Abstract
IMPORTANCE
Stroke is a major complication of surgical aortic valve replacement.
OBJECTIVE
To determine the effectiveness and safety of cerebral embolic protection devices in reducing ischemic central nervous system injury during surgical aortic valve replacement
DESIGN, SETTING and PARTICIPANTS
A parallel-group trial conducted in 18 North American centers, randomizing patients with calcific aortic stenosis undergoing surgical aortic valve replacement between March 2015 and July 2016.
INTERVENTIONS
Use of one of two cerebral embolic protection devices (suction-based extraction;n=118 or intra-aortic filtration device;n=133) versus a standard aortic cannula (n=132) at the time of surgical aortic valve replacement.
MAIN OUTCOMES AND MEASURES
The primary endpoint was freedom from clinical or radiographic central nervous system (CNS) infarction at 7±3 days post-procedure. Secondary endpoints included a composite of clinical ischemic stroke, acute kidney injury, and death ≤30 days after surgery; delirium; mortality; serious adverse events and neurocognition.
RESULTS
Among 383 randomized patients (mean age 73.9, 38.4% women), freedom from CNS infarction at 7 days did not differ between suction-based extraction and controls (32.0 vs. 33.3%,difference −1.3,95%CI−13.8, 11.2) nor between intra-aortic filtration and controls (25.6% vs 32.4%, −6.9,95%CI−17.9, 4.2). Clinical stroke occurred in 5.1% suction-based extraction vs 5.8% controls (−0.7, 95%CI −6.5, 5.1) and 8.3% intra-aortic filtration vs. 6.1% controls (2.2, 95%CI −4.1, 8.4). Delirium trajectories (baseline to day 7) differed for suction-based extraction vs. control (p= 0.03) and intra-aortic filtration vs. control (p=0.02); by day 7, 6.3% of suction-based extraction vs 15.3% of control patients (−9.1, 95%CI −17.1, −1.0), and 8.1% of intra-aortic filtration vs 15.6% of control patients (−7.4, 95%CI−15.5, 0.6) experienced delirium. The 30-day composite endpoint did not differ between suction-based extraction and controls (21.4% vs 24.2%; −2.8, 95%CI −13.5,7.9) nor between intra-aortic filtration and controls (33.3% vs 23.7%; 9.7, 95%CI −1.2,20.5). Neurocognitive outcomes were not different between groups except for less decline in executive function in intra-aortic filtration vs controls (p=0.05).
CONCLUSIONS AND RELEVANCE
The incidence of CNS infarction after surgical aortic valve replacement was not altered by either embolic protection device. Potential benefits for delirium reduction, cognition and symptomatic stroke merit larger trials with longer follow-up.
The prevalence of aortic stenosis increases with aging and the expanding number of U.S. patients requiring surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) is estimated to be 100,000 per year.1,2 Steady improvements in patient selection, surgical techniques, and perioperative management have improved survival and quality of life for these patients. However, concerns remain about the incidence of central nervous system (CNS) infarction, a serious complication of SAVR. Recent studies documented a high incidence of radiographic brain infarcts –up to 60% on post-operative magnetic resonance imaging (MRI) scans, although the vast majority are subclinical.3,4 In a large cohort study, 17% of SAVR patients (enrolled 2008–2012) experienced clinical stroke, about 25% of which were moderate or severe5. Whereas the effect of perioperative stroke on survival, quality of life, and cost is well-established,6,7 the effect of silent (non-symptomatic) cerebral infarcts on these outcomes is unknown.
These concerns stimulated the development of embolic protection devices. Currently two devices are approved in the U.S. The Embol-X (Edwards Lifesciences, CA) intra-aortic filtration device has been shown to be safe and capture emboli, but not reduce stroke in a mostly low-risk CABG population8. The CardioGard (CardioGard, Israel) device extracts both particulate and gaseous emboli through a suction sideport of the aortic cannula. A small trial of this device in SAVR patients reported a significant reduction in total volume and number of radiographically-detected brain lesions, but the effect on cognitive outcomes was not evaluated9. As such, more rigorous data are needed on the value of embolic protection devices in reducing ischemic CNS injury, documented by clinical and radiographic means.10
This trial evaluates the effectiveness and safety of these embolic protection devices in a high-risk setting for CNS infarction, namely SAVR, and expands the evidence base by including clinical, radiographic, and cognitive outcomes within 90 days.
METHODS
TRIAL DESIGN AND OVERSIGHT
This trial, conducted at 18 centers, compared one of two approved embolic protection devices to a standard aortic cannula. There was a Coordinating Center, an events adjudication committee (EAC), and a National Institutes of Health (NIH)-appointed Data and Safety Monitoring Board overseeing trial progress. Participating institutional review boards approved the protocol; all patients gave written informed consent.
PATIENTS AND INTERVENTIONS
The trial randomized patients ≥ 60 years of age undergoing SAVR for AS with minimal or no deficits on the pre-operative NIH Stroke Scale (NIHSS;i.e. score ≤1) and modified Rankin scale (mRS;i.e. ≤ 2) within 7 days of randomization. Key exclusion criteria included prior clinical stroke <3 months of randomization, cardiac catheterization, cerebral and/or aortic arch angiography <3 days of planned SAVR, and active endocarditis. Patients were randomized immediately after sternotomy and stratified by center and procedure (isolated AVR versus AVR+CABG±MVrepair). Patients were followed from March 2015 to December 2016.
The EMBOL-X® device uses intra-aortic filtration to remove particulate emboli, with a heparin-coated polyester mesh filter (intra-aortic filtration device). Filter size was determined by measuring size of the distal ascending aorta (CT scan or intraoperative measurement). The CardioGard® device extracts particulate and air emboli through a suction sideport located posterior to the main port of an aortic perfusion cannula (suction-based extraction device). In the control group, a standard aortic perfusion cannula was used (see Appendix).
ENDPOINTS
Patients were assessed at baseline and days 1, 3, 7, 30 and 90 post-operatively, with investigators blinded to endpoint data. The primary endpoint was freedom from clinical or radiographic CNS infarction at 7 (± 3) days post procedure. Radiographic CNS infarcts were identified using 1.5 or 3.0 T diffusion-weighted magnetic resonance imaging (DW-MRI). Imaging-based stroke ascertainment was supplemented by serial neurological assessment using the NIHSS (days 1, 3, 7), and reporting of ischemic stroke adverse events detected during hospitalization. All MRIs were read by a core lab and stroke events with clinical findings (NIHSS≥2) were adjudicated by an EAC, consisting of vascular neurologists. The composite secondary endpoint was clinical ischemic stroke (including newly MRI-detected CNS infarcts associated with focal findings by NIHSS <7 days post-operatively), acute kidney injury (AKI), or death ≤30 days after surgery. Other secondary endpoints included volume and number of radiographic brain lesions, delirium (3D-CAM/CAM-ICU) ≤ 7 days post-operatively, modified Rankin scale, Barthel index, Geriatric Depression Scale, survival, serious adverse events, and readmissions within 90 days. Quality of life (SF-12; physical and mental sub-scales), and cognition in 6 domains (verbal memory, visual memory, executive functioning, visuospatial/constructional praxis, attention, and information processing speed) were assessed at 90 days. See Appendix for definition and assessment of outcomes.
STATISTICAL ANALYSIS
Patients were randomized with equal allocation into one of two embolic protection device groups and one control group. Random permuted blocks of size 3, 6 and 9 were used. The randomization sequence was generated by a trial statistician and randomization assignment was controlled centrally through a web-based data collection system and performed in the OR. A sample size of 165 patients in each group ensured that each comparison had a power of approximately 90% to detect a difference of 17.5% from an assumed control rate of 50% in the incidence of post-operative CNS infarcts between groups, which corresponds to a 35% reduction in risk. A single interim analysis was planned and performed and was based on group sequential-monitoring with Lan–DeMets efficacy boundaries defined by an O’Brien–Fleming spending function. The consideration of halting for futility was pre-specified if conditional power was below 20% (Appendix). Intention-to-treat chi-squared tests, performed at the nominal 0.05 level (two-sided), were used to test hypotheses about differences between devices and controls. No adjustment was made to the Type I Error rate as these two planned comparisons are separate comparisons of each device group to a shared control. At the interim analysis, randomization, but not follow-up, was halted due to low conditional power of observing any between-group differences for the primary endpoint. At that point, 383 patients had been randomized.
