Abstract
Study question
Are boys who are born to mothers who use analgesics during pregnancy at increased risk of cryptorchidism compared to those born to mothers who do not take analgesia?
Summary answer
In this systematic review and meta-analysis of 10 published studies, we observed only weak evidence of an association between analgesia use during pregnancy and risk of cryptorchidism in the son.
What is known already
Concentrations of analgesia relevant to human exposure have been implicated as causing endocrine disturbances in the developing foetal testis. However, when viewed collectively there appears to be conflicting evidence regarding an association between maternal use of analgesics and development of cryptorchidism.
Study design, size, duration
A systematic review and meta-analysis of studies on analgesia use during pregnancy and risk of cryptorchidism was performed. The search terms used were (analges* OR paracetamol OR acetaminophen) AND (cryptorchidism OR cryptorchism OR undescended test* OR non-descended test* OR non descended test*) for the databases Ovid Medline, Embase, Scopus and Web of Science. The search included all published articles up until 23rd May 2016 and no limits were set in terms of language.
Participants/materials, setting, methods
Abstracts were screened by one reviewer to remove irrelevant studies, with a 10% random sample of these verified by a second reviewer. The full text of all remaining papers was obtained and assessed by two reviewers to identify those which met our inclusion criteria. Abstracts included in the final analysis included studies which reported associations between the exposure (analgesia) and the outcome (cryptorchidism), with no limit on study design. Studies were only included if data was provided from which summary associations (odds ratios or relative risks) and their 95% confidence limits could be calculated, or if these summary associations were provided by the authors themselves. For each included study, two reviewers (JG and VS) independently extracted study meta-data in line with PRISMA recommendations. We assessed study quality and potential for bias using the criteria outlined in the Newcastle-Ottawa Quality Assessment Scale, but did not determine a quality score. Two reviewers independently assessed study quality against these criteria.
Main results and the role of chance
After screening 350 manuscripts, 10 were included in our review (five case-control studies, five cohort studies). We observed weak evidence of an association between ever-use of analgesia and risk of cryptorchidism (pooled crude odds ratio (OR): 1.11, 95% CI 1.00-1.23), with case-control studies revealing a marginally stronger association (1.23, 95% CI 0.85-1.78) than cohort studies (1.09, 95% CI 0.97-1.22). We observed weak evidence of a dose-response relationship between increasing weeks of analgesia exposure and risk of cryptorchidism, as well as weak evidence of an effect of timing on analgesia exposure and risk of cryptorchidism. Assessment of study quality via the Newcastle-Ottawa criteria revealed little (if any) evidence of substantial bias that may have meaningfully affected a given study’s results.
Limitations, reasons for caution
While confounding does not appear to be important, misclassification of the exposure is possibly an important source of measurement error in this context. The systematic review is open to reporting bias. Owing to scant data, no meta-analyses for two key questions (relating to dose-response and timing of exposure) could be performed. Medications were grouped based on their common effect and this offers little insight into the relation between specific types of analgesia and cryptorchidism. Finally, there are limitations in assuming that analgesia use reported by mothers is synonymous with actual intrauterine exposure.
Wider implications of the findings
The ubiquity of analgesia use during pregnancy makes this exposure particularly important from a population health perspective. Nine of the ten studies were conducted in Europe or USA, limiting generalizability of our observations. While the observations from our systematic review and meta-analysis suggest that analgesia use during pregnancy is not strongly associated with cryptorchidism development in the son, they also highlight the need for further detailed assessments of the relationship between maternal analgesia use and risk of cryptorchidism.
Study funding/competing interest(s)
This study was funded by the Health Research Council of New Zealand (reference #: 14/052). The authors have no conflict of interest to declare.
Registration number
CRD42016041414
Keywords: Cryptorchidism, cryptorchism, analgesia, painkiller, paracetamol, congenital abnormality, pregnancy
Introduction
Although rare in absolute terms, cryptorchidism – failure of the testes to descend permanently into their terminal scrotal position – is one of the most common congenital anomalies to affect boys (Ansell et al., 1992). Cryptorchidism is one of the few known risk factors for testicular cancer, wherein males who have suffered cryptorchidism are nearly five times more likely to develop testicular cancer than those who have not (relative risk: 4.8, 95% CI 4.0-5.7) (Dieckmann & Pichlmeier, 2004). Cryptorchidism is also a risk factor for sub-fertility: men who have a history of cryptorchidism are twice as likely to be sub-fertile (10.5%) compared to those without cryptorchidism (5.4%) (Lee et al., 1996). Risk factors for cryptorchidism remain unclear, but are thought to be related to disruptions to normal endocrine function sometime during the two phases of testicular descent (8-15 weeks and 25-35 weeks gestation, respectively) (Hutson et al., 2016).
The use of medicines to relieve pain – collectively referred to as analgesics – is ubiquitous: a recent study of nearly 40,000 Norwegian adults observed that 47% took an over-the-counter analgesic at least once per week, with the most common of these being paracetamol (also known as acetaminophen) (Dale et al., 2015). Use is also common in pregnancy: in a study of 47,000 mothers giving birth to boys, Jensen et al. (2010) observed that 55% had taken either acetaminophen, ibuprofen or acetylsalicylic acid at some point during pregnancy.
Analgesics have been implicated as endocrine disruptors, and it has been shown that concentrations relevant to human exposure can cause endocrine disturbances in the foetal testis (Mazaud-Guittot et al., 2013). Several studies have observed a positive association between maternal use of analgesics and subsequent development of cryptorchidism in their sons (Berkowitz & Lapinski, 1996; Jensen et al., 2010; Kristensen et al., 2011; Snijder et al., 2012). For example, Snijder et al. (2012) observed that women who used mild analgesics during their second trimester had more than twice the odds of giving birth to sons who had cryptorchidism (adjusted odds ratio (OR): 2.12, 95% CI 1.17-3.83), and that up to 24% of all cryptorchidism cases in their cohort could be attributed to the maternal use of mild analgesics during pregnancy.
