Abstract
Degeneration of the intevertebral disc represents a significant musculoskeletal disease burden. Although spinal fusion has some efficacy in pain management, spine biomechanics is ultimately compromised. In addition, there is inherent limitation of hardware-based IVD replacement prostheses, which underscores the importance of biological approaches to disc repair. In this study, we have seeded multipotent, adult human mesenchymal stem cells (MSCs) into a novel biomaterial amalgam to develop a biphasic construct that consisted of electrospun, biodegradable nanofiber scaffold (NFS) enveloping a hyaluronic acid (HA) hydrogel center. The seeded MSCs were induced to undergo chondrogenesis in vitro in the presence of transforming growth factor-β for up to 28 days. The cartilaginous HANFS construct architecturally resembled a native intervertebral disc, with an outer annulus fibrosus (AF)-like region and inner nucleus pulposus (NP)-like region. Histological and biochemical analyses, immunohistochemistry, and gene expression profiling revealed the time-dependent development of chondrocytic phenotype of the seeded cells. Taken together, these findings suggest the prototypic potential of MSC-seeded HANFS constructs for the tissue engineering of biological replacements of degenerated IVD.
INTRODUCTION
Degeneration of the intervertebral disc (IVD) is a common and significant source of morbidity in our society. From 25% to 80% of adults over the course of their life will experience an episode of significant low back pain1 and as many as 90% of these people will improve without formal treatment.2 However, for those with low back pain that does not resolve spontaneously, surgical management may be indicated.3 Historically, surgical interventions have focused on fusion of the involved IVD levels, which may eliminate pain but does not attempt to restore disc functions. Over 200,000 spinal fusions were performed in the United States in 2003 in an attempt to address the pain associated with lumbar disc degeneration, up from 134% in 1993.4
Spinal fusion is not a benign procedure; it significantly alters the biomechanics of the spine, which can cause adjacent level degeneration.5,6 As a result, there has been increasing interest in the concept of IVD replacement in the past decade.7–10 The replacement of the IVD holds tremendous potential as an alternative to spinal fusion for the treatment of degenerative disc disease (DDD) by offering an effective, motion-preserving alternative.
At the present time, two lumbar total disc replacement (TDR) implants (Charite, DePuy Spine; and Prodisc-L, Synthes Spine) are approved for human use in the United States and another two implants (Maverick, Medtronic; and Flexi-core, Stryker Spine) are far along in the Food and Drug Administration (FDA) approval process.5–8 These disc replacement technologies are all designed to preserve flexion, extension, and lateral bending motions and restore disc space height; however, they do little to address compressive forces and their longevity is unknown, but inherently limited due to their inability to biointegrate and subsequently remodel. Furthermore, metal on polyethylene, and to a lesser degree metal on metal implants, generate wear debris that can cause cellular reactions that lead to osteolysis and other potentially deleterious effects.11 In light of these drawbacks, a cell-based tissue engineered replacement disc offers a potentially promising treatment alternative for DDD, by combining the height restoration and motion preserving attributes of metallic implants with the ability for permanent biointegration.
Cell-based tissue engineering is an emerging field that involves seeding cells into a biomaterial scaffold in order to fabricate functional biological substitutes for the replacement of lost or damaged tissues.12 For successful cell-based tissue engineering, cells should ideally interact with an appropriate scaffolding material that closely mimics the structural, biological, and mechanical functions of native extracellular matrix (ECM).13 In order to achieve this goal, a scaffold material must be structurally, biochemically and biomechanically biocompatible with the seeded cells. The electrospinning method has been used to fabricate three-dimensional, porous, nano-scale fiber-based scaffolds for various tissue engineering applications.13–16 Electrospun nanofibrous scaffolds (NFS) have recently been recognized as a novel biomaterial that closely mimics the architectural scale and morphology of native fibrillar collagen, including that in the native IVD ECM. The combined characteristics of high porosity (90%), favorable mechanical properties, high surface area-to-volume ratio, and a wide range of possible pore sizes (5 to 475 μm)17 make NFS a promising and effective component for functional tissue engineering. Over the last several years, we have engineered a number of different bioengineered tissue constructs using biodegradable polymers seeded with various cell types, including adult human multipotent mesenchymals stem cells (MSCs).18–23
To develop a tissue engineering solution to IVD degeneration, the engineered construct must exhibit characteristics of structural support and discrete tissue architecture that mimics that of the IVD, i.e., annulus fibrosus (AF) and nucleus pulposus (NP). For this purpose, in this study, we have combined poly(L-lactic acid) (PLLA) NFS and hyaluronic acid (HA) hydrogel to produce an amalgam (HANFS). The addition of HA is intended to improve structural support and to enhance biocompatibility during cell differentiation and proliferation.24–28
We report here the use of the novel HANFS seeded with human bone-marrow derived MSCs to construct an engineered IVD composed of both AF- and NP-like components.
