Abstract
Background
During the 2014–15 US influenza season, expanded genetic characterization of circulating influenza A(H3N2) viruses was used to assess the impact of genetic variability of influenza A(H3N2) viruses on influenza vaccine effectiveness (VE).
Methods
A novel pyrosequencing assay was used to determine genetic group based on hemagglutinin (HA) gene sequences of influenza A(H3N2) viruses from patients enrolled US Flu Vaccine Effectiveness network sites. Vaccine effectiveness was estimated using a test-negative design comparing vaccination among patients infected with influenza A(H3N2) viruses and uninfected patients.
Results
Among 9710 enrollees, 1868 (19%) tested positive for influenza A(H3N2); genetic characterization of 1397 viruses showed 1134 (81%) belonged to one HA genetic group (3C.2a) of antigenically drifted H3N2 viruses. Effectiveness of 2014–15 influenza vaccination varied by A(H3N2) genetic group from 1% (95% confidence interval [CI], −14% to 14%) against illness caused by antigenically drifted A(H3N2) group 3C.2a viruses versus 44% (95% CI, 16% to 63%) against illness caused by vaccine-like A(H3N2) group 3C.3b viruses.
Conclusion
Effectiveness of 2014–15 influenza vaccination varied by genetic group of influenza A(H3N2) virus. Changes in hemagglutinin genes related to antigenic drift were associated with reduced vaccine effectiveness.
Keywords: influenza, pyrosequencing, influenza vaccine, vaccine effectiveness
INTRODUCTION
The 2014–2015 influenza season was characterized by widespread circulation of influenza A(H3N2) viruses, with high levels of outpatient illness and influenza-associated hospitalization, especially for adults aged ≥65 years [1]. Characterization of influenza A(H3N2) viruses that circulated in the US indicated that the majority were antigenically different (drifted) from the A(H3N2) vaccine component of 2014–2015 Northern Hemisphere seasonal influenza vaccines [2]. Antigenic relatedness of vaccine strains and circulating viruses is traditionally determined by the hemagglutination inhibition (HI) assay, which measures inhibition of virus-mediated agglutination of red blood cells by reference antisera from ferrets inoculated with vaccine viruses [3]. During the 2014–15 season, changes in the properties of many A(H3N2) viruses resulted in insufficient hemagglutination activity for characterization by HI [1]. In response, sequencing of hemagglutinin (HA) genes was increasingly used to determine genetic relatedness of antigenic variates of influenza A(H3N2) viruses [4]. Few studies have evaluated differences in influenza vaccine effectiveness according to genetic relatedness among influenza viruses [5, 6].
The multisite US Flu Vaccine Effectiveness network estimates influenza vaccine effectiveness (VE) each season; interim estimates of VE were 23% (95% confidence interval [CI] = 8%–36%) among patients enrolled November 10, 2014—January 2, 2015 when most influenza illnesses were due to A(H3N2) viruses [7]. To improve our ability to characterize the effect of antigenically drifted A(H3N2) viruses on influenza VE, we used a newly developed, high throughput pyrosequencing assay (unpublished data) to genetically characterize A(H3N2) viruses directly from respiratory specimens collected from patients enrolled in the Flu VE study. We report VE for the most common H3N2 genetic groups circulating in the US during 2014–15, and for the subset of A(H3N2) viruses that could not be genetically characterized. In addition, to better understand the genetic variability and geographic and temporal distribution of circulating A(H3N2) viruses in the United States during 2014–15, we describe the A(H3N2) genetic groups among U.S. surveillance viruses during the 2014–2015 influenza season.
