Abstract
There is a large, global unmet need for the development of countermeasures to combat intracellular pathogens. The development of novel antimicrobials is expensive, slow, and typically focuses on selective inhibition of proteins encoded by a single pathogen, thereby providing a narrow spectrum of coverage. The repurposing of approved drugs targeting host functions required for microbial infections represents a promising alternative. This review summarizes progress and challenges in the repurposing of approved drugs as host-targeted broad-spectrum agents for the treatment of intracellular pathogens. These strategies include targeting both cellular factors required for infection by various viruses, obligate intracellular bacteria, and/or protozoa as well as factors that modulate the host immune response to these microbial infections. The repurposed approach offers complementary means to develop therapeutics against existing and emerging intracellular microbial threats.
Keywords: Antimicrobial drugs, Antiviral drugs, Repurposing, Broad-spectrum antivirals, Host-targeted drugs, Kinase inhibitors
There is an urgent need for new strategies to combat hundreds of human disease-causing intracellular pathogens, including viruses, obligate intracellular bacteria, and protozoa. Currently approved antiviral drugs treat fewer than ten viral infections. While antibacterial agents are more widely available, only a minority of them penetrate mammalian cells and achieve a therapeutic level in the appropriate intracellular compartment to treat intracellular bacterial infections1. The narrow spectrum of coverage of most antimicrobials that target microbial functions, particularly viruses, is another major limitation. Given the high average cost (over two billion dollars) and long timeline (8–12 years) to develop a new drug, the scalability of targeting microbes individually is limited. Lastly, resistance typically emerges rapidly when conventional drugs that target microbial functions are used as monotherapy.
An attractive solution to overcome these limitations is to repurpose already approved drugs that target host functions required for the lifecycle of intracellular pathogens as antimicrobial agents. Off-label use of approved drugs requires significantly less capital and time and diminishes the clinical risks, because such drugs were already rigorously tested (toxicity, pharmacokinetics, dosing, etc.) for their primary indication. Since some host functions are hijacked by various viruses, intracellular bacteria, and/or protozoa, they may represent targets for broad-spectrum antimicrobials. This approach can therefore increase the efficiency of antimicrobial development, improve drug access for patients, and facilitate readiness for future outbreaks of emerging pathogens. Additionally, host-targeted approaches may have the added benefit of a higher genetic barrier to the emergence of resistance.
One promising drug category for repurposing as antimicrobials is inhibitors of host kinases, such as those widely approved for the treatment of cancer and inflammatory conditions2. A large number of host kinases are co-opted by intracellular pathogens and thus represent attractive targets for broad-spectrum antimicrobial therapy3. Indeed, recent efforts have demonstrated the therapeutic potential of kinase inhibitors to combat various intracellular microbial infections. The anticancer epidermal growth factor receptor (EGFR) and/or ERBB2 kinase inhibitors gefitinib, erlotinib, and lapatinib demonstrate in vitro activity against a number of viruses including hepatitis C virus (HCV), human cytomegalovirus (HCMV), and poxvirus4, 5. Gefitinib and lapatinib also inhibit HCV and HCMV infections in a mouse and guinea pig model, respectively4, 5, and reduce replication of Mycobacterium Tuberculosis (Mtb) in the lungs of infected mice6. The antiviral effect of these drugs is mediated in part by inhibition of signaling in the entry stage and/or in F-actin-mediated intracellular viral trafficking 7, 8. Enhanced antimicrobial immune effect via inhibition of EGFR/p38 mitogen associated protein kinase signaling plays a role in their anti-Mtb activity6.
Another class of approved kinase inhibitors demonstrating a broad antimicrobial coverage is the cellular Abelson tyrosine kinase (c-Abl) inhibitors, such as imatinib and nilotinib. These drugs inhibit replication of Ebola virus (EBOV), dengue virus (DENV), Middle Eastern respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) in cultured cells and reduce vaccinia virus load, spread, and overall mortality in a mouse model9–12. Moreover, imatinib treatment impairs entry and intracellular survival of Mtb and Mycobacterium marinum in infected macrophages and reduces the bacterial load and associated pathologies in mice infected with antibiotic-sensitive or resistant Mtb13. Notably, when co-administered with rifamycins, imatinib displays synergy, indicating its potential utility in combination anti-Mtb drug regimens13. The molecular targets underlying the antimicrobial effect of c-Abl inhibitors remain to be validated. Moreover, while inhibition of actin motility is proposed as a mechanism of antiviral action of these drugs12, the precise mechanism and mode of antibacterial action remain unknown.