MATERIALS AND METHODS
Materials and reagents
The materials and reagents in this study were obtained from the following sources: poly (L-lactic acid) (PLLA) (MW=50,000), and poly (2-hydroxyethyl methacrylate) (poly-HEMA), Polysciences (Warrington, PA); chloroform, N,N-dimethyformamide (DMF), Fisher Scientific (Pittsburgh, PA); Dulbecco’s Modified Eagle’s Medium (DMEM, high glucose), phosphate buffered saline (PBS), and penicillin-streptomycin, Gibco BRL Life Technologies (Grand Island, NY); fetal bovine serum (FBS; selected lots), Atlanta Biologicals (Atlanta, GA); Hank’s Balanced Salt Solution (HBSS), BioSource International (Camarillo, CA); ITS-plus Premix, BD Biosciences (Bedford, MA); recombinant human transforming growth factor-β1 (TGF-β1), R&D Systems (Minneapolis, MN); Trizol reagent, glycogen, and SuperScript First-Strand Synthesis System kit for RT-PCR, Invitrogen (Carlsbad, CA); collagen type I (SP1.D8) and collagen type II antibody (II-6B3), aggrecan antibody (1-C-6), and cartilage proteoglycan link protein antibody (8-A-4), Developmental Studies Hybridoma Bank (Iowa City, IA); Broad Spectrum Histostain-SP Kit, Zymed Laboratories (San Francisco, CA); Blyscan™ sulfated glycosaminoglycan assay kit, Accurate Chemical & Scientific (Westbury, NY); RediPlate™ 96 PicoGreen dsDNA Assay, Molecular Probes (Eugene, OR); and all other reagents, Sigma Chemical (St. Louis, MO).
MSC harvest and culture
Human bone marrow derived MSCs were obtained from patients undergoing lower extremity reconstructive surgery as previously described29, and with approval by Institutional Review Board (Walter Reed Army Medical Center, Washington, DC). Briefly, bone marrow was harvested from the interior of the femoral neck and head or the intramedullary canal of long bones by using a bone curet and transferred to 50 mL conical tubes containing DMEM supplemented with 10% FBS and antibiotics (50 μg/mL of streptomycin, 50 IU of penicillin/mL). The marrow-containing tube was vigorously vortexed and a 10-cc syringe fitted with an 18-G needle was used to aspirate the homogenized bone marrow solution while leaving large debris such as bone chips to settle to the bottom of the tube.
The collected bone marrow was centrifuged at 1,000 rpm for 5 minutes, and the resultant cell pellet reconstituted in culture medium and plated in 150 cm2 cell culture flasks. Non-adherent hematopoietic and red blood cells were removed during medium changes leaving adherent MSCs attached to the culture flask. Cells were grown in a medium composed of DMEM supplemented with 10% FBS and antibiotics, and maintained at 37°C in a humidified, 5% CO2 atmosphere. Cells were consistently removed using 0.05% trypsin/EDTA solution when they reached approximately 80% confluence, and the culture medium was replaced every three days. Cells obtained at passages 1 to 3 were used in this study.
Electrospinning and construct fabrication
NFSs were fabricated by the electrospinning process as described previously16. Briefly, a solution of the biodegradable polymer, PLLA, was prepared at 0.145 g/mL by dissolving 1.6 g of PLLA in the organic solvent mixture composed of 10 mL of chloroform and 1 mL of DMF followed by vortex-mixing overnight at room temperature. The polymer solution was placed in a vertically fixed 20 mL glass syringe fitted with a 10 cm, 18-G needle. A 16 kilovolt electric field was applied at a distance of 20 cm between an aluminum foil sheet covering a copper plate and the needle tip resulting in a 0.8 kv/cm charge density (voltage/distance) on the polymer solution. After 11 mL of polymer solution was totally consumed at the rate of 1.8 mL/hr, an electrospun PLLA mat measuring 144 cm2 with a thickness of approximately 1 mm is formed homogeneously on the aluminum foil. The mat was then removed, placed in a vacuum chamber for at least 48 hours to remove organic solvent residue, and then stored in a desiccator.
The electrospun mat was cut into 1-cm2 units and both sides of the scaffold were sterilized by ultraviolet irradiation in a laminar flow hood for 30 minutes. To provide a hydrophilic surface conducive for efficient cell attachment, scaffolds were pre-wetted by immersion in HBSS for 24 hours. MSCs grown in 150 cm2 cell culture flasks were trypsinized, counted, and plated at a density of 400,000 cells/cm2 onto the surface of pre-wetted scaffolds that were placed in 24-well culture plates pre-coated with 0.3% poly-HEMA to prevent cell attachment to the tissue culture polystyrene surface. Cells were seeded onto one side of NFS and incubated at 37°C for 2 hours to allow MSCs to diffuse into and adhere to the scaffold. Two hours later, the other side was seeded with cells and incubated for additional 2 hours. During the 4 hours of incubation, 20 μL of serum-containing culture medium was applied to each cellular scaffold every 30 minutes to prevent desiccation of the constructs.