The primary endpoint analysis employed an iterative hot-deck multiple imputation approach, assuming a non-ignorable missing data mechanism (Appendix). The proportions of patients who experienced the composite clinical endpoint were compared using chi-squared tests. Volume of brain infarcts were compared by permutation test. Negative binomial models were used to analyze number of brain infarcts, instead of protocol-defined zero-inflated Poisson regression models, because model fit was better. All-cause mortality was analyzed using the log-rank test and differences in adverse event rates were tested using Poisson regression. The incidence of delirium at days 1, 3 and 7 were compared using chi-squared tests and the trajectory of delirium incidence over time was compared using generalized estimating equations (GEE) logistic regression models. Decline in scores for cognitive domains (decrease of 0.5 standard deviation (SD) at 90 days) were compared between groups using logistic regression models, adjusting for baseline scores. Quality of Life (QoL) was assessed using t-tests, and depression, modified Rankin scores(> 2), and Barthel Index scores (≤80) were assessed using chi-square tests (see online supplement for the protocol). All analyses were conducted using SAS version 9.4 (SAS, Cary, NC).
RESULTS
PATIENTS
A total of 870 patients were screened and found eligible (Figure 1), with 383 randomized to intra-aortic filtration (n=133), suction-based extraction (n=118), or a shared control group (n=132). As the trial began with randomization to intra-aortic filtration or control, the first 12 control patients served as controls for intra-aortic filtration only since the suction-based extraction device was not then clinically available. With the addition of the suction-based extraction device to the trial, the subsequent 120 control patients were part of the shared control group. The randomized groups had similar baseline characteristics (Table 1). Preoperatively, 36.3% of suction-based extraction patients and 25.7% of their controls, as well as 29.8% of intra-aortic filtration patients and 25.8% of their controls had severe cognitive impairment (below 2 SDs from the mean of an age-standardized population) in one or more of the domains specified in Table 1.
Figure 1. CONSORT Diagram.
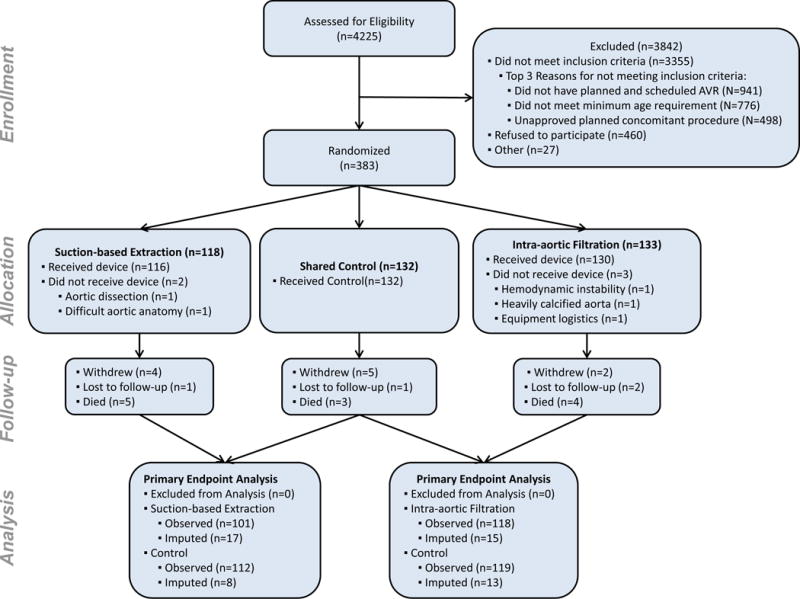
As the trial began with randomization to intra-aortic filtration or control, the first 12 control patients served as controls for intra-aortic filtration only and 120 patients were common to both control groups.
Table 1.
Baseline and Operative Characteristics
| Suction-based Extraction (N=118) |
Controla (N=120) |
Intra-aortic Filtration (N=133) |
Controla (N=132) |
|
|---|---|---|---|---|
| Baseline Characteristicsb | ||||
| Male sex | 69 (58.5) | 77 (64.2) | 81 (60.9) | 86 (65.2) |
| White | 108 (91.5) | 107 (89.2) | 126 (94.7) | 118 (89.4) |
| Age – yrs | 74.6 (6.8) | 73.4 (6.7) | 73.6 (6.6) | 73.6 (6.7) |
| Medical and surgical history | ||||
| Diabetes | 48 (40.7) | 36 (30.0) | 36 (27.1) | 37 (28.0) |
| Renal insufficiency | 15 (12.7) | 13 (10.8) | 18 (13.5) | 14 (10.6) |
| Myocardial infarction | 16 (13.6) | 8 (6.7) | 15 (11.3) | 10 (7.6) |
| Atrial fibrillation | 14 (11.9) | 16 (13.3) | 13 (9.8) | 16 (12.1) |
| Stroke or TIA | 16 (13.6) | 8 (6.7) | 11 (8.3) | 8 (6.1) |
| SF-12c | ||||
| Physical Health Composite Score | 41.4 (10.6) | 40.5 (11.2) | 40.1 (11.0) | 40.2 (11.2) |
| Mental Health Composite Score | 53.2 (9.3) | 52.9 (9.3) | 52.9 (9.6) | 52.9 (9.4) |
| Severe Cognitive Impairmentd | ||||
| Verbal Memory | 16/114 (14.0) | 14/116 (12.1) | 19/127 (15.0) | 16/128 (12.5) |
| Executive Function | 18/98 (18.4) | 15/106 (14.2) | 21/119 (17.6) | 18/117 (15.4) |
| Auditory-Verbal Simple Attention | 5/115 (4.3) | 6/116 (5.2) | 4/127 (3.1) | 6/128 (4.7) |
| Visuomotor/Information Processing Speed | 9/109 (8.3) | 9/113 (8.0) | 11/123 (8.9) | 9/125 (7.2) |
| At least one deficit | 37/102 (36.3) | 28/109 (25.7) | 36/121 (29.8) | 31/120 (25.8) |
| White Matter Lesion Volume (mm3) | 4592 (2433, 8377) | 4719 (2201, 9776) | 6303 (2686, 10027) | 4704 (2265, 9776) |
| Presence of Large Cortical Lesionse | 1/96 (1.0) | 2/107 (1.9) | 2/109 (1.8) | 2/114 (1.8) |
| Maximum Atheroma Gradef | 2.5 (0.7) | 2.4 (0.6) | 2.3 (0.7) | 2.3 (0.6) |
| Operative Characteristicsb | ||||
| Surgical Procedure | ||||
| Isolated AVR | 67 (56.8) | 73 (60.8) | 77 (57.9) | 80 (60.6) |
| AVR & CABG | 50 (42.4) | 47 (39.2) | 51 (38.3) | 52 (39.4) |
| AVR & MV Repair ± CABG | 1 (0.8) | 0 | 5 (3.8) | 0 |
| Concomitant proceduresg | 17 (14.4) | 19 (15.8) | 23 (17.3) | 20 (15.2) |
| Duration of cardiopulmonary bypass – min | 104.9 (39.6) | 102.2 (40.2) | 109.1 (42.4) | 101.7 (39.8) |
| Debris Captured in Filter(s) | 79/106 (74.5) | - | 115/116 (99.1) | - |
| Type of Debris Captured | ||||
| Calcification | 30/106 (28.3) | - | 23/116 (19.8) | - |
| Valve Tissue and/or Arterial Wall | 53/106 (50.0) | - | 113/116 (97.4) | - |
| Platelet-Rich Thrombus | 55/106 (51.9) | - | 39/116 (33.6) | - |
| Other | 8/106 (7.5) | - | 13/116 (11.2) | - |
As the trial began with randomization to Intra-aortic Filtration or control, the first 12 control patients served as controls for Intra-aortic Filtration only and 120 patients were common to both control groups.
Categorical measures are presented as the number of patients and (%). If the denominator is not equal to the group sample size, data is presented as the number of patients/the number observed (%). White matter lesion volume is presented as median (IQR) and all other continuous measures are presented as mean (standard deviation).
