Abstract
Background
Exposure to pollution from motor vehicles in early life may increase susceptibility to common pediatric infections.
Methods
We estimated associations between residential exposure to primary fine particulate matter (PM2.5), nitrogen oxides (NOx), and carbon monoxide (CO) from traffic during the first year of life and incident pneumonia, bronchiolitis, and otitis media events by age two years in 22,441 children from the Kaiser Air Pollution and Pediatric Asthma Study, a retrospective birth cohort of children born during 2000–2010 and insured by Kaiser Permanente Georgia. Time to first clinical diagnosis of each outcome was defined using medical records. Exposure to traffic pollutants was based on observation-calibrated estimates from A Research LINE-source dispersion model for near surface releases (RLINE) and child residential histories. Associations were modeled using Cox proportional hazards models, with exposure as a continuous linear variable, a natural-log transformed continuous variable, and categorized by quintiles.
Results
During follow-up 2,181 children were diagnosed with pneumonia, 5,533 with bronchiolitis, and 14,373 with otitis media. We observed positive associations between early-life traffic exposures and all three outcomes; confidence intervals were widest for pneumonia as it was the least common outcome. For example, adjusted hazard ratios for a 1-unit increase in NOx on the natural log scale (a 2.7-fold increase) were 1.19 (95% CI 1.12, 1.27) for bronchiolitis, 1.17 (1.12, 1.22) for otitis media, and 1.08 (0.97, 1.20) for pneumonia.
Conclusions
Our results provide evidence for modest, positive associations between exposure to traffic emissions and common pediatric infections during early childhood.
Keywords: pneumonia, bronchiolitis, otitis media, air pollution, traffic
Introduction
Pneumonia, bronchiolitis, and otitis media are common pediatric infections with a large economic burden. Pneumonia is the second leading cause of infant mortality globally and in 2009 was the leading cause of pediatric hospitalization in the U.S. with associated medical costs amounting to almost $1 billion1, 2. In 2002, an estimated 149,000 children under the age of two in the U.S. were hospitalized for bronchiolitis resulting in $543 million in direct medical costs and $1.4 billion in hospital charges3. An estimated 8.7 million children are diagnosed with otitis media annually in the U.S., with an associated cost of $2.88 billion in health care utilization 4.
Due to their size, inhalation rates, incomplete development of the respiratory and immune systems, and time spent outdoors, children are more vulnerable to urban air pollutants than adults5. Recent epidemiologic evidence suggests traffic-related air pollution may be a risk factor for respiratory infections and related comorbidities in infants and children6–12. Secondary pollutants including ozone and particulate matter were found to be significantly associated with increased risk for bronchitis, pneumonia, and otitis media in a case crossover study of Georgia pediatric emergency department visits during 2002–200813. Jedrychowski and colleagues reported a dose-response relationship between recurrent bronchiolitis and pneumonia infections and PM2.5 at the child’s prenatal residence in Krakow, Poland14. In British Columbia, Canada, infants living within 50 meters of a highway had a 6% higher risk of bronchiolitis diagnosis, and infants living within 150 m of a highway had a 14% higher risk of hospitalization for respiratory syncytial virus (RSV) bronchiolitis infection; however, the confidence intervals for both estimates included the null15, 16. Another proximity study observed a statistically significant increased risk of bronchiolitis clinical encounters for infants living in areas of dense traffic, but found chronic PM2.5 exposure alone was not meaningfully associated with infant bronchiolitis17. A meta-analysis of 10 European birth cohorts found elevated and statistically significant associations between NO2 and PM10 with pneumonia in early childhood, but not PM2.518. Reductions in traffic emissions over a 20-year period [1993–2012] were associated with decreases in pediatric bronchitis diagnosis and hospitalizations in Southern California19. Modest, positive associations between NOx, PM2.5, and PM10 with otitis media among children have been reported in several studies18, 20, 21,22,23 but not others 11,24.
In this paper, we report estimated associations between fine particulate matter (PM2.5), carbon monoxide (CO), and nitrogen oxides (NOx) concentrations from traffic during the first year of life and childhood pneumonia, otitis media, and bronchiolitis by age two in a cohort of insured children in Atlanta, Georgia.
