Abstract
Purpose
Policy reforms in the Affordable Care Act encourage health care integration to improve quality and lower costs. We therefore examined the association between system-level integration and longitudinal costs of cancer care.
Methods
We used linked SEER-Medicare data to identify patients aged 66–99 years diagnosed with either prostate, bladder, esophageal, pancreatic, lung, liver, kidney, colorectal, breast, or ovarian cancer from 2007–2012. We attributed each patient to one or more phases of care (i.e., initial, continuing, and end-of-life), based on time from diagnosis until death or end of study interval. For each phase, we aggregated all claims with the primary cancer diagnosis and identified patients treated in an integrated delivery network (IDN), as defined by Becker’s Top 100. We then determined if care provided in an IDN was associated with decreased payments across cancers and for each individual cancer by phase and across phases.
Results
We identified 428,300 patients diagnosed with one of ten common cancers. Overall, there were no differences in phase-based payments in IDNs and non-IDNs. Average adjusted annual payments, by phase, for IDN and non-IDNs were: Initial $14,194 and $14,421, p=0.672; Continuing: $2,051 vs. $2,099, p=0.566; End-of-life: $16,257 vs. $16,232, p=0.948. However, in select cancers, we observed lower payments in IDNs. For bladder cancer, payments at end-of-life were lower for IDNs ($11,041 vs $12,331, p=0.008). Of the 4 cancers with the lowest 5-year survival rates (pancreatic, lung, esophageal and liver), average expenditures during the initial and continuing care phases were lower for patients with liver cancer treated in IDNs.
Conclusions
For patients with ten common malignancies, treatment in an IDN is generally not associated with lower costs during any phase of cancer care.
Introduction
Health care reforms in the Affordable Care Act aim to promote greater integration of local health care delivery systems. Broadly speaking, system-level integration includes the integration and coordination of acute, ambulatory, extended care, and community health services. In addition, care providers in an integrated system often work together with shared incentives and infrastructure, such as common electronic medical records [1]. Proponents for greater system-level integration argue that much of the duplicative and low-value care identified to date stems from our current health care system’s disjointed nature: communication between providers is frequently lacking, there is ineffective care coordination, and care providers are not aligned with a common goal. The aims of an integrated delivery system, therefore, are to provide high-quality, evidence-based care with greater coordination and less duplication across the health care continuum.
Proponents of system-level integration highlight existing data that fully integrated delivery systems perform better from the perspective of both cost and quality, at least for ambulatory and preventive care [2, 3]. It is unclear, however, whether such systems achieve similar cost efficiencies for patients requiring complex, multi-disciplinary specialty care such as that required by patients with cancer. Cancer is a prime condition to evaluate the effects of integrated health care delivery for patients with complex medical conditions, as care is frequently fragmented with the provision of extraneous services [4]. In addition, cancer care is complex, with differing needs across the cancer continuum. Patients frequently require care that spans several disciplines (e.g., medicine, social-work, psychological) with multiple providers in a variety of care settings (i.e., hospital, home, and outpatient settings). The acuity of treatment also varies depending on cancer characteristics, time from diagnosis, and time until death.
Through its focus on increased care coordination, easier information sharing and provision of an entire spectrum of services, integration has the potential to improve health care quality and decrease costs for complex cancer care [5, 6]. For example, a patient who receives a cancer diagnosis in an optimally integrated delivery system may obtain treatment in the same system, without repetitive diagnostic testing. Prior-to, during, and after the initial treatment, the patient’s co-morbidities and specific care needs will be well known to the providers and addressed appropriately. If a hospitalization occurs, the discharge plan will be carefully coordinated with follow-up arranged prior to discharge [7]. Furthermore, if problems should arise, the system will have better care coordination where a patient’s concerns can be efficiently addressed. The care coordination and decreased utilization would continue throughout the continuing phase and could potentially play a particularly important role at the end-of-life and for patients with aggressive cancers, where repeat diagnostics and in-patient hospitalizations could be decreased, and patients’ end-of-life goals better addressed, such as the potential desire for hospice care. Ultimately, if health care integration is successful, patients receiving care within these systems would have decreased readmissions, complications, and redundancies in care, all leading to cost savings. In contrast, system level integration may not change specialist provider behavior or patient care, resulting in no cost savings.
