Abstract
Classical estrogen receptor signaling mechanisms involve estradiol binding to intracellular nuclear receptors (estrogen receptor-α (ERα) and estrogen receptor-β (ERβ)) to promote changes in protein expression. Estradiol can also exert effects within seconds to minutes, however, a timescale incongruent with genomic signaling. In the brain, estradiol rapidly potentiates stimulated dopamine release in the striatum of female rats and enhances spontaneous rotational behavior. Furthermore, estradiol rapidly attenuates the K+- evoked increase of GABA in dialysate. We hypothesize that these rapid effects of estradiol in the striatum are mediated by ERα located on the membrane of medium spiny GABAergic neurons. This experiment examined whether over-expression of ERα in the striatum would enhance the effect of estradiol on rotational behavior and the K+- evoked increase in GABA in dialysate. Ovariectomized female rats were tested for rotational behavior or underwent microdialysis experiments after unilateral intrastriatal injections of a recombinant adeno-associated virus (AAV) containing the human ERα cDNA (AAV.ERα) into the striatum; controls received either the same vector into areas outside the striatum or an AAV containing the human alkaline phosphatase gene into the striatum (AAV.ALP). Animals that received AAV.ERα in the striatum exhibited significantly greater estradiol-induced contralateral rotations compared to controls and exhibited behavioral sensitization of contralateral rotations induced by a low dose of amphetamine. ERα over-expression also enhanced the inhibitory effect of estradiol on K+- evoked GABA release suggesting that disinhibition of dopamine release from terminals in the striatum resulted in the enhanced rotational behavior.
Keywords: Estradiol, dopamine, rotational behavior, microdialysis, striatum, adenoassociated viral vector, estrogen receptor
INTRODUCTION
Estradiol rapidly enhances stimulated dopamine (DA) release from striatum in vitro (Becker, 1990a; Xiao and Becker, 1998; Xiao et al., 2003) or in vivo (Becker, 1990b; Castner et al., 1993). This rapid effect of estradiol on striatal DA is thought to contribute to the enhanced acquisition of cocaine self-administration and enhanced motivation to take cocaine found in female rats following estradiol treatment (Jackson et al., 2006; Becker and Hu, 2008; Hu and Becker, 2008).
DA release in striatum is inhibited by intrinsic GABA input (Smolders et al., 1995; Whitehead et al., 2001), so we hypothesized that estradiol enhances DA release in the striatum indirectly via inhibition of GABA input onto DA terminals. In whole-cell clamp recordings, application of physiological concentrations of 17β-estradiol decreases Ca2+ current mediated by L-type Ca2+ channels in acutely dissected medium spiny neurons within seconds of application (Mermelstein et al., 1996). Since inhibition of L-type Ca2+ channels on cell bodies decreases neurotransmitter release from neurons (Vigh and Lasater, 2004), and striatal medium spiny neurons are GABAergic, this would suggest that estradiol can inhibit GABA release. In microdialysis experiments estradiol significantly attenuates the K+-evoked increase in GABA in dialysate from the striatum (Hu et al., 2006) supporting this hypothesis.
Activation of membrane-associated ERα and/or ERβ may mediate some of the rapid effects of estradiol in striatum, and other brain regions, through a novel mechanism (Boulware et al., 2007; Micevych and Mermelstein, 2008). ERα and/or ERβ can associate with caveolin in the extracellular membrane of cells to mediate rapid responses to estradiol. For example, extracellular estradiol can rapidly activate the mitogen-activated protein kinase (MAPK) pathway, one of the primary mediators of the intracellular signal transduction cascade triggered by estradiol binding to ERα at the membrane (Razandi et al., 2003; Wade and Dorsa, 2003). Whether estradiol acts in the striatum via ERα to influence behavior or GABA release remains to be determined.
These experiments investigate the effects of over-expression of ERα in the striatum. Female rats have an endogenous asymmetry in the ascending DA system, so they turn in circles away from the striatum with greater DA activity (Jerussi and Glick, 1976; Becker et al., 1982), and turn more during behavioral estrus than on other days of the cycle (Becker et al., 1982). We took advantage of this estradiol-modulated behavioral and neurochemical asymmetry to investigate whether over-expression of ERα in striatum would enhance the effect of estradiol on this behavior. We also investigate whether ERα over-expression would enhance the effect of estradiol on the K+-evoked increase in GABA in dialysate from striatum. The results demonstrate that increasing expression of ERα unilaterally in the striatum enhances the rapid behavioral effect of estradiol and induces greater attenuation of K+-evoked GABA release after estradiol treatment compared to control animals. Furthermore, we demonstrate that in the striatum of adult ovariectomized rats there is low-level expression of ERα protein. These results indicate that endogenous ERα may mediate rapid effects of estradiol on GABA activity and striatal-mediated behaviors observed in the female rat.
Methods
Animals
Adult female Sprague-Dawley rats (Harlan, Indianapolis, IN) were maintained on a 14:10 light-dark cycle with soy-free rat chow (Teklad #2014, Harlan rat chow, Madison, WI) and water available ad lib. Rooms were maintained at a constant temperature of 20–21°C. For the behavioral experiments animals were housed in groups of 2–3 with lights on at 6:30 pm. For the microdialysis experiments animals were individually housed after guide cannula implantation, and lights in the colony room were on at 5:30 am. All experiments were conducted in accordance with the National Institute of Health (NIH) guidelines on a protocol approved by the University of Michigan Committee for Use and Care of Animals.