The approved anticancer drugs sunitinib and erlotinib display potent activity against viruses from six viral families, including DENV and EBOV in cultured cells14. Combinations of these drugs reduce morbidity and mortality of dengue and Ebola infected mice and are advancing into clinical studies for dengue and possibly Ebola in future outbreaks (ClinicalTrials.gov NCT02380625)14. Inhibition of the cellular kinases adaptor protein 2 (AP2)-associated protein kinase 1 (AAK1) and cyclin G-associated kinase (GAK), regulators of adaptor protein-mediated intracellular viral trafficking, is an important mechanism of action (MOA) of this antiviral approach14.
Three approved immunosuppressive drugs that inhibit the phosphatidylinositol 3′-kinase–Akt–mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, leflunomide, everolimus, and sirolimus, reduce viremia and disease progression in patients with BK virus nephropathy, when used individually or in combination15. Additionally, leflunomide demonstrates activity against HCMV both in vitro and in a rat model16. While direct suppression of viral replication is proposed as one underlying mechanism of this approach, the precise MOA and molecular targets remain unknown.
Inhibitors of cyclophilin, a host factor that facilitates protein folding, represent another class of drugs, beyond kinase inhibitors, that shows promise as antimicrobials. Cyclosporine, an immunosuppressant cyclophilin inhibitor, disrupts replication of multiple viruses, including HCV, DENV, other flaviviruses, and human immunodeficiency virus (HIV) in vitro17, 18. Two experimental, non-immunosuppressive cyclophilin inhibitors, alisporivir and SCY-635, also inhibit multiple RNA viruses in cultured cells, yet their effect is mouse models is variable19. Alisporivir significantly reduced HCV viral load in chronically infected patients19, however a clinical hold was placed on the Phase III trial due to toxicity. Cyclosporine also inhibits the erythrocytic stages of Plasmodium falciparum in vitro and rodent malaria in a mouse model20. Nevertheless, it remains unknown whether this effect results in part from inhibition of the host cyclophilin or solely the parasitic protein.
Another candidate for repurposing is nitazoxanide, a drug approved for the treatment of parasite-induced diarrhea. It inhibits replication of multiple viruses including HCV, flaviviruses, respiratory viruses, HIV, and hepatitis B virus (HBV) in cultured cells21. Nitazoxanide blocks maturation of the influenza hemagglutinin, but the target remains unknown. In the case of HCV, it is thought to activate protein kinase R leading to phosphorylation of eukaryotic initiation factor 2α, which then blocks viral replication21. In a phase II trial, nitazoxanide modestly reduced the time to resolution of symptoms of flu and is currently being evaluated in a phase III trial for this indication22. Whereas addition of nitazoxanide to peginterferon-ribavirin improved the sustained virologic response in HCV patients in a phase II trial, no such improvement was observed in a phase III trial in genotype 4 infected patients23. Additionally, nitazoxanide is effective against clinical Mtb isolates, including those that are resistant to standard anti-tuberculosis drugs24. Disruption of membrane potential and pH homeostasis in Mtb is one proposed MOA24. A phase II clinical trial is currently ongoing to determine the efficacy of 14-day nitazoxanide treatment prior to initiation of standard anti-tuberculosis therapy.
Metformin, a drug approved for the treatment of type 2 diabetes, inhibits the intracellular growth of Mtb (including drug resistant strains) and enhances the efficacy of approved anti-Mtb drugs in cell culture25. In a murine model, metformin mildly reduces Mtb load in target organs and improves lung pathology when used individually or in combination drug treatment25. Notably, metformin demonstrated efficacy in decreasing disease severity and improving clinical outcome as an adjunct therapy in retrospective cohorts of tuberculosis patients with diabetes and also reduced the incidence of latent tuberculosis in diabetic patients25. Activation of the adenosine monophosphate–activated protein kinase (AMPK), which regulates cellular immune functions, is involved in mediating the antibacterial effect25. Beyond tuberculosis, a clinical study that aims to evaluate the size of HIV reservoirs upon addition of metformin to antiretroviral regimens is currently ongoing.