The SF-12 composite scores are normed as T-scores (mean=50, SD=10); a higher score indicates a better health state
Severe cognitive impairment is defined as falling below 2 SD from the mean of an age-standardized population. Cognition was measured using a battery of tests including Hopkins Verbal Learning Test – Revised (HVLT-R), Controlled Oral Word Association Test (COWA), Trail Making Test – Parts A & B, Medical College of Georgia Complex Figures, and Wechsler Adult Intelligence Scale – 3rd Revision (WAIS-III, Digit Symbol Coding Subtest and Digits Span Subtest)
A large cortical lesion is defined as having a chronic infarct involving cortical gray matter of maximum diameter greater than or equal to 4.0 cm
Atheroma was examined in the ascending aorta and the aortic arch and graded according to Katz. The maximum grade is reported. Katz’s grade ranges from 1 to 5 with 1 representing normal to mild intimal thickening and 5 indicating any thickness with mobile component.31
The most common concomitant procedures were annular enlargement for valve placement, ascending aorta repair or replacement, and left atrial appendage ligation
Most patients underwent either isolated SAVR (58%) or a combined SAVR and CABG procedure (41%). Cardiopulmonary bypass times were similar in both treatment groups and controls. Three patients randomized to intra-aortic filtration and two patients randomized to suction-based extraction did not receive the designated device, due to anatomic constraints and hemodynamic instability. Embolic debris was captured in 99% of patients undergoing intra-aortic filtration and 75% of patients undergoing suction-based extraction (Table 1).
FREEDOM FROM CLINICAL AND RADIOGRAPHIC CNS INFARCTS
Within 7 days post-randomization, freedom from CNS infarction at 7 days (using imputation for missing data) did not differ between suction-based extraction and controls (32.0 vs. 33.3%, difference −1.3, 95%CI −13.8,11.2) nor between intra-aortic filtration and controls (25.6% vs 32.4%, −6.9, 95% CI −17.9, 4.2). Among patients who met the primary endpoint, the vast majority did not show clinical evidence of stroke (91% suction-based extraction vs. 90% controls; 87% intra-aortic filtration vs 90% controls). Endpoint analyses stratified by procedure and site are provided in the Appendix.
The proportion of suction-based extraction device patients (with or without an MRI) who had clinically apparent stroke was 5.1% vs 5.8% among control patients (−0.7, 95%CI −6.5, 5.1), whereas for intra-aortic filtration patients it was 8.3% vs. 6.1% in controls (2.2, 95%CI−4.1, 8.4). Seventy-six percent of clinically apparent strokes were detected by post-operative day 3, and, in a post-hoc analysis, there were fewer severe strokes in embolic protection device patients within this 3-day time window (Table 2).
Table 2.
Clinical EndPoints, Serious Adverse Events and Hospitalizations
| Suction-based Extraction (N=118) |
Control (N=120) |
Absolute Difference (95% CI)a | P-Valueb | Intra-aortic Filtration (N=133) |
Control (N=132) |
Absolute Difference (95% CI)a | P-Valueb | |
|---|---|---|---|---|---|---|---|---|
| Clinical Endpoints in first 7 Days | Percent (Standard Error) | Percent (Standard Error) | ||||||
| Primary Endpointc -Imputed | 68.0 (4.5) | 66.7 (4.4) | 1.3 (−11.2, 13.8) | 0.84 | 74.4 (4.1) | 67.6 (4.5) | 6.9 (−4.2, 17.9) | 0.22 |
| no./total no. of patients (%) | no./total no. of patients (%) | |||||||
| Primary Endpointc -Observed | 68/101 (67.3) | 73/112 (65.2) | 2.1 (−10.6, 14.9) | 0.74 | 86/118 (72.9) | 78/119 (65.5) | 7.3 (−4.4, 19.1) | 0.22 |
| Death ≤ 7 daysd | 0/117 (0.0) | 1/120 (0.8) | −0.8 (−2.5, 0.8) | >0.99 | 1/133 (0.8) | 1/131 (0.8) | 0.0 (−2.1, 2.1) | >0.99 |
| Radiographic Infarcts on dwMRIe | 66/101 (65.3) | 71/111 (64.0) | 1.4 (−11.5, 14.3) | 0.83 | 83/115 (72.2) | 76/118 (64.4) | 7.8 (−4.1, 19.7) | 0.20 |
| Clinically Apparent Stroke ≤ 7 daysd,f | 6/117 (5.1) | 7/120 (5.8) | −0.7 (−6.5, 5.1) | 0.81 | 11/133 (8.3) | 8/131 (6.1) | 2.2 (−4.1, 8.4) | 0.50 |
| Severity of Clinically Apparent Stroke ≤ 7 days | ||||||||
| Severe (NIHSS >20) | 1/6 (16.7) | 3/7 (42.9) | - | - | 1/11 (9.1) | 3/8 (37.5) | - | - |
| Moderate to severe (NIHSS 16–20) | 0/6 | 0/7 | - | 0/11 | 0/8 | - | ||
| Moderate (NIHSS 5–15) | 2/6 (33.3) | 1/7 (14.3) | - | - | 5/11 (45.5) | 2/8 (25.0) | - | - |
| Mild (NIHSS 0–4) | 3/6 (50.0) | 3/7 (42.9) | - | - | 5/11 (45.5) | 3/8 (37.5) | - | - |
| Clinically Apparent Stroke ≤ 3 days | 4/6 (66.7) | 6/7 (85.7) | - | - | 8/11 (72.7) | 7/8 (87.5) | - | - |
|  Severe (NIHSS >20) | 0/4 | 3/6 (50.0) | - | - | 1/8 (12.5) | 3/7 (42.9) | - | - |
| Moderate to severe (NIHSS 16–20) | 0/4 | 0/6 | - | 0/8 | 0/7 | - | ||
| Moderate (NIHSS 5–15) | 1/4 (25.0) | 1/6 (16.7) | - | - | 5/8 (62.5) | 2/7 (28.6) | - | - |
| Mild (NIHSS 0–4) | 3/4 (75.0) | 2/6 (33.3) | - | - | 2/8 (25.0) | 2/7 (28.6) | - | - |
| Delirium | ||||||||
| Day 1 | 19/111 (17.1) | 16/110 (14.5) | 2.6 (−7.0, 12.2) | 0.60 | 27/121 (22.3) | 18/119 (15.1) | 7.2 (−2.6, 17.0) | 0.15 |
| Day 3 | 15/108 (13.9) | 13/113 (11.5) | 2.4 (−6.4, 11.2) | 0.59 | 15/117 (12.8) | 18/124 (14.5) | −1.7 (−10.4, 7.0) | 0.70 |
| Day 7 | 7/112 (6.3) | 17/111 (15.3) | −9.1 (−17.1, −1.0) | 0.03 | 10/123 (8.1) | 19/122 (15.6) | −7.4 (−15.5, 0.6) | 0.07 |
| Clinical Endpoint at 30 Daysd | ||||||||
| Compositeg | 25/117 (21.4) | 29/120 (24.2) | −2.8 (−13.5, 7.9) | 0.61 | 44/132 (33.3) | 31/131 (23.7) | 9.7 (−1.2, 20.5) | 0.08 |
| Clinically Apparent Stroke | 6/117 (5.1) | 7/120 (5.8) | −0.7 (−6.5, 5.1) | 0.81 | 11/132 (8.3) | 8/131 (6.1) | 2.2 (−4.0, 8.5) | 0.49 |
| Death | 4/117 (3.4) | 2/120 (1.7) | 1.8 (−2.3, 5.8) | 0.44 | 3/132 (2.3) | 2/131 (1.5) | 0.7 (−2.6, 4.0) | >0.99 |
| AKI (Stage 1–3, Serious & Non-Serious) | 19/117 (16.2) | 24/120 (20.0) | −3.8 (−13.6, 6.0) | 0.45 | 35/132 (26.5) | 25/131 (19.1) | 7.4 (−2.7, 17.5) | 0.15 |
| no. events (rate per 100 patient months) | no. events (rate per 100 patient months) | |||||||
| Serious Adverse Events by 90 Days | ||||||||