Rats were ovariectomized (OVX) under isoflurane (Baxter Healthcare Corporation, Deerfield, IL) anesthesia (Hu and Becker, 2003) and those undergoing behavioral testing were tested for rotational behavior two weeks later with 0.85 mg/kg d-amphetamine sulfate (1 hour test on 3 consecutive days). Rats then received intra-striatal injections of the viral vector (adeno-associated virus with ERα; AAV.ERα; N=14; see below for details) or control construct (adeno-associated virus with the enzyme alkaline phosphatase; AAV.ALP; N=9) into three sites, unilaterally in the striatum contralateral to preferred direction of turning (i.e., into the dominant striatum). On histological examination, four of the animals in the AAV.ERα group did not have ER expression in striatum (the expression was only in cortex) and data from these animals are included with the control group (final groups AAV.ERα; N=10, AAV.ALP; N=13).
Adeno-Associated Viral Vectors (AAV)
Experimental animals received an AAV containing the human ERα cDNA AAV.ERα. Control animals received an AAV harboring the human placental alkaline phosphatase gene (AAV.ALP). The expression of the transgenes was driven by the chicken beta-actin/CMV hybrid promoter (CAG). In addition, an internal ribosomal entry site (IRES) element provides a bicistronic expression of enhanced green fluorescent protein (EGFP) from both constructs to permit visualization of transduced neurons. The expression cassettes were flanked by AAV inverted terminal repeats (ITR) derived from psub201 (Samulski et al., 1987). To prepare virus stocks the vector plasmids were packaged into AAV-2 particles using helper-free plasmid transfection system in 293 cells. The vectors were purified using heparin affinity chromatography (Clark et al., 1999) and dialyzed against PBS. Genomic titers were determined by quantitative PCR (Veldwijk et al., 2002) and adjusted to 1012 particles per ml.
Injection of viral vector unilaterally into striatum
OVX female rats underwent stereotaxic surgery under ketamine (75 mg/kg, i.p.) and medetomidine hydrochloride (0.5 mg/kg, i.p.) anesthesia. Small burr holes were drilled through the skull immediately above the dominant striatum (determined by AMPH-induced behavior as described above) with a drill mounted on a stereotaxic arm. Three holes were drilled and a microsyringe was slowly lowered over 2 min to the following coordinates: 1): Ant +1.0, Lat ±2.0, Ventral 2.7; 2) Ant +0.15, Lat ±3.5, Ventral 3.0; 3) Ant –0.75, Lat±3.8, Ventral 3.0. At each site, 1 μl of the AAV was injected over 7–10 minutes, the needle was left in place for an additional 2 minutes and then the needle was slowly raised.
Behavioral testing
Animals were tested repeatedly for rotational behavior. As discussed above, in unlesioned female rats there is an endogenous asymmetry in turning behavior seen during the dark phase of the cycle or with a low dose of amphetamine (AMPH) (Jerussi and Glick, 1976; Becker et al., 1982). We hypothesized that enhanced expression of ERα unilaterally in the striatum would induce a greater asymmetry in the striatum, and we would see greater rotational behavior after estradiol or in response to AMPH in animals with enhanced expression of the ERα transgene. Thus, animals were repeatedly tested for rotational behavior after acute estradiol, oil, and/or AMPH injection.
Estradiol-induced turning
Three weeks after the viral vector had been injected into the brain, animals were repeatedly tested in automated rotometers for turning behavior (Hu and Becker, 2003). Testing was initiated during the first hour after lights off in the colony. There was a 1 hr habituation session, then animals received 5 pg estradiol benzoate (EB) in 0.1 ml peanut oil or 0.1 ml peanut oil by s.c. injection, followed by a 1 hr test session. Rotational behavior was recorded by computer at 15 min intervals.
Animals received 3 tests/week (2 with EB, 1 with oil) every other day for 2 weeks, then had 1 week off when they were tested with AMPH (see below), followed by 1 additional week of testing with EB and oil for a total of 6 EB tests and 3 oil tests. The first 15 min of the habituation periods and post-injection test periods were used for analyses, since animals exhibited the greatest activity during these periods.
AMPH-induced turning
On three consecutive days, 5 weeks after the viral vector had been injected into the striatum, animals received EB or oil (EB on days 1 and 3, oil on day 2). Animals were injected with EB or oil, placed in the automated rotometers, and thirty min later animals received 0.85 mg/kg AMPH (i.p.); rotational behavior was recorded for 1 hour.
Serum concentrations of estradiol at the time of the test were determined in independent groups of OVX animals of the same size using an immunofluorescent assay: 184.5 ± 38.3 (17β-estradiol in pg/ml ± SEM; day 1 and 3) and 109.5 ± 19.2 (day 2). These values were not significantly different from each other, and were within the range of values reported for this strain of rat during proestrus (Butcher et al., 1974). Thus, on all 3 days animals had elevated serum estradiol concentrations relative to OVX animals, which we measured as 23.6±2.5 pg/ml (Hu et al., 2004).
Guide Cannula and Microdialysis Probe Implantation
At least 4 weeks after insertion of the viral vector, a separate group of rats were anesthetized using a combination of ketamine and medetomidine hydrochloride anesthesia as outlined previously. Using aseptic surgical techniques, guide cannulae were implanted stereotaxically through the skull (from Bregma skull flat in mm, AP: +0.2 mm; ML: +/− 3.2 mm; DV: −2.25 mm) and secured with cranioplastic cement. A stylet was placed in the guide cannula to keep the cannula patent.
After a minimum one-week recovery period, the animal was lightly anesthetized with isoflurane (Baxter Healthcare Corporation, Deerfield, IL), and a microdialysis probe (4 mm active length CMA/11, CMA/Microdialysis AB, Chelmsford, MA) was inserted into the guide cannula. The animal was placed inside a Raturn (BioAnalytical Systems Inc., West Lafayette, IN) bowl and artificial cerebral spinal fluid (aCSF: 145mM NaCl, 2.68 mM KCl, 1.01 mM MgSO4*7H2O, 1.22 mM CaCl2, pH 7.3) was perfused through the probe at 0.1 pl/min overnight.
