Abstract
Objective
Juvenile idiopathic arthritis (JIA) is comprised of seven heterogeneous categories of chronic childhood arthritides. About 5% of children with JIA have rheumatoid factor (RF) positive arthritis, which phenotypically resembles adult rheumatoid arthritis (RA). Our objective was to compare and contrast the genetics of RF-positive polyarticular JIA with RA, and selected other JIA categories, to more fully understand the pathophysiological relationships of inflammatory arthropathies.
Methods
RF-positive polyarticular JIA cases (n=340) and controls (n=14,412) were genotyped using the Immunochip array. Single nucleotide polymorphisms (SNPs) were tested for association using a logistic regression model adjusting for admixture proportions. Weighted genetic risk scores (wGRS) of published RA and JIA risk loci were calculated and their ability to predict RF-positive polyarticular JIA were compared.
Results
As expected, the HLA region was strongly associated with RF-positive polyarticular JIA (p=5.51×10−31). Nineteen of 44 RA risk loci and 6 of 27 oligoarticular/RF-negative polyarticular JIA risk loci were associated (p<0.05) with RF-positive polyarticular JIA. The RA wGRS predicted RF-positive polyarticular JIA (AUC=0.71) better than the oligoarticular/RF-negative polyarticular JIA wGRS (AUC=0.56). RF-positive polyarticular JIA was also genetically more similar to RA patients with age at onset <30 years compared to RA onset >70 years.
Conclusions
RF-positive polyarticular JIA is genetically more similar to adult RA than to the most common JIA categories and thus appears to be a childhood-onset presentation of autoantibody positive RA. These findings suggest common disease mechanisms, which could lead to novel therapeutic targets and shared treatment strategies.
Introduction
Juvenile idiopathic arthritis (JIA) is a heterogeneous collection of chronic arthropathies with distinct clinical and laboratory features, but all manifest with arthritis in one or more joints and present before the 16th birthday. The International League of Associations for Rheumatology (ILAR) criteria for JIA recognize seven JIA categories (1). There is robust evidence for genetic factors conferring susceptibility to all forms of JIA (2). Without a clearer understanding of the genetic similarities and distinctions, the clinically different categories must be studied separately. Unfortunately, this stratification results in smaller sample sizes and reduced power to detect association. Thus the JIA Consortium for Immunochip (JACI) was formed with the intent to bring together the large sample sizes required for investigation of the rarer JIA categories. The Immunochip is a custom microarray designed by the Immunochip Consortium to fine-map autoimmune disease-associated loci from 11 autoimmune phenotypes including adult rheumatoid arthritis (RA) (3). The Immunochip assays 196,524 variants representing ~186 loci, including dense coverage of the major histocompatibility complex (MHC) region. Investigation of children with the most common categories of JIA, oligoarticular/RF-negative polyarticular JIA, which comprise approximately 70% of all cases in children of European descent, resulted in the identification of 17 loci associated with JIA at genome-wide levels of significance. In addition, 11 loci showed suggestive evidence of association (4).
About 5% of children with JIA demonstrate the presence of RF and antibodies directed against citrullinated peptides, such as anti-cyclic citrullinated peptide (CCP) antibodies, characteristic biomarkers observed in adults with seropositive RA. These children and young people tend to present at a later age-of-onset compared to oligoarticular/RF-negative polyarticular JIA, and often tend to have erosive disease with worse long-term outcomes. Thus, children with RF-positive polyarticular JIA phenotypically resemble adults with RA and could be considered to have childhood onset RA. In contrast to the robust genetic studies that include large cohorts in RA and oligoarticular/RF-negative polyarticular JIA, studies of children with RF-positive polyarticular JIA have been limited to small-scale candidate gene studies. These include investigations of association with the shared epitope encoding HLA-DRB1 alleles as well as several candidate loci associated with RA (5;6). To date, a systematic analysis of RF-positive polyarticular JIA genetic risk has not been completed, largely due to the lack of sufficiently sized cohorts.
To progress beyond this limitation in cohort size and also advance the understanding of RF-positive polyarticular JIA, we have used the Immunochip to compare and contrast the genetics of RF-positive polyarticular JIA to other categories of JIA, and RA. This may provide a greater understanding of the genetic architecture of RF-positive polyarticular JIA.
