus infection. J Neuroimmunol. 2008;193:28–37. doi: 10.1016/j.jneuroim.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman GA, Chappelow AV, Anderson RJ, Rotenstreich Y, Derlacki DJ. Short term intervisit variability of erg amplitudes in normal subjects and patients with retinitis pigmentosa. Retina. 2005;25:1014–1021. doi: 10.1097/00006982-200512000-00010. [DOI] [PubMed] [Google Scholar]
- Forooghian F, Macdonald IM, Heckenlively JR, Héon E, Gordon LK, Hooks JJ, Detrick B, Nussenblatt RB. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2008;146:489–495. doi: 10.1016/j.ajo.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AR, Gordon LK, Heckenlively JR, Davis JL, Goldstein DA, Lowder CY, Nussenblatt RB, Butler NJ, Dalal M, Jayasundera T, Smith WM, Lee RW, Adamus G, Chan CC, Hooks JJ, Morgans CW, Detrick B, Sen HN. Consensus on the diagnosis and management of nonparaneoplastic autoimmune retinopathy using a modified Delphi approach. Am J Ophthalmol. 2016;168:183–190. doi: 10.1016/j.ajo.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. Am J Ophthalmol. 2014;157:266–272. doi: 10.1016/j.ajo.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckenlively JR, Jordan BL, Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 1999;127:565–573. doi: 10.1016/s0002-9394(98)00446-2. [DOI] [PubMed] [Google Scholar]
- Hirano T. IL-6 and autoimmune disease. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:717–730. doi: 10.2183/pjab.86.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- Hooks JJ, Percopo C, Wang Y, Detrick B. Retinal and retinal pigment epithelial cell autoantibodies are produced during coronavirus retinopathy. J Immunol. 1993;151:3381–3389. [PubMed] [Google Scholar]
- Hooks J, Nagineni C, Hooper L, Hayashi K, Detrick B. IFN-beta provides immuno-protection in the retina by inhibiting ICAM-1 and CXCL9 in retinal pigment epithelial cells. J Immunol. 2008;180(6):3789–3796. doi: 10.4049/jimmunol.180.6.3789. [DOI] [PubMed] [Google Scholar]
- Hunter AC, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immun. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Islam SA, Medoff BD, Luster AD. Chemokine and chemokine receptor analysis. In: Detrick B, Schmitz J, Hamilton R, editors. Manual of Molecular and Clinical Laboratory Immunology. 8th ed. ASM Press; Washington, DC: 2016. pp. 343–353. [Google Scholar]
- Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin 17A in uveitis patients. J Ocul Immunol Inflammn. 2013;21:434–439. doi: 10.3109/09273948.2013.815786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, Harris NL, Junt T, Odermatt B, Clavien PA, Pircher H, Akira S, Hengartner H, Zinkernagel RM. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MT, Hooper LC, Kump L, Hayashi K, Nussenblatt R, Hooks JJ, Detrick B. Interferon-gamma and adhesion molecules (E-selection and sICAM-1) are detected in the sera from patients with retinal vasculitis and are induced in retinal vascular endothelial cells by TLR-3 signaling. Clin Exp Immunol. 2007;147:71–80. doi: 10.1111/j.1365-2249.2006.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, Fraeyman A, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J, Proost P. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Lab Investig. 2006;86:902–916. doi: 10.1038/labinvest.3700453. [DOI] [PubMed] [Google Scholar]
- Mo FM, Proia AD, Johnson WH, Cyr D, Lashkari K. Interferon-inducible protein 10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4226–4236. doi: 10.1167/iovs.09-3910. [DOI] [PubMed] [Google Scholar]
- Morrell CN, Srivastava K, Swaim A, Lee MT, Chen J, Nagineni C, Hooks JJ, Detrick B. Beta interferon suppresses the development of experimental cerebral malaria. Infect Immun. 2011;79:1750–1758. doi: 10.1128/IAI.00810-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Kommineni VK, William A, Detrick B, Hooks JJ. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol. 2012;227:116–126. doi: 10.1002/jcp.22708. [DOI] [PubMed] [Google Scholar]
- Niewold TB. Connective tissue diseases: targeting type 1 interferon in systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:377–378. doi: 10.1038/nrrheum.2016.83. [DOI] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B, et al. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6 and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Jirawuthiworavong GV, Heckenlively JR, Gordon LK. Frequency of anti-retinal antibodies in normal human serum. Neuroophthalmology. 2008;28:5–11. doi: 10.1097/WNO.0b013e318167549f. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Newcombe J, Cuzner ML, Woodroffe MN. Expression of interferon–gamma inducible chemokine, IP-10 and MIG and their receptor, CXCR3. In: Multiple Sclerosis. Neuropath and Appl Neurobio. 2000;26:133–142. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Oh-I K, Suzuki J, Hattori T, Takeuchi A, Okunuki Y, Usui Y, Usui Elevated serum levels of CXCL9/monokine induced by interferon-gamma and CXCL10/interferon-gamma-inducible protein-10 in ocular sarcoidosis. Invest Ophthalmol Vis Sci. 2006;47:1063–1068. doi: 10.1167/iovs.05-0966. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kishimoto T. Targeting Interleukin-6: all the way to treat autoimmunity and inflammatory diseases. Int J Biol Sci. 2012;8:1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen F, Kuiper J, Nierkens S, Imhof SM, Radstake T, de Boer J. Systemic inflammatory immune signatures in a patient with CRB1 linked retinal dystrophy. Expert Rev Clin Immunol. 2016;12:1359–1362. doi: 10.1080/1744666X.2016.1241709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