However, when viewed collectively there appears to be conflicting evidence regarding this relationship, with several authors finding no (Mori et al., 1992; Wagner-Mahler et al., 2011) or weak/limited (Davies et al., 1986; Philippat et al., 2011; Rebordosa et al., 2008) evidence of an association. Thus, the following questions remain unanswered: are boys who are born to mothers who use analgesia during pregnancy at increased risk of cryptorchidism compared to those born to mothers who do not take analgesia? If an association exists, does the risk of cryptorchidism increase with increasing levels of maternal analgesia use? Finally, does the timing of maternal analgesia use (e.g. by trimester) affect the risk of cryptorchidism?
In order to address these questions, we have conducted a systematic review and meta-analysis of studies of maternal analgesia use and development of cryptorchidism in the son.
Materials and Methods
Search strategy
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). The review was registered with the International Prospective Register of Systematic Reviews (PROSPERO, registration ID. CRD42016041414), describing in advance the aims and methods of our investigation (Gurney et al., 2016).
Eligibility criteria
The PICOS (Patient/Participant, Intervention, Comparator, Outcome, Study design) criteria used to construct this review are outlined in Supplementary Table S1. Abstracts in the final analysis included studies which reported associations between the exposure (analgesia) and the outcome (cryptorchidism), with no limit on study design. Studies were only included if data were provided from which summary associations (ORs or relative risks) and their 95% CIs could be calculated, or if these summary associations were provided by the authors themselves.
Information sources
A systematic review was conducted on 23rd May 2016 for all articles published up until that time. No limits were set in terms of language used during the initial abstract search. Our search was conducted using Ovid Medline, Embase, Scopus and Web of Science databases. The reference list of those studies which were considered eligible for inclusion (see Study selection and data extraction, below) were scanned for additional relevant studies. International experts in the field (KM, LR) scanned the list of those studies which met our inclusion criteria in order to identify any studies that had been missed by our search.
Search terms
Using a Boolean approach, we searched the electronic databases for each possible combination of the following keywords (* indicates ‘explosion’ term): (analges* OR paracetamol OR acetaminophen) AND (cryptorchidism OR cryptorchism OR undescended test* OR non-descended test* OR non descended test*) (Supplementary Table S2). References were collected and logged in EndNote vX7.1 (Thomson Reuters, NY, USA).
Study selection and data extraction
Screening of abstracts
Figure 1 shows the flow chart for study identification and inclusion. Duplicate papers were removed prior to abstract screening. Abstracts were screened by one reviewer (JG) to remove irrelevant studies, with a 10% random sample of these verified by a second reviewer (VS). The full text of all remaining papers was obtained and assessed by two reviewers (JG and VS) to identify those meeting our inclusion criteria. Any disagreements about inclusion were resolved by referral to a third reviewer (DS). All papers that were considered relevant during the abstract screening process but ineligible for inclusion in our final analysis are listed, along with justification(s) for why they were ultimately excluded (Supplementary Table S3).
Figure 1.
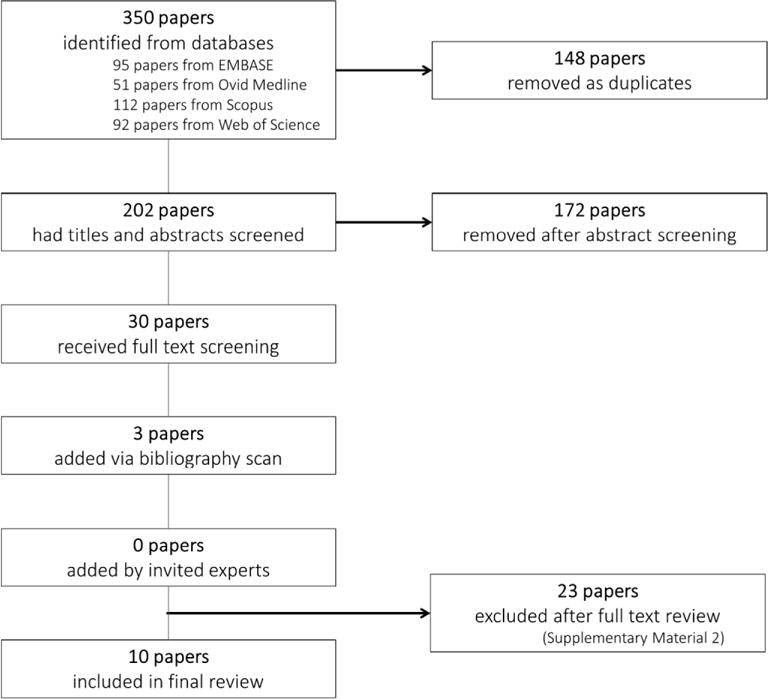
Flow chart for study identification and inclusion in a systematic review and meta-analysis of analgesia use during pregnancy and risk of cryptorchidism.
Data extraction
For each included study, two reviewers (JG and VS) independently extracted study meta-data in line with PRISMA recommendations. Data items for which there was any disagreement between the two reviewers were referred to a third reviewer (DS).
Assessment of risk of bias (individual and across studies)
In the absence of a gold standard, it has been recommended that any tools used to measure study quality should be as specific as possible to the given topic, and involve a simple checklist as opposed to a scale or score (Sanderson et al., 2007). Given these factors, we assessed study quality and potential for bias using the criteria outlined in the Newcastle-Ottawa Quality Assessment Scale (Alam et al., 2010; Wells et al., 2004), but did not determine a quality score (Burkey et al., 2014). Two reviewers (JG and VS) independently assessed study quality against these criteria, with disagreements resolved by referral to a third reviewer (DS).