In this context, we hypothesized that treatment in integrated delivery networks (IDNs) will be associated with lower costs for patients with cancer. Using Medicare claims data, we examined this question by estimating the costs of cancer care over its continuum (i.e., initial, continuing and end-of-life phases) for patients treated in IDNs compared to non-integrated health networks. We specifically examined differences in payments for patients at the end-of-life and who have cancer diagnoses with low 5- year survival rates (pancreatic, esophageal, lung, and liver).
Methods
Data sources
We utilized linked Surveillance, Epidemiology and End Results (SEER)-Medicare data from July 2007 through 2012. SEER-Medicare is a patient-level dataset that links Medicare claims with information about clinical characteristics, patient demographics, and outcomes from the SEER registries. Claims from this dataset are divided into five files: MEDPAR (readmissions, index, skilled nursing facility [SNF]), Carrier (Professional), Outpatient, Home Health, and Hospice payment files. We used all five files for our analyses.
Study population
Our study cohort included patients aged 66–99 years who were diagnosed with one of ten cancers (prostate, bladder, esophageal, pancreatic, lung, liver, kidney, colorectal, breast, or ovarian) between 2007 through 2012. We initially identified these patients in SEER’s Patient Entitlement and Diagnosis Summary File (PEDSF) using the ICD-0-3 cancer site recode for the cancer of interest. We then confirmed the diagnosis by only including patients with the relevant ICD-0-3 histology codes for each cancer. We excluded cases where the diagnosis was noted only by autopsy or on the death certificate. We further excluded patients without continuous Medicare Parts A and B enrollment from 12 months prior to diagnosis until end of study interval or death, and patients who participated in a Medicare Health Maintenance Organization (HMO).
For each patient, we determined patient demographic and cancer characteristics, including gender, age, marital status, race, year of diagnosis, and cancer stage, using the SEER (PEDSF) file. Age was defined as the diagnosis date minus the birth date and was made a categorical variable. We limited race to White, Black, and other. Based on the health system to which the patient was attributed, we used the American Hospital Association Annual Survey data to determine the number of hospital beds, teaching status, hospital ownership, and geographic region, which was categorized in the following locations: Northeast, Midwest, South, and West.
Attribution of patients to phases of care
In this analysis, we identified three phases of care, as described previously [8]. The phases of care are the initial, continuing, and end-of-life. Individual patients were attributed to one or more phases of care depending on length of survival from cancer diagnosis to death or end of study period. To do this, we used the PEDSF file to determine days of survival by subtracting the diagnosis date from the date of death. Patients surviving < 12 months after diagnosis were attributed only to the end-of-life phase. Those surviving >12 months but less than 24 months were attributed to the initial and end-of-life phases, and the remainder were attributed to the initial, end-of-life and continuing care phases. The 12 months during end-of-life were given precedence, then the 12 months for the initial phase, and last, the continuing phase. Depending upon days of survival after diagnosis, each patient could be attributed to either the end-of-life phase, the initial and end-of-life, or all three phases. Patients who survived throughout the study interval were attributed to the initial and continuing phases of care. Each patient-phase dyad was evaluated distinctly from the other phases with the exception of the overall estimates, where only patients present in all three phases were included in our analyses.
Identification of patients treated in integrated delivery systems
In order to identify patients treated in highly integrated delivery systems, we undertook a two-step process. First, we attributed patients to a health system. Second, we identified the highly integrated health care systems.
In order to attribute each patient to a health care system, we attributed each patient to a physician and then to a health system for each phase of cancer care. This attribution occurred in three main steps. First, each patient was assigned to the physician who had the plurality of cancer- related claims (i.e., the number of claims not total reimbursement) for each cancer phase. For this step, we limited physician ICD-9, Health Care Financing Administration (HCFA) Provider specialty codes, and Berenson-Eggers Type of Service (BETOS) codes to those claims involving direct patient care. Second, we attributed each physician to a health care system. To do this, we assigned each physician to the health system where the majority of his or her patients were admitted annually. Third, we attributed each patient to a health system for each cancer phase. We did this by calculating the “dominant” year, or year with the greatest number of claims, by cancer phase. Each patient’s phase of cancer care was then attributed to the health system, by using the dominant year of care and the physician attribution to the health system (i.e., step 2).