Statins, drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR), represent another potential class for repurposing as host-targeted antimicrobials. Various statins inhibit replication of HCV and DENV in vitro26, 27. Nevertheless, while several retrospective studies demonstrated that statins reduce relapse rates in patients infected with HCV when added to peginterferon-ribavirin, this effect was quite variable27. The mechanism of anti-HCV action of statins is mediated in part by their effect on lipid biosynthesis, as their antiviral activity in cells is reversed upon addition of mevalonate or geranylgeraniol, and resistance to these drugs coincides with an increase in HMG-CoAR level27. Due to their ability to restore endothelial stability, statins were also proposed as a strategy to treat infections associated with endothelial dysfunction. While no formal study has been conducted, it was reported that atorvastatin in combination with an angiotensin receptor blocker reduced mortality in 100 Ebola patients in Sierra Leone28.
The antimalarial and anti-inflammatory drug chloroquine also shows some promise as an antimicrobial agent. In a non-human primate dengue model, chloroquine reduces viremia, cytokine production, and organ damage29. However, when studied in dengue patients, it reduced pain and improved daily activity performance, yet it did not reduce disease duration or viremia30, 31. In mice infected with Zika virus, chloroquine increases the life span and reduces vertical transmission and fetal brain infection32. Moreover, it reduces acute lung injury, morbidity, and mortality in a mouse model of avian influenza A H5N133. The mechanism of antiviral activity of chloroquine is multifaceted and thought to involve alterations in the intracellular pH causing impaired fusion and cleavage of the prM protein during flaviviral release and/or inhibition of autophagy32, 33. Chloroquine may also show utility as an add-on to anti-tuberculosis regimens, as it enhances the intracellular killing of Mtb in human macrophages by inhibiting the BCRP-1 (breast cancer resistance protein-1)-mediated active efflux of the anti-tuberculosis drugs isoniazid and pyrazinamide34.
Collectively, these data provide a proof-of-concept for the useful expansion of potential antimicrobial drug targets by focusing on host functions and for the therapeutic potential of repurposed drugs targeting these functions to combat intracellular pathogens. Further benefit is provided by the higher genetic barrier to resistance associated with many of these host-targeted approaches. Whereas emergence of resistance can complicate treatment with host-targeted approaches, as in the case of the chemokine receptor 5 antagonist maraviroc35, the time to resistance with other host-targeted approaches is longer and the level of resistance lower relative to typical direct-acting antimicrobials monotherapy. This is exemplified by treatment of DENV with sunitinib/erlotinib combinations, Mtb with nitazoxanide, and HCV with cyclophilin inhibitors14, 19, 24. Targeting host proteins that are not under the genetic control of microbes and simultaneously inhibiting several host targets with the same drug or drug combination are thought to facilitate this increase in the resistance barrier of these approaches.
Nevertheless, repurposing of host-targeted drugs as antimicrobials faces its own challenges. Cellular proteins function in a complex network of interactions and their inhibitors are often non-selective. Thus, the mechanism of antimicrobial action of host-targeted approaches often remains elusive and the molecular targets underlying this effect unvalidated. For example, erlotinib’s effect on HCV infection was first attributed solely to its effect on EGFR, its cancer target, yet it was later demonstrated that inhibition of GAK, another target that is inhibited by erlotinib with a comparable potency to EGFR, also plays a role8, 14. For other drugs, such as nitazoxanide and chloroquine, the MOA is even less clear and appears to be pathogen specific21, 24, 32–34.