Patients and methods
All JIA cases had a diagnosis of polyarticular JIA by the ILAR classification criteria (1) and were positive for RF and/or anti-CCP antibodies. The ILAR criteria do not include any recommendation for CCP testing, hence, CCP is not routinely tested in pediatric rheumatology cohorts. We do have CCP data on 73 subjects (~20%). Of those tested, the prevalence of CCP positivity is 79%. Among cases who were RF positive, 78% were also positive for CCP, comparable to the ~59% of published reports of CCP positivity in RF-positive polyarticular JIA in the literature (7). Cases were ascertained at institutions in the United States (US), United Kingdom (UK), Germany, Canada and Norway. Genotyping was performed using the Illumina Immunochip genotyping array. There were 421 RF-positive polyarticular JIA cases and 16,403 controls before quality control (QC). Standard SNP and sample QC was performed as previously described in the total JIA cohort (4;8). Details of cohorts can be found in the supplementary information.
For comparison with different age-at-onset groups of RA cases, UK RA cases genotyped on the Immunochip array were available from a cohort described previously (9). RA cases were selected if they fell into two age-at-onset categories, early-onset RA (age 16-29 years) n=370 and later-onset RA (age ≥ 70 years) n=259. In total, 8,675 controls from the RA cohort overlapped with the UK controls used for the JIA cohorts. To preserve independence, these controls were randomly split into two groups (Supplementary Table 1).
To test for SNP association with RF-positive polyarticular JIA, a logistic regression model was computed using Caucasian admixture proportions calculated by the program ADMIXTURE (10) as covariates. The additive genetic model was the primary analysis unless there was significant departure from additivity, upon which the most associated genetic model was used. For markers on the X chromosome, the logistic model was stratified by gender and inference was based on the resulting weighted inverse normal meta-analysis. Imputation of SNP genotypes was completed using IMPUTE2 with the 1000 Genomes Phase 1 integrated reference panel (11). To test for association with the imputed data, a logistic regression model with admixture adjustment was computed on the imputed allele dosage. Only SNPs that passed standard imputation QC and had information score >0.5 and confidence score >0.9 were considered for association analysis. For each region we reported the strongest associated genotyped SNP. If there was an imputed SNP that showed stronger association than the genotyped SNP, then both SNPs were reported; imputed SNPs required at least two SNPs in strong linkage disequilibrium to also exhibit association. Regional plots of association were computed using LocusZoom (12).
The 45 non-HLA risk loci associated with RA using the Immunochip (9) and the 27 oligoarticular/RF-negative polyarticular JIA non-HLA risk loci (4) were assessed to determine if they were also associated with RF-positive polyarticular JIA in our cohort.
Two weighted genetic risk scores (wGRS) were calculated: the first used the RA risk loci (9) and the second used the oligoarticular/RF-negative polyarticular JIA risk loci (4). The RA wGRS analysis started with the 46 SNPS (including HLA) (p< 5 × 10−8) associated with RA as published by Eyre et al (9). However, no proxies (r2>0.8) were available for rs13397 at IRAK1, rs2240336 at PADI4, rs39984 at GIN1 or rs10683701 at KIF5A, therefore the number of SNPs in the wGRS was 42 SNPs. The HLA region was captured through the HLA-DRB1 tag SNP rs660895 (13).
The JIA wGRS analysis started with the 28 SNPs (including HLA) (p< 1 × 10−6) associated with oligoarticular/RF-negative polyarticular JIA as reported by Hinks et al (4). However, no proxies were available for rs7909519 at IL2RA; rs2266959 at UBE2L3 and rs7069750 at FAS, so the final number of SNPs in the wGRS was 25 SNPs. The HLA association was captured using the top SNP (rs7775055) in the region.