Statistical analysis
In those instances where it was feasible to conduct meta-analyses, we used crude ORs and their 95% CIs for this analysis. This was for two reasons. First, the included studies varied in their reporting of risk estimates, with some reporting ORs and one study reporting hazard ratios (HRs) (although we note that given the rarity of the outcome risk ratios, ORs and HRs will give approximately the same estimate). Second, there was heterogeneity among studies in terms of adjustment for confounding, with some studies not adjusting for confounding whatsoever.
Since not all studies provided crude ORs, we calculated these estimates and their 95% CIs from tabulated data provided in the manuscripts. Using a random-effects model, we applied inverse-variance weighted methods for combining results across included studies to arrive at a final summary OR (and associated 95% CIs). We tested for evidence of heterogeneity among studies using both the X2 test (with a lower p-value indicating high inter-study heterogeneity) (Kirkwood & Sterne, 2003) and I2 index (0% indicating no inter-study heterogeneity) (Alam et al., 2010). We also generated and visually inspected funnel plots for evidence of publication bias.
Meta-analysis was completed in Stata v11.2 (StataCorp, Texas, USA) using the metan and metafunnel functions (Sterne et al., 2006).
Results
The systematic review identified 10 manuscripts that reported associations between analgesia use during pregnancy and risk of cryptorchidism in the son (Figure 1), of which five were case-control studies (Banhidy et al., 2007; Berkowitz & Lapinski, 1996; Davies et al., 1986; Mori et al., 1992; Wagner-Mahler et al., 2011) and five were cohort studies (Jensen et al., 2010; Kristensen et al., 2011; Philippat et al., 2011; Rebordosa et al., 2008; Snijder et al., 2012). It is worth noting that three of the included (cohort) studies were conducted over a similar period of time using the Danish National Birth Cohort as a basis for cohort development (Jensen et al., 2010; Kristensen et al., 2011; Rebordosa et al., 2008).
Studies were largely conducted among European populations, with eight studies conducted in Europe, one in the USA and one in Japan (Table 1). In terms of outcome measurement, the vast majority (8 of 10) used clinical diagnosis either at birth or shortly afterward, with only two of the included studies (Jensen et al., 2010; Mori et al., 1992) using orchidopexy to define the occurrence of cryptorchidism. Due to disease rarity, many studies had a small sample of cryptorchid cases, with the number of cases ranging from n=35 (Kristensen et al., 2011) to n=2,051 (Banhidy et al., 2007).
Table 1.
Papers included in meta-analysis of association between maternal use of analgesia during pregnancy and cryptorchidism, with study meta-data.
| Author | Year of publication |
Study design |
Study period |
Location of study |
Sample size | Source of case / outcome data |
Source of control / total cohort |
Exclusion criteria |
Method
of outcome measurement |
Timing
of outcome measurement |
Method
of exposure measurement |
Timing
of exposure measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banhidy et al.1 | 2007 | CCS | 1980 – 1996 | Hungary | 23,721 cases with congenital abnormalities
(2,051 with crypt) 45,946 controls (38,151 matched healthy and 834 with Downs Syndrome) |
Hungarian Congenital Abnormality Registry | Hungarian Birth Registry | Mild congenital abnormalities not included | Clinical diagnosis (reported to the Registry by obstetrician/paediatrician) | Reported during the first three months since birth | Maternal self-report (questionnaire),
medical note review/antenatal logbook Face to face interview for non-respondents |
Retrospective
questionnaire 2nd and 3rd gestational months |
| Berkowitz et al.2 | 1996 | Nested CCS | 1987 –1990 | New York City hospital |
140 ‘late descenders’; 63
cases with crypt at one-year 203 controls |
Hospital obstetrics service – all boys born during study period | Hospital obstetrics service – the next male infant born after case during study period | All multiple births | Clinical diagnosis | F/U clinical exam at 3 months and 1 year from expected delivery date | Parental questionnaire | Retrospective questionnaire administered
during post-partum stay Medications during the index pregnancy |
| Davies et al.3 | 1986 | CCS | 1978 - 1986 | UK | 83 cases 129 controls |
Hospital notes -crypt boys born in a single hospital and confirmed in Urological clinic | Two boys born just after each case – on the same day | All multiple births | Clinical diagnosis | Unspecified | Maternal face to face or phone Interview – GP confirmation of medical/pregnancy history | Retrospective |
| Mori et al.4 | 1992 | CCS | 1978 - 1986 | Japan | 104 crypt 104 matched controls |
Discharge records or medical notes of two hospitals | Outpatients records at same two hospitals | Any multiple birth | Orchidopexy | Throughout childhood | Self-report questionnaire or face to face interview | Retrospective, throughout childhood (average child age 6.2 years) |
| Wagner-Mahler et al.5 | 2011 | Nested CCS |
2002 – 2005 | France | 95 cases 188 controls |
Hospital records | Hospital records | Born before 34 weeks of amenorrhoea, stillbirth | Clinical diagnosis | Birth | Self-report questionnaire | Retrospective, during hospital stay after birth |
| Jensen et al.6 | 2010 | Cohort | 1996 - 2002 | Denmark | 47,400 women 980 crypt (565 with orchidopexy) |
Danish National Patient Registry | Danish National Birth Cohort | Mother not intending to carry to term, not live birth, all multiple births, boys or mothers not uniquely identifiable | Clinical diagnosis Orchidopexy |
Throughout childhood | Self-report questionnaire, then phone interview post-birth | Prospective, interviews at 17 and 32 gestational weeks; retrospective interviews 6 and 18 months after birth |
| Kristensen et al.7 | 2011 | Cohort | 1997-2001 | Denmark and Finland |
Denmark: 491 mothers Finland: 1,463 mothers |
Hospital records | Hospital records | Non-usual residence in Denmark or Finland among parents and grandparents of the son | Clinical diagnosis | Denmark: Birth Finland: 18 months |
Denmark: phone interview Finland: self-administered questionnaire |
Prospective, third trimester |
| Philippat et al.8 | 2011 | Cohort | 2003 -2006 | France | 1st and 2nd trimester:
895 Whole pregnancy: 903 38 crypt cases |
Hospital records from two hospital maternity units (Eden mother-child cohort) | Hospital records from two hospital maternity units (Eden mother-child cohort) | Any multiple birth | Clinical diagnosis | Within 3 days of birth | Questionnaire by midwives | Prospective, first and second trimesters |
| Rebordosa et al.9 | 2008 | Cohort | 1996 – 2003 | Denmark | 88,142 women | Danish National Patient Registry | Danish National Birth Cohort | Not intended to carry to term, non-Danish speaking, stillbirths, abortions (spontaneous and induced), ectopic pregnancies, hydatiform mole, twins, and triplets | Clinical diagnosis | Throughout childhood | Self-report questionnaire, and phone interviews | Prospective, only first trimester results presented for cryptorchidism |
| Snijder et al.10 | 2012 | Cohort | 2002 - 2006 | Netherlands | 3,184 women 68 crypt cases 3,094 normal boys |
Determined during one of 10 visits to child health centres between 0-48 months | ‘Generation R’ birth cohort | Lived outside of study area, had incomplete analgesic information | Clinical diagnosis | Between birth and 2 years of age | Self-report questionnaire | Prospective, three times during pregnancy (12, 20 and 30 gestational weeks) |
CCS = case-control study.