Microdialysis and determination of GABA concentrations in dialysate
Experiments began 15–18 hours after implantation of the dialysis probe. aCSF was perfused through the probe at 1 μl/min, and on-line analysis of the dialysate was performed using capillary electrophoresis with laser-induced fluorescence detection (CE-LIF), which has been described previously (Bowser and Kennedy 2001). Briefly, dialysate was mixed online with a derivatization solution (10 mM o-phthaldialdehyde, 40 mM β-mercaptoethanol, 36 mM sodium borate, 0.81 mM hydroxypropyl- β-cyclodextrin, and 10% methanol (v/v) at pH 9.5) and allowed to react for 90 s. The derivatized dialysate was then electrokinetically injected onto the separation capillary (10 μm i.d., 150 μm o.d. and 9.5 cm in length) with a flow-gated interface. Cross flow buffer (40 mM sodium tetraborate with 0.9 mM HPBCD at pH 9.5) prevented leakage of dialysate onto the separation capillary during each separation. The applied voltage for separation equated to an electric field of - 2.22 kV/cm. Fluorescence detection was performed off-column with the aid of a sheath-flow cuvette. The sheath flow buffer consisted of 40 mM sodium tetraborate at pH 9.5. Fluorescence was induced with the 351 nm laser line of an argon ion laser (Enterprise 622 argon ion laser; Coherent, Inc., Santa Clara, CA), and fluorescence emission (450 nm) was collected orthogonally to the incident beam. Data was collected using software written in Labview 5.0 (National Instruments, Austin, TX). The detection limit for GABA in these assays was 15.1±0.7 nM.
For each experiment, 50 electropherograms were collected at 15 sec interval to establish basal levels before a subcutaneous injection of 5 mu;g EB (N = 19) or vehicle (peanut oil, 0.1 mL, N = 17). Electropherograms were collected for 30 minutes after vehicle/EB administration to determine the effect of EB on basal GABA release. Then high potassium aCSF (75 mM K+) was perfused through the probe for 10 minutes. The ionic strength of the aCSF was maintained by lowering the sodium concentration to 72.7mM. Electropherograms collected during the 10 min stimulation and 30 min post-stimulation period were used to determine effects of estradiol on stimulated GABA release.
Once the dialysis experiment was complete, Flurogold (Molecular Imaging Products, Ann Arbor, MI) was perfused through the probe for 10 minutes to mark the location of the dialysis probe in the tissue. After explant the probe was placed in ethanol for 5 minutes and then in aCSF. The probe was then calibrated using GABA standards that were heated to 37°C.
Immunocytochemistry (ICC)
At the completion of testing, animals were intracardially perfused with 4% paraformaldehyde and brains were stored in 30% sucrose at 4°C. Sections were cut at 35 mu;m and stored in cryoprotectant solution (30% ethylene glycol, 30% sucrose in 0.1 M phosphate buffered saline (PBS), pH 7.2) at –20°C until ICC. Then, sections were washed 4 X 5 min w/PBS, followed by NaBH4 (1%) in PBS for 30 min. After additional washes, sections were incubated in blocking serum with 4% normal goat serum (NGS), 0.2% Triton X-100 for 15–60 min, then transferred to incubate with the primary antibody at 4 °C for 48 hours (ERα H222 antibody; courtesy Dr. G. Greene, University of Chicago) was used at 1:5000; antibody to green fluorescent protein (AbCam, Inc., Cambridge, MA ) was used at 1:25,000 to confirm expression of AAV.ALP and AAV.ERα in dialysis experiments). Sections were washed and then incubated in the secondary antibody (1:600), 2% NGS, and 0.2% tritonX-100 for 1 hour. After several washes the sections were transferred to the Vectastain ABC reagent (Vectastain Elite ABC kits, Vector Labs, Burlingame, CA) solution for 1 hour. After washing, sites of antibody-antigen binding were visualized with the chromagen diaminobenzidine. Sections were mounted, dried, dehydrated and coverslipped.
Western Blots
Immunoprecipitation and Western blot analysis for ERα were carried out using striatal tissue from female rats, at 3 weeks post-OVX, according to previously published methods (Singh et al., 1999; Toran-Allerand et al., 2002). Females were OVX to remove the endogenous source of estradiol which can induce variation in the number and intracellular location of ER. Western blots were derived from at least four independent experiments, a representative of which is shown in Figure 5. The specificity of the signal is determined by the apparent molecular weight of the protein detected, along with direct comparison with the appropriate protein lysate. Negative controls to test for the specificity of the interactions are carried out by immunoprecipitation of the pre-cleared protein lysates with mouse IgG, instead of specific antibodies.
Figure 5.
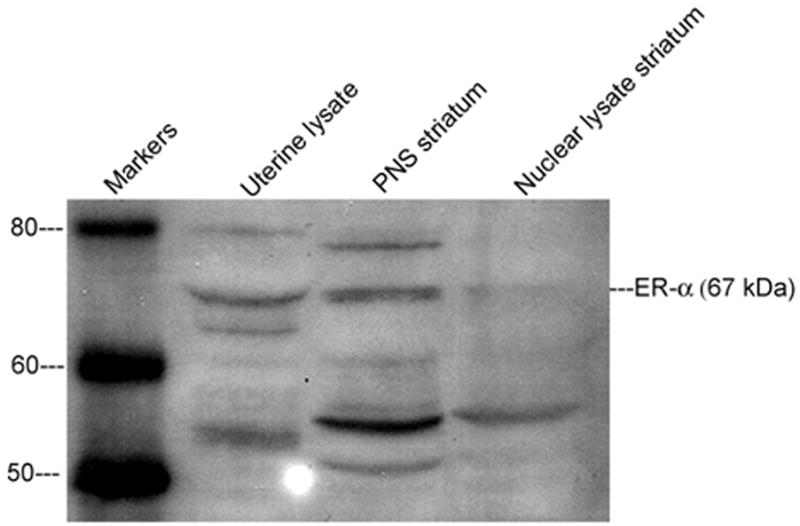
Western Blot using an antibody to ER-α (UBI, C-1355) with 100 Gg protein from striatal tissue obtained from female rats 3 weeks post-OVX showing the presence of ER-α in the membrane fraction (labeled PNS) from striatum
Statistical Analyses
Behavioral data were analyzed by two-way analysis of variance with pairwise comparisons by the Bonferroni correction. Microdialysis data was analyzed by 2-way ANOVA with repeated measures and planned paired T-tests.