| Acute Kidney Injuryh | 3 (0.9) | 4 (1.2) | −0.3 (−1.8, 1.3) | 0.74 | 14 (3.8) | 4 (1.1) | 2.7 (0.4, 4.9) | 0.02 |
| Stage 1 | 1 (0.3) | 0 (0.0) | 0.3 (−0.3, 0.9) | 0.32 | 3 (0.8) | 0 (0.0) | 0.8 (−0.1, 1.7) | 0.08 |
| Stage 2 | 0 (0.0) | 2 (0.6) | −0.6 (−1.4, 0.2) | 0.16 | 4 (1.1) | 2 (0.5) | 0.5 (−0.8, 1.8) | 0.42 |
| Stage 3 | 2 (0.6) | 2 (0.6) | 0 (−1.2, 1.2) | 0.97 | 7 (1.9) | 2 (0.5) | 1.3 (−0.2, 2.9) | 0.10 |
| Bleeding | 8 (2.5) | 6 (1.8) | 0.7 (−1.5, 2.9) | 0.55 | 5 (1.3) | 6 (1.6) | −0.3 (−2.0, 1.5) | 0.75 |
| Cardiac Arrhythmias | 31 (9.5) | 25 (7.4) | 2.1 (−2.3, 6.6) | 0.35 | 57 (15.3) | 30 (8.1) | 7.2 (2.3, 12.1) | 0.004 |
| Cardiac arrest | 0 (0.0) | 1 (0.3) | −0.3 (−0.9, 0.3) | 0.32 | 6 (1.6) | 2 (0.5) | 1.1 (−0.4, 2.6) | 0.16 |
| Sustained ventricular arrhythmia with defibrillation or cardioversion | 0 (0.0) | 0 (0.0) | - | - | 1 (0.3) | 0 (0.0) | 0.3 (−0.3, 0.8) | 0.32 |
| Sustained supraventricular arrhythmia with drug treatment or cardioversion | 25 (7.7) | 22 (6.5) | 1.2 (−2.9, 5.2) | 0.58 | 43 (11.6) | 25 (6.8) | 4.8 (0.4, 9.1) | 0.03 |
| Conduction abnormalities or sustained bradycardia with permanent pacemaker placement | 6 (1.8) | 2 (0.6) | 1.3 (−0.4, 2.9) | 0.15 | 7 (1.9) | 3 (0.8) | 1.1 (−0.6, 2.7) | 0.21 |
| Major Infectioni | 10 (3.1) | 12 (3.6) | −0.5 (−3.3, 2.3) | 0.73 | 15 (4.0) | 15 (4.1) | 0 (−2.9, 2.9) | 0.98 |
| Myocardial Infarction | 0 (0.0) | 0 (0.0) | − | − | 3 (0.8) | 0 (0.0) | 0.8 (−0.1, 1.7) | 0.08 |
| Neurological Dysfunction | 4 (1.2) | 10 (3.0) | −1.7 (−3.9, 0.5) | 0.12 | 7 (1.9) | 12 (3.3) | −1.4 (−3.7, 0.9) | 0.24 |
| Transient Ischemic Attack | 0 (0.0) | 1 (0.3) | −0.3 (−0.9, 0.3) | 0.32 | 0 (0.0) | 1 (0.3) | −0.3 (−0.8, 0.3) | 0.32 |
| Ischemic Strokej | 3 (0.9) | 3 (0.9) | 0 (−1.4, 1.5) | 0.97 | 4 (1.1) | 4 (1.1) | 0 (−1.5, 1.5) | 0.99 |
| Toxic Metabolic Encephalopathy | 1 (0.3) | 1 (0.3) | 0 (−0.8, 0.8) | 0.98 | 2 (0.5) | 1 (0.3) | 0.3 (−0.6, 1.2) | 0.57 |
| Seizure | 0 (0.0) | 2 (0.6) | −0.6 (−1.4, 0.2) | 0.16 | 0 (0.0) | 3 (0.8) | −0.8 (−1.7, 0.1) | 0.08 |
| Other | 0 (0.0) | 3 (0.9) | −0.9 (−1.9, 0.1) | 0.08 | 1 (0.3) | 3 (0.8) | −0.5 (−1.6, 0.5) | 0.31 |
| Renal Failure | 0 (0.0) | 2 (0.6) | −0.6 (−1.4, 0.2) | 0.16 | 1 (0.3) | 2 (0.5) | −0.3 (−1.2, 0.6) | 0.56 |
| Respiratory Failure | 3 (0.9) | 9 (2.7) | −1.8 (−3.8, 0.3) | 0.09 | 8 (2.2) | 11 (3.0) | −0.8 (−3.1, 1.5) | 0.48 |
| Heart Failure | 4 (1.2) | 8 (2.4) | −1.1 (−3.2, 0.9) | 0.27 | 2 (0.5) | 9 (2.4) | −1.9 (−3.7, −0.1) | 0.03 |
| Venous Thromboembolism Eventk | 1 (0.3) | 2 (0.6) | −0.3 (−1.3, 0.7) | 0.58 | 4 (1.1) | 2 (0.5) | 0.5 (−0.8, 1.8) | 0.42 |
| All Serious Adverse Events | 91 (28.0) | 112 (33.3) | −5.3 (−13.7, 3.2) | 0.22 | 157 (42.2) | 126 (34.2) | 8.0 (−0.8, 16.9) | 0.08 |
| Hospitalization | ||||||||
| Readmissions | 24 (8.4) | 20 (6.8) | 1.7 (−2.8, 6.1) | 0.47 | 30 (9.3) | 23 (7.1) | 2.2 (−2.3, 6.6) | 0.34 |
| Quality of Life at 90 Days | ||||||||
| Mean (sd) | Mean (sd) | |||||||
| SF12 Composite Mental Health Scorel | 55.4 (8.2) | 55.1 (8.1 | 0.4 (−1.8, 2.6) | 0.74 | 55.2 (10.2 | 54.8 (8.0) | 0.3 (−2.0, 2.7) | 0.77 |
| SF12 Composite Physical Health Scorel | 44.9 (8.3) | 44.7 (8.4 | 0.1 (−2.2, 2.4) | 0.92 | 43.0 (10.8 | 44.2 (9.0) | −1.2 (−3.7, 1.3) | 0.36 |
| no./total no. of patients (%) | no./total no. of patients (%) | |||||||
| Geriatric Depression Scale > 10m | 5/104 (4.8) | 7/108 (6.5) | −1.7 (−7.9, 4.5) | 0.60 | 13/122 (10.7) | 9/119 (7.6) | 3.1 (−4.2, 10.3) | 0.40 |
| Functional Status at 90 Days | ||||||||
| Decline in Overall Neurocognitionn | 24/81 (29.6) | 27/86 (31.4) | OR 1.1 (0.5, 2.2) | 0.82 | 28/98 (28.6) | 31/96 (32.3) | OR 0.8 (0.4, 1.5) | 0.54 |
| Modified Rankin Scale >2o | 7/110 (6.4) | 4/112 (3.6) | 2.8 (−2.9, 8.5) | 0.34 | 5/127 (3.9) | 5/123 (4.1) | −0.1 (−5.0, 4.7) | >0.99 |
| Barthel Index ≤80p | 2/105 (1.9) | 3/109 (2.8) | −0.8 (−4.9, 3.2) | >0.99 | 2/123 (1.6) | 4/120 (3.3) | −1.7 (−5.6, 2.2) | 0.44 |
| Mortality – no. | 5 | 3 | HR 1.7 (0.4, 7.2) | 0.45 | 4 | 3 | HR 1.3 (0.3, 5.9) | 0.71 |
Instead of absolute difference, the odds ratio is given for decline in overall neurocognition as the analysis is based on a logistic model adjusting for baseline score and the hazard ratio is given for mortality as the analysis was to time to event;
P values were calculated by means of the chi-square test or Fisher’s exact test for the clinical end points and the Modified Rankin Scale, Barthel Index, and Geriatric Depression Scale endpoints, Poisson regression for serious adverse events and hospitalizations, t-tests for SF-12 scores, a logistic regression model of decline adjusted for baseline score for neurocognitive decline, and the log rank test for mortality;
The primary endpoint consists of radiographic infarcts on 7-day dwMRI and/or clinically apparent stroke ≤ 7 days post-surgery. Deaths are counted as treatment failures. The denominator for the observed is the number of patients with a non-missing MRI image and/or evidence of a clinical stroke or death before 7 days.
2 patients withdrew from the trial prior to 7 days and are not included in the denominators; 1 additional patient withdrew prior to 30 days;
334 dwMRIs were evaluated by the core lab. 210 (63%) were collected using a 1.5 T scanner and 124 (37%) were 3 T.;
Five patients had evidence of a clinical infarction and either did not undergo dwMRI or had no lesions on their dwMRI, (2 in Intra-aortic Filtration, 1 in the shared control, 2 in Suction-based Extraction)
The composite clinical endpoint consists of clinically apparent stroke, acute kidney injury (AKI), or death within 30 days of surgery;
The composite clinical endpoint includes clinically apparent strokes, as well as death and AKI, that are expressed in events per patient. By comparison, the AKI AE within 90 days reflects the number of events per unit time. The composite clinical endpoint includes both serious and non-serious AKI adverse events whereas the AKI rate at 90 days are only the reported serious AKI AEs per the protocol. See supplemental appendix for AKI stage definitions.