Next, we measured health care integration using Becker’s Hospital Review list of the top 100 most integrated health delivery systems. The designation of a top 100 integrated delivery system was determined by Becker’s Hospital Review, based on metrics reported by IMS Health, a health care analytics firm. Ratings measure 33 attributes of a health system in 8 domains, including: overall integration, integrated technology, hospital utilization, access, available services, contract capabilities, financial stability and services[9]. We defined an integrated delivery system as a dichotomous variable if the health system was listed in Becker’s Hospital Review top 100 most integrated delivery systems. This rating methodology has been previously used to identify and measure health care integration [9–11].
Calculating payments according to phase of care
For each patient-phase of care dyad, we aggregated all standard payments for claims with a primary diagnosis code for the corresponding cancer for each Medicare data file (Medicare provider analysis and review (MEDPAR), Carrier Claims (NCH), outpatient, home health, and hospice). We estimated the average payment per beneficiary in each data file and then for each beneficiary across all files. To account for differences in Medicare reimbursement based on geography, teaching status, and disproportionate share payments, we price-standardized all payments using methods previously described by our research team [12]. These methods were adapted from the Dartmouth Institute for Health Policy and Clinical Practice and the Medicare Payment Advisory Commission (MedPAC).
Statistical Analyses
To determine whether payments for cancer care were lower in an integrated delivery system compared to a non-integrated system, we fit phase-specific generalized estimating equation models with the gamma distribution and log link for each cancer type and across all ten cancers. We fit this model in order to better account for costs having a non-normal distribution and for our analyses occurring at the individual patient level, where patients receive care at different hospitals, which are in different health systems. We first fit the models with only IDN as a covariate. Next, we fit similar phase-specific models for each of the ten cancers, but adjusted for patient (age, sex, number of Charlson co-morbidities, marital status), hospital (geographic region, number of beds, urban/rural, hospital ownership, surgical volume), and cancer (stage, grade) characteristics. We paid particular attention to end-of-life spending and cancers with the lowest 5-year survival rate: pancreatic, lung, esophageal, and liver [13].
All analyses were performed using SAS v 9.4 (Cary, NC) and using the 5% significance level. The University of Michigan’s institutional review board deemed this study exempt from review.
Results
We identified 428,300 patients who were diagnosed with one of ten cancers from 2006 through 2011, of which 140,383 (33%) received care from an integrated delivery network.