To calculate the wGRS for an individual, the natural log of the reported odds ratio was multiplied by the number of risk alleles for each SNP and summed. Individuals with missing genotypes were assigned (imputed) a score based on the expectation from the allele frequency and assuming Hardy-Weinberg Equilibrium. Logistic regression was used to compare each wGRS between cases and controls. In addition, receiver operator characteristic (ROC) curves defined by the sensitivity and specificity of each wGRS were generated and the area under the curve (AUC) calculated. The GRS analysis did not include the imputed genotype data. Analysis was performed using STATA v13.1. We tested whether there was a difference between the areas under the two ROC curves using DeLong’s method as implemented in SAS.
Results
After QC there were 340 RF-positive polyarticular JIA cases (mean onset age: 10.2 ± 4.2 years) and 14,412 controls (Table 1). For the X chromosome analysis, the breakdown by gender was 292 female cases and 8,002 female controls; 48 male cases and 6,410 male controls.
Table 1.
Breakdown of RF-positive polyarticular JIA case and control cohort by population before and after quality control (QC)
| Population | Pre QC Cases |
Pre QC Controls |
Post QC Cases |
Post QC Controls |
|---|---|---|---|---|
| US | 272 | 5985 | 222 | 4408 |
| UK | 104 | 8940 | 94 | 8579 |
| Germany | 15 | 489 | 1 | 480 |
| Norway | 14 | 989 | 13 | 945 |
| Canada | 16 | – | 10 | – |
| Total | 421 | 16403 | 340 | 14412 |
Despite the modest sample size, association with the HLA region was identified, the most significant association was at rs3129769, near HLA-DRB1 (p=5.51×10−31), a SNP in strong linkage disequilibrium (LD) (r2=0.88) with the HLA-DRB1 SNP reported in RA (rs660895, p=2.14×10−29). These SNPs are tagging the HLA-DRB1*0401 classical allele (14). There was no significant association of the most associated SNP in the HLA region reported in the oligoarticular/RF-negative polyarticular JIA Immunochip study, rs7775055 (p=0.08).
The most significantly associated loci identified in the oligoarticular/RF-negative polyarticular JIA and RA Immunochip study were assessed for association with RF-positive polyarticular JIA. Of the 27 non-HLA SNPs most strongly associated with oligoarticular/RF-negative polyarticular JIA (4), six showed evidence for association with RF-positive polyarticular JIA (p<0.05) (Supplementary Table 2). Of the 44 SNPs (not including HLA and KIF5A region, the latter is a deletion polymorphism and not analyzed in this study) most strongly associated with RA (9), 19 showed evidence for association with RF-positive polyarticular JIA (p<0.05) (Supplementary Table 3).
The wGRS generated using the top RA loci was compared with the wGRS generated using the top oligoarticular/RF-negative polyarticular JIA loci to see which best predicted RF-positive polyarticular JIA cases compared to controls. The wGRS generated using the top RA loci from Eyre et al (9), showed statistically significant improved prediction of RF-positive polyarticular JIA cases than that generated using the top oligoarticular/RF-negative polyarticular JIA loci (AUC=0.71 versus AUC=0.58, respectively; p=8.26 × 10−33; Figure 1). The RA wGRS showed comparable prediction of RF-positive polyarticular JIA and early-onset RA cases (AUC=0.75) (Figure 2a; p=0.25) but was less effective in predicting later-onset RA (AUC=0.62) compared to RF-positive polyarticular JIA (Figure 2b; p=1.65 × 10−5). This suggests that the RF-positive polyarticular JIA genetic profile looks more similar to younger RA cases than older.
Figure 1. Comparison of wGRS models calculated using RA wGRS or oligoarticular/RF-negative polyarticular JIA wGRS in RF-positive polyarticular JIA.