Major infection is defined as a new clinical infection accompanied by pain, fever, drainage and/or leukocytosis that is treated by anti-microbial agents (non-prophylactic). A positive culture from the infected site or organ should be present unless strong clinical evidence indicates the need for treatment despite negative cultures.;
The composite clinical endpoint includes clinically apparent strokes, as well as death and AKI, that are expressed in events per patient. By comparison, the ischemic stroke AE within 90 days reflects the number of events per unit time. The composite clinical endpoint includes both serious and non-serious stroke adverse events and stroke events that were identified based on focal findings on serial NIHSS assessments. Whereas the ischemic stroke rate at 90 days are only the reported serious stroke AEs per the protocol.;
Venous Thromboembolism consists of Deep Vein Thrombosis, Pulmonary Embolism and Other;
SF-12 is given as mean (sd). The SF-12 composite scores are normed as T-scores (mean=50, SD=10); a higher score indicates a better health state
A Geriatric Depression Scale score of 10 or below indicates absence of depression, while a score of 11 or higher is indicative of depression;
Decline is defined as number of patients whose Z-score at day 90 decreased by 0.5 standard deviations relative to their baseline score. Z-Scores were computed relative to the study population at baseline adjusting for age, education and gender.;
A modified Rankin Scale score > 2 corresponds to ≥moderate disability (3–5) or death (6);
A Barthel Index score ≤80 corresponds to ≥mild to moderate disability
NUMBER AND VOLUME OF CNS INFARCTS
Patients frequently showed evidence of multiple new CNS infarcts. The mean number of infarcts did not differ between suction-based extraction and control patients (2.4 (3.7) vs. 2.3 (3.8);p=0.88), nor between intra-aortic filtration and control patients (2.8 (4.3) vs. 2.7 (4.7);p=0.78). While there were no between-group differences in total lesion volume (mean 178.5 mm3 suction-based extraction vs. 476.4 mm3 controls, p=0.28; mean 321.3 mm3 intra-aortic filtration vs. 484.4 mm3 controls, p=0.49); Figure 2 depicts patients’ total lesion volume by decile. Infarct volumes were larger in patients with clinically apparent strokes than those with silent infarcts (mean 1688 vs 236 mm3;median 91 vs 39 mm3;p=0.001).
Figure 2. Distribution of lesion volume by randomization arm at day 7.
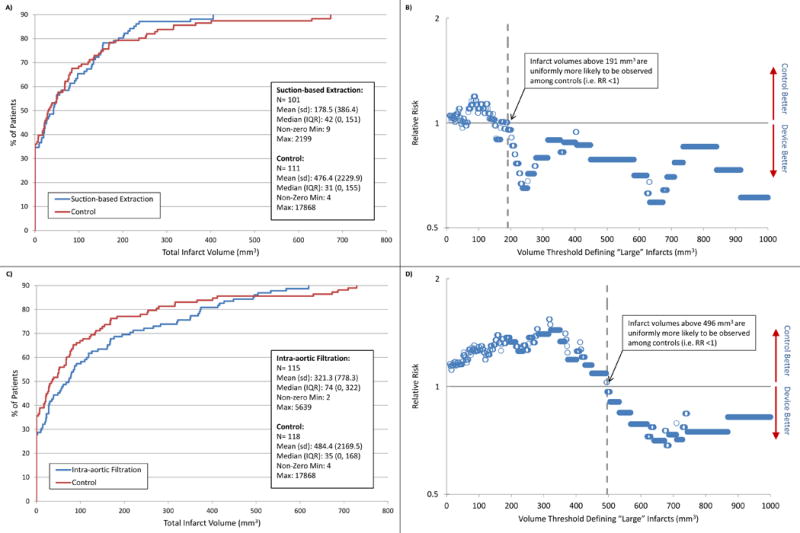
Panels A and C depict deciles of the observed infarct volume distributions for the suction-based extraction device and intra-aortic filtration device respectively, compared to control. Panel A shows, for example, that the 80th percentile of radiographic infarcts were <191mm3 for the suction-based extraction device and <236mm3 for controls. Panel C shows that the 80th percentile of infarcts were <374mm3 for intra-aortic filtration device and <273mm3 for controls. The plotted points in panels B and D depict the natural log of relative risk of “large” volume infarcts for each of the treatment devices compared to their controls as the cut-off value defining “large” varies from 10 to 1000 or above on the x-axis. Values <0 (below the solid line) favor the embolic protection device, and values >0 (above the solid line) favor the control.
COMPOSITE CLINICAL ENDPOINT, ADVERSE EVENTS AND HOSPITALIZATION
The proportion of patients experiencing death, clinically apparent ischemic stroke or acute kidney injury within 30 days of surgery was 21.4% in suction-based extraction patients vs. 24.2% in control patients (−2.8,95%CI −13.5,7.9), and 33.3% in intra-aortic filtration vs. 23.7% in control patients (9.7,95%CI−1.2,20.5). The individual components of the composite 30-day endpoint were not different across groups (Table 2).
Mortality, composite neurologic events and overall serious adverse events at 90 days did not differ across groups (Table 2). Intra-aortic filtration patients experienced significantly more acute kidney injury events and cardiac arrhythmias than controls (14 vs 4;2.7;95% CI 0.4,4.9 and 57 vs 30;7.2;95%CI 2.3,12.1, respectively).
Mean length of stay (LOS) during the index hospitalization did not differ between suction-based extraction and control patients (9.8(6.7) vs 10.3(6.1); −0.5; 95%CI −2.1,1.1), nor did mean ICU stay (4.0(3.5) vs 4.2(3.3); −0.1;95%CI −1.0,0.7). Similarly, mean LOS did not differ between intra-aortic filtration and control patients (10.4(7.0) vs 10.3(6.2); 0.1;95%CI −1.5,1.7), nor did mean ICU stay (4.5(3.5) vs 4.1(3.2); 0.4;95%CI −0.5,1.2). Readmission rates were not different between both treatment and control arms.
DELIRIUM, QUALITY OF LIFE AND COGNITION
There were significant differences in the trajectories (baseline to day 7) of suction-based extraction vs. control (p=0.03) and intra-aortic filtration vs. control (p=0.02; Figure 3). At day 7 post-operatively, 6.3% of suction-based extraction vs 15.3% of control patients (−9.1;95%CI −17.1,−1.0), and 8.1% of intra-aortic filtration vs 15.6% of control patients (−7.4;95%CI−15.5,0.6) experienced delirium. The SF-12 composite physical and mental health scores did not differ between devices and their controls (Table 2). Moreover, no differences were observed in the overall cognition scores between the two devices and their respective controls (Table 2), but decline in overall cognition scores was greater for patients experiencing clinically apparent stroke than for other patients (71.4% vs 28.0%;43.5;95%CI 19.2,67.7). With the exception of executive function, which showed less decline in intra-aortic filtration vs control patients (p=0.05), there were no differences among the neurocognitive domain comparisons (eTable 1).
Figure 3. Delirium over time.