An unadjusted comparison of the costs of cancer care by cancer type and phase, within an IDN compared to non-integrated delivery systems is demonstrated in Appendix Table 1. In the initial phase, only prostate cancer was significantly lower in an IDN compared to a non-IDN ($9,389 vs. $10,422, p<0.001). There were no statistically significant differences in the continuing phase. At the end-of-life, bladder cancer care was lower in an IDN compared to a non-IDN ($10,895 vs. $12,044, p=0.007).
Appendix Table 1.
Unadjusted annual costs of cancer care for each phase of cancer, by cancer type
| Initial | Continuing | End-of-life | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-IDN | IDN | p-Value | Non-IDN | IDN | p-Value | Non-IDN | IDN | p-Value | |
| Prostate | $10,422 | $9,389 | <0.001 | $1,133 | $1,155 | 0.314 | $6,463 | $6,591 | 0.343 |
| Bladder | $7,011 | $6,811 | 0.686 | $1,805 | $1,812 | 0.422 | $12,044 | $10,895 | 0.007 |
| Kidney | $9,631 | $9,419 | 0.248 | $1,062 | $1,042 | 0.994 | $11,105 | $10,557 | 0.475 |
| Pancreatic | $23,495 | $24,459 | 0.360 | $3,929 | $4,930 | 0.062 | $18,121 | $18,509 | 0.059 |
| Lung | $17,003 | $17,124 | 0.138 | $3,373 | $3,213 | 0.223 | $16,183 | $16,062 | 0.196 |
| Esophageal | $21,086 | $21,581 | 0.889 | $2,577 | $2,568 | 0.918 | $19,639 | $19,564 | 0.605 |
| Liver | $12,446 | $11,716 | 0.392 | $3,499 | $2,948 | 0.298 | $11,989 | $11,779 | 0.303 |
| Colorectal | $19,864 | $19,957 | 0.003 | $2,708 | $2,600 | 0.675 | $19,788 | $20,080 | 0.124 |
| Breast | $12,229 | $12,379 | 0.097 | $1,332 | $1,283 | 0.689 | $9,027 | $9,028 | 0.250 |
| Ovarian | $18,257 | $18,238 | 0.585 | $4,540 | $4,806 | 0.224 | $17,796 | $18,063 | 0.917 |
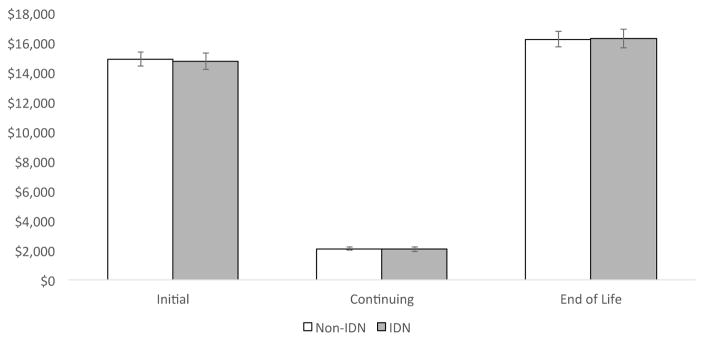
Overall, there were no significant phase-based differences in costs between highly integrated delivery systems and those that were non-integrated. Average annual costs in the initial phase, continuing and end-of-life phases for IDN and non-IDNs were as follows: Initial $14,194 and $14,421, p=0.672; Continuing: $2,051 vs. $2,099, p=0.566; End-of-life: $16,257 vs. $16,232, p=0.948 (Figure 1).
Figure 1.
Adjusted* average annual costs of cancer care across 10 cancer types for IDN vs. non-IDN
*Adjusted for patient (age, sex, number of Charlson co-morbidities, marital status), hospital (geographic region, number of beds, urban/rural, hospital ownership, surgical volume), and cancer (stage, grade) characteristics
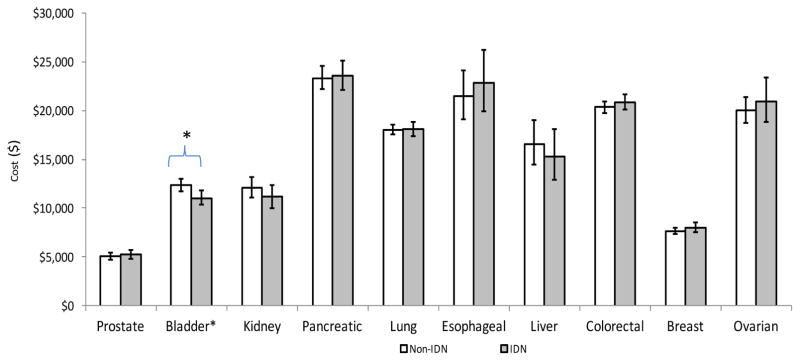
However, in a few select cancers and phases, we observed lower costs for those treated in an IDN. Figure 2 displays the end-of-life costs for integrated versus non-integrated health delivery systems for each of the ten cancers. Only bladder cancer realized cost savings when care was provided in an IDN at the end-of-life compared to a non-integrated network ($11,041 vs $12,331, p=0.008).
Figure 2.