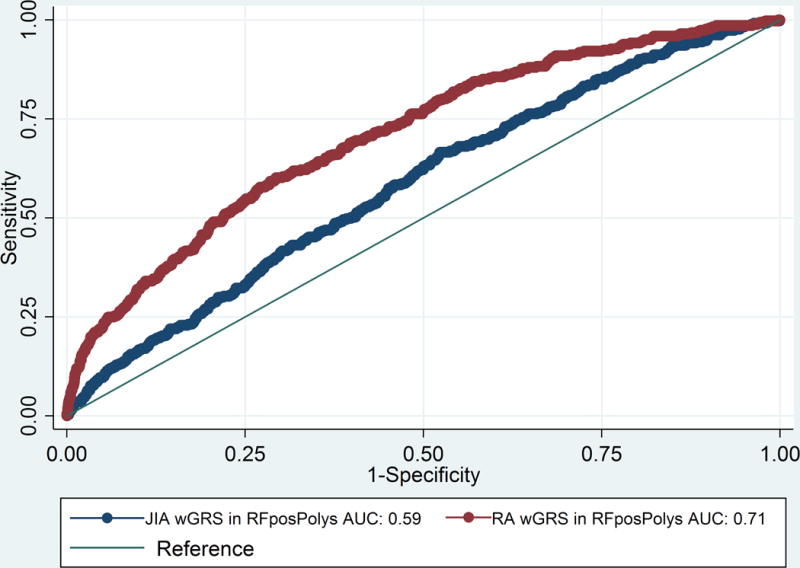
JIA, Juvenile idiopathic arthritis (oligoarticular/RF-negative polyarticular JIA); RA, rheumatoid arthritis; ROC, receiver operator characteristic; wGRS, weighted genetic risk score; AUC, area under the curve.
Figure 2. (a) Comparison of wGRS models calculated using RA wGRS in RF-positive polyarticular JIA to that with early-onset (16-29 yrs) (b) Comparison of wGRS models calculated using RA wGRS in RF-positive polyarticular JIA to that with later-onset (≥70 yrs) RA.
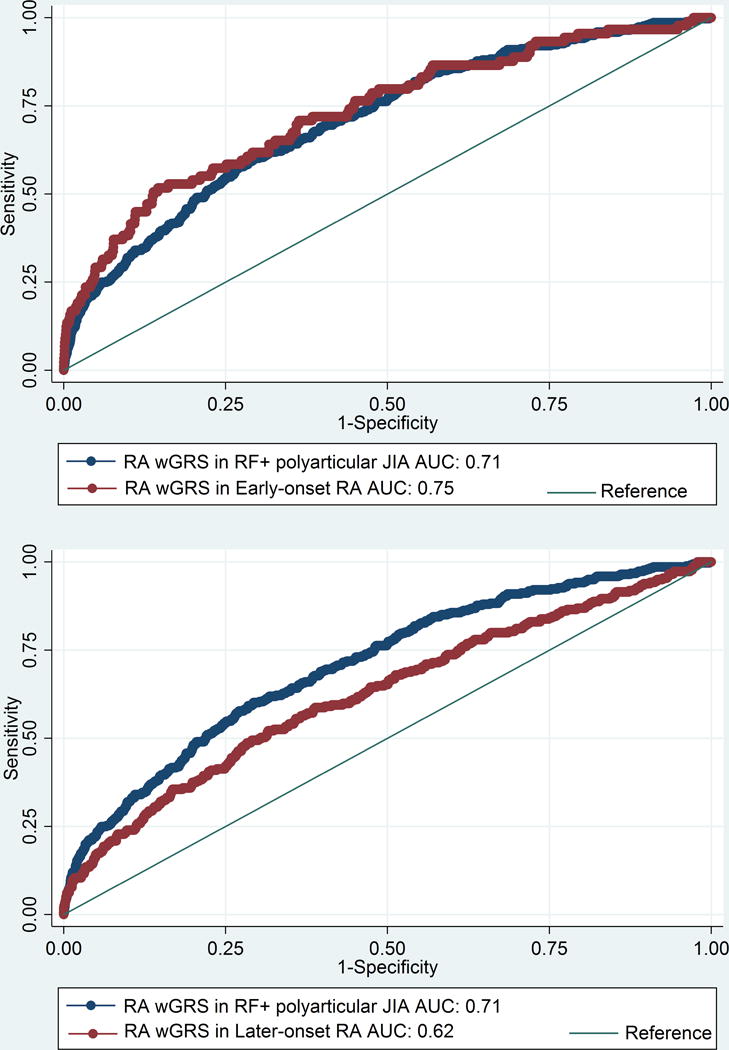
JIA, Juvenile idiopathic arthritis (oligoarticular/RF-negative polyarticular JIA); RA, rheumatoid arthritis; ROC, receiver operator characteristic; wGRS, weighted genetic risk score; AUC, area under the curve.