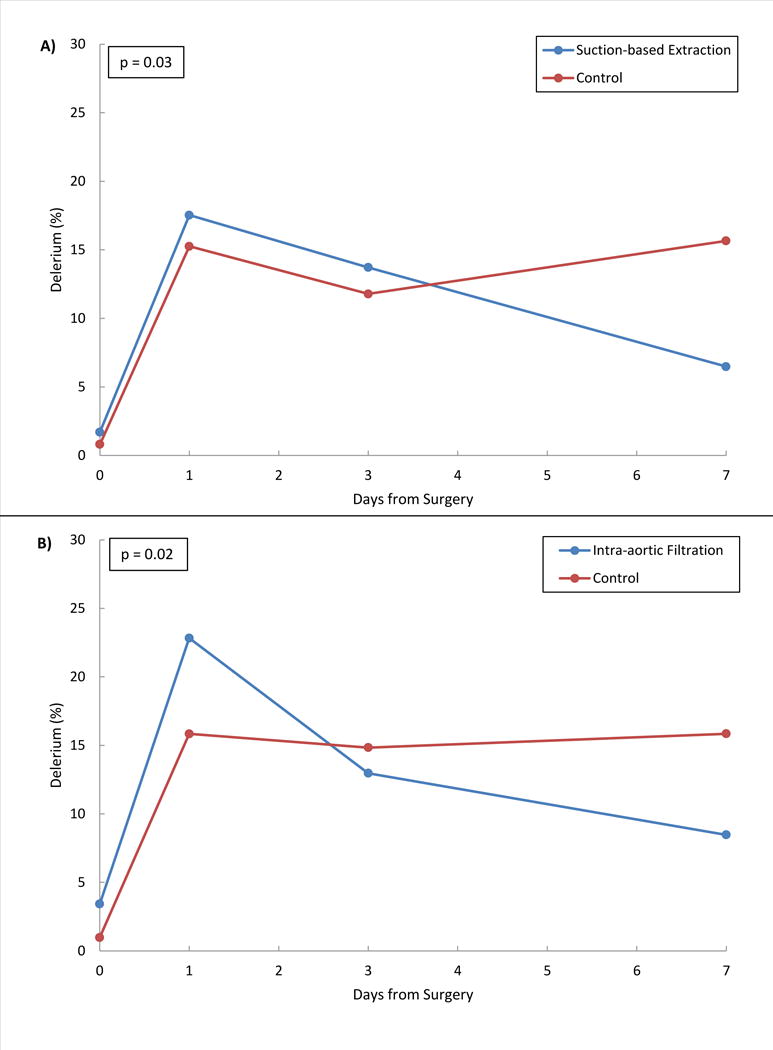
Delirium was measured by CAM assessment at baseline, days 1, 3 and 7. For both treatment devices vs. controls, the probability of delirium over time was modeled using GEE. Panel A gives the model estimated incidence of delirium at baseline, days 1, 3 and 7 for the suction-based extraction device vs. control and panel B gives estimates for the intra-aortic filtration device vs. control. In both models, the interaction term between days from surgery and randomization group was statistically significant (p<0.05) indicating that the incidence of delirium over time was significantly different between treatment interventions and their controls.
DISCUSSION
Stroke is a known complication of SAVR, most commonly due to emboli from aortic valve excision and debridement, aortic manipulation, cardiopulmonary bypass, and introduction of air. Developing effective methods to prevent embolization and reduce adverse clinical outcomes has been challenging. Despite the fact that debris was captured in most patients who received a protection device, rates of clinical and radiographic infarction were not reduced. Nearly two thirds of patients who underwent SAVR suffered clinical or radiographic stroke. However, the vast majority of these events was only detectable by post-operative DW-MRI, with just 9% of patients exhibiting clinical findings. Neither the number of MRI lesions nor total lesion volume differed between patients receiving either of the embolic protection devices and their controls. However, the infarct volume pattern suggested a possible differential effect of devices compared to controls, with larger volume infarcts more numerous in control patients. This observation may be important as the risk of clinically evident stroke increases with infarct volume.
The reported incidence of newly-detected clinical and radiographic infarcts after cardiac surgery has varied across observational studies likely in relation to cohort size, patient characteristics, approaches to clinical assessment, and imaging techniques.3,4,11–15 The volume of radiographic infarcts seen in this study, the vast majority of which were small (median infarct volume 48.7 mm3), is concordant with that reported in prior studies using MRI after SAVR.5, 9,16 Imaging studies in the general population have reported that clinically silent brain infarction, including even micro-infarction (<3 mm), is associated with dementia, mortality, and risk of future stroke and death, but it is not clear whether these same associations pertain to peri-procedural infarcts that are clinically silent.10,14,17–20
The proportion of patients with clinical stroke findings at day 7 (with or without MRI evidence of infarction) did not differ between suction-based extraction and control patients (5.1% vs 5.8%) nor between intra-aortic filtration and control patients (8.3% vs 6.1%). The majority of clinical stroke findings, however, was detected by post-operative day 3, suggesting that many peri-operative strokes may be preventable with embolic protection devices. In a post-hoc analysis, there were numerically fewer severe clinical strokes (NIHSS>20) among embolic protection device recipients compared to controls at days 0–3. Given that severity at onset, defined by NIHSS, is one of the strongest predictors of long-term outcome after acute stroke, a reduction in the risk of severe strokes in the early perioperative period might be a tangible benefit of embolic protection devices during SAVR, and may justify a larger trial.21
The rate of clinically apparent stroke (6.5%) in this trial is much higher than the 1–3% stroke incidence (7 days) reported in several prior studies,22,23 and is most likely attributable to active ascertainment with early repeated neurologic assessments. However, there was a lower incidence of stroke with clinical findings than the 17% seen in a previously reported large cohort study, perhaps related to a treatment shift of high-risk SAVR patients to TAVR that occurred during the course of this trial.5 There was a large difference in this trial between strokes that were observed in the course of clinical care and those detected through protocol-specified, routine NIHSS screening, suggesting that many clinical events are undetected or underreported in practice.
Delirium, a disturbance of consciousness and cognition that can develop acutely after surgery, is common, occurring in 11 to 46% of post-operative patients.24 Prior studies have shown that cerebral embolization is associated with delirium, a condition that is costly and associated with poor outcomes, including prolonged hospitalization, readmissions, long-term cognitive decline and mortality.25–27 Longitudinally (over 7 days post-operatively), there was a significant difference between recipients of treatment devices and their controls. At day 7 (when peri-operative medications are less of a factor), fewer device patients experienced in-hospital delirium than controls and the difference achieved statistical significance with the suction-based extraction device. This difference may be related to the fact that, in addition to particulate matter, the suction-based device also extracts gaseous micro-emboli, which have been shown to affect neuropsychological functioning in cardiac surgery patients early post-operatively.28
Over 25% of patients had severe cognitive impairment on baseline testing in one domain, compared to age-adjusted norms, especially in verbal memory and executive functioning. These domains are associated with late onset Alzheimer’s-related pathology and cerebrovascular disease. Similar findings have been reported withTAVR.29 Given the advanced age of AS patients and increased incidence of pre-procedural cerebrovascular disease, higher rates of postoperative cognitive impairment in SAVR and TAVR populations is not unexpected. These findings underscore the importance of evaluating cognitive impairment during pre-procedural risk assessment given its association with postoperative delirium and late-onset dementia.18,27
Baseline performance-adjusted cognitive outcomes at 90-days did not differ significantly between treatments and controls, with the exception of reduced executive functioning decline in the intra-aortic filtration group. This finding requires more investigation, but might relate to the lower incidence of delirium observed in the device groups compared to controls.
There was evidence of an increased incidence of acute kidney injury and a higher rate of cardiac arrhythmias with intra-aortic filtration patients compared with their controls. These findings merit further investigation, but need to be interpreted in the context of multiple adverse event statistical comparisons that increase the likelihood of finding abnormalities due to random variation alone.
This trial has several limitations. Whereas the trial subscribes to the latest imaging recommendations for assessing neurologic outcomes in the post-cardiac surgery setting,30 the significance of the many small and clinically silent lesions identified by DW-MRI cannot be established within the confines of this trial. Additionally, while the optimal timeframe to assess the effectiveness of an intraoperative embolic protection device is within the first few days post-procedure, 7-day imaging is more feasible for cardiac surgery patients. As a result, this study may have captured radiographic and clinical CNS infarctions not related to intra-operative embolization (e.g., from post-operative atrial fibrillation), thus overestimating infarct burden, whereas smaller lesions may have normalized by 7 days, underestimating infarct burden. Moreover, while the NIHSS has been validated as a measure of stroke severity, it does not inherently inform whether a clinical stroke has occurred. However, clinical stroke endpoint adjudication was performed by experienced vascular neurologists who reviewed trial data to determine whether an event occurred. It is also important to note that because randomization was halted due to low conditional power for the primary endpoint, diminishing the power to detect differences for secondary endpoints. In addition, the long term effects on cognition may not have been fully evident within the trial’s 90-day follow-up.
Conclusions
The incidence of CNS infarction after surgical aortic valve replacement was not altered by either embolic protection device. Potential benefits for delirium reduction, cognition and symptomatic stroke merit larger trials with longer follow-up.
Supplementary Material
Key Points.
Question
Can embolic protection devices reduce the incidence of stroke after surgical aortic valve replacement?