Another challenge is that the antimicrobial effect observed in vitro often cannot be reproduced in vivo. For example, several multi kinase inhibitors, such as lestaurtinib, display in vitro activity against Plasmodium falciparum, but no significant effect in malaria-infected mice36. Moreover, as exemplified by chloroquine for dengue treatment, a promising effect in animal models cannot guarantee effect in patients29–31. Toxicity is another major concern. The Src- and c-Abl-inhibitor dasatinib demonstrates broad-spectrum antiviral activity in cultured cells, yet in a murine model of vaccinia virus it induces immunosuppression rather than protection12, 37. It is thus important to investigate the antimicrobial activity and safety of these drugs in animal models of other infections. Nevertheless, for some drugs it may be feasible to find a therapeutic window where the drug level is sufficient to inhibit microbial replication with minimal cellular toxicity. Shifting from indications requiring long-term therapy (e.g., months to years for cancer) to a shorter duration sufficient to treat most acute infections should help limit toxicity. In contrast, the utility of some of these approaches for treating chronic infections requiring longer duration treatment may be more limited. This is exemplified by the unexpected occurrence of pancreatitis during the Phase III trial with alisporivir in chronically infected HCV patients. Nevertheless, drugs that are extensively used in humans, such as metformin and chloroquine, are more likely to be safe add-ons to standard regimens even for chronic infections.
In summary, these examples provide a proof-of-concept for the potential feasibility of repurposing approved drugs as host-targeted broad-spectrum therapies to combat intracellular microbial pathogens. To effectively meet the clinical needs posed by emerging intracellular pathogens, such approaches may find utility in combination with novel host-targeted approaches and microbial-targeted strategies.
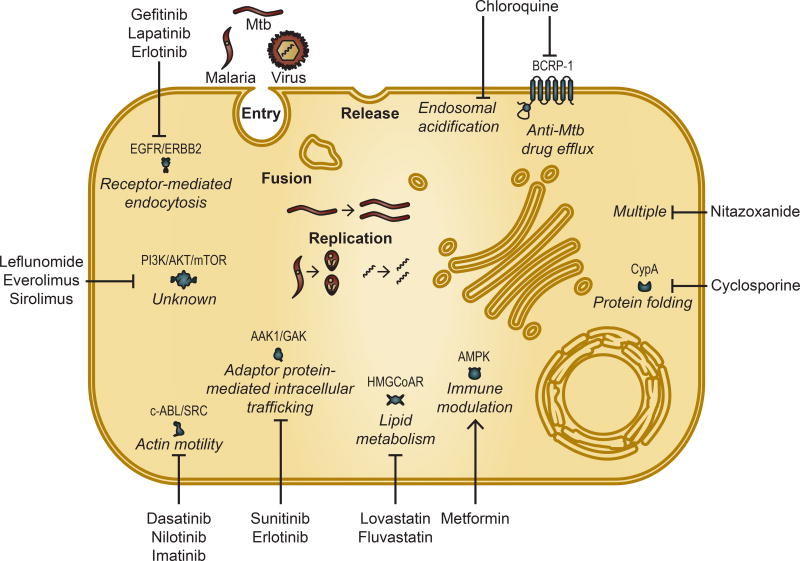
Figure 1.
Approved host-targeted drugs can be repurposed as countermeasures against intracellular pathogens including viruses, obligate intracellular bacteria such as Mtb, and protozoa such as malaria. Depicted are representative stages of the intracellular pathogen lifecycle that are often targeted by antimicrobials. Shown are examples of repurposed drugs and the relevant corresponding pathway and target protein(s), if known. Only targets that are validated as either antimicrobial and/or molecular targets underlying the antimicrobial effect are shown. CypA (Cyclophilin A). Blunt arrows represent inhibition, whereas pointed arrow represents activation.
Acknowledgments
This research was supported by grants from the NIH (1U19 AI10966201), DoD/CDMRP (PRMRP, PR151090), American Cancer Society (RSG-14-11 0-01-MPC), Stanford Bio-X and Stanford SPARK program to S.E. The authors acknowledge all the contributions in the field that could not be included in the review.
Footnotes
Disclosure Statement. No competing financial interests exist.