Assessment of study quality via the Newcastle-Ottawa criteria revealed little (if any) evidence of substantial bias that may have meaningfully affected a given study’s results (Supplementary Tables S4 and S5). There was some relatively minor divergence between cases and controls in terms of response rate, with the differences in non-response proportions between cases and controls ranging from 13% to 19%. The use of self-report to measure exposure status is discussed later in this manuscript.
We observed a high degree of heterogeneity among studies in terms of adjustment for confounding. Table 2 shows the variables that were controlled for, in order of frequency across studies.
Table 2.
Variables included as confounders in the reviewed manuscripts.
Ever-use of analgesia
A total of 10 (five case-control and five cohort) studies were included in our assessment of the association between ever-use of any analgesia during pregnancy and development of cryptorchidism (Table 3). The adjusted measures of association presented by these studies ranged from 1.00 (Mori et al., 1992) to 1.93 (Berkowitz & Lapinski, 1996) for case-control studies, and 0.74 to 1.43 (Kristensen et al., 2011) for cohort studies. Where both crude and adjusted estimates were presented, adjustment for confounding had little if any impact.
Table 3.
Extracted data relating to ever-use of analgesia during pregnancy.
| Author | Study design | Level of exposure / comparator | Level of exposure / comparator | Measure of relative risk (OR/RR/HR) |
Reported crude
OR/RR/HR (95% CI) |
Reported adjusted
OR/RR/HR (95% CI) |
Number of exposed vs. non-exposed cases | Number of exposed vs. non-exposed controls/cohort | Adjustment for confoundinga |
|---|---|---|---|---|---|---|---|---|---|
| Banhidy et al.1 | CCS | Dipyrone | Ever-use of Dipyrone during 2nd or 3rd months of pregnancy | OR | – | Matched controls 1.2, 95% CI 0.8 – 1.8 | 43 exposed 2,008 non-exposed (2% exposed) | 736 exposed 37,415 non-exposed (2% exposed) | Controls matched for sex, week of birth,
district of parent’s residence Additional adjustment for maternal employment status, influenza or cold in 2nd and/or 3rd month of pregnancy |
| Boys with Down Syndrome 0.7, 95% CI 0.4 – 1.2 | Downs unmatched but adjusted as above, plus maternal age and birth order | ||||||||
| Berkowitz et al.2 | Nested CCS | Any analgesia | Ever use during pregnancy | OR | Cryptorchid cases: 1.89, 95% CI 1.07-3.34 | Cryptorchid cases: 1.93, 95% CI 1.03-3.62 | Cryptorchid cases: 37 exposed 26 non-exposed (59% exposed) | 87 exposed* 116 non-exposed* (43% exposed) | Controls matched on age of
son Additional adjustment for ethnicity (‘Asian women’), swollen legs or feet, Family history of cryptorchidism, Low birthweight, use of cola drinks during pregnancy, consumption of > 1 cup of tea per day |
| Late descenders: 1.29, 95% CI 0.84-1.98 | – | Late-descenders: 69 exposed 71 non-exposed (49% exposed) | – | ||||||
| Davies et al.3 | CCS | Use of analgesics during pregnancy | Ever use during pregnancy (further detail not provided) | OR (stated as ‘Relative Risk’) | 1.77 (no confidence intervals provided) | – | 23 exposed 60 non-exposed (29% exposed) | 23 exposed 106 non-exposed (18% exposed) | Controls matched on age of son |
| Mori et al.4 | CCS | Use of analgesics during pregnancy | Ever use during pregnancy | OR | – | 1.00, 95% CI 0.20-37.85 | 1 exposed 103 non-exposed (0% exposed) | 1 exposed 103 non-exposed (0% exposed) | Controls matched for age of son, year of admission for orchidopexy and birth year |
| Wagner-Mahler et al.5 | Nested CCS | Any use of Aspirin and/or Paracetamol | Ever use during pregnancy | – | – | – | 17 exposed 78 non-exposed (18% exposed) | 45 exposed 143 non-exposed (24% exposed) | Controls matched in terms of the age, place of birth, Gestational Age, birth weight and the country of birth of the parents |
| Jensen et al.6 | Cohort | Acetaminophen, ibuprofen, and acetylsalicylic acid | Ever-use of either
acetaminophen, ibuprofen or acetylsalicylic Acid (Among other exposures) |