Methods
Ambient air quality model
We modeled hourly concentrations of primary PM2.5 (μg/m3), CO (ppm), and NOx (ppb) contributed by mobile sources for 2002–2011 in metropolitan Atlanta at 250 meter resolution using A Research LINE-source dispersion model for near surface releases (RLINE)25. This model is designed to estimate air quality emissions from traffic in the direct vicinity of the roadway by numerically integrating point source emissions while also accounting for local meteorological conditions that affect dispersion patterns. Model inputs included emissions data for roadway segments based on 2010 traffic data from the Atlanta Regional Commission’s Atlanta Roadside Emissions Exposure Study and surface meteorology data for 2002–2010 from AERMET, the meteorological processors of AERMOD26,27. We created annual averages from the hourly estimates, and these averages were used in the epidemiologic analyses. The averages were calibrated using observational data from stationary air pollution monitors to adjust for overestimation of spatial gradients. Estimates of NOx and CO were calibrated directly to observations because an estimated 73% and 88% of these pollutants, respectively, are contributed by mobile sources28. A smaller proportion of PM2.5 is contributed by mobile sources, so primary PM2.5 was calibrated to source apportionment estimates based on monitoring data that were created using a chemical mass balance model with gas constraints29. Annual average pollutant concentrations were created for years 2002–2011. Because the spatial characteristics of the study area (number/location of highways, traffic density, etc.) did not change meaningfully between 2000 and 2002, we assigned the year 2002 estimates to the study years prior to 2002, i.e., 2000 and 200128. RLINE does not include mechanisms forming secondary PM2.5, so only the associations with the primary portion of the PM2.5 are assessed here. Further details about the creation of these air pollution estimates are available28.
KAPPA cohort data
The Kaiser Air Pollution and Pediatric Asthma Study (KAPPA) is a retrospective birth cohort of children insured by Kaiser Permanente Georgia (KPGA) Health Maintenance Organization who were born between 2000 and 2010 in metropolitan Atlanta, Georgia. There were 24,608 children in the KAPPA cohort and 22,441 were included in this analysis. Children were excluded if they were not enrolled in KPGA at day 29 of life (the start of the outcome period of interest) (n=489); were diagnosed with pneumonia, bronchiolitis, or otitis media in the first 28 days of life (n=223); had no residential history information in the first year of life (n=721); or had one or more residence during the exposure period outside the region for which pollutant concentrations were available (n=734).
The three health outcomes examined in this study were childhood pneumonia (ICD-9 codes 480–486), otitis media (ICD-9 codes 382.XX), and acute bronchitis and bronchiolitis (ICD-9 codes 466.XX). Because 80% of the events in the acute bronchitis and bronchiolitis outcome group were bronchiolitis (ICD-9 codes 466.1X), we refer to this outcome henceforth as “bronchiolitis.” For each outcome we followed children from day 29 of life (to exclude neonatal infections) until time of the first diagnosis, censorship (e.g., they ceased to be insured by the HMO), or the child’s second birthday. Ambient concentrations of primary PM2.5, CO, and NOx from traffic were assigned to each child based on residential location. We calculated separate estimates for each outcome using residences between the child’s birth date and date of diagnosis of the outcome of interest, censorship, or their first birthday (whichever came first). When a child moved during the exposure period we calculated a time-weighted average of the estimated concentrations at each location.
Description of covariates
We adjusted for neighborhood socioeconomic status (SES), city region, child race, child sex, maternal asthma, maternal education, maternal prenatal smoking, birth year and maternal age in the analyses. Because the RLINE estimates are for annual averages, there is no seasonality in exposure concentrations, and hence no confounding by season. Neighborhood SES was characterized at the census block group level by demographic clusters created by the Georgia Department of Public Health30. These clusters were created using 2010 U.S. Census data on 25 variables related to age, income, family structure, housing, education attainment and employment. Neighborhood SES was determined for each child based on residence at birth. City region described the location of the child’s residence in Atlanta: inside metropolitan Atlanta (defined as inside the I-285 highway that surrounds the city), less than or equal to 16 kilometers outside I-285, and more than 16 kilometers outside I-285. Categorizations of other covariates were: child race (white, black, other, unknown), maternal asthma status (no, yes, missing), maternal education (less than 12th grade, high school of GED, at least some college, missing), maternal smoking during pregnancy from birth certificate data (no, yes, missing), birth year indicator variables (2000–2010), maternal age dichotomized at the mean, and child sex (male, female).