RESULTS
Rotational Behavior
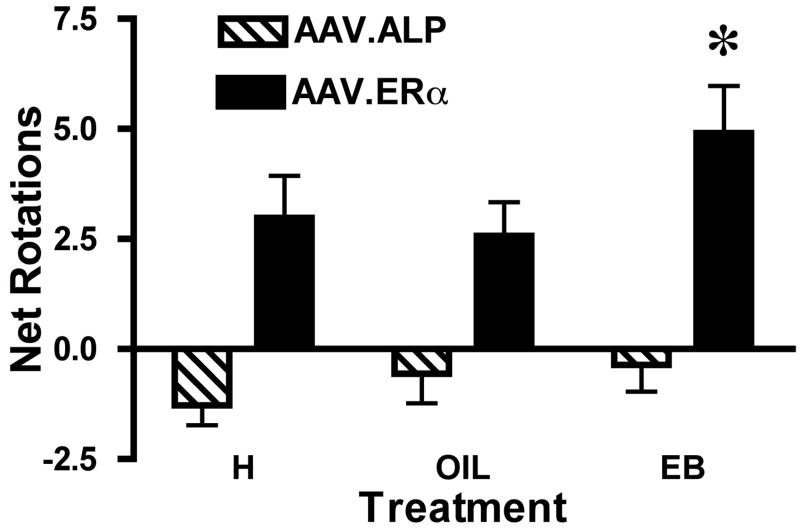
Animals that received the AAV.ERα in the dominant striatum made more net rotations (turns contralateral to the striatum with the AAV.ERα minus turns ipsiversive), both during the first 15 min (Figure 1) and throughout the hour after receiving EB (data not shown), than did the control group (F(1,21)=14.48, p<0.001). These rats also turned more after the EB injection than after vehicle or during the habituation period (p<0.01; Figure 1). The control animals did not exhibit a significant asymmetry in terms of net rotations. Thus, the introduction of the AAV.ERα in the striatum enhanced the slight endogenous asymmetry in the striatum, and transgene expression resulted in turning after treatment with EB.
Figure 1.
Full turns (360 degrees) made contralateral to the striatum with the AAV.ERα or AAV.ALP transgene minus turns in the opposite direction during the first 15 min of the habituation period (H), the first 15 min after animals received 0.1 ml peanut oil (O; s.c.) or the first 15 min after 5 pg estradiol benzoate in oil (EB, s.c.). * Animals with the AAV.ERα transgene expressed unilaterally in the striatum turned significantly more (p<0.01) after EB than during the habituation period or after O and turned more than the control animals (p<0.01).
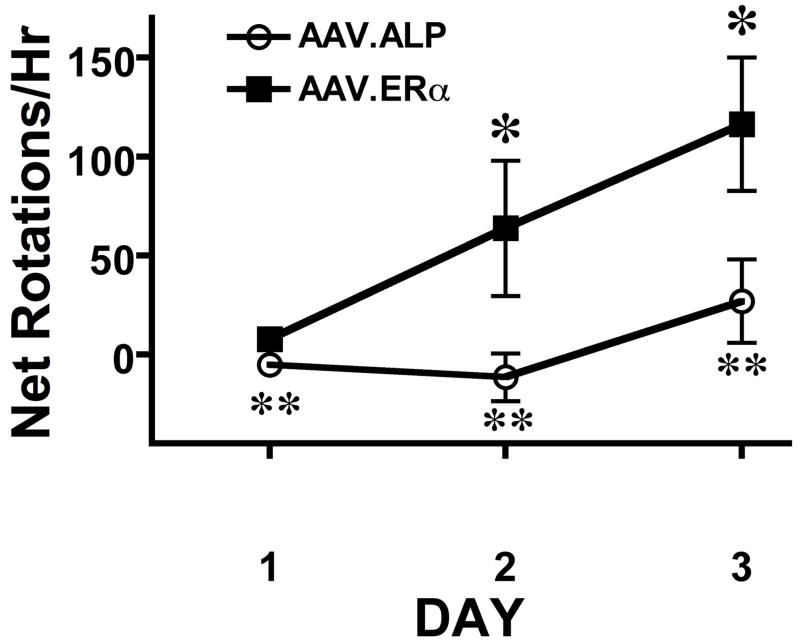
When animals were tested with 0.85 mg/kg AMPH (Figure 2) the AAV.ERα group again exhibited significantly more turning contralateral to the striatum with the AAV.ERα than did the AAV.ALP group (Group effect: F[1,20]=7.097, p<0.15; Time effect F[2,2]=8.462, p<0.001; Group X Time:F[2,40]=3.363, p<0.05). As can be seen in Figure 2, there was only modest behavioral activation on the first day of testing in both groups, yet the AAV.ERα group exhibited greater rotational behavior than the control group (P<0.015). On the second and third days of testing the AAV.ERα group also exhibited significantly more turning than did the AAV.ALP group (p<0.002). There was also significant sensitization of rotational behavior in the AAV.ERα group with animals turning more on day 3 than they did on day 1 (p<0.008) or day 2 (p<0.03).
Figure 2.