References
- 1.McOrist S. Obligate intracellular bacteria and antibiotic resistance. Trends Microbiol. 2000;8:483–486. doi: 10.1016/s0966-842x(00)01854-0. [DOI] [PubMed] [Google Scholar]
- 2.Gross S, Rahal R, Stransky N, Lengauer C, Hoeflich KP. Targeting cancer with kinase inhibitors. The Journal of clinical investigation. 2015;125:1780–1789. doi: 10.1172/JCI76094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating JA, Striker R. Phosphorylation events during viral infections provide potential therapeutic targets. Rev Med Virol. 2012;22:166–181. doi: 10.1002/rmv.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langhammer S, Koban R, Yue C, Ellerbrok H. Inhibition of poxvirus spreading by the anti-tumor drug Gefitinib (Iressa) Antiviral Res. 2011;89:64–70. doi: 10.1016/j.antiviral.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Schleiss M, Eickhoff J, Auerochs S, Leis M, Abele S, Rechter S, Choi Y, Anderson J, Scott G, Rawlinson W, Michel D, Ensminger S, Klebl B, Stamminger T, Marschall M. Protein kinase inhibitors of the quinazoline class exert anti-cytomegaloviral activity in vitro and in vivo. Antiviral Res. 2008;79:49–61. doi: 10.1016/j.antiviral.2008.01.154. [DOI] [PubMed] [Google Scholar]
- 6.Stanley SA, Barczak AK, Silvis MR, Luo SS, Sogi K, Vokes M, Bray MA, Carpenter AE, Moore CB, Siddiqi N, Rubin EJ, Hung DT. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014;10:e1003946. doi: 10.1371/journal.ppat.1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 8.Lupberger J, Zeisel M, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee C, Turek M, Gorke S, Royer C, Fischer B, Zahid M, Lavillette D, Fresquet J, Cosset F-L, Rothenberg SM, Pietschmann T, Patel A, Pessaux P, Doffol M, Raffelsberger W, Poch O, McKeating J, Brino L, Baumert T. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nature medicine. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia M, Cooper A, Shi W, Bornmann W, Carrion R, Kalman D, Nabel GJ. Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci Transl Med. 2012;4:123ra124. doi: 10.1126/scitranslmed.3003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, Johnson RF, Olinger GG, Jr, Jahrling PB, Laidlaw M, Johansen LM, Lear-Rooney CM, Glass PJ, Hensley LE, Frieman MB. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrobial agents and chemotherapy. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark MJ, Miduturu C, Schmidt AG, Zhu X, Pitts JD, Wang J, Potisopon S, Zhang J, Wojciechowski A, Hann Chu JJ, Gray NS, Yang PL. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein. Cell Chem Biol. 2016;23:443–452. doi: 10.1016/j.chembiol.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves PM, Smith SK, Olson VA, Thorne SH, Bornmann W, Damon IK, Kalman D. Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J Virol. 2011;85:21–31. doi: 10.1128/JVI.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, Salgame P, Shinnick TM, Kalman D. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 2011;10:475–485. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekerman E, Neveu G, Shulla A, Brannan J, Pu S-Y, Wang S, Xiao F, Barouch-Bentov R, Bakken RR, Mateo R, Govero J, Nagamine CM, Diamond MS, De Jonghe S, Herdewijn P, Dye JM, Randall G, Einav S. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. The Journal of clinical investigation. 2017;127 doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JW, Javaid B, Kadambi PV, Gillen D, Harland R, Thistlewaite JR, Garfinkel M, Foster P, Atwood W, Millis JM, Meehan SM, Josephson MA. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352:1157–1158. doi: 10.1056/NEJM200503173521125. [DOI] [PubMed] [Google Scholar]