HR | – | Clinically-diagnosed cryptorchidism: 1.06 (95% CI 0.93-1.21) | Clinically-diagnosed cryptorchidism: 467 exposed 428 non-exposed (52% exposed) | Clinically-diagnosed cryptorchidism: 195,326 PYAR exposed 190,405 PYAR unexposed (51% exposed) | Adjusted for Maternal age, household occupation, time to pregnancy, parity before birth of index boy, treatment of infertility, maternal smoking. |
| Orchidopexy-confirmed cryptorchidism: 1.11 (95% CI 0.93-1.32) | Orchidopexy-confirmed cryptorchidism: 276 exposed 242 non-exposed (53% exposed) | Orchidopexy-confirmed cryptorchidism: 196,556 PYAR exposed 191,606 PYAR non-exposed (51% exposed) | |||||||
| Kristensen et al.7 | Cohort | Mild analgesics (combined paracetamol, ibuprofen, and acetylsalicylic acid) | Ever use during
pregnancy (Among other exposures) |
OR | Denmark: 1.45, 95% CI 0.75-2.79 | Denmark: 1.43, 95% CI 0.73-2.79 | 27 exposed 15 non-exposed (64% exposed) | 249 exposed 200 non-exposed (55% exposed) | Adjusted for disease reported during pregnancy (indication to treat), use of other medications during pregnancy, gestational age |
| Finland: 0.80, 95% CI 0.40-1.60 | Finland: 0.74, 95% CI 0.35-1.57 | 13 exposed 22 non-exposed (37% exposed) | 606 exposed 822 non-exposed (42% exposed) | ||||||
| Philippat et al.8 | Cohort | Any use of Asprin, Paracetamol or Ibuprofen | Ever use during
pregnancy (Among other exposures) |
OR | 1.5, 95% CI 0.45-7.5 | 1.1, 95% CI 0.31–3.6 | 35 exposed 3 non-exposed (92% exposed) | 804 exposed 99 non-exposed (89% exposed) | Adjusted for gestational duration (defined from the date of the last menstrual period), center, maternal age, parity, maternal smoking, and educational level |
| – | With pre-term babies excluded: 1.6, 95% CI 0.35-6.8 | 33 exposed 2 non-exposed (94% exposed) | 776 exposed 96 non-exposed (89% exposed) | ||||||
| Rebordosa et al.9 | Cohort | Ever-use of acetaminophen during each trimester of pregnancy (only first trimester reported for cryptorchidism) | HR | 1.21 (no confidence intervals provided) | 1.24, 95% CI 0.79-1.94 | 33 exposed 63 non-exposed (34% exposed) | 26,424 exposed 61,718 non-exposed (30% exposed) | Adjusted for mother’s age, birth year, birth order and history of chronic diseases | |
| Snijder et al.10 | Cohort | Any use of mild analgesics (combined paracetamol and other painkillers, including NSAIDS and aspirin) | Ever use during pregnancy | OR | – | 1.25, 95% CI 0.73-2.13) | 23 exposed 45 non-exposed (34% exposed) | 956 exposed 2,228 non-exposed (30% exposed) | Adjusted for maternal age, educational level, BMI at intake, general health, use of co-medication, underlying diseases and fever during pregnancy |
Adjustment for confounding achieved via either control matching or inclusion as covariates in regression models.
Calculated from tabulated data in manuscript.
OR: odds ratio, RR: relative risk, HR: hazard ratio
Crude ORs calculated for the purposes of meta-analysis ranged from 0.69 (Wagner-Mahler et al., 2011) to 1.90 (Berkowitz & Lapinski, 1996) for case-control studies, and 0.80 to 1.45 (Kristensen et al., 2011) for cohort studies (Figure 2). Meta-analysis of crude ORs revealed little evidence of a strong association between ever-use of analgesia during pregnancy and risk of cryptorchidism in the son (pooled crude OR: 1.11, 95% CI 1.00-1.23; case-control studies: 1.23, 95% CI 0.85-1.78; cohort studies 1.09, 95% CI 0.97-1.22). While there was low heterogeneity among cohort studies (I2 0%, p=0.820), there was some evidence of moderate heterogeneity among case-control studies (I2 44%, p=0.128). When studies were combined, there was low overall heterogeneity across studies (I2 0%, p=0.46). A funnel plot revealed no strong evidence of publication bias (Supplementary Figure S1).
Figure 2.
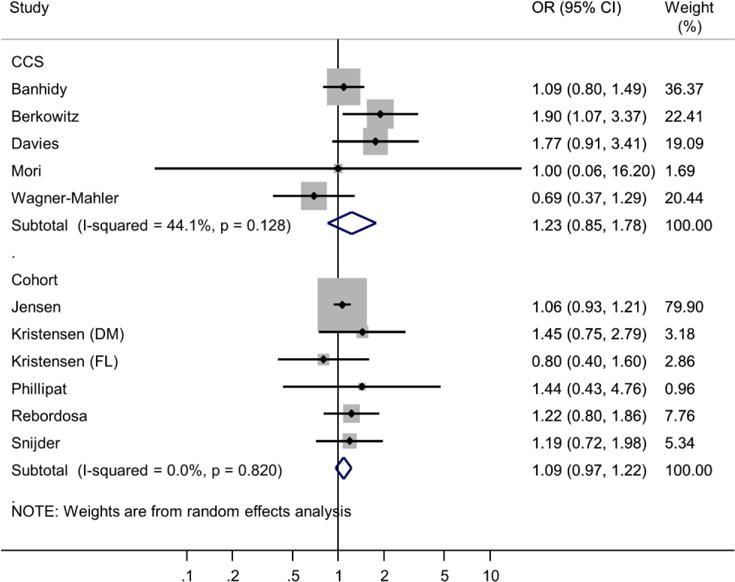
Meta-analysis of ever-use of analgesia during pregnancy and risk of cryptorchidism in the son, by study type (case-control studies and cohort studies). OR: odds ratio.
Dose-response
Two studies, both of which were cohort studies, presented data on a possible dose-response association between use of analgesia during pregnancy and development of cryptorchidism (Jensen et al., 2010; Kristensen et al., 2011).