The HANFS amalgam was created by injecting approximately 250 μL of a 2 × 107 cells/ml HA slurry into the center of the NFS square using a 23-G needle (Fig. 1). This created a pressurized pouch with an inner core of HA and nanofibrous elements surrounded by a sheath of dense NFS.
Figure 1.
Schematic diagram describing HANFS construction. After isolation and expansion, MSCs are loaded onto the nanofibrous scaffold NFS (A) to ensure uniform cell distribution (B). A 250 μl aliquot of hyaluronic acid (HA)/MSC slurry is injected into the center of the NFS (C) to create the NP and tension the NFS layer to approximate the AF (D).
To induce an IVD cell-like phenotype, all cell-seeded cultures were maintained in culture medium composed of DMEM, 10 μg/ml of TGF-β1, 100 nM dexamethasone, 50 μg/mL ascorbate 2-phosphate, 100 μg/mL sodium pyruvate, 40 μg/mL proline, antibiotics (50 μg/ml of streptomycin, 50 IU of penicillin/mL), and ITS-plus Premix diluted 1:100 for final concentrations of 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenious acid, 5.33 μg/mL linoleic acid, and 1.25 mg/mL bovine serum albumin. Cell culture medium was replaced every three days.
Histological analysis
Engineered constructs were washed in PBS, fixed in 4% phosphate-buffered paraformaldehyde at 4°C for 30 minutes, dehydrated through a graded series of ethanol, infiltrated with Histo-Clear (American Mastertech Scientific), embedded in paraffin, and sectioned at 10 μm thickness. For histological analysis, sections were deparaffinized in Histo-Clear, rehydrated using a graded series of ethanol, and stained with hematoxylin and eosin (H&E) and Alcian blue (pH 1.0).
Immunohistological analysis
Immunohistochemical analysis was used to detect aggrecan, collagen types I, II and IX, and cartilage proteoglycan link protein. To detect collagen types I, II and IX, sections were pre-digested with 300 U/mL of hyaluronidase at 37°C for 15 minutes before incubation in 15 μg/mL of the respective antibodies. For the detection of aggrecan and link protein, sections were pre-digested with 1.5 U/mL of chondroitinase for 15 minutes at 37°C, and then incubated in 10 μg/mL of aggrecan antibody at 37°C for 1 hour or in 6 μg/mL of link protein antibody at 4°C overnight. Broad Spectrum alkaline phosphatase-conjugated secondary antibodies were used for immunodetection and developed using BCIP-NTB (Broad Spectrum Histostain-SP kit). Tissue sections without treatment with primary antibodies served as controls.
Scanning Electron Microscopy
Cultured cell-seeded HANFS constructs were harvested, washed in PBS, fixed in 2.5% glutaraldehyde for 20 minutes, dehydrated in a series of graded concentrations of ethanol, and vacuum-dried. Dehydrated constructs were cut, sputter-coated with gold using a sputter coating unit (MED010; BAL-TEC, Liechtenstein). Top and cross-sectional views were imaged at an accelerating voltage of 20 kV using a Hitachi Model-4500 scanning electron micrscope (Japan).
RNA isolation and RT-PCR analysis
Total RNA was isolated from HANFS constructs with 800 μL Trizol reagent. After addition of 160 μl chloroform to the homogenized samples, RNA was precipitated using 400 μL of isopropanol. RNA pellets were dissolved in 20 μL of RNase- and DNase-free water and RNA yields were estimated based on A260. First strand cDNA was reverse transcribed (RT) from 3 μg of total RNA using the SuperScript First-Strand Synthesis System kit, and gene-specific amplicans were prodcued by polymerase chain reaction (PCR) using the oligonucleotide primers shown in Table 1, for collagen types I, II, IX, X, and XI, cartilage oligomeric matrix protein (COMP), and glyceraldehydes-3-phosphate dehydrogenase (GAPDH).
TABLE 1.