Outside the HLA region, no other region reached genome-wide significance, however there was suggestive association for 13 regions (p<1×10−4) Imputed SNP results are included when the imputed SNP had a better imputed P value than the most significant directly genotyped SNP in the region (Supplementary Table 4, Supplementary Figures 1 and 2). Supplementary Table 4 denotes imputed SNPs with a “b” superscript. Of the 13 regions most strongly associated with RF-positive polyarticular JIA, 5 contained SNPs (or SNPs in LD, r2>0.8) with some previous evidence for association with RA (9).
Discussion
This represents the largest genetic study for RF-positive polyarticular JIA to date. We provide evidence that this uncommon category of JIA, which is phenotypically similar to adult seropositive RA, is also genetically more similar to adult RA than to the most common JIA categories, which lack the characteristic biomarkers (RF and anti-CCP). The results of the wGRS analysis generated from the top RA associated loci better predicted RF-positive polyarticular JIA case-control status than the wGRS generated from the oligoarticular/RF-negative polyarticular JIA top hits.
We investigated whether any of the previously associated RA (9) or oligoarticular/RF-negative polyarticular JIA loci (4) showed evidence for association with RF-positive polyarticular JIA. Nineteen of the 44 SNPs reaching genome-wide significance thresholds with RA show evidence for association with RF-positive polyarticular JIA (p<0.05). There appears to be less overlap with the oligoarticular/RF-negative polyarticular JIA loci since only six of the 27 oligoarticular/RF-negative polyarticular JIA SNPs show evidence for association with RF-positive polyarticular JIA. Formal testing for a difference in the two proportions using the likelihood ratio test was suggestive but not statistically significant (p=0.0676).
As might be expected, the most significant association was within the HLA region, and the SNP is in strong LD (r2=0.88) with the most associated HLA SNP in RA. We have previously reported the HLA associations for all the categories of JIA (8) and found that RF-positive polyarticular JIA has distinct HLA associations compared to the other categories of JIA. The HLA-DRB1 amino acid position 13, is most strongly associated with RF-positive polyarticular JIA, with a histidine residue driving the association. This is the same HLA association as found in RA (8;13). A glycine residue at this same amino acid position drives the association in oligoarticular/RF-negative polyarticular JIA. This supports separation of RF-positive polyarticular JIA from the other JIA categories and confirms that RF-positive polyarticular JIA is more similar to RA than to other JIA categories (8).
Other than the HLA region we were unable to identify novel loci meeting genome-wide levels of significance. This may be expected, as despite being the largest genetic study to date for RF-positive polyarticular JIA, our study is still relatively underpowered to detect odds ratios of ~1.1-1.2, as are often observed in autoimmune diseases. We have identified 13 regions which have a p-value of < 1×10−4, which will need validation in an independent cohort to confirm. The strongest non-HLA association for RF-positive polyarticular JIA was rs9610687 which lies upstream of the RAC2 gene. Mutations with RAC2 are associated with neutrophil immunodeficiency syndrome. Polymorphisms within the IL2RB gene, close to RAC2, have previously been associated with oligoarticular/RF-negative polyarticular JIA (4) and with RA (9). However the oligoarticular/RF-negative polyarticular JIA-associated SNP (rs2284033) is ~500kb from the RF-positive polyarticular JIA-associated SNP. The oligoarticular/RF-negative polyarticular JIA-associated SNP in IL2RB was not significantly associated with RF-positive polyarticular JIA (p=0.70). In RA the most associated SNP (rs3218251) in this region again lies in the IL2RB gene, and this SNP is not in LD with the oligoarticular/RF-negative polyarticular JIA-associated SNP.
Although this study has numerous important findings, there are some important limitations. Firstly, the RA cases included in the wGRS analysis are a mixture of both seronegative and seropositive RA (though the biggest proportion are seropositive (68% CCP positive)), potentially diluting or masking effect sizes.
Secondly, the RA UK cases and controls included in these analyses are part of the Eyre et al RA Immunochip study(9), and this lack of independence could artificially inflate the predictive scores of the wGRS. A more recent genetic study in RA published by Okada et al (15), identified 101 genetic regions associated with RA. Many of these regions were not covered on the Immunochip array and so it was not possible to use these in the wGRS analysis (9).