Findings
This randomized trial shows that, despite debris capture in the majority of patients receiving embolic protection devices, freedom from CNS infarction at 7 days did not differ between suction-based extraction and controls (32.0 vs. 33.3%, difference −1.3 [95%CI −13.8, 11.2]) nor between intra-aortic filtration and controls (25.6% vs 32.4%,difference −6.9, [95% CI −17.9, 4.2]).
Meaning
The incidence of CNS infarction after SAVR was not altered by either of two embolic protection devices.
Acknowledgments
Cardiothoracic Surgical Trials Network (CTSN)
The members of the Cardiothoracic Surgical Trials Network (CTSN) involved in this trial are as follows: National Heart, Lung and Blood Institute: Dennis Buxton, Nancy L. Geller, Catherine Burke, Albert Lee, Tyrone Smith; Canadian Institutes of Health Research: Ilana Kogan Gombos; Data Coordinating Center: International Center for Health Outcomes and Innovation Research at Icahn School of Medicine at Mount Sinai, Kinjal Shah, Stephanie Pan, Alishba Aslam, Helena Chang, Melissa Chase, Kayla Dellefratte, Seth Goldfarb, Lopa Gupta, Katherine Kirkwood, Edlira Dobrev, Ron Levitan, Andrea Ratner, Milerva Santos, Nancy Sledz-Joyce, Xia Ye; Clinical Site Investigators: Baylor Research Institute: Amanda Fenlon, Katherine Harrington, Kelly Hutcheson, Melissa Johnson, Jessica Jones, Megan Kolb, Sarah Lam, Lucy Miranda, Jackie Ward, Renessa Whitman, Brittany Zingler, William Ryan, Robert L. Smith, Pedro Nosnik, Justin Whisenant; Cleveland Clinic Foundation: Edward G. Soltesz, Stephanie Mick, Irene Katzan, Brian Strippy, Shoi Smith, Michelle Garcia, Mary Alice Bowman; Columbia University: Michael Argenziano (PI), Michael Borger, Hiroo Takayama, Lyn Goldsmith, Nadia Sookraj, Talaya McCright-Gill, Sowmya Sreekanth; Dartmouth-Hitchcock Medical Center: Jock N. McCullough (PI), Joseph P. DeSimone, Anthony W. DiScipio, Henry Stokes, Amanda St. Ivany, Gaylin Petty, Jim Hart Duke University: John H. Alexander, Carmelo A. Milano, Donald D. Glower, Stacey Welsh, Sarah Casalinova, Victoria Johnson, Derek Smith, Greg Tipton; Emory University: Robert Guyton, Omar Lattouf, Michael Halkos, Kim Baio, Tamara Prince, Natascha Cook, Alexis A. Neill; Hôpital Laval: François Dagenais, Robert Laforce, Jr., Kim O’Connor, Gladys Dussault, Manon Caouette, Hugo Tremblay, Nathalie Gagne, Patricia Landry; Mission Hospital: John G. Short, Reid D. Taylor, Tracy Nanney, Holly Aubart, Kristin Cross, Leslie McPeters, Christina Riggsbee, Lucy Rixey; Montefiore-Einstein Heart Center: Joseph J. DeRose, Jr., Daniel J. Goldstein, Ricardo A. Bello, William Jakobleff, Kathryn Kirchoff, Richard Zampolin, Rebecca Meli, Juan Garcia, Jon Goldenberg, Lauren Kealy; Montreal Heart Institute: Denis Bouchard, Michel Carrier, Jean François Tanguay, Pierre Pagé, Céline Odier, Filippo Codemartiri, Jonathan Lacharité, Sophie Robichaud; NIH Heart Center at Suburban Hospital: Keith A. Horvath (PI), Philip C. Corcoran, Michael P. Siegenthaler, Mandy Murphy, Margaret Iraola, Ann Greenberg, Greg Kumkumian, Mark Milner, Zurab Nadareishvili; Ohio State University Medical Center: Bryan A. Whitson (PI), Juan Crestellano, Xuan Nguyen, Mohit Datta, Asia McDavid, Denise Fadorsen; Toronto General Hospital: Maral Ouzounian (PI), Terry Yau, Cheryl Jaigobin, Narinder Paul, Walter Kucharczyk, Nishit Fumakia, Shakira Christie; University of Alberta Hospital: John C. Mullen (PI), Asvina Bissonauth, Alexandra Hripko; University of Maryland: Bradley Taylor (PI), James Gammie, John Cole, Robert Morales, Kristen Mackowick, Stephanie Deasey, Julia Collins; University of Pennsylvania: Jesse Raiten, Cen Zhang, Mary Lou Mayer, Caitlin McDonald, Holley Fok, Breanna Maffei, Stephen Cresse, Christine Gepty; University of Southern California: Vaughn A. Starnes, David Shavalle, Christi Heck, Amy Hackmann, Craig Baker, Fernando Fleischman, Mark Cunningham, Meng Law, Edward Lozano, Michelle Hernandez, Sylvia Ramos; University of Virginia: Irving L. Kron, Karen Johnston, Ravi K. Ghanta, Leora Yarboro, Joe Carrera, Nicole Chiota-McCollum, Sandra Burks, Mike Cosner, China Green, Samantha Loya, Helen Kim, Jennifer Phillips, Rachel Simon; Protocol Review Committee: David A. Bull (Chair); Patrice Desvigne-Nickens, Executive Secretary; Dennis O. Dixon, Rebecca Gottesman, Mark Haigney, Richard Holubkov, Constantino Iadecola, Alice Jacobs, Eric M. Meslin, John M. Murkin, John A. Spertus; Data and Safety Monitoring Board: Frank Sellke (Chair); Cheryl L. McDonald, Executive Secretary; John Canty, Neal Dickert, Dennis O. Dixon, John S. Ikonomidis, KyungMann Kim, David O. Williams, Clyde W. Yancy, Seemant Chaturvedi, Marc Chimowitz; Medical Monitors: James C. Fang, Wayne Richenbacher; Overall Event Adjudication Committee: Vivek Rao (Chair); Rachel Miller, Jennifer Cook, David D’Alessandro, Frederick Han, Sean Pinney, Mary N. Walsh; EAC neurology sub-committee: David Greer (Chair); Koto Ishida, Christian Stapf; Echo Core Lab, Massachusetts General Hospital: Judy Hung, Xin Zeng, David Hung, Sudarat Satitthummanid;
MRI Core Lab, University of Pennsylvania: Guray Erus, Lauren Karpf, Lisa Desiderio;
Neurocognitive Core Lab, Duke University Medical Center: Joseph P. Mathew, Michael L. James, Yanne Toulgoat-Dubois, Rachele Brassard; Histopathology Core Lab, CVPath Institute, Inc., Gaithersburg, MD: Renu Virmanu, Maria E. Romero, Ryan Braumann.
Funding/Support:
The neuroprotection trial was supported by a cooperative agreement (U01 HL088942) funded by the National Institutes of Neurological Disorders and Stroke, and National Heart Lung and Blood Institute of the National Institutes of Health (NIH), Bethesda, MD, and the Canadian Institutes for Health Research.
Role of the Funder/Sponsor
The NIH representatives were involved in study design, management and review of the manuscript. The companies provided no financial support but did provide training in device use. The device manufacturers had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript or the decision to submit for publication.
Footnotes
ClinicalTrials.Gov REGISTRATION: NCT02389894
Author Contributions
Drs. Parides and Overbey had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Michael J. Mack, Department of Cardiothoracic Surgery, Baylor Research Institute, Baylor Scott & White Health, Plano, TX
Michael A. Acker, Department of Surgery, Division of Cardiovascular Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA.