Human gene-specific primer sequences for RT-PCR analysis
| Gene | Primer sequences | Expected Product Size (bp) |
|---|---|---|
| Aggrecan | 5′-TGAGGAGGGCTGGAACAAGTACC-3′ 5′-GGAGGTGGTAATTGCAGGGAACA-3′ |
350 |
| Collagen type I | 5′-GGTGTAAGCGGTGGTGGTTAT-3′ 5′-GCTGGGATGTTTTCAGGTTGG-3′ |
335 |
| Collagen type II | 5′-CAGGTCAAGATGGTC-3′ 5′-TTCAGCACCTGTCTCACCA-3′ |
377 |
| Collagen type IX | 5′-GGGAAAATGAAGACCTGCTGG-3′ 5′-CGAAAAGGCTGCTGTTTGGAGAC-3′ |
516 |
| Collagen type X | 5′-GCCCAAGAGGTGCCCCTGGAATAC-3′ 5′-CCTGAGAAAGAGGAGTGGACATAC-3′ |
703 |
| Collagen type XI | 5′-GGAAAGGACGAAGTTGGTCTGC-3′ 5′-TTCTCCACGCTGATTGCTACCC-3′ |
590 |
| Cartilage oligomeric matrix protein (COMP) | 5′-AAGGACACAGATAAGGACGG-3′ 5′-CACTGTTGGGCACTGTAGG-3′ |
286 |
| Glyceraldehyde-3- phosphate dehydrogenase (GAPDH) | 5′-GGGCTGCTTTTAACTCTGGT-3′ 5′-GCAGGTTTTTCTAGACGG-3′ |
702 |
GAPDH gene expression was used as an internal control for mRNA loading. Thirty-two cycles were used to amplify all gene sequences, and the PCR products were electrophoretically analyzed with ethidium bromide staining.
Sulfated glycosaminoglycan assay
Sulfated glycosaminoglycan (GAG) content was quantified using a commercially available assay kit (Blyscan). sGAGs were extracted from HANFS cultures following a method modified from a previous report.30 Briefly, samples were harvested, washed with PBS, and digested with 300 μg/mL papain in 20 mM sodium phosphate, pH 6.8, 5 mM EDTA, 2 mM DTT at 60°C for 12 hours. The extract was cleared by centrifugation and analyzed for sGAG and DNA. For sGAG, the sample was reacted with the Blyscan dye reagent, 1, 9-dimethylmethylene blue (DMMB), for 30 minutes. Unbound dye was removed by centrifugation, and bound dye was released from the insoluble sGAG-dye complex, and quantified spectrophotometrically on the basis of A656. The total amount of sGAG was estimated from a standard curve generated using chondroitin 4-sulfate as a standard. Results were expressed as the ratio of sGAG amount to DNA amount. For DNA, the extract was assayed using the RediPlate 96 PicoGreen dsDNA methods (Invitrogen, Carlsbad, CA).
Statistical analysis
Data collected from quadruplicate samples are expressed as mean ± SD, and analyzed statistically with a two-tailed Student’s t test, with significance determined at p < 0.05.
RESULTS
Generation of an AF region
The HANFS construct fabricated here approximated the two regions of the native IVD, the NP and AF. The exterior or cortical region consists of non-woven electrospun nanofibers that became tensioned after HA injection (Fig. 1). MSCs were loaded into this region prior to HA injection to ensure uniform distribution of cells. Histological and immunohistochemical staining was performed at 7, 14 and 28 days and confirmed both uniform distribution of cell loading as well as cell morphology changes during the experimental period. H&E staining demonstrated uniform cell loading in the AF region at the early time points. At later time points, the cells began to elongate and became layered in a concentric fashion, similar to the microarchitecture of the native AF, which is organized in a series of concentric fibrous rings that impart much of the tensile strength to the IVD.31 Increased cartilaginous ECM deposition was also detected with alcian blue staining in the histological sections, with complete filling of the pores within the NFS in the AF region by Day 28 (Fig. 2). Increased sulfated proteoglycan content in the ECM was detected over the culture period as seen by increasing alcian blue staining on histological sections, and as noted by significant increases in sGAG content of the constructs as determined using the DMMB assay (see below).
Figure 2.
Alcian blue and haematoxylin and eosin staining of HANFS constructs at 7, 14 and 28 days of culture. The outer region (or AF-like region) and the interior region (or NP-like region) both demonstrate increasing cellularity and proteoglycan deposition during the experimental period. Scale bar = 100 μm.
Generation of an NP region
Initially, the NP region appeared to be sparsely populated with cells and showed little ECM deposition (Fig. 2). Later in the culture period, after deposition of more ECM, cells were detected in greater numbers and they appeared rounded and encapsulated, notably different in morphology from the layered cells in the cortical AF region of the construct. Over time in culture, the adjacent AF and NP regions assumed the different histological features described above, with the AF completely encapsulating the NP region.
ECM production
Alcian blue staining, which revealed a sulfated proteoglycan-rich ECM, showed increasing intensity in the HANFS construct through the 28 day culture period, with the most intense staining localized to a ring-like zone in the AF region (Fig. 2). Alcian blue staining of the NP appeared amorphous without distinct organization. This staining pattern correlated with the intended architectural design of the construct, i.e., a cross section consisting of an organized ring-like structural barrier enveloping a relatively amorphous center. It was noteworthy that an integrated transition formed between the two regions in these constructs, and approximated that for the AF and NP regions in native human IVD, where there is no distinct division between the two regions.