When investigating cryptorchidism in Danish and Finnish cohorts, Kristensen et al. (2011) observed a dose-response association between analgesia use during pregnancy in their Danish cohort (adjusted ORs: 1-2 weeks use [compared to 0 weeks] 1.5, 95% CI 0.63-3.55; >2 weeks use 2.47, 95% CI 1.02-5.96) but not in their Finnish cohort (1-2 weeks use 1.21, 95% CI 0.50-2.90; >2 weeks use 0.56, 95% CI 0.13-2.45). Jensen et al. (2010) also observed a weak dose-response association, although this association marginally attenuated at the most extreme exposure level (adjusted HRs: 1 week [compared to 0 weeks] 1.09, 95% CI 0.89-1.34; 2-4 weeks 1.01, 95% CI 0.81-1.28; 5-8 weeks 1.32, 95% CI 0.97-1.78; 9-12 weeks 1.35, 95% CI 0.88-2.05; >12 weeks 1.23, 95% CI 0.90-1.66).
Timing of analgesia exposure
Four (cohort) studies assessed timing of analgesia use and risk of cryptorchidism (Jensen et al., 2010; Kristensen et al., 2011; Philippat et al., 2011; Snijder et al., 2012). Where both crude and adjusted estimates were presented adjustment for confounding had only marginal impact.
When investigating risk of cryptorchidism in both a Danish and a Finnish cohort, Kristensen et al. (2011) observed a stronger association between use of analgesia during the 2nd trimester (adjusted OR: Danish cohort 2.30, 95% CI 1.12-4.73; Finnish cohort 1.21, 95% CI 0.53-2.76) compared to the first (Danish cohort 1.48, 95% CI 0.66-3.34; Finnish cohort 0.77, 95% CI 0.26-2.27). Similarly, Jensen et al. (2010) observed a stronger association for analgesia use during the 2nd trimester (adjusted HR: 1.17, 95% CI 0.89-1.54) compared to the first (0.94, 95% CI 0.75-1.17), but also observed a small attenuation of this association for use during the 3rd trimester (1.08, 95% CI 0.87-1.33). Snijder et al. (2012) observed a stronger association for analgesia use during mid-pregnancy (crude OR: 2.04, 95% CI 1.15-3.62) compared to early (0.97, 95% CI 0.38-2.48) and late pregnancy (1.56, 95% CI 0.80-3.03).
Discussion
Our meta-analysis suggests that ever-use (compared to never-use) of analgesia during pregnancy is weakly associated with the development of cryptorchidism (pooled crude OR: 1.11, 95% CI 1.00-1.23). However, this lack of a clear association may reflect poor exposure specificity, since ever-use pools those who may have been exposed only once with those who may have been routinely exposed. This would result in misclassification of the exposure, which is likely to bias the observed associations towards the null.
On the other hand, it is also possible that in retrospective studies mothers with affected sons might overestimate their exposure relative to those with unaffected sons – which could explain the marginally-stronger association observed for the case-control studies. However, the difference between these two point estimates is not large (1.23 versus 1.09), suggesting that recall bias is not of substantial importance in this context.
We observed remarkable heterogeneity in the prevalence of ever-use between study populations – ranging from near-0% prevalence up to 94% prevalence, with considerable variation in between (Table 3). While there was a tendency for this prevalence to be higher in cohort studies (compared to case-control studies), this was not strongly associated with study design. This heterogeneity between studies could reflect wide variation in analgesia ever-use between study populations; however, it could also reflect a validity problem with this unit of measurement – in which self-report (particularly retrospective) does not adequately capture the true prevalence of analgesia ever-use. Also, we must accept that there are limitations in assuming that analgesia use reported by mothers is synonymous with actual intrauterine exposure by the foetus.
While there were few studies that had investigated a possible dose-response relationship, we did observe a pattern of increasing risk of cryptorchidism with increasing number of weeks of analgesia exposure. However, as with ever-use there is likely to be misclassification of the exposure. Ideally, we would measure dosage and frequency of use over the course of a woman’s pregnancy; but in this case, the exposure was simply the number of weeks during which any given amount of analgesia was taken.
Finally, we did observe a pattern of increased cryptorchidism risk when analgesia was consumed during the middle of pregnancy (largely coinciding with the 2nd trimester) relative to early- and late-pregnancy (Jensen et al., 2010; Kristensen et al., 2011; Snijder et al., 2012). Both Jensen et al. (2010) and Phillipat et al. (2011) observed a somewhat higher risk of cryptorchidism among those exposed to analgesia during early- to mid-pregnancy than those exposed at any time during pregnancy, suggestive of a pathway by which analgesia may disrupt the first phase of testicular descent; however, the risk estimates (adjusted ORs: 1.14 and 1.20, respectively) and their associated confidence intervals suggest that evidence of this scenario is weak. The issues relating to poor exposure measurement are also relevant here.
It was our original intention to conduct separate stratified analyses for those boys diagnosed by clinical examination and those diagnosed via corrective orchidopexy, in an effort to understand whether the relationship between analgesia use and cryptorchidism differed according to disease severity and/or persistence. However, the vast majority (8 of 10) of the included studies used clinical diagnosis at birth (or shortly afterward). Thus, we were unable to examine this relationship in any meaningful way.
Plausibility of analgesia as a cause of cryptorchidism
The biological plausibility of analgesia exposure during pregnancy as a risk factor for cryptorchidism in the son rests on the premise that this exposure disturbs the normal endocrine processes responsible for testicular descent in utero. Similar versions of the mechanism by which this disruption is proposed to occur have been put forward in the literature: namely, that cyclo-oxygenase (COX)-inhibiting analgesia taken during the critical ‘male programming window’ (sometime between 8 and 15 weeks gestation) diminishes normal androgen activity (Kristensen et al., 2012), causing a potentially critical disruption to this first phase of testicular descent by interfering with prostaglandin synthesis (Jensen et al., 2010; Kristensen et al., 2011; Snijder et al., 2012).