Statistical modeling
We used Cox proportional hazards (PH) regression to estimate associations between first year of life exposure to primary PM2.5, CO and NOx from traffic emissions and time to first diagnosis of pneumonia, bronchiolitis, or otitis media (up until age two). The PH assumption was evaluated using Kaplan Meier log-log curves, goodness of fit (GOF) tests using Schoenfeld residual p values, and extended Cox models to test each variable’s interaction with survival time. Variables satisfying the PH assumption according to (at least) 2 of these three approaches in univariate models were deemed to satisfy the PH assumption. Variables not satisfying the PH assumption were further evaluated in adjusted PH models. We used stratified Cox models to accommodate variables that did not satisfy the PH assumption. As expected, the pollutant exposure distributions were right skewed, which motivated an examination of modeling exposures as continuous linear variables (scaled to 1 μg/m3 PM2.5, 20 ppb NOx, and 1 ppm CO), as natural log-transformed continuous variables, and by quintiles. Data were analyzed using SAS 9.4 (Cary, NC) allowing for non-independence due to sibling clustering via the robust sandwich estimator implemented by the “covs(aggregate)” statement in PROC PHREG.
Results
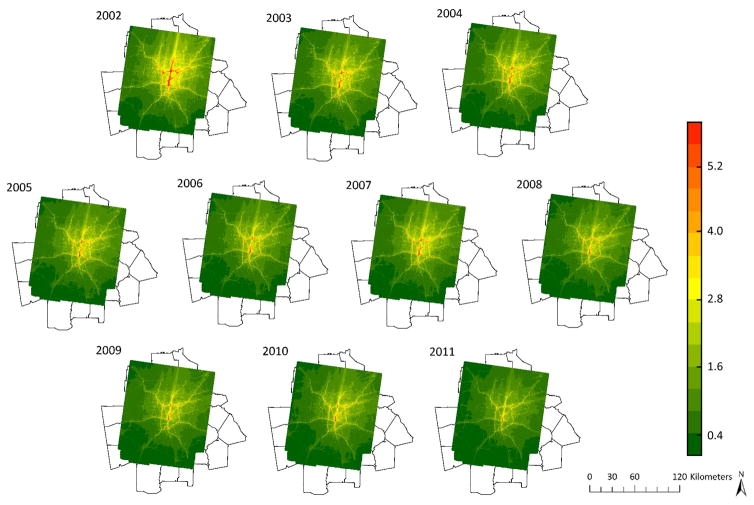
Annual average estimates for primary PM2.5, NOx, and CO from traffic are shown in Figure 1, eFigure 1, and eFigure 2. Concentrations of the three examined air pollutants decreased over the course of the study period. Descriptive statistics on first year of life air pollution exposures from traffic are shown in Table 1. Pollutant concentrations were highly correlated because they were modeled using the same emissions data.
Figure 1.
2002–2011 Primary PM2.5 (μg/m3) concentrations contributed by mobile sources.
Table 1.
First year of life exposure to primary PM2.5, NOx and CO from traffic (N=22,441)
| Pollutant | Minimum | Median (IQR) | Maximum |
|---|---|---|---|
| PM2.5 (μg/m3) | 0.06 | 1.41 (0.93) | 13.76 |
| NOx (ppm) | 0.01 | 0.06 (0.04) | 0.59 |
| CO (ppm) | 0.10 | 0.59 (0.38) | 5.13 |
Exposures were estimated separately for each outcome, but were extremely similar across outcomes. To avoid unnecessary repetition only exposures calculated for pneumonia are displayed.
Cohort characteristics are shown in Table 2. The cohort was racially diverse, with 39.5% of children classified as white, 34.9% as black, and the remaining classified as unknown or other race. The majority of children were born to mothers who attended at least some college and lived in neighborhoods classified as high SES. Out of the three outcomes examined by the second birthday, otitis media was the most common; during follow-up 2,181 children (9.7%) were diagnosed with pneumonia, 5,533 children (24.7%) were diagnosed with bronchiolitis, and 14,374 children (64.1%) were diagnosed with otitis media. Males were more likely than females to be diagnosed with a respiratory or ear infection by age two. Examining differences by race, black children were more likely to be diagnosed with pneumonia, and white children were more likely to be diagnosed with bronchiolitis or otitis media. Seasonal variation in diagnosis was observed, with the highest proportion of diagnoses occurring in winter for all outcomes (38% of pneumonia cases, 44% of bronchiolitis cases, and 34% of otitis media cases).
Table 2.