Animals with the AAV.ERα transgene expressed unilaterally in the striatum exhibited sensitization of asymmetrical rotational behavior when treated with 5 μg EB followed 30 min later with 0.85 mg/kg d-amphetamine (AMPH). Rotational behavior contralateral to the side with the ERα transgene (minus turns in the other direction) was recorded for 1 hour after animals received AMPH. *In the animals with AAV.ERα transgene asymmetrical rotational behavior induced by AMPH was greater on day 3 than on day 1 (p<0.008) or 2 (p<0.03). ** In the animals with AAV.ERα transgene asymmetrical rotational behavior induced by AMPH was greater than the control animals on all days (p<0.015).
The AAV.ALP group did not exhibit behavioral sensitization of rotational behavior contralateral to the side of the transgene implant. They did, however, show activation induced by AMPH (Table 1), and both groups exhibited an increase in the total number of rotations (contralateral + ipsilateral) over the 3 days of AMPH testing indicating sensitization of overall activity (Table 1). There were no differences between the groups in total rotations on any day.
Table 1.
Mean number of rotations in both directions induced by 0.85 mg/kg AMPH during 1 hour of rotational behavior testing.
| Group | Total Rotations Day 1 of AMPH | Total Rotations Day 2 of AMPH | Total Rotations Day 3 of AMPH |
|---|---|---|---|
| AAV.ERα | 14.6±5.21 | 172.3±44.2** | 141.7±26.1* |
| AAV.ALP | 16.6±7.4 | 128.2±27.1** | 93.2±12.4* |
Mean ± SEM
P<0.05 compared with Day 1;
P<0.001 compared with Day 1.
Microdialysis
During the microdialysis experiments the basal extracellular concentration of GABA did not differ among the four groups (F 1, 11 = 1.353, P= 0.2694; Table 1). Furthermore, the basal extracellular GABA concentration was not significantly affected by treatment with EB or vehicle. Post-K+ stimulation baseline values were also not statistically different (Table 2).
Table 2. The effects of viral vector expression, hormonal treatment, and exposure to K+ on striatum dialysate concentrations of GABA.
Electropherograms were collected and the mean was obtained before (basal) and after (post-injection) an injection of 5 9μg EB or vehicle to OVX female rats. There was no main effect of treatment (F1,539 = 4.34, p = 0.061) on basal GABA concentration, and no interaction between treatment and time of sample collection (F49,539 = 0.68, p = 0.949). Following recovery from the K+ stimulation the GABA dialysate concentration was also determined. Basal extracellular GABA concentrations in dialysate were compared with pre-injection and pre-stimulation basal GABA concentrations in same rats. Two way ANOVA with repeated measures showed that that there was no main effect of treatment (F1,550 = 4.13, p = 0.067) on post-stimulation of basal GABA concentration and no interaction between treatment and time of sample collection (F50,550 = 0.75, p = 0.894).
| Group | Basal GABA in μM ± SEM | GABA Post-EB Injection in μM ± SEM | GABA Post recovery from K+ Stimulation in μM ± SEM |
|---|---|---|---|
| AAV.ALP + OIL | 0.11 ± 0.01 | 0.109 ± 0.008 | 0.088 ± 0.009 |
| AAV.ALP + EB | 0.14 ± 0.03 | 0.12 ± 0.02 | 0.11± 0.02 |
| AAV.ERα + OIL | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.08± 0.01 |
| AAV.ERα + EB | 0.10 ± 0.02 | 0.07 ± 0.01 | 0.07± 0.02 |
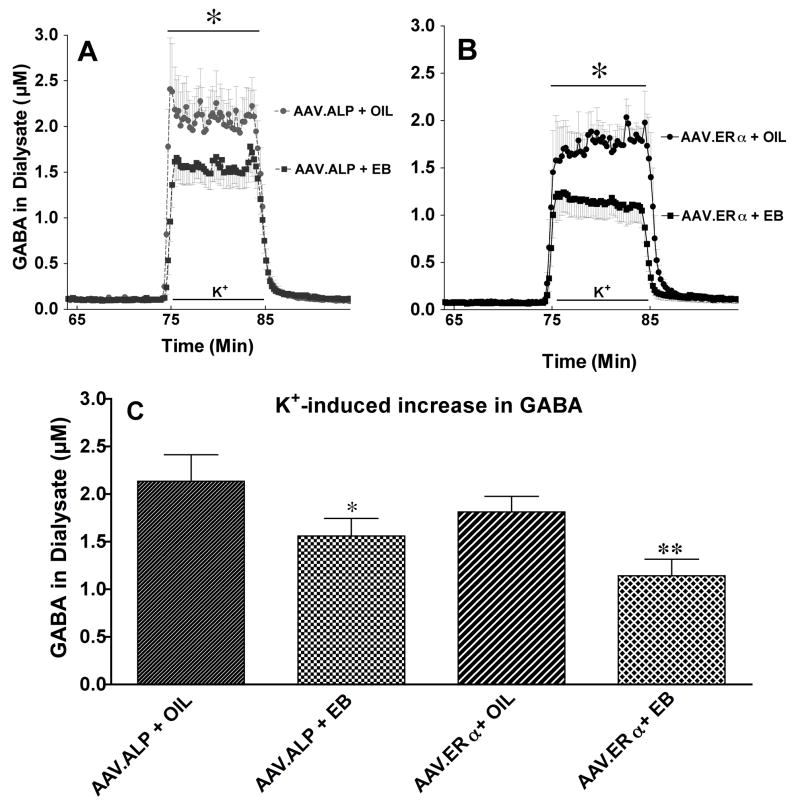
Local perfusion of 75 mM K+ through the dialysis probe for 10 minutes produced a transient increase of GABA in the extracellular fluid in all four groups of animals. Treatment with EB significantly attenuated the K+-induced increase in extracellular GABA concentration in dialysate (Figure 3A & B). When results were analyzed by two-way ANOVA with repeated measures, there was a main effect of treatment (F 3,29 = 4.28, p<0.013), a treatment X time interaction (F 29, 426 = 2.94, p<0.0001); and an effect of time of sample collection (F 29, 142 = 126.37, p<0.0001).