Immunohistochemical staining for known IVD ECM components showed the presence of collagen type I, II, collagen type IX (not shown), aggrecan and link protein. The staining pattern was similar to that seen with alcian blue staining, with intensity increasing over the 28-day culture period (Fig. 3). Substantial deposition collagen types I, II and IX, aggrecan and link protein was first noted in the immediate pericellular area, with increasing signal in the intercellular matrix over 28 days. The presence of these ECM components was consistent with the cartilage phenotype of a native IVD. Specifically, collagen types II and IX indicated the presence of a fibrillar collagen network, in conjunction with a proteoglycan-rich matrix as shown by the intense staining for aggrecan and link protein.
Figure 3.
Immunohistochemistry of HANFS constructs at 7, 14 and 28 days. Antibodies for collagen type I (col I), collagen type II (col II), aggrecan (agg) and link protein (Link) were used to probe the constructs. The intensity of immunostaining intensity increased over the experimental period in both the AF-like and NP-like regions of the construct. Scale bar = 100 μm.
Scanning electron microscopy revealed uniform cell distribution in the NFS, similar to that observed in our previous cartilage tissue engineering studies.18 Matrix accumulation was apparent in both the AF and NP regions and continued to increase over time, filling the small pores between the nanofibers and the larger void within the NP region (Fig. 4). Nanofiber architecture remained intact for the duration of the experiment and ultimately became intimately associated with the ECM produced by the seeded cells.
Figure 4.
Scanning electron microscopy of HANFS constructs at 7, 14 and 28 days. Cells initially adhered to individual nanofibers in both the AF and NP regions of the construct, and increasing amounts of ECM was deposited as a function of time. Scale bar = 10 μm.
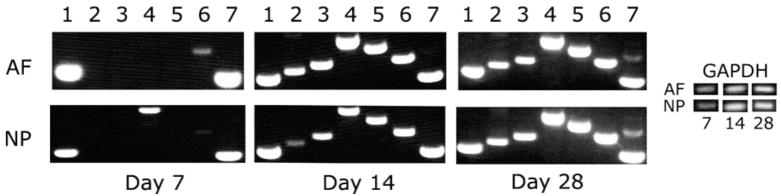
RT-PCR was performed to assess the expression of ECM genes characteristic for the cartilage phenotype of the IVD. Specifically, collagen types I, II, IX, X, and XI, aggrecan and COMP were all found to be expressed by Day 14, with collagen type I and COMP expression occurring as early as Day 7 (Fig. 5). Of particular significance was the initiation and maintenance of collagen type II and type IX expression. Expression of collagen types II and IX generally required higher density cell culture in a three dimensional microenvironment.19, 32; these conditions were thus apparently achieved in the HANFS constructs.
Figure 5.
mRNA expression of the AF and NP regions of the HANFS construct at day 7, 14 and 28 in culture, analyzed by RT-PCR. Lane 1= col I, 2=col II, 3=col IX, 4=col X, 5=col XI, 6=aggrecan, 7=cartilage oligomeric matrix protein (COMP). GAPDH level was used as a control.
Production of sGAG was also detected in the engineered constructs. The DMMB assay showed sGAG accumulation as early as Day 7, and the levels increased over the 28-day culture period (Fig. 6).
Figure 6.
Quantitative analysis of sGAG production in entire HANFS construct at 7, 14 and 28 days using the DMMB-based assay. The most significant increases in proteoglycan production are noted between the 7 and 14 day time points. Values are mean ± S.D. *, p < 0.05, n = 3)
DISCUSSION
In this study, we report the successful production of a biphasic cartilaginous tissue construct, using a novel HANFS biomaterial amalgam seeded with adult human MSCs. The biphasic architecture and ECM distribution and composition of the constructs resemble those of native IVD, suggesting that these constructs have the potential to develop and mature into IVD-like tissues. While cell-based tissue engineering has been considered a promising approach to tissue repair and regeneration, applications to IVD have been limited. While other investigators have engineered portions of the IVD,33 this is the first construct to be developed as a complete unit, possessing both AF and NP regions. Since the IVD fails as a unit, an ideal biological solution must address the entire disc, not just one component. Furthermore, the fundamental challenge for partial IVD constructs has been their inability to restore the complete IVD as a functionally integrated unit.33, 34 In this study, we have demonstrated in vitro that MSCs loaded on a HANFS scaffold can produce a bioengineered cartiliaginous tissue that exhibits the distinct anatomical architecture and biosynthetic features of the native human IVD.