Descriptive statistics for children born between 2000–2010 and enrolled in Kaiser Permanente in the Atlanta, Georgia metropolitan area (n=22,441)
| Characteristic (n, %) | Pneumonia (%) | Bronchiolitis (%) | Otitis Media (%) |
|---|---|---|---|
| % Diagnosed | 9.7 | 24.7 | 64.1 |
|
| |||
| Child Sex | |||
| Male (11,409, 50.8%) | 10.6 | 27.8 | 66.0 |
| Female (11,032, 49.2%) | 8.9 | 21.4 | 62.0 |
| Neighborhood SES1 | |||
| Highest SES (14,012, 62.4%) | 9.6 | 25.9 | 65.6 |
| Urban/Suburban (2,225, 9.9%) | 10.1 | 20.6 | 57.9 |
| Rural, average to low SES (1,087, 4.8%) | 11.8 | 24.9 | 63.8 |
| Lowest SES (5,114, 22.8%) | 9.4 | 23.0 | 62.6 |
| Child Race | |||
| White (8,857, 39.5%) | 9.6 | 27.4 | 68.2 |
| Black (7,833, 34.9%) | 10.7 | 25.1 | 63.7 |
| Other2 (2,720, 12.1%) | 8.2 | 17.8 | 58.7 |
| Unknown (3,031, 13.5%) | 8.9 | 21.8 | 57.7 |
| Maternal Asthma Status | |||
| No (17,736, 79.0%) | 9.5 | 24.2 | 63.7 |
| Yes (2,450, 10.9%) | 11.8 | 30.8 | 71.2 |
| Missing (2,255, 10.0%) | 9.4 | 21.7 | 58.5 |
| Maternal Education | |||
| At least some college (13,270, 59.1%) | 9.6 | 25.3 | 65.6 |
| Less than 12th grade (280, 1.2%) | 14.3 | 22.9 | 66.8 |
| High School or equivalent (2,564, 11.4%) | 9.6 | 25.8 | 62.3 |
| Missing (6,327, 28.2%) | 9.9 | 22.9 | 61.4 |
| Prenatal Smoking Status | |||
| No (17,636, 78.6%) | 9.8 | 24.9 | 64.8 |
| Yes (450, 2.0%) | 12.0 | 27.8 | 64.2 |
| Missing (4,355, 19.4%) | 9.3 | 23.5 | 61.2 |
| City Region3 | |||
| Metro Atlanta (2,348, 10.5%) | 9.5 | 20.4 | 60.6 |
| ≤16 km from metro Atlanta (9,629, 42.9%) | 9.8 | 23.0 | 62.7 |
| >16 km from metro Atlanta (10,464, 46.6%) | 9.7 | 27.2 | 66.1 |
| Maternal Age | |||
| Less than 28, or missing (6,963, 31.0%) | 10.0 | 23.1 | 60.9 |
| Between 28 and 31 (4,435, 19.8%) | 9.6 | 26.5 | 65.3 |
| Between 31 and 35 (5,922, 26.4%) | 9.3 | 26.2 | 66.5 |
| Greater than 35 (5,121, 22.8%) | 9.9 | 23.4 | 64.4 |
Neighborhood SES classified using demographic clusters created by Georgia Department of Public Health.
Includes Asian, American Indian, Alaska Native, Native Hawaiian or other Pacific Islander, and children identifying with more than one racial group.
Metro Atlanta defined as inside the I-285 perimeter that encircles Atlanta.
Neighborhood SES violated the proportional hazards assumption for both bronchiolitis and otitis media, child race violated the PH assumption for bronchiolitis, and city region violated the PH assumption for otitis media. No variables violated the PH assumption for pneumonia. We therefore implemented stratified Cox models for the bronchiolitis and otitis media analyses.
Although the magnitude of the hazard ratios varied, overall conclusions were consistent from Cox models when exposure was modeled as a continuous linear variable (eTable 1) and as a natural log-transformed continuous variable (Table 3). For a log increase in exposure, unadjusted hazard ratios for all pollutants with pneumonia, bronchiolitis and otitis media ranged from 0.95 to 1.05 with confidence intervals including the null (HR=1.0) in all but one instance (Table 3). The association estimates were elevated after statistical adjustment for covariates. In the adjusted models for bronchiolitis, the hazard ratios ranged from 1.16 (95% CI 1.08, 1.25) for a 2.7-fold increase in CO (a 1-unit increase on the natural log scale), to 1.23 (95% CI 1.15, 1.32) for a 2.7-fold increase in PM2.5. Adjusted hazard ratios were similar for otitis media. The adjusted HRs for pneumonia were also positive, but 95% confidence intervals were wider because pneumonia was the least common outcome. For example, the adjusted HR for pneumonia for a 2.7-fold increase in log-transformed PM2.5 was 1.08 (95% CI 0.97, 1.20).
Table 3.