Adjusted** average annual costs of cancer care for IDN vs. non-IDN hospitals at end of life
* p <0.05
**Adjusted for patient (age, sex, number of Charlson co-morbidities, marital status), hospital (geographic region, number of beds, urban/rural, hospital ownership, surgical volume), and cancer (stage, grade) characteristics
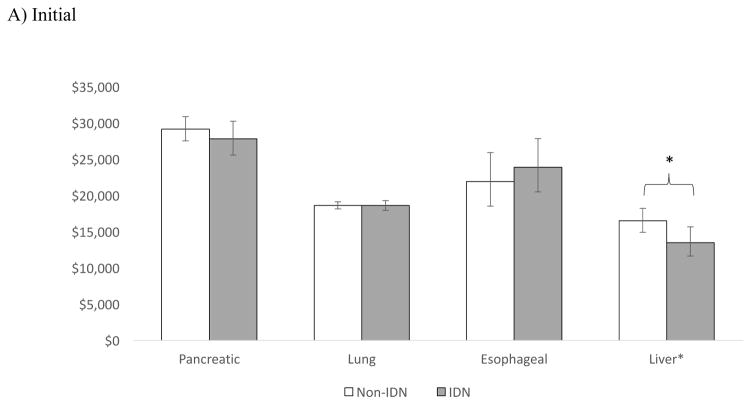
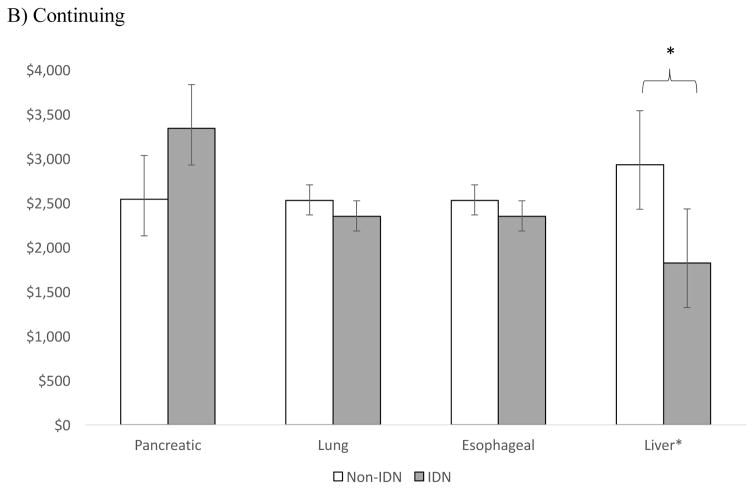
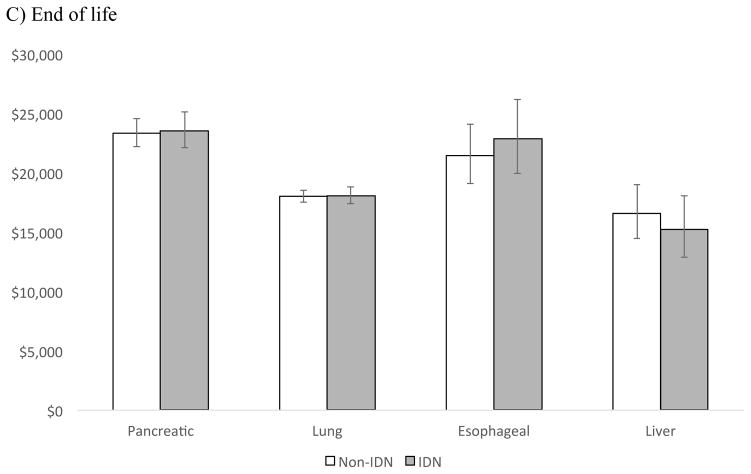
Of the four cancers with the lowest 5-year survival rates: pancreatic, lung, esophageal, and liver [13], only patients with liver cancer in the initial and continuing phase had lower average annual expenditures when receiving care in an integrated delivery system compared to a non-integrated system (Figure 3). The cost differentials for liver cancer by phase were: $2,985 (p=0.016) for the initial phase, $1,107 (p=0.002) for the continuing, and $1,301 at end-of life (p=0.472).
Figure 3.
Adjusted** average annual costs of cancer for IDN vs. Non-IDN hospitals in A) Initial B) Continuing and C) End of life phase, for cancers with the lowest 5- year survival
*p <0.05
** Adjusted for patient (age, sex, number of Charlson co-morbidities, marital status), hospital (geographic region, number of beds, urban/rural, hospital ownership, surgical volume), and cancer (stage, grade) characteristics
Discussion
In this study, we examined payments for ten cancer types according to health system integration. Our findings demonstrate few payment differences between IDN and non-IDNs. Namely, after adjusting for patient, hospital, and cancer characteristics, there were no differences in phase-based payments for cancer care across the ten cancers combined, and few differences in phase-based payments for individual malignancies. Collectively, these findings suggest that cancer care in IDNs is not associated with lower total costs of care.