The current ILAR classification criteria (1) are based on clinical features and family history, and it is not always straightforward to assign children to a category. In addition, there still remains heterogeneity, especially in terms of prognosis, between and within the categories of JIA. In time, clear delineation of the genetics of JIA categories may contribute to a more refined classification system. Whilst it has been recognized for many years that RF-positive polyarticular JIA is clinically and serologically similar to adult RA, there have been no systematic investigations of possible genetic overlap between these phenotypes of inflammatory arthritis. One reason for this is that several JIA categories are rare, and large-scale international collaborations such as this, and the one established for systemic onset JIA, another rare category (16), are necessary to build up sample sizes for genetic studies of these phenotypes.
We have now shown that RF-positive polyarticular JIA is genetically more similar to adult RA than to the oligoarticular/RF-negative polyarticular JIA categories. Demonstrating that RF-positive polyarticular JIA genetically appears to be a childhood-onset presentation of RA supports further investigation of this phenotype, and the factors influencing an early onset presentation. Broadly, our results suggest that genetic profiling might enhance our ability to classify and understand the different phenotypes of inflammatory arthritis. Our results also provide a rationale for studying both diseases together and for translating therapeutic trials of successful pharmacological agents from adult RA to RF-positive polyarticular JIA and vice-versa.
Supplementary Material
Acknowledgments
We thank Paul Gilbert for preparing UK JIA case samples for genotyping and Mary Ryan for preparing US JIA and the Cincinnati local control samples.
Genotyping of the US JIA, German JIA and respective control collections was supported by RC1-AR-058587 and U01-AI-067150S1. In addition, patient recruitment and DNA preparation in the US was largely funded by N01-AR-42272, P01-AR-048929 and P30-AR-473639, with contributions from the Rheumatology Research Foundation, the Arthritis Foundation, The Val A. Browning Charitable Foundation in Salt Lake City and the Marcus Foundation Inc. in Atlanta, GA as well as NIH grants K23-AR-50177 and R01-AR-060893. The Federal Ministry of Education and Research, Germany (BMBF grants 01GM0907 and 01 ZZ 0403 supported patient recruitment and sample preparation in Germany. We acknowledge support from the Wake Forest School of Medicine Center for Public Health Genomics and from the NIH for computing resources and data analysis (R01-AR-057106).
Genotyping of the UK JIA cases samples was supported by the Arthritis Research UK grant reference number 20385. This work is supported by the National Institute for Health Research Biomedical Research Centre. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The Arthritis Research UK centre for Genetics and Genomics laboratory is supported by the Manchester Academic Health Sciences Centre (MAHSC). The CAPS study was funded by Arthritis Research UK grant reference 20542. The CHARMS study was funded by Sparks UK, reference 08ICH09, Arthritis Research UK grant reference 20164) and the Medical Research Council, reference MR/M004600/1, and supported by the National Institute for Health Research (NIHR) Biomedical Research Centres at Great Ormond Street Hospital for Children NHS Foundation Trust, and University College London Hospitals Trust, and the NIHR-Clinical Research Network (CRN). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The authors would like to acknowledge the assistance given by IT Services and the use of the Computational Shared Facility at The University of Manchester.
Patient recruitment and DNA preparation in Canada was supported by funding from the Canadian Institutes of Health Research (FRN-82517), The Canadian Arthritis Society and the Canadian Arthritis Network.
We acknowledge Nils Thomas Songstad and Nina Moe for patient recruitment in the Norwegian subcohort of the Nordic JIA study, to Kristin Rian for technical support, and Helse Nord Research Grants for funding. The HUNT study is a collaboration between the HUNT Research Centre (Faculty of Medicine and Health Sciences, NTU - Norwegian University of Science and Technology), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health. Johanna Hadler, Katie Cremin, Karena Pryce, and Jessica Harris are acknowledged for excellent technical assistance. Funding: the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU).