Hazard ratios per natural log increase in primary PM2.5, NOx and CO from traffic and child outcomes by age two (n=22,441)
| Outcome | Exposure | Unadjusted HR (95% CI) |
Adjusted1,2 HR (95% CI) |
Adjusted,3 Females Only HR (95% CI) |
Adjusted,3 Males Only HR (95% CI) |
|---|---|---|---|---|---|
| Pneumonia | PM2.5 | 0.96 (0.89, 1.04) | 1.08 (0.97, 1.20) | 1.04 (0.89, 1.23) | 1.10 (0.95, 1.26) |
| NOX | 0.95 (0.88, 1.02) | 1.08 (0.97, 1.20) | 1.04 (0.90, 1.21) | 1.10 (0.96, 1.26) | |
| CO | 0.95 (0.87, 1.03) | 1.06 (0.95, 1.18) | 1.03 (0.87, 1.21) | 1.07 (0.93, 1.24) | |
|
| |||||
| Bronchiolitis | PM2.5 | 1.00 (0.95, 1.05) | 1.23 (1.15, 1.32) | 1.13 (1.03, 1.25) | 1.32 (1.20, 1.44) |
| NOX | 1.01 (0.96, 1.06) | 1.19 (1.12, 1.27) | 1.10 (1.01, 1.21) | 1.27 (1.16, 1.38) | |
| CO | 0.97 (0.92, 1.02) | 1.16 (1.08, 1.25) | 1.07 (0.97, 1.18) | 1.24 (1.13, 1.36) | |
|
| |||||
| Otitis media | PM2.5 | 1.03 (1.00, 1.06) | 1.17 (1.11, 1.22) | 1.14 (1.07, 1.21) | 1.19 (1.12, 1.26) |
| NOX | 1.05 (1.02, 1.08) | 1.17 (1.12, 1.22) | 1.14 (1.08, 1.21) | 1.19 (1.13, 1.26) | |
| CO | 1.02 (0.99, 1.06) | 1.15 (1.10, 1.21) | 1.13 (1.06, 1.20) | 1.17 (1.10, 1.25) | |
Adjusted models controlled for child sex, child race, maternal asthma, maternal age, neighborhood SES, city region, maternal education, maternal prenatal smoking, and year of birth.
Stratified Cox models selected based on a priori modeling strategy for bronchiolitis and otitis media outcomes: bronchiolitis models were stratified on neighborhood SES and child race; otitis media models were stratified on neighborhood SES and city region.
Adjusted model, data limited to only females (or only males).
Hazard ratios represent the relative change in hazard rate per 2.7-fold increase in mobile source pollutant concentration.
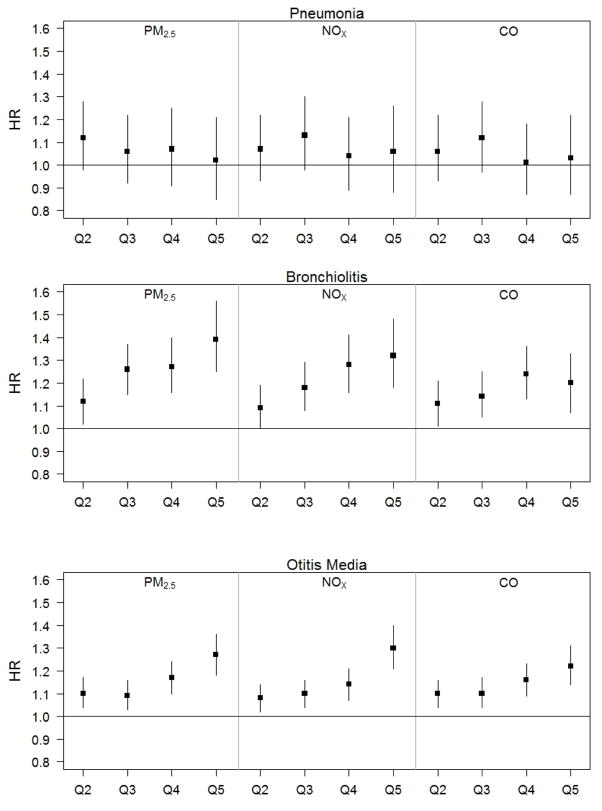
When exposure was modeled using quintiles, we observed a general trend where the association estimates between primary PM2.5, NOx, and CO from traffic and bronchiolitis and otitis media by age 2 tended to increase as the exposure quintiles increased (Figure 2). This pattern was not evident for pneumonia. For bronchiolitis and otitis media, the shape of the exposure-response relationship across quintiles varied slightly, with the HRs increasing more rapidly for bronchiolitis than for otitis media.
Figure 2.
Adjusted hazard ratios and 95% confidence intervals per quintile of primary PM2.5, NOx and CO from traffic and pneumonia, bronchiolitis, and otitis media by age 2 (using quintile 1 as the reference group). Numeric results for this figure are available in eTable 2.