However, biological plausibility and aetiological reality are not synonymous concepts. Firstly, as noted earlier evidence is unclear regarding the relationship between the timing of analgesia use and cryptorchidism development – somewhat contradicting the hypothesis that analgesia use during the ‘male programming window’ and cryptorchidism development are strongly related. Secondly, there are similarly plausible arguments that suggest maternal analgesia use might cause other congenital anomalies – but there is little evidence that this is actually the case. For example, Rebordosa et al. (2008) compared 26,000 children who were exposed to acetaminophen during early pregnancy with 62,000 children who were not, and found no increased risk of congenital abnormality, regardless of whether they were ever-exposed (adjusted HR: 1.01, 95% CI 0.93-1.08) or exposed for more than 4 weeks during pregnancy (adjusted HR, compared to never-exposed: 1.04. 95% CI 0.89-1.21).
The weak associations reported here must also be viewed in the context of other research which has found that low-dose aspirin may be protective against other health outcomes, including pre-eclampsia (Henderson et al., 2014), as well as the ongoing debate regarding whether pre-conceptual aspirin use may (or may not) prevent pregnancy loss in high-risk populations (Schisterman et al., 2014).
The role of confounding
Adjustment for confounding among those studies that presented both crude and adjusted risk estimates generally had little impact on the strength of the given association. For example, Jensen et al.,(2010) reported near-identical crude and adjusted HRs despite adjusting for a number of potential confounders. The authors also conducted multiple sensitivity analyses with further potential confounders to their models, but again observed very little variation in terms of association strength (Jensen et al., 2011). This suggests that the relationship between analgesia use and risk of cryptorchidism is not noticeably affected by confounding.
Limitations
Our review has limitations. Firstly, like all systematic reviews the current manuscript is open to reporting bias, wherein those studies that observe the strongest effect are more likely to be published. Secondly, because of scant data, we were unable to conduct meta-analyses for two of our key study questions (relating to dose-response and timing of exposure). Thirdly, nine of the ten included studies were conducted in either Europe or the US, limiting the generalisability of our observations to non-Western populations.
Lastly, the current review grouped together medications based on their common effect – i.e. analgesia taken to alleviate pain. This grouping is largely in keeping with the idea that COX-inhibiting analgesia diminishes normal androgen activity by interfering with prostaglandin synthesis; however, it is still possible that these medications may be heterogeneous with respect to both the means by, and extent to, which they may affect testicular descent. For example, Jensen et al. observed a moderate association between acetaminophen exposure and cryptorchidism, but little evidence of such an effect from ibuprofen and acetylsalicylic acid (Jensen et al., 2010). It was our initial intention to stratify our analyses by medication group, but this was abandoned when it was discovered that many of the included manuscripts conflated these medications into an ‘any-use’ category (see Table 3). Thus, the current review offers little insight into the relationship between specific types of analgesia and cryptorchidism development.
Conclusions
We observed weak evidence of an association between ever-use of analgesia and risk of cryptorchidism. Due to the likely mismeasurement of the exposure we cannot rule out the possibility that this measure underestimates the association between a non-trivial dose of analgesic during pregnancy and cryptorchidism. We observed weak evidence of a dose-response relationship between increasing weeks of analgesia exposure and risk of cryptorchidism, but finer-grained assessments (including measurement of actual dosage and frequency of use) are required to substantiate this relationship. We observed weak evidence of an association between the timing of analgesia exposure and risk of cryptorchidism, whereby those boys exposed during early- to mid-pregnancy appeared to be marginally more at risk than those who were ever-exposed; however, risk estimates were close to the null and CIs were wide. This review highlights the need for further detailed assessments of the relationship between maternal analgesia use and risk of cryptorchidism.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Health Research Council of New Zealand (reference #: HRC 14/052).
Footnotes
Authors’ Roles
Jason Gurney led conception and design, led the analysis and interpretation of data, drafted the manuscript, and revised content based on feedback. Lorenzo Richiardi assisted with conception and design, assisted with interpretation of data, and provided critical revision of drafts. Katherine McGlynn assisted with interpretation of data, and provided critical revision of drafts. Virginia Signal acted as the second reviewer, and also extracted meta-data from the included manuscripts. Diana Sarfati assisted with interpretation of data and provided critical revision of drafts. She also acted as the third (mediating) reviewer in all cases where discrepancies between JG and VS occurred.
Conflict of interest
The authors have no conflict of interest to declare.
Supplementary Figure S1 Funnel plot, with study variance (i.e. SE of the log-odds ratio, vertical axis) plotted against measure of association (i.e. log-odds ratio, horizontal axis).
Contributor Information
Jason Gurney, Department of Public Health, University of Otago, Wellington, New Zealand.
Lorenzo Richiardi, Cancer Epidemiology Unit, University of Turin, Turin, Italy.
Katherine A. McGlynn, Division of Cancer Epidemiology & Genetics, National Cancer Institute, Maryland, USA
Virginia Signal, Department of Public Health, University of Otago, Wellington, New Zealand.
Diana Sarfati, Department of Public Health, University of Otago, Wellington, New Zealand.