While multiple biological approaches have been considered for the treatment of DDD, including biologics- and gene-based therapies, we will focus our discussion on tissue-engineered solutions and their comparison to current TDR options.35–37 The current tissue engineering approaches for IVD replacement primarily focus on regenerating a specific component of the IVD.38–42 Specifically, the NP was the first and continues to be the most frequent target for cell-based tissue engineered solutions for IVD pathology.43,44 The aim of the approach reported here is the introduction of in vitro cultured IVD using an MSC-based tissue engineering approach to restore function of the degenerated IVDs. Sato et al. have developed a true tissue engineered construct to regenerate injured portions of the IVD, in that it is composed of allogeneic AF cells loaded on a three-dimensional collagen scaffold, rather than simply injecting a slurry of cultured cells.45 This construct was used to fill lacunae created following laser vaporization of the NP in Japanese white rabbits. At 12 weeks, 90% of the transplanted cells were alive, and this construct preserved disc height significantly better than controls. The annular hole created by the laser fiber was filled in with AF cells and a hyaline-like ECM by the completion of the experiment. The biocompatibility and successful integration of this experimental construct provide optimism for the more challenging endeavor presented here to regenerate an entire IVD, rather than restoring regions resected or lost during treatment of herniated NP.
Meisel et al. have taken cell-based therapies to the next step using autologous disc chondrocyte transplantation (ADCT), reporting results at 2 year follow-up in the first 28 patients (12 received ADCT) enrolled in an unblinded randomized (no control) multi-center clinical trial (EuroDisc Study). This study compares discectomy alone to combined discectomy and ADCT in patients presenting with a single level herniated NP but minimal to no DDD changes. No complications or adverse reactions were reported. The only significant improvement in Oswestry Low Back Pain Disability Questionaire score (the primary outcome measure) came immediately after discectomy (69–71% improvement in total score) for both treatment groups. However, the investigators did point out that results after surgery immediately plateaued in the discectomy alone group but slightly increased (median total score of 9 at 3 months, improved to 2 by 2 years) in the ADCT treated patients over the study period. The most promising finding in the study was that ADCT treated discs appeared to be rehydrating, demonstrating 41% normal fluid content compared to only 25% in the discectomy alone group. This study suggests that transplanted ADCTs remain viable and restore proteoglycan and collagen type II production, thus correlating to the findings from their previous canine model.46 While the results of this clinical study may not be dramatic, the fact that cell-based IVD therapies are being clinically tested shows promise for future cell-based therapies. Although the approaches and components used to tissue engineer an IVD (complete or partial) have so far been unique for each study, it is generally believed that biology, rather than metallurgy or prosthetic development, is ultimately critical for an ideal solution for DDD.
In the future, bioengineered disc replacements should outperform traditional metal and synthetic devices currently used in spine surgery. Unlike metal and synthetic polymer devices, a bioengineered replacement disc will not release exogenous wear particles over the life of the implant. Currently, approved TDRs employ polyethylene as a bearing, which will release submicron sized debris particles as a function of wear. In total joint arthroplasty, these particles elicit biological reactions that lead to osteolysis and aseptic loosening of the prosthesis. Newer TDRs have adopted a metal-on-metal design. While the wear rates of these constructs are significantly lower than those for metal-on-polyethylene prostheses, the long-term biological effects, both local and systemic, of metal ions created when this type of prosthesis wears are still unknown. Additionally, metal ions from spinal implants, while to a significantly lower degree, have been demonstrated to incite wear-induced osteolysis.47, 48
In contrast, bioengineered IVDs have the potential for minimal to no antigencity, and most importantly, can biointegrate. Ideally, the bioengineered IVD should be capable of remodeling and repair. Thus, unlike metal prosthesis that begin to wear from the time they are implanted, a biointegrated disc may hold the potential to maintain and improve its function with time. Bioengineered IVDs should therefore be the ultimate goal for treatment of DDD.
Bioengineering an IVD requires the combination of an ideal cell source, biomaterial scaffold, and enabling biological signals. As noted above, previous IVD tissue engineering studies, have investigated the proliferative and regenerative potential of mature NP and AF cells.40 These cell types have the disadvantage of being scarce and difficult to safely harvest.33 The amount of harvestable NP cells is greatly reduced in degenerated IVDs and harvesting requires injury to the annulus (needle puncture, typically). Most pragmatic of concerns is that autologous harvesting may not be financially and commercially sustainable. A very viable option is thus the use of MSCs. A number of studies have demonstrated the potential use of MSCs in IVD tissue engineering with promising results.49–52 MSCs may provide a more ideal cell source for the regeneration of the two distinct regions of the IVD for the following reasons: (1) they are easily accessible for harvest, with bone marrow being a frequently used source; (2) they possess extensive self-renewal and expansion capability; (3) they possess little to no immunogenicity; and (4) most importantly, as demonstrated in this study, they are capable of differentiating into cells phenotypically and biosynthetically similar to those found in AF and NP regions of the IVD. These characteristics make MSCs well suited for cell-based tissue engineering of an IVD.
In this study, the MSCs were seeded into a novel HANFS construct; the cells proliferated, differentiated and produced a proteoglycan-rich ECM with a protein expression profile similar to that of a native IVD. While there are many biocompatible polymers used in IVD tissue engineering45,53,54, we have found that electro-spun PLLA nanofibers, with the unique characteristic of nanofiber size that exponentially increases the surface area of the scaffold, allow for efficient cell loading and generation of an environment highly conducive to chondrogenesis. The efficient production of a proteoglycan-rich matrix within the NFS is critical for IVD tissue engineering, since diminished proteoglycan production and disc dehydration are central to DDD.