Annetine C. Gelijns, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Jessica R. Overbey, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Michael K. Parides, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Jeffrey N. Browndyke, Department of Psychiatry & Behavioral Sciences, Duke University Medical Centers, Durham, NC
Mark A. Groh, Cardiovascular and Thoracic Surgery, Mission Health and Hospitals, Asheville, NC
Alan J. Moskowitz, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Neal O. Jeffries, Office of Biostatistics Research, National Heart, Lung, and Blood Institute, Bethesda, MD
Gorav Ailawadi, Division of Thoracic and Cardiovascular Surgery, University of Virginia School of Medicine, Charlottesville, VA
Vinod H. Thourani, Clinical Research Unit, Division of Cardiothoracic Surgery, Emory University School of Medicine, Atlanta, GA
Ellen G. Moquete, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Alexander Iribarne, Cardiac Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH
Pierre Voisine, Institut Universitaire de Cardiologie de Québec, Hôpital Laval, Quebec, QC, Canada
Louis P. Perrault, Montréal Heart Institute, University of Montréal, Montréal, QC, Canada
Michael E. Bowdish, Department of Surgery, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA
Michel Bilello, Department of Radiology, University of Pennsylvania, Philadelphia, PA
Christos Davatzikos, Department of Radiology, University of Pennsylvania, Philadelphia, PA
Ralph F. Mangusan, Cardiovascular and Thoracic Surgery, Mission Health and Hospitals, Asheville, NC
Rachelle A. Winkle, Department of Cardiothoracic Surgery, Baylor Research Institute, Baylor Scott & White Health, Plano, TX
Peter K. Smith, Division of Cardiovascular and Thoracic Surgery, Department of Surgery, Duke University Medical Center, Durham, NC
Robert E. Michler, Department of Cardiothoracic Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, New York, NY
Marissa A. Miller, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD
Karen L. O’Sullivan, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Wendy C. Taddei-Peters, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD
Eric A. Rose, Department of Cardiac Surgery, Mount Sinai Health System, New York, NY
Richard D. Weisel, Peter Munk Cardiac Centre and Division of Cardiovascular Surgery, Toronto General Hospital, University Health Network and the Division of Cardiac Surgery, University of Toronto
Karen L. Furie, Department of Neurology, Rhode Island Hospital, The Miriam Hospital and Bradley Hospital, The Warren Alpert Medical School of Brown University, Providence, RI
Emilia Bagiella, International Center for Health Outcomes and Innovation Research (InCHOIR), the Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, NY
Claudia Scala Moy, National Institutes of Neurological Disorders and Stroke, Bethesda, MD
Patrick T. O’Gara, Cardiovascular Division, Brigham and Women’s Hospital, Boston
Steven R. Messé, and Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA
References
- 1.Adult Cardiac Surgery Database Executive Summary 10 Years - STS Period Ending 3/31/2016. STS National Database. (Accessed February, 2017, at http://www.sts.org/sites/default/files/documents/2016Harvest2_ExecutiveSummary_new.pdf)
- 2.Grover FL, Vemulapalli S, Carroll JD, et al. 2016 Annual Report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Ann Thorac Surg. 2017;103(3):1021–1035. doi: 10.1016/j.athoracsur.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Floyd TF, Shah PN, Price CC, et al. Clinically silent cerebral ischemic events after cardiac surgery: their incidence, regional vascular occurrence, and procedural dependence. Ann Thorac Surg. 2006;81(6):2160–6. doi: 10.1016/j.athoracsur.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 4.Alassar A, Soppa G, Edsell M, et al. Incidence and mechanisms of cerebral ischemia after transcatheter aortic valve implantation compared with surgical aortic valve replacement. Ann Thorac Surg. 2015;99(3):802–8. doi: 10.1016/j.athoracsur.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 5.Messé SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: Results from a prospective cohort. Circulation. 2014;129(22):2253–2261. doi: 10.1161/CIRCULATIONAHA.113.005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puskas JD, Winston AD, Wright CE, et al. Stroke after coronary artery operation: Incidence, correlates, outcome, and cost. The Annals of Thoracic Surgery. 2000;69(4):1053–1056. doi: 10.1016/s0003-4975(99)01569-6. [DOI] [PubMed] [Google Scholar]
- 7.Salazar JD, Wityk RJ, Grega MA, et al. Stroke after cardiac surgery: Short- and long-term outcomes. Ann Thorac Surg. 2001;72(4):1195–1201. doi: 10.1016/s0003-4975(01)02929-0. discussion 1201–2. [DOI] [PubMed] [Google Scholar]
- 8.Banbury MK, Kouchoukos NT, Allen KB, et al. Emboli capture using the Embol-X intraaortic filter in cardiac surgery: a multicentered randomized trial of 1,289 patients. Ann Thorac Surg. 2003;76(2):508–15. doi: 10.1016/s0003-4975(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 9.Bolotin G, Huber CH, Shani L, et al. Novel emboli protection system during cardiac surgery: a multi-center, randomized, clinical trial. Ann Thorac Surg. 2014;98(5):1627–33. doi: 10.1016/j.athoracsur.2014.06.061. discussion 1633–4. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st Century. A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolz E, Gerriets T, Kluge A, Klövekorn WP, Kaps M, Bachmann G. Diffusion-weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: implications for future neuroprotective trials? Stroke. 2004;35(4):888–92. doi: 10.1161/01.STR.0000120306.82787.5A. [DOI] [PubMed] [Google Scholar]
- 12.Cook DJ, Huston J, 3rd, Trenerry MR, Brown RD, Jr, Zehr KJ, Sundt TM., 3rd Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83(4):1389–95. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 13.Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39(5):1427–33. doi: 10.1161/STROKEAHA.107.502989. [DOI] [PubMed] [Google Scholar]
- 14.Patel N, Horsfield MA, Banahan C, et al. Impact of perioperative infarcts after cardiac surgery. Stroke. 2015;46(3):680–6. doi: 10.1161/STROKEAHA.114.007533. [DOI] [PubMed] [Google Scholar]
- 15.Knipp SC, Matatko N, Schlamann M, et al. Small ischemic brain lesions after cardiac valve replacement detected by diffusion-weighted magnetic resonance imaging: Relation to neurocognitive function. Eur J Cardiothorac Surg. 2005;28(1):88–96. doi: 10.1016/j.ejcts.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Uddin A, Fairbairn TA, Djoukhader IK, et al. Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement: effect on health-related quality of life. Circ Cardiovasc Interv. 2015;8(3):e001913. doi: 10.1161/CIRCINTERVENTIONS.114.001913. [DOI] [PubMed] [Google Scholar]
- 17.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126–9. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 18.Patel N, Minhas JS, Chung EM. Risk factors associated with cognitive decline after cardiac surgery: a systematic review. Cardiovasc Psychiatry Neurol. 2015;2015:370612. doi: 10.1155/2015/370612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel N, Minhas JS, Chung EM. The presence of new MRI lesions and cognitive decline after cardiac surgery: a systematic review. J Card Surg. 2015;30(11):808–12. doi: 10.1111/jocs.12643. [DOI] [PubMed] [Google Scholar]
- 20.Meller SM, Baumbach A, Brickman AM, Lansky AJ. Clinical implications for diffusion-weighted MRI brain lesions associated with transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2014;83(3):502–8. doi: 10.1002/ccd.24904. [DOI] [PubMed] [Google Scholar]
- 21.König IR, Ziegler A, Bluhmki E, et al. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke. 2008;39(6):1821–6. doi: 10.1161/STROKEAHA.107.505867. [DOI] [PubMed] [Google Scholar]
- 22.Shahian DM, O’Brien SM, Filardo G, et al. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 3–valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S43–S62. doi: 10.1016/j.athoracsur.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien SM, Shahian DM, Filardo G, et al. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco K, Litaker D, Locala J, Bronson D. The cost of delirium in the surgical patient. Psychosomatics. 2001;42(1):68–73. doi: 10.1176/appi.psy.42.1.68. [DOI] [PubMed] [Google Scholar]
- 26.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottesman RF, Grega MA, Bailey MM, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338–44. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doganci S, Gunaydin S, Kocak OM, Yilmaz S, Demirkilic U. Impact of the intensity of microemboli on neurocognitive outcome following cardiopulmonary bypass. Perfusion. 2013;28(3):256–62. doi: 10.1177/0267659112470693. [DOI] [PubMed] [Google Scholar]
- 29.Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69(4):367–377. doi: 10.1016/j.jacc.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Lansky AJ, Messé SR, Brickman AM, et al. Proposed standardized neurological endpoints for cardiovascular clinical trials: An academic research consortium initiative. J Am Coll Cardiol. 2017;69(6):679–691. doi: 10.1016/j.jacc.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Glas KE, Swaminathan M, Reeves ST, et al. Guidelines for the Performance of a Comprehensive Intraoperative Epiaortic Ultrasonographic Examination: Recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists; Endorsed by the Society of Thoracic Surgeons. J Am Society of Echocardiography. 2007;20(11):1227–1235. doi: 10.1016/j.echo.2007.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