Figure 3.
Effect of AAV.ALP or AAV.ERα and EB or OIL pretreatment on K+-evoked GABA in dialysate from striatum. A. The time course of the effect of AAV.ALP and EB or OIL pretreatment on K+- evoked GABA in dialysate from striatum. B. The time course of the effect of AAV.ERα and EB or OIL pretreatment on K+- evoked GABA in dialysate from striatum. For A & B: The bar depicts the period during which K+ was dialyzed into striatum through the dialysis probe. * There was a main effect of treatment (F 3,29 = 4.28, p<0.013), a treatment X time interaction (F 29, 426 = 2.94, p<0.0001); and an effect of time of sample collection (F 29, 142 = 126.37, p<0.0001). C. Effect of AAV.ALP or AAV.ERα and EB or OIL pretreatment on the mean increase in K+- evoked GABA in dialysate from striatum. *AAV.ALP+EB less than AAV.ALP+OIL (p<0.043). ** AAV.ERα + EB less than AAV.ERα + OIL (p<0.002), and AAV.ALP + EB (p<0.02). There was no significant difference between the oil treated groups.
T-tests were planned a priori to compare differences between the group pairings. Importantly, the AAV.ERα +EB group had significantly lower stimulated GABA release compared to the AAV.ALP +EB (t = 2.335, p<0.02) indicating that AAV.ERα enhanced the response to EB (Figure 3). As previously shown, EB attenuated the K+-induced increase in GABA in both the AAV.ALP and AAV.ERα groups (AAV.ALP +EB vs. AAV.ALP +OIL, t = 2.036, p<0.043; AAV.ERα +EB vs. AAV.ERα +OIL group, t = 3.191, p<0.002). There were no significant differences between the AAV.ERα +OIL and the AAV.ALP +OIL groups.
Immunocytochemistry
The expression of the AAV.ERα transgene in striatal neurons as indicated by immunocytochemistry for ERα was confined primarily to the dorsolateral striatum exclusively on the side of the injection. Expression was quite variable in terms of the number of cells expressing the transduced genes, but expression was observed in all animals receiving the AAV.ERα. Expression of ERα was localized to the membrane/cytosol region of neurons, with most cells showing little nuclear staining (Figure 4, top) and expression extending beyond the cell body into the surrounding processes. Animals that received AAV.ALP exhibited expression of green fluorescent protein (GFP) in the striatum (data not shown). For the microdialysis experiments, only animals with GFP expression around the dialysis probe (Figure 4, bottom) were included.
Figure 4.
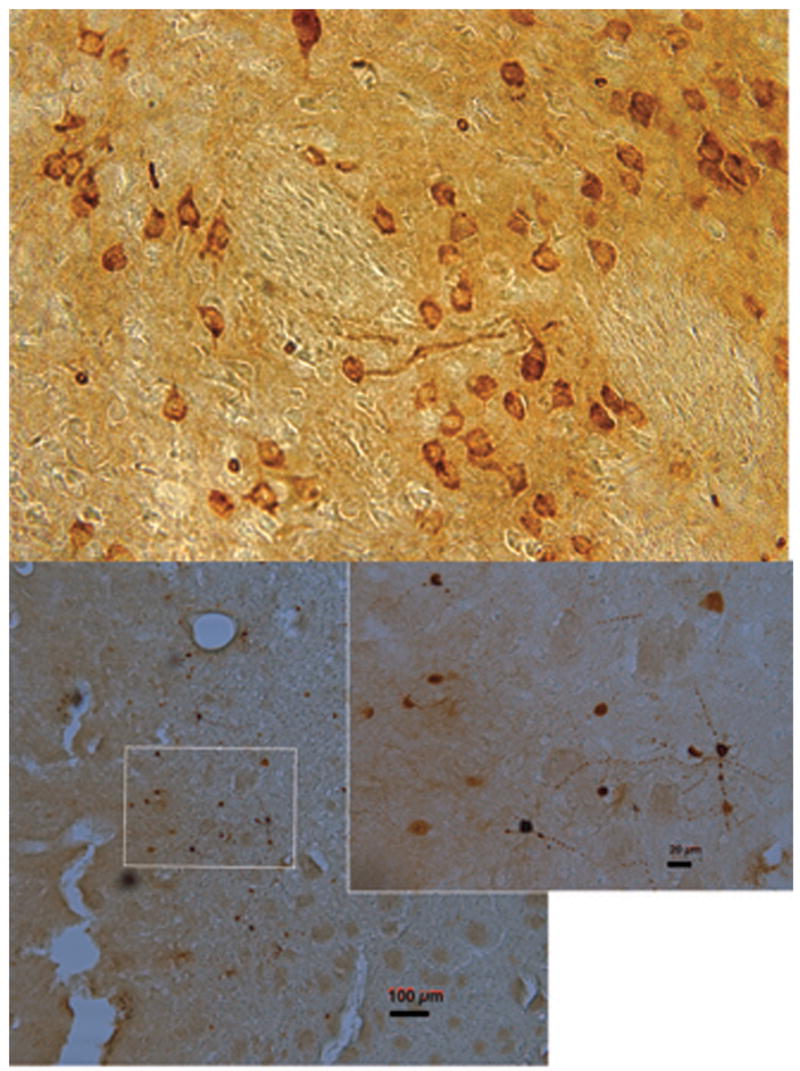
Expression of ERα (top) and green fluorescent protein (GFP; bottom) in striatum after adeno-associated viral vector injection. Top is a representative image from an animal in the behavioral study. The bottom image is from an animal in the dialysis study, inset depicts cells with expression of GFP adjacent to the track. Magnification 40X, calibration bar lower right is 100 μm; and in inset 20 μm.