The biphasic HANFS scaffold and IVD growth medium directed region-specific differentiation of MSCs into cells with phenotypes and biosynthetic activities resembling those of the two distinct regions in the native IVD. Our preliminary studies reported here thus demonstrate the potential for this specific construct to be a prototype for further research into the creation of an ideal tissue engineered IVD.
Future studies will evaluate the mechanical properties of the MSC-seeded HANFS constructs and to optimize the culture conditions. It is likely that exposure of the constructs to mechanical stimulation during culture will improve ECM production, metabolism, and the mechanical characteristics of the tissue engineered IVD.55,56 In addition, a number of growth factors have been shown to play a role in IVD development, maintenance and repair.57–60 The importance of these growth factors in IVD tissue engineering will be studied to optimize culture conditions and construct function.
CONCLUSION
Degenerative disc disease is a widespread problem with no biologic solution, and for which current surgical options have produced variable results. The search for a biologic solution for DDD is in its infancy. Currently, research is focused on proof-of-concept investigations, such as the study reported here. We have demonstrated here that a tissue construct that resembles the overall gross, histological, biochemical, and biosynthetic properties of a native IVD can be engineered by implanting human MSCs into a novel HANFS scaffold.
Acknowledgments
This work is supported by the Intramural Research Program of the NIAMS, NIH (ZO1 AR41131).
References
- 1.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine. 2006;31:2724. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 2.Manchikanti L. Epidemiology of low back pain. Pain Physician. 2000;3:167. [PubMed] [Google Scholar]
- 3.Fritzell P, Hagg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cowan JA, Jr, Dimick JB, Wainess R, Upchurch GR, Jr, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59:15. doi: 10.1227/01.NEU.0000219836.54861.CD. [DOI] [PubMed] [Google Scholar]
- 5.Panjabi M, Henderson G, Abjornson C, Yue J. Multidirectional testing of one- and two-level ProDisc-L versus simulated fusions. Spine. 2007;32:1311. doi: 10.1097/BRS.0b013e318059af6f. [DOI] [PubMed] [Google Scholar]
- 6.Huang RC, Girardi FP, Cammisa FP, Jr, Lim MR, Tropiano P, Marnay T. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine. 2005;30:1407. doi: 10.1097/01.brs.0000166528.67425.0e. [DOI] [PubMed] [Google Scholar]
- 7.Zigler J, Delamarter R, Spivak JM, Linovitz RJ, Danielson GO, 3rd, Haider TT, Cammisa F, Zuchermann J, Balderston R, Kitchel S, Foley K, Watkins R, Bradford D, Yue J, Yuan H, Herkowitz H, Geiger D, Bendo J, Peppers T, Sachs B, Girardi F, Kropf M, Goldstein J. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 8.McAfee PC, Cunningham B, Holsapple G, Adams K, Blumenthal S, Guyer RD, Dmietriev A, Maxwell JH, Regan JJ, Isaza J. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30:1576. doi: 10.1097/01.brs.0000170561.25636.1c. [DOI] [PubMed] [Google Scholar]
- 9.Le Huec JC, Mathews H, Basso Y, Aunoble S, Hoste D, Bley B, Friesem T. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36:315. doi: 10.1016/j.ocl.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Valdevit A, Errico TJ. Design and evaluation of the FlexiCore metal-on-metal intervertebral disc prosthesis. Spine J. 2004;4:276S. doi: 10.1016/j.spinee.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Shuff C, An HS. Artificial disc replacement: the new solution for discogenic low back pain? Am J Orthop. 2005;34:8. [PubMed] [Google Scholar]
- 12.Langer R, Vacanti JP. Tissue engineering. Science (New York, NY) 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 13.Venugopal J, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11:847. doi: 10.1089/ten.2005.11.847. [DOI] [PubMed] [Google Scholar]
- 14.Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Abukawa H, Terai H, Hannouche D, Vacanti JP, Kaban LB, Troulis MJ. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61:94. doi: 10.1053/joms.2003.50015. [DOI] [PubMed] [Google Scholar]
- 17.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 18.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 22.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 23.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomaterialia. 2006;2:377. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003;28:446. doi: 10.1097/01.BRS.0000048672.34459.31. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer M, Boudriot U, Pfeiffer D, Ishaque N, Goetz W, Wilke A. Intradiscal application of hyaluronic acid in the non-human primate lumbar spine: radiological results. Eur Spine J. 2003;12:76. doi: 10.1007/s00586-002-0478-7. [DOI] [PubMed] [Google Scholar]
- 26.Stern S, Lindenhayn K, Schultz O, Perka C. Cultivation of porcine cells from the nucleus pulposus in a fibrin/hyaluronic acid matrix. Acta Orthop Scand. 2000;71:496. doi: 10.1080/000164700317381207. [DOI] [PubMed] [Google Scholar]