Sample recruitment was supported in part by Grants Number N01AR62277, GM103510, AI082714, AR053483 from NIAMS/NIGMS/NIAID/NIH and from grant number 1296353 from the Texas Scottish Rite Hospital for Children. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of these institutes or NIH. PAN was supported by the Fundación Bechara. SR is supported by grants from the National Institutes of Health (1R01AR063759 (SR), 5U01GM092691-05 (SR), 1UH2AR067677-01, U19 AI111224-01), and the Doris Duke Charitable Foundation Grant #2013097.
We would like to thank the WTSI Genotyping Facility and in particular Emma Gray, Sue Bumpstead, Doug Simpkin and Hannah Blackburn for typing some of the UK samples.
We acknowledge use of DNA from the UK Blood Services collection of common controls (UKBS-CC collection), which is funded by the Wellcome Trust grant 076113/C/04/Z and by US National Institute for Health Research program grant to the National Health Service Blood and Transplant (RP-PG-0310-1002). We acknowledge the use of DNA from the British 1958 Birth Cohort Collection, which is funded by the UK Medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/01. Genotyping of control samples was supported, in part, by grants from the Juvenile Diabetes Research Foundation International (JDRF) and the NIH (U01 DK062418).
We thank Peter K. Gregersen at the Feinstein Institute for providing U.S. control genotyping from the Genotype and Phenotype registry (www.gapregistry.org) supported by National Institutes of Health grant RC2AR059092. We thank the NIDDK IBD Genetics Consortium for providing North American control genotyping supported by the National Institutes of Health grants DK062431, DK062422, DK062420, DK062432, DK062423, DK062413, and DK062429.
We gratefully acknowledge contributions from physicians at CCHMC and collaborating clinics. We also acknowledge the assistance of Bronte Clifford and Lori Ponder for patient recruitment and coordination of clinical information at Cincinnati Children’s Hospital Medical Center, the University of Utah and at Emory University, respectively. The Cincinnati normal control DNA collection was supported and made available by Cincinnati Children’s Hospital Medical Center.
Footnotes
DR. ANNE HINKS (Orcid ID : 0000-0001-8843-3967)
Reference List
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 2.Prahalad S, Zeft AS, Pimentel R, Clifford B, McNally B, Mineau GP, et al. Quantification of the familial contribution to juvenile idiopathic arthritis. Arthritis Rheum. 2010;62(8):2525–9. doi: 10.1002/art.27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther. 2011;13(1):101. doi: 10.1186/ar3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinks A, Cobb J, Marion MC, Prahalad S, Sudman M, Bowes J, et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45(6):664–9. doi: 10.1038/ng.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prahalad S, Conneely KN, Jiang Y, Sudman M, Wallace CA, Brown MR, et al. Susceptibility to childhood-onset rheumatoid arthritis: investigation of a weighted genetic risk score that integrates cumulative effects of variants at five genetic loci. Arthritis Rheum. 2013;65(6):1663–7. doi: 10.1002/art.37913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prahalad S, Thompson SD, Conneely KN, Jiang Y, Leong T, Prozonic J, et al. Hierarchy of risk of childhood-onset rheumatoid arthritis conferred by HLA-DRB1 alleles encoding the shared epitope. Arthritis Rheum. 2012;64(3):925–30. doi: 10.1002/art.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tebo AE, Jaskowski T, Davis KW, Whiting A, Clifford B, Zeft A, et al. Profiling anti-cyclic citrullinated peptide antibodies in patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2012;10(1):29. doi: 10.1186/1546-0096-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinks A, Bowes J, Cobb J, Ainsworth HC, Marion MC, Comeau ME, et al. Fine-mapping the MHC locus in juvenile idiopathic arthritis (JIA) reveals genetic heterogeneity corresponding to distinct adult inflammatory arthritic diseases. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44(12):1336–40. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–7. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44(3):291–6. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bakker PI, McVean G, Sabeti PC, Miretti MM, Green T, Marchini J, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38(10):1166–72. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ombrello MJ, Arthur VL, Remmers EF, Hinks A, Tachmazidou I, Grom AA, et al. Genetic architecture distinguishes systemic juvenile idiopathic arthritis from other forms of juvenile idiopathic arthritis: clinical and therapeutic implications. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