Previous investigators have demonstrated that IDNs provide higher quality primary care and ambulatory services at lower costs [10, 14, 15]. However, the argument for IDNs providing cost savings for acute inpatient surgery is poor, with a recent investigation of in-hospital care for coronary artery bypass graft, back surgery, hip replacement, and colectomy demonstrating similar outcomes and costs between IDN and non-IDNs, with the exception of decreased readmissions for colectomy and a 4% episode cost savings for patients undergoing hip replacement surgery [10]. For prostate cancer, receiving care in an IDN resulted in small decreases in rates of prostate cancer treatment, but not in potential overtreatment [9]. Our findings are novel in that we evaluate the costs of treatment for a complex diagnosis (i.e., cancer) over its entire care continuum (i.e., from diagnosis to end-of-life). While we found disparities in IDN and non-IDNs for bladder cancer at the end-of-life and liver cancer at the initial and continuing phases, it is unclear if these are meaningful. Overall, payments for cancer care seem similar across different levels of delivery system integration.
A potential reason for our findings of no payment differences in IDNs, is that we evaluated system-level integration. The benefits of system-level integration, including the potential for improved communication and shared electronic health records may not be facile enough to respond to the manifold needs of patients with complex medical conditions. Perhaps a more micro- level of integration, such as service-line specific integration, will better align providers with improved communication, decreased redundancy, increased care coordination, and a common goal, while still remaining nimble enough to provide patient-centered care. The “Come Home” project, a medical home for oncology patients, is an example of a service-line approach to cancer care. Some participants in this model have demonstrated significant increases in telephone triaging, reductions in ED visits, hospital admissions and outpatient visits, and high levels of patient satisfaction [16, 17].
Our study has several limitations. First, we estimated payments for cancer care only for the Medicare population. As a result, our findings may not be generalizable to younger patients with cancer. Nonetheless, estimates from the Medicare program are policy relevant given the burden of cancer in this population, including an incidence rate that is ten times higher, and a death rate from cancer that is sixteen times greater, than for patients < 65 years [18]. Second, the definition of IDN varies and we only applied one definition, Becker’s top 100, to our analyses. This definition of IDNs has been previously used in major medical journals and is readily quantifiable [3, 9, 11]. In addition, all claims for patients attributed to an IDN may not have occurred within an IDN. However, this scenario is similar to other Medicare payment programs (accountable care organizations and Value Based Purchasing), where patients and hospitals are held accountable for claims that occur outside of their facilities. Third, the initial and end-of-life phases were based on 12-month time periods. Since some patients may not have been in the respective phase for the entire 12-months (e.g., the patient died 20 months after diagnosis), payments in these phases may be underestimated. Fourth, we only include claims with a primary diagnosis code of the diagnosed malignancy. This approach may underestimate the true costs of cancer care, but it ensures specificity for cancer-related expenditures and has been used in prior work [19]. Last, we did not evaluate the composition of payments (e.g., readmissions compared to physical therapy or psychological counseling) and the quality of care provided, which could potentially demonstrate differences across IDNs and non-IDNS.
These limitations not withstanding, our findings have important implications for hospital administrators, policymakers, and payers. For hospital administrators, our findings suggest that system-level integration does not decrease costs of care for all patients. For complex patients, perhaps service-line integration may be more successful. Similarly, from a policy and payer perspective, driving improvement into complex specialty based care may require a focus on the micro-level (i.e., service-line) integration, rather than on the macro- (i.e. system) level. Unlike policy initiatives that focus on PCPs and aim to drive system-level integration (e.g., accountable care organizations), service-line integration may be better able to impact the decisions made by specialists and their interactions in the health care network. Two examples of service-line approaches recently introduced by the Centers for Medicare and Medicaid Innovation (CMMI) are bundled payments and the Oncology Care Payment Model (OCM). Bundled payments focus on an acute inpatient episode and post-discharge care. In contrast, the OCM provides a service-line approach to managing costs and quality for patients with cancer. For patient receiving chemotherapy, physician groups participating in the OCM agree to provide increased care coordination with performance and financial liability for the potential of economic reward. Understanding the effects of the OCM payment model will provide significant insight into whether more micro-level integration is higher impact than what we observed for more macro-level integration. Moving forward, research on quality and costs in the OCM payment model and oncology medical home will be necessary to determine if more micro- level integrated care delivery systems are beneficial. In addition, evaluating top performing providers, regardless of their IDN affiliation, could provide insight into improving oncology care. Last, it is perhaps worthwhile to evaluate different delivery system models, such as cancer-focused systems (i.e., National Cancer Institute designation).