- 27.Inkinen RI, Lammi MJ, Agren U, Tammi R, Puustjarvi K, Tammi MI. Hyaluronan distribution in the human and canine intervertebral disc and cartilage endplate. Histochem J. 1999;31:579. doi: 10.1023/a:1003898923823. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Toole BP, Dealy CN, Kosher RA. Hyaluronan in limb morphogenesis. Dev Biol. 2007;305:411. doi: 10.1016/j.ydbio.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesti LJ, Caterson EJ, Wang ML, Chang R, Chapovsky F, Hoek JB, Tuan RS. TGF-β1 calcium signaling increases α5 integrin expression in osteoblasts. J Orthop Res. 2002;20:1042. doi: 10.1016/S0736-0266(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 30.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 31.Williams KD, Park AL. Lower Back Pain and Disorders of Intervertebral Discs. In: Canale ST, Campbell WC, editors. Campbell’s Operative Orthopaedics. St. Louis: Mosby; 2003. [Google Scholar]
- 32.Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16:447. doi: 10.1007/s00586-006-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilke HJ, Heuer F, Neidlinger-Wilke C, Claes L. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur Spine J. 2006;15(Suppl 3):S433. doi: 10.1007/s00586-006-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida K, Doita M, Takada T, Kakutani K, Miyamoto H, Shimomura T, Maeno K, Kurosaka M. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine. 2006;31:1415. doi: 10.1097/01.brs.0000219945.70675.dd. [DOI] [PubMed] [Google Scholar]
- 36.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, Gilbertson LG, Kang JD. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 37.Wallach CJ, Kim JS, Sobajima S, Lattermann C, Oxner WM, McFadden K, Robbins PD, Gilbertson LG, Kang JD. Safety assessment of intradiscal gene transfer: a pilot study. Spine J. 2006;6:107. doi: 10.1016/j.spinee.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006;15(Suppl 3):S406. doi: 10.1007/s00586-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, Lin TW, Lin WC, Huang TY, Deng WP. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 40.Le Visage C, Yang SH, Kadakia L, Sieber AN, Kostuik JP, Leong KW. Small intestinal submucosa as a potential bioscaffold for intervertebral disc regeneration. Spine. 2006;31:2423. doi: 10.1097/01.brs.0000238684.04792.eb. [DOI] [PubMed] [Google Scholar]
- 41.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 42.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 43.Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15(Suppl 3):S397. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maldonado BA, Oegema TR., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 45.Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, Fujikawa K. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine. 2003;28:548. doi: 10.1097/01.BRS.0000049909.09102.60. [DOI] [PubMed] [Google Scholar]
- 46.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 47.von Knoch M, Jewison DE, Sibonga JD, Sprecher C, Morrey BF, Loer F, Berry DJ, Scully SP. The effectiveness of polyethylene versus titanium particles in inducing osteolysis in vivo. J Orthop Res. 2004;22:237. doi: 10.1016/j.orthres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Hallab NJ, Cunningham BW, Jacobs JJ. Spinal implant debris-induced osteolysis. Spine. 2003;28:S125. doi: 10.1097/00007632-200310151-00006. [DOI] [PubMed] [Google Scholar]
- 49.Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, Shapiro IM. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29:2627. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 50.Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ. Stem cell regeneration of the nucleus pulposus. Spine J. 2004;4:348S. doi: 10.1016/j.spinee.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 51.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 52.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 53.Wilda H, Gough JE. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglass composite films. Biomaterials. 2006;27:5220. doi: 10.1016/j.biomaterials.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Rong Y, Sugumaran G, Silbert JE, Spector M. Proteoglycans synthesized by canine intervertebral disc cells grown in a type I collagen-glycosaminoglycan matrix. Tissue Eng. 2002;8:1037. doi: 10.1089/107632702320934137. [DOI] [PubMed] [Google Scholar]
- 55.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Relat Res. 1977:101. [PubMed] [Google Scholar]
- 56.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982:296. [PubMed] [Google Scholar]
- 57.Imai Y, Okuma M, An HS, Nakagawa K, Yamada M, Muehleman C, Thonar E, Masuda K. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007;32:1197. doi: 10.1097/BRS.0b013e3180574d26. [DOI] [PubMed] [Google Scholar]
- 58.Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007;32:1174. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 59.Tsai TT, Guttapalli A, Oguz E, Chen LH, Vaccaro AR, Albert TJ, Shapiro IM, Risbud MV. Fibroblast growth factor-2 maintains the differentiation potential of nucleus pulposus cells in vitro: implications for cell-based transplantation therapy. Spine. 2007;32:495. doi: 10.1097/01.brs.0000257341.88880.f1. [DOI] [PubMed] [Google Scholar]
- 60.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]