Discussion
These results provide evidence for modest, positive associations between exposure to traffic emissions and bronchiolitis and otitis media diagnoses in the first two years of life. The estimated associations with pneumonia were positive but less elevated, and the confidence intervals were wider. Our findings add to evidence from earlier studies suggesting that early life exposures to traffic may increase susceptibility to childhood infections.
Misclassification of residential location in the KAPPA study is likely a small concern because the Kaiser Permanente Georgia HMO retains information on previous addresses, which enabled us to create time-weighted air pollution metrics. Although there is imprecision in the date of address change, simulations performed using KAPPA data to investigate the consequences of exposure measurement error due to residential mobility suggest that in this cohort this source of error likely causes only a small (2–10%) bias towards the null31. The calibrated RLINE estimates were shown in Zhai et al. (2016) to have good accuracy and precision; the calibration reduced normalized mean bias for all pollutants when compared to raw RLINE estimates (29% to 0.3% for PM2.5, 22% to −1% for CO, and 303% to 43% for NOx). A limitation of this model is that traffic emissions data were only available for 2010. Although we used the network of regulatory monitors to calibrate the pollutant concentrations for the earlier years of the study, our air quality model would not have captured meaningful variability in traffic dynamics or intensity in the early years of our study period (2002–2009). However, we do not expect large changes in the spatial distribution of emissions to have occurred during or prior to this period as there were no major changes in freeways or major highways (calibration captures temporal changes, including impacts from the recession and emission controls). Additionally, due to the high correlation between estimates of PM2.5, NOx, and CO, we were unable to isolate their individual impacts. Our epidemiologic results are indicative of associations between traffic exposure and early life outcomes in this cohort rather than of the specific pollutants.
The outcomes of interest in this study were defined using ICD-9 codes from clinical diagnosis instead of parental self-report, which lessens the potential for recall bias and outcome misclassification. This is a strength of our study; much of the prior literature has relied either on parental self-report 6, 24, 32, 33, 34, 35 or on emergency department visits and hospitalizations11,15, 20, 23, 36, 37 which do not capture the less-severe morbidities that are treated in pediatric care offices. Even with these clinical records, however, it is very likely that some children had one or more of the outcomes but were never seen by a doctor particularly due to the similar symptoms that these conditions can have to cold or flu. As such, the true incidence of these pediatric conditions is likely somewhat higher than what was captured by the medical records.
We used Cox proportional hazards regression to analyze the data, wherein we considered children to be at risk for illness until the date of their illness diagnosis or censorship (which occurred when a child reached age two or when they were no longer insured by Kaiser Permanente Georgia). Due to skewed exposure distributions, we modeled exposure as an un-transformed continuous variable, transformed by the natural log, and by quintile to allow for a potential linear or non-linear relationship between exposure and outcome. Results from all modeling techniques led to similar conclusions.
The KAPPA cohort is not a random sample of children, as membership was limited to children with health insurance through Kaiser Permanente Georgia HMO. Broadly, the KAPPA children tended to be of higher SES than the general population, with 59% of mothers having at least some college education and 62% of children residing in neighborhoods classified as having the highest of the four SES categories (Table 2). We do not know if the estimated associations with traffic pollutants would have been different had our cohort consisted of a more socioeconomically diverse group of children. However, it is probable that certain factors that could plausibly lessen the exposure effects in this cohort, e.g., air conditioning use and good nutrition, were likely more common among KAPPA cohort children. One advantage of a relatively uniformly high socioeconomic cohort is that risk of bias from residual confounding by socioeconomic factors might be lower than if the cohort consisted of a more diverse sample of Atlanta children.
In this large urban birth cohort, we observed positive associations between concentrations of primary PM2.5, NOx, and CO from traffic emissions and childhood bronchiolitis and otitis media diagnoses. Associations with pneumonia were also positive, although the effect estimates were of relatively smaller magnitude, and the confidence intervals were wider. Our study, which integrates calibrated RLINE outputs with a rich dataset of pediatric clinical encounters from Kaiser Permanente Georgia, provides further evidence regarding the associations between traffic pollution and pediatric respiratory disease.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by grant R834799 from the U.S. Environmental Protection Agency, grants R03HD084884-01 and T32HD052460 from the National Institutes of Health, and grant 5T03OH008609 from the U.S. Centers for Disease Control and Prevention. This publication’s contents are solely the responsibility of the grantee and do not necessarily represent the official view of the US EPA. Further US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
Description of process for obtaining data and code: Kaiser Permanente data are not available for redistribution. Parties interested in pursuing a data use agreement should contact the Center for Health Research Southeast Division of the Kaiser Foundation Health Plan, Inc.