Acknowledgments
This project was supported by the National Cancer Institute (1-R01-CA-174768-01-A1 to DCM & 5-T32-CA-180984-03 to DRK).
Footnotes
Conflicts of Interest: JMD, DCM: Grant contract from the Blue Cross Blue Shield of Michigan (BCBSM) for the Michigan Value Collaborative (MVC) and the Michigan Urological Surgery Improvement Collaborative (MUSIC)
CE: Grant contract from BCBSM for MVC
References
- 1.Zuckerman AM. Becker's Hospital Review. 2014. Systemness: The Next Frontier for Integrated Health Delivery. [Google Scholar]
- 2.Hollander P, et al. Quality of care of Medicare patients with diabetes in a metropolitan fee-for-service primary care integrated delivery system. Am J Med Qual. 2005;20(6):344–52. doi: 10.1177/1062860605280205. [DOI] [PubMed] [Google Scholar]
- 3.Rittenhouse DR, et al. Small and medium-size physician practices use few patient-centered medical home processes. Health Aff (Millwood) 2011;30(8):1575–84. doi: 10.1377/hlthaff.2010.1210. [DOI] [PubMed] [Google Scholar]
- 4.Skolarus TA, Zhang Y, Hollenbeck BK. Understanding fragmentation of prostate cancer survivorship care: implications for cost and quality. Cancer. 2012;118(11):2837–45. doi: 10.1002/cncr.26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickell NA, et al. Lost opportunities: physicians' reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25(18):2516–21. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 6.Chubak J, et al. Providing care for cancer survivors in integrated health care delivery systems: practices, challenges, and research opportunities. J Oncol Pract. 2012;8(3):184–9. doi: 10.1200/JOP.2011.000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27(3):759–69. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 8.Mariotto AB, et al. Projections of the Cost of Cancer Care in the United States: 2010–2020. JNCI Journal of the National Cancer Institute. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollenbeck BK, et al. Implications of evolving delivery system reforms for prostate cancer care. Am J Manag Care. 2016;22(9):569–75. [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DC, et al. Anticipating the effects of accountable care organizations for inpatient surgery. JAMA Surg. 2013;148(6):549–54. doi: 10.1001/jamasurg.2013.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyweide DJ, et al. Relationship of primary care physicians' patient caseload with measurement of quality and cost performance. Jama. 2009;302(22):2444–50. doi: 10.1001/jama.2009.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb DJ. Prices Don't Drive Regional Medicare Spending Variations. Health affairs (Project Hope) 2010;29(3):537–543. doi: 10.1377/hlthaff.2009.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SEER. Age-Adjusted SEER Incidence and U.S. Death Rates and 5- Year Relative Survival (Percent) By Primary Cancer Site, Sex, and Time Period. 2014. [Google Scholar]
- 14.Mehrotra A, Epstein AM, Rosenthal MB. Do integrated medical groups provide higher-quality medical care than individual practice associations? Ann Intern Med. 2006;145(11):826–33. doi: 10.7326/0003-4819-145-11-200612050-00007. [DOI] [PubMed] [Google Scholar]
- 15.Weeks WB, et al. Higher health care quality and bigger savings found at large multispecialty medical groups. Health Aff (Millwood) 2010;29(5):991–7. doi: 10.1377/hlthaff.2009.0388. [DOI] [PubMed] [Google Scholar]
- 16.Sprandio JD. Oncology Patient-Centered Medical Home. Journal of Oncology Practice. 2012;83(Suppl):47s–49s. doi: 10.1200/JOP.2012.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters TM, et al. Community Oncology Medical Homes: Physician-Driven Change to Improve Patient Care and Reduce Costs. J Oncol Pract. 2015;11(6):462–7. doi: 10.1200/JOP.2015.005256. [DOI] [PubMed] [Google Scholar]
- 18.Berger NA, et al. Cancer in the Elderly. Transactions of the American Clinical and Climatological Association. 2006;117:147–156. [PMC free article] [PubMed] [Google Scholar]
- 19.Skolarus TA, et al. The economic burden of prostate cancer survivorship care. J Urol. 2010;184(2):532–8. doi: 10.1016/j.juro.2010.03.136. [DOI] [PubMed] [Google Scholar]