Western Blots
Western blots were performed as a positive control to measure endogenous ERα expression in the striatum. The blot indicated a low-level expression of ERα in the membrane fraction from striatum of OVX rats (Figure 5). ERα expression was higher in the cytosol/membrane fraction than the nuclear extract taken from homogenized striatal tissue.
Discussion
The expression of recombinant ERα in the dorsolateral striatum resulted in enhanced turning contralateral to the side of the AAV.ERα expression compared with animals with AAV.ALP. Furthermore, animals with the AAV.ERα exhibited a greater estradiol-dependent attenuation of the K+-induced increase in GABA relative to controls. Coupling these behavioral and neurochemical results to the western blot results showing expression of endogenous ERα in striatum, these data suggest that endogenous ERα in striatum may enhance the striatal DA response to estradiol, and this effect may be mediated by an attenuation of GABA release resulting in a release of inhibition on DA terminals.
Striatal GABAergic neurons are known to have recurrent collaterals that have synapses in close proximity to DA terminals (Sizemore et al., 2004). GABA release onto GABAB receptors located on DA presynaptic terminals tonically inhibits DA release in the striatum (Smolders et al., 1995; Charara et al., 2000). This inhibition of DA release mediated via GABAB receptors on DA terminals is thought to be due to inactivation of voltage-dependent Ca2+ channels on the presynaptic terminal (Cardozo and Bean, 1995). Combining these anatomical results along with electrophysiology (Mermelstein et al., 1996) and microdialysis (Hu et al., 2006) data showing the inhibitory effects of estradiol on GABAergic neurons suggests a linkage between the DA enhancing effects of estradiol and the reduction in GABA release. The two fold increase in attenuation of K+ - evoked GABA seen with ERα overexpression suggests that this receptor plays a role in linking these two neurotransmitter systems in the striatum. The decrease in GABA release is hypothesized to result in a release of inhibition, which enhances DA release when DA neurons are activated (for discussion see (Becker and Hu, 2008; Becker and Taylor, 2008)). Thus, when behaviorally active rats receive estradiol, we postulate that there is an enhanced release of DA on the side of the striatum with the AAV.ERα, relative to the other striatum, and animals turn in circles away from the more active side.
Behavioral sensitization to AMPH occurs due to long-term changes in DA neurons. The activation of DA systems is necessary to induce sensitization with AMPH, and behavioral sensitization is prevented by co-treatment with DA antagonists, given either systemically or directly into the ventral tegmental area (Kuczenski and Leith, 1981; Vezina and Stewart, 1989; Weiss et al., 1989). The evidence implicating D1 receptors is quite strong, although the involvement of D2 receptors is more controversial (White and Wolf, 1991; Stewart and Badiani, 1993). When AAV.ERα injected animals treated with estradiol receive AMPH, they exhibit sensitization of rotational behavioral in the direction away from the striatum with the transgene. Both groups showed enhanced total rotations on the second test day even though they did not receive additional estradiol treatment prior to AMPH. This is likely due to the combined effects of the neural changes associated with sensitization and the residual serum estradiol which was still elevated at the time of the second AMPH test. Interestingly, only the AAV.ERα animals showed a greater rotational behavior in the direction away from the side of the transgene expression. We hypothesize that the sensitization relative to the side of the transgene in the animals with the AAV.ERα is caused by enhanced post-synaptic changes due to either greater DA release on the side of the transgene expression, or the effects of ERα transgene expression in the post-synaptic cell, or both processes. The control animals did not exhibit sensitization of rotational behavior contralateral to the control transgene implant, but they did exhibit an increase in total rotations. Thus, both groups showed behavioral sensitization, but in the AAV.ERα group the transgene apparently enhanced activity in the striatum on the side containing the transgene resulting in an asymmetry in the striatal response to AMPH. Since rotational behavior is related to the magnitude of the asymmetry in striatal DA (Robinson and Becker, 1986), this is indirect evidence of enhanced DA activity.
Studies to identify where estradiol acts in the brain of adult rats have previously reported that the striatum is not an area that concentrates estradiol in the nucleus or that expresses ERα mRNA (Pfaff and Keiner, 1973; Shughrue et al., 1997). On the other hand, ERα mRNA is present in the striatum during development at days 10–12 in the female rat, and there is specific binding to ER at this time (Toran-Allerand et al., 1992). In animals expressing AAV.ERα shown in Fig. 4, it is intriguing that there is little nuclear localization of the ERα. This suggests that the protein is treated differently in the striatum than in other brain regions (e.g., in the ventromedial hypothalamus) where expression is seen primarily in the nucleus (Musatov et al., 2007). Immunocytochemistry for ERα protein in the striatum of control rats finds low levels of protein immunoreactvity associated with striatal neuronal membranes of females (preliminary results from the Becker laboratory), but with the high background seen in striatum, images are not conclusive in animals not expressing ERα. Our results from Western blots indicate that there is a low level of endogenous ERα expression in striatum.
It is now apparent that all steroid hormones can have both long-term and rapid effects on target tissues (Hammes, 2003). The intracellular receptors mediating the long-term effect of steroid hormones through initiation of transcription have been known for many years (e.g., (Greene et al., 1986)). Alternate forms of estradiol receptors have also been identified that include ERβ (Kuiper et al., 1996) and ER-X (Toran-Allerand et al., 2002; Toran-Allerand, 2004, 2005). Furthermore, there is now evidence for functional interaction between the membrane and nuclear receptors (Levin, 2005; Pedram et al., 2006).