References
- Alam SS, Cantwell MM, Cardwell CR, Cook MB, Murray LJ. Maternal body mass index and risk of testicular cancer in male offspring: A systematic review and meta-analysis. Cancer Epidemiology. 2010;34:509–515. doi: 10.1016/j.canep.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell PE, Bennet V, Bull D, Jackson MB, Pike LA, Pike MC, Chilvers CED, Dudley NE, Gough MH, Griffiths DM, et al. Cryptorchidism: A prospective study of 7500 consecutive male births, 1984–8. Archives of Disease in Childhood. 1992;67(7):892–899. doi: 10.1136/adc.67.7.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banhidy F, Acs N, Puho E, Czeizel AE. A population-based case-control teratologic study of oral dipyrone treatment during pregnancy. Drug Saf. 2007;30(1):59–70. doi: 10.2165/00002018-200730010-00006. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Lapinski RH. Risk factors for cryptorchidism: A nested case-control study. Paediatric and Perinatal Epidemiology. 1996;10(1):39–51. doi: 10.1111/j.1365-3016.1996.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Burkey MD, Feirman S, Wang H, Choudhury SR, Grover S, Johnston FM. The association between smokeless tobacco use and pancreatic adenocarcinoma: A systematic review. Cancer Epidemiology. 2014;38(6):647–653. doi: 10.1016/j.canep.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale O, Borchgrevink PC, Fredheim OMS, Mahic M, Romundstad P, Skurtveit S. Prevalence of use of non-prescription analgesics in the Norwegian HUNT3 population: Impact of gender, age, exercise and prescription of opioids. BMC Public Health. 2015;15(1):1–9. doi: 10.1186/s12889-015-1774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TW, Williams DRR, Whitaker AH. Risk factors for undescended testis. International Journal of Epidemiology. 1986;15(2):197–201. doi: 10.1093/ije/15.2.197. [DOI] [PubMed] [Google Scholar]
- Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World Journal of Urology. 2004;22(1):2–14. doi: 10.1007/s00345-004-0398-8. [DOI] [PubMed] [Google Scholar]
- Gurney J, Signal V, Sarfati D. Analgesia use during pregnancy and risk of cryptorchidism: a systematic review and meta-analysis (ID: CRD42016041414) 2016 doi: 10.1093/humrep/dex047. from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016041414. [DOI] [PMC free article] [PubMed]
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Thorup JM, Beasley SW. Descent of the testis. 2nd. Springer; 2016. [Google Scholar]
- Jensen MS, Henriksen TB, Rebordosa C, Thulstrup AM, Toft G, Sorensen HT, Bonde JP, Olsen J. Analgesics during pregnancy and cryptorchidism: additional analyses. Epidemiology. 2011;22(4):610–612. doi: 10.1097/EDE.0b013e31821eca69. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Rebordosa C, Thulstrup AM, Toft G, Sorensen HT, Bonde JP, Henriksen TB, Olsen J. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology. 2010;21(6):779–785. doi: 10.1097/EDE.0b013e3181f20bed. [DOI] [PubMed] [Google Scholar]
- Kirkwood BR, Sterne JA. Essential Medical Statistics. 2nd. Blackwell Science Ltd; 2003. [Google Scholar]
- Kristensen DM, Hass U, Lesne L, Lottrup G, Jacobsen PR, Desdoits-Lethimonier C, Boberg J, Petersen JH, Toppari J, Jensen TK, et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod. 2011;26(1):235–244. doi: 10.1093/humrep/deq323. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Lesné L, Le Fol V, Desdoits-Lethimonier C, Dejucq-Rainsford N, Leffers H, Jégou B. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. International Journal of Andrology. 2012;35(3):377–384. doi: 10.1111/j.1365-2605.2012.01282.x. [DOI] [PubMed] [Google Scholar]
- Lee PA, O’Leary LA, Songer NJ, Coughlin MT, Bellinger MF, LaPorte RE. Paternity after unilateral cryptorchidism: A controlled study. Pediatrics. 1996;98(4 PART 1):676–679. [PubMed] [Google Scholar]
- Mazaud-Guittot S, Nicolaz CN, Desdoits-Lethimonier C, Coiffec I, Maamar MB, Balaguer P, Kristensen DM, Chevrier C, Lavoué V, Poulain P, et al. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. Journal of Clinical Endocrinology and Metabolism. 2013;98(11):E1757–E1767. doi: 10.1210/jc.2013-2531. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Davies TW, Tsukamoto T, Kumamoto Y, Fukuda K. Maternal and other factors of cryptorchidism–a case-control study in Japan. Kurume Medical Journal. 1992;39(2):53–60. doi: 10.2739/kurumemedj.39.53. [DOI] [PubMed] [Google Scholar]
- Philippat C, Giorgis-Allemand L, Chevrier C, Cordier S, Jégou B, Charles MA, Slama R. Analgesics During Pregnancy and Undescended Testis. Epidemiology. 2011;22(5):747–749. doi: 10.1097/EDE.0b013e318225bf33. [DOI] [PubMed] [Google Scholar]
- Rebordosa C, Kogevinas M, Horváth-Puhó E, Nørgård B, Morales M, Czeizel AE, Vilstrup H, Sørensen HT, Olsen J. Acetaminophen use during pregnancy: effects on risk for congenital abnormalities. American Journal of Obstetrics and Gynecology. 2008;198(2):178.e171–178.e177. doi: 10.1016/j.ajog.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. International Journal of Epidemiology. 2007;36(3):666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, Lynch AM, Perkins NJ, Mumford SL, Galai N. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. The Lancet. 2014;384(9937):29–36. doi: 10.1016/S0140-6736(14)60157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder CA, Kortenkamp A, Steegers EAP, Jaddoe VWV, Hofman A, Hass U, Burdorf A. Intrauterine exposure to mild analgesics during pregnancy and the occurrence of cryptorchidism and hypospadia in the offspring: The Generation R Study. Human Reproduction. 2012;27(4):1191–1201. doi: 10.1093/humrep/der474. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Bradburn MJ, Egger M. Stata Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd. Wiley; 2006. Meta-analysis; pp. 347–369. [Google Scholar]
- Wagner-Mahler K, Kurzenne JY, Delattre I, Bérard E, Mas JC, Bornebush L, Tommasi C, Boda-Buccino M, Ducot B, Boullé C, et al. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. International Journal of Andrology. 2011;34(5 PART 2):e499–e510. doi: 10.1111/j.1365-2605.2011.01211.x. [DOI] [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, P T. Quality Assessment Scales for Observational Studies. Ottawa Health Research Institute; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