References
- 1.Zhang S, Sammon P, King I, Andrade A, Toscano C, Araujo S, et al. Cost of management of severe pneumonia in young children: Systematic analysis. Journal of Global Health. 2016;6:1–15. doi: 10.7189/jogh.06.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Williams D, Arnold S, Ampofo K, Bramley A, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. The New England Journal of Medicine. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelletier A, Mansbach J, Camargo C. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418–2423. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, Shapiro N, Bhattacharyya N. Incremental health care utilization and costs for acute otitis media in children. The Laryngoscope. 2014;124:301–305. doi: 10.1002/lary.24190. [DOI] [PubMed] [Google Scholar]
- 5.Leith Sly J, Carpenter D. Special vulnerability of children to environmental exposures. Reviews on environmental health. 2012;27:151–157. doi: 10.1515/reveh-2012-0024. [DOI] [PubMed] [Google Scholar]
- 6.Esposito S, Galeone C, Lelii M, Longhi B, Ascolese B, Senatore L, et al. Impact of air pollution on respiratory diseases in children with recurrent wheezing or asthma. BMC Pulmonary Medicine. 2014;14:130–138. doi: 10.1186/1471-2466-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuertes E, MacIntyre E, Agius R, Beelen R, Brunekreef B, Bucci S, et al. Associations between particulate matter elements and early-life pneumonia in seven birth cohorts: Results from the ESCAPE and TRANSPHORM projects. International Journal of Hygiene and Environmental Health. 2014;217:819–829. doi: 10.1016/j.ijheh.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Lu C, Deng Q, Yu C, Sundell J, Ou C. Effects of ambient air pollution on the prevalence of pneumonia in children: Implication for national ambient air quality standards in China. Indoor Built Environment. 2014;23:259–269. [Google Scholar]
- 9.Rice M, Rifas-Shiman S, Oken E, Gillman M, Ljungman P, Litonjua A, et al. Exposure to traffic and early life respiratory infection: A cohort study. Pediatric Pulmonology. 2015;50:252–259. doi: 10.1002/ppul.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan P, Bernstein D, Lockey J, Reponen T, Levin L, Grinshpun S, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. American Journal of Respiratory and Critical Care Medicine. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strickland M, Hao H, Hu X, Chang H, Darrow L, Liu Y. Pediatric emergency visits and short-term changes in PM2.5 concentrations in the U.S. state of Georgia. Environmental Health Perspectives. 2016;124:690–696. doi: 10.1289/ehp.1509856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira S, Stein R, Ferraro A, Pastro L, Pedro S, Lemos M, et al. Urban air pollutants are significant risk factors for asthma and pneumonia in children: The influence of location on the measurement of pollutants. Archivos de Bronconeumologia (English Edition) 2012;48:389–395. doi: 10.1016/j.arbres.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Q, Liu Y, Mulholland J, Russell A, Darrow L, Tolbert P, et al. Pediatric emergency department visits and ambient air pollution in the U.S. State of Georgia: A case- crossover study. Environmental Health. 2016;15:115–122. doi: 10.1186/s12940-016-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jedrychowski W, Perera F, Spengler J, Mroz E, Stigter L, Flak E, et al. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. International Journal of Hygiene and Environmental Health. 2013;216:395–401. doi: 10.1016/j.ijheh.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karr C, Rudra C, Miller K, Gould T, Larson T, Sathyanarayana S, et al. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environmental Research. 2009;109:321–327. doi: 10.1016/j.envres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karr C, Demers P, Koehoorn M, Lencar C, Tamburic L, Brauer M. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. American Journal of Respiratory and Critical Care Medicine. 2009;180:995–1001. doi: 10.1164/rccm.200901-0117OC. [DOI] [PubMed] [Google Scholar]
- 17.Girguis M, Strickland M, Hu X, Liu Y, Chang H, Belanoff C, et al. Chronic PM2. 5 exposure and risk of infant bronchiolitis and otitis media clinical encounters. International Journal of Hygiene and Environmental Health. 2017;220:1055–1063. doi: 10.1016/j.ijheh.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIntyre E, Gehring U, Mölter A, Fuertes E, Klümper C, Krämer U, et al. Air pollution and respiratory infections during early childhood: An analysis of 10 European birth cohorts within the ESCAPE Project. Environmental Health Perspectives. 2014;122:107–113. doi: 10.1289/ehp.1306755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berhane K, Chang C, McConnell R, Gauderman W, Avol E, Rapapport E, et al. Association of changes in air quality with bronchitic symptoms in children in California, 1993–2012. The Journal of the American Medical Association. 2016;315:1491–1501. doi: 10.1001/jama.2016.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kousha T, Castner J. The Air Quality Health Index and emergency department visits for otitis media. Journal of Nursing Scholarship. 2016;48:163–171. doi: 10.1111/jnu.12195. [DOI] [PubMed] [Google Scholar]