The recent finding that there are membrane progestin receptors in fish, with homologous genes found in humans and mice raises the possibility that there may be other families of receptors involved in the rapid signaling effects of other steroid hormones as well (Zhu et al., 2003a; Zhu et al., 2003b). These novel progestin receptors code proteins that appear to have seven transmembrane domains characteristic of G protein-coupled receptors. Further research will be required to determine if there are estrogen receptors homologous to these progestin receptors.
Results reported here are consistent with a role for ERα in mediating the rapid behavioral and neurochemical effects of estradiol in the striatum. It is possible that other ERs may participate in the endogenous effects of estradiol in the striatum. The results reported here demonstrate that over-expressed ERα enhances the behavioral and neurochemical effects of estradiol that are usually seen. These results support the idea that ERα is involved in the effect of estradiol on striatal-mediated behaviors.
Acknowledgments
DA12677, NSF #IBN9816673, and NS048141 to JBB; NSF agreement #IBN-9876754; R37 EB003320 to RTK. MH and KNS were supported by the NIH under the Ruth L. Kirschstein National Research Service Award T32 DA007267. Thanks to Dr. Geoffrey Greene (University of Chicago) for his gift of the H222 estrogen receptor antibody. Many thanks to Dr. Thomas Insel (NIMH) for his comments on an earlier version of this manuscript and for his encouragement and support throughout this project.
References Cited
- Becker JB. Direct effect of 17β-estradiol on striatum: sex differences in dopamine release. Synapse. 1990a;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990b;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB, Taylor JR. Sex differences in motivation. In: Becker JB, Berkley K, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: from Genes to Behavior. Oxford, UK: Oxford University Press; 2008. pp. 177–199. [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. Journal of Neuroscience. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol -17-β throughout the 4-day estrous cycle of the rat. Endocrinol. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Cardozo D, Bean B. Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. Journal of Neurophysiology. 1995;74:1137–1148. doi: 10.1152/jn.1995.74.3.1137. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- Charara A, Heilman T, Levey A, Smith Y. Pre- and postsynaptic localization of GABA(B) receptors in the basal ganglia in monkeys. Neuroscience. 2000;95:127–114. doi: 10.1016/s0306-4522(99)00409-1. [DOI] [PubMed] [Google Scholar]
- Clark K, Liu X, McGrath J, Johnson P. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Hammes SR. The further redefining of steroid-mediated signaling. Proceedings of the National Academy of Science USA. 2003;100:2168–2170. doi: 10.1073/pnas.0530224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: Effect of estradiol dose or chronic estradiol administration. Drug and Alcohol Dependence. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jerussi TP, Glick SD. Drug-induced rotation in rats without lesions: behavioral and neurochemical indices of a normal asymmetry in nigro-striatal function. Psychopharmacology (Berl) 1976;47:249–260. doi: 10.1007/BF00427609. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Leith NJ. Chronic amphetamine: Is dopamine a link in or mediator of the development of tolerance or reverse tolerance? Pharmacol Biochem Behav. 1981;15:405–413. doi: 10.1016/0091-3057(81)90270-7. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci, US. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Molecular Endocrinology. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Mermelstein PG. Membrane Estrogen Receptors Acting Through Metabotropic Glutamate Receptors: An Emerging Mechanism of Estrogen Action in Brain. Mol Neurobiol Epub Aug. 2008;2:2008. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Molecular Endocrinology. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the rat. Comp Neur. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Molecular and Cellular Biology. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Samulski R, Chang L, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue P, Lane M, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Guan XP, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: Convergence of estrogen and neurotrophin signaling pathways. Journal of Neuroscience. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore GM, Co C, Koves TR, Martin TJ, Smith JE. Time-dependent recovery from the effects of 6-hydroxydopamine lesions of the rat nucleus accumbens on cocaine self-administration and the levels of dopamine in microdialysates. Psychopharmacology. 2004;171:413–420. doi: 10.1007/s00213-003-1596-6. [DOI] [PubMed] [Google Scholar]
- Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y. Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. European Journal of Pharmacology. 1995;284:83–91. doi: 10.1016/0014-2999(95)00369-v. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Annals of the New York Academy of Sciences. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Miranda RC, Hochberg RB, MacLusky NJ. Cellular variations in estrogen receptor mRNA translation in the developing brain: evidence from combined [125I]estrogen autoradiography and non-isotopic in situ hybridization histochemistry. Brain Res. 1992;576:25–41. doi: 10.1016/0006-8993(92)90606-a. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan XP, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Nethrapalli IS, Tinnikov AA. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldwijk M, Topaly J, Laufs S. Development and optimization of a real-time quantitative PCR-based method for the titration of AAV-2 vector stocks. Mol Ther. 2002;6:272–278. doi: 10.1006/mthe.2002.0659. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Vigh J, Lasater E. L-type calcium channels mediate transmitter release in isolated, wide-field retinal amacrine cells. Vis Neurosci. 2004;21:129–134. doi: 10.1017/s095252380404204x. [DOI] [PubMed] [Google Scholar]
- Wade CNB, Dorsa DM. Estrogen activation of cyclic adenosine 5 ′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacol Biochem Behav. 1989;34:655–661. [PubMed] [Google Scholar]
- White FJ, Wolf ME. Psychomotor stimulants. In: Pratt J, editor. The Biological Bases of Drug Tolerance and Dependence. New York: Academic Press; 1991. pp. 153–197. [Google Scholar]
- Whitehead KJ, Rose S, Jenner P. Involvement of intrinsic cholinergic and GABAergis innervation in the effect of NMDA on striatal dopamine efflux and metabolism as assessed by microdialysis in freely moving rats. Eur J Neurosci. 2001;14:851–860. doi: 10.1046/j.0953-816x.2001.01702.x. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29:379–391. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jackson LR, Becker JB. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology. 2003;77:239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proceedings of the National Academy of Science USA. 2003a;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proceedings of the National Academy of Science USA. 2003b;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]