- 16.Waldman WJ, Knight DA, Blinder L, Shen J, Lurain NS, Miller DM, Sedmak DD, Williams JW, Chong AS. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42:412–418. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- 17.Qing M, Yang F, Zhang B, Zou G, Robida JM, Yuan Z, Tang H, Shi PY. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob Agents Chemother. 2009;53:3226–3235. doi: 10.1128/AAC.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J Virol. 1995;69:2451–2461. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin K, Gallay P. Curing a viral infection by targeting the host: the example of cyclophilin inhibitors. Antiviral Res. 2013;99:68–77. doi: 10.1016/j.antiviral.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy JR, Baqar S, Baker RH, Roberts E, Nickell SP, Cole GA. Stage-selective inhibition of rodent malaria by cyclosporine. Antimicrob Agents Chemother. 1988;32:462–466. doi: 10.1128/aac.32.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossignol JF. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, Bardin M, Rossignol JF Group, U. S. N. I. C. S. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohla MA, El-Said H, El-Fert A, Ehsan N, Ezzat S, Taha H. Impact of nitazoxanide on sustained virologic response in Egyptian patients with chronic hepatitis C genotype 4: a double-blind placebo-controlled trial. Eur J Gastroenterol Hepatol. 2016;28:42–47. doi: 10.1097/MEG.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 24.Shigyo K, Ocheretina O, Merveille YM, Johnson WD, Pape JW, Nathan CF, Fitzgerald DW. Efficacy of nitazoxanide against clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2013;57:2834–2837. doi: 10.1128/AAC.02542-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singhal A, Jie L, Kumar P, Hong GS, Leow MK-S, Paleja B, Tsenova L, Kurepina N, Chen J, Zolezzi F, Kreiswirth B, Poidinger M, Chee C, Kaplan G, Wang YT, De Libero G. Metformin as adjunct antituberculosis therapy. Science Translational Medicine. 2014;6:263ra159–263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell C, Lebreton A, Young Ng C, Lim JY, Liu W, Vasudevan S, Labow M, Gu F, Gaither LA. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Simon TG, Butt AA. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World J Gastroenterol. 2015;21:8293–8303. doi: 10.3748/wjg.v21.i27.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedson DS, Jacobson JR, Rordam OM, Opal SM. Treating the Host Response to Ebola Virus Disease with Generic Statins and Angiotensin Receptor Blockers. MBio. 2015;6:e00716. doi: 10.1128/mBio.00716-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farias KJ, Machado PR, Muniz JA, Imbeloni AA, da Fonseca BA. Antiviral activity of chloroquine against dengue virus type 2 replication in Aotus monkeys. Viral Immunol. 2015;28:161–169. doi: 10.1089/vim.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges MC, Castro LA, da Fonseca BAL. Chloroquine use improves dengue-related symptoms. Memorias do Instituto Oswaldo Cruz. 2013;108:596–599. doi: 10.1590/0074-0276108052013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vianney T, Nguyet NM, Toi PV, Lee SJ, Farrar J, Wills B, Hien TT, Simmons CP. A Randomized Controlled Trial of Chloroquine for the Treatment of Dengue in Vietnamese Adults. Plos Neglect Trop D. 2010;4 doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiryaev SA, Mesci P, Pinto A, Fernandes I, Sheets N, Shresta S, Farhy C, Huang C-T, Strongin AY, Muotri AR, Terskikh AV. Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis. Scientific Reports. 2017;7:15771. doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matt U, Selchow P, Dal Molin M, Strommer S, Sharif O, Schilcher K, Andreoni F, Stenzinger A, Zinkernagel AS, Zeitlinger M, Sander P, Nemeth J. Chloroquine enhances the antimycobacterial activity of isoniazid and pyrazinamide by reversing inflammation-induced macrophage efflux. Int J Antimicrob Agents. 2017;50:55–62. doi: 10.1016/j.ijantimicag.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Haqqani AA, Tilton JC. Entry inhibitors and their use in the treatment of HIV-1 infection. Antiviral Res. 2013;98:158–170. doi: 10.1016/j.antiviral.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Lotharius J, Gamo-Benito FJ, Angulo-Barturen I, Clark J, Connelly M, Ferrer-Bazaga S, Parkinson T, Viswanath P, Bandodkar B, Rautela N, Bharath S, Duffy S, Avery VM, Mohrle JJ, Guy RK, Wells T. Repositioning: the fast track to new anti-malarial medicines? Malar J. 2014;13:143. doi: 10.1186/1475-2875-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Wispelaere M, LaCroix AJ, Yang PL. The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase. J Virol. 2013;87:7367–7381. doi: 10.1128/JVI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]