- 21.MacIntyre E, Karr C, Koehoorn M, Demers P, Tamburic L, Lencar C, et al. Residential air pollution and otitis media during the first two years of life. Epidemiology. 2011;22:81–89. doi: 10.1097/EDE.0b013e3181fdb60f. [DOI] [PubMed] [Google Scholar]
- 22.Rovers M, de Kok I, Schilder A. Risk factors for otitis media: An international perspective. International Journal of Pediatric Otorhinolaryngology. 2006;70:1251–1256. doi: 10.1016/j.ijporl.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Zemek R, Szyszkowicz M, Rowe B. Air pollution and emergency department visits for otitis media: A case-crossover study in Edmonton, Canada. Environmental Health Perspectives. 2010;118:1631–1636. doi: 10.1289/ehp.0901675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Q, Lu C, Li Y, Chen L, He Y, Sundell J, et al. Association between prenatal exposure to industrial air pollution and onset of early childhood ear infection in China. Atmospheric Environment. 2017;157:18–26. [Google Scholar]
- 25.Community Modeling and Analysis System. [last accessed September 6, 2016];R-LINE: A Research LINE-source dispersion model for near-surface releases. https://www.cmascenter.org/r-line/
- 26.Atlanta Regional Commission. [last accessed May 6, 2016];Atlanta Roadside Emissions Exposure Study (AREES) 2017 http://atlantaregional.com/environment/air/arees-near-road-emissions.
- 27.Cimorelli A, Perry S, Venkatram A, Weil J, Paine R, Wilson R, et al. AERMOD: A dispersion model for industrial source applications. Part I: General model formulation and boundary layer characterization. Journal of Applied Meteorology. 2005;44:682–693. [Google Scholar]
- 28.Zhai X, Russell A, Sampath P, Mulholland J, Kim B, Kim Y, et al. Calibrating R-LINE model results with observational data to develop annual mobile source air pollutant fields at fine spatial resolution: Application in Atlanta. Atmospheric Environment. 2016;147:446–457. [Google Scholar]
- 29.Marmur A, Unal A, Mulholland J, Russell A. Optimization-based source apportionment of PM2. 5 incorporating gas-to-particle ratios. Environmental Science and Technology. 2005;39:3245–3254. doi: 10.1021/es0490121. [DOI] [PubMed] [Google Scholar]
- 30.Georgia Department of Public Health, Office of Health Indicators for Planning (OHIP) [last accessed April 11, 2016];Online Analytical Statistical Information System, Demographic Clusters of Georgia: Accessing the Georgia Department of Public Health’s Data Warehouse. https://oasis.state.ga.us/gis/demographiccluster/DemoClusters2011.htm.
- 31.Pennington A, Strickland M, Klein M, Zhai X, Russell A, Hansen C, et al. Measurement error in mobile source air pollution exposure estimates due to residential mobility during pregnancy. Journal of Exposure Science and Environmental Epidemiology. 2017;27:513–520. doi: 10.1038/jes.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grover G, Berkowitz C, Lewis R. Parental recall after a visit to the emergency department. Clinical Pediatrics. 1994;33:194–201. doi: 10.1177/000992289403300401. [DOI] [PubMed] [Google Scholar]
- 33.MacIntyre C, McIntyre P, Cagney M. Community-based estimates of incidence and risk factors for childhood pneumonia in Western Sydney. Epidemiology and Infection. 2003;131:1091–1096. doi: 10.1017/s0950268803001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nystad W, Skrondal A, Magnus P. Day care attendance, recurrent respiratory tract infections and asthma. International Journal of Epidemiology. 1999;28:882–887. doi: 10.1093/ije/28.5.882. [DOI] [PubMed] [Google Scholar]
- 35.Williams B, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. The Lancet Infectious Diseases. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 36.Karr C, Lumley T, Shepherd K, Davis R, Larson T, Ritz B, et al. A case-crossover study of wintertime ambient air pollution and infant bronchiolitis. Environmental Health Perspectives. 2006;114:277–281. doi: 10.1289/ehp.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karr C, Lumley T, Schreuder A, Davis R, Larson T, Ritz B, et al. Effects of subchronic and chronic exposure to ambient air pollutants on infant bronchiolitis. American Journal of Epidemiology. 2007;165:553–560. doi: 10.1093/aje/kwk032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.