MATERIALS AND METHODS
US Flu VE Network
US Flu VE network methods have been previously described.[8–10] Briefly, patients aged ≥6 months seeking outpatient medical care for an acute respiratory illness (ARI) within 7 days of illness onset were enrolled at study sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin during periods of influenza virus circulation at each site. Presence of a high-risk medical condition was defined as documentation of any International Classification of Diseases, Ninth Revision (ICD-9) diagnostic code associated with specified high-risk conditions[11] in the patient’s medical record during the 12 months before enrollment. Receipt of 2014–2015 influenza vaccine was confirmed by review of medical records or immunization registries; patients who self-reported vaccination not captured by medical records or registries were assumed to be vaccinated if plausible information on date and location of vaccine receipt was provided. Receipt of prior season (2013–2014) influenza vaccine was obtained from medical records or immunization registries only. We excluded patients vaccinated 0–13 days before illness onset and those aged 6 months to 8 years who received only one dose of 2014–2015 influenza vaccine for whom two doses were recommended by the Advisory Committee on Immunization Practices (ACIP).[11]
Respiratory specimens (combined nasal and oropharyngeal swabs for patients aged ≥2 years; nasal swabs only for patients aged 6–23 months) were collected and tested for influenza viruses at Flu VE network laboratories by rRT-PCR.[12] A subset of influenza-positive specimens from each network site, including the first 10 positive specimens identified and up to five positive specimens every two weeks during the enrollment period, were sent to National Influenza Surveillance Reference Centers for virus isolation and propagation for antigenic characterization by HI. Given challenges with antigenic characterization of many A(H3N2) viruses, A(H3N2)-positive respiratory specimens containing high levels of influenza virus RNA (defined as rRT-PCR cycle threshold value ≤ 30) were sent to CDC for pyrosequencing directly from respiratory specimens. All A(H3N2)-positive specimens from the Pennsylvania Flu VE site were processed by pyrosequencing as, in contrast to other locations, early testing suggested widespread circulation of vaccine-like strains.
Virologic Surveillance
Distribution of influenza A(H3N2) viruses in the United States before and during the 2014–15 influenza season was examined using virologic surveillance data for influenza A(H3N2) viruses identified by U.S. public health laboratories from March 1, 2014 through April 10, 2015 and submitted to CDC for virus characterization. CDC requested that state public health laboratories submit the first ten influenza-positive specimens of any virus type or subtype and up to five positive specimens every two weeks throughout the season to CDC. For A(H3N2) viruses that did not achieve sufficient hemagglutination titers for antigenic characterization by HI, genetic characterization was used to determine HA genetic group and antigenic properties were inferred based on the subset of viruses from the same HA genetic group that could be characterized by HI assays.[1]
Genetic characterization
Analyses of full-length HA sequences from influenza A(H3N2) viruses collected from January 1 through December 1, 2014, and characterized at CDC were used to design a high-throughput pyrosequencing assay to screen A(H3N2) viruses for genetic markers associated with six major HA genetic groups (3C.2, 3C.2a, 3C.2b, 3C.3, 3C.3a, and 3C.3b). Viral RNA was extracted from clinical specimens in a 96-well plate format using MagNA Pure 96 nucleic acid isolation system (Roche Diagnostics, Basel, Switzerland). A fragment of the HA1 coding region, encompassing nucleotides 370 to 645, was amplified using RT-PCR and subjected to sequence analysis using a PyroMark Q96ID sequencing platform (Qiagen, Valencia, CA), essentially as described.[13] Three pyrosequencing primers were utilized to generate pyrograms for three target HA regions encompassing the following nucleotides: (1) 412–431; (2) 456–481; and (3) 559–571. Sequencing results were analyzed using IdentiFire software (Qiagen, Valencia, CA) and a purpose-built library containing the signature sequences of target regions. Unique combination of short signature sequences within each target region allows identification of 3C.2, 3C.2a, 3C.2b, 3C.3, 3C.3a, and 3C.3b clades (Protocol for the pyrosequencing assay is available upon request, Email: fluantiviral@cdc.gov).
In addition, for a subset of viruses, full-length HA sequences were obtained from whole genome sequencing, performed as previously described.[14] Sequence data were deposited to the Global Initiative on Sharing All Influenza Data (GISAID). A(H3N2) viruses were classified into previously described HA genetic groups based on phylogenetic analysis.[15]
2014–2015 vaccine components and genetic groups
The A(H3N2) reference virus for 2014–2015 Northern Hemisphere influenza vaccines was A/Texas/50/2012(H3N2), HA genetic group 3C.1. For antigenic characterization of circulating A(H3N2) viruses, CDC used a panel of ferret antisera raised against egg- and cell-propagated reference viruses including A/Texas/50/2012, A/Michigan/15/2014 (group 3C.2a), A/Louisiana/39/2013 (group 3C.2b), A/New York/39/2012 (group 3C.3), A/Pennsylvania/09/2015 (group 3C.3b) and A/Switzerland/9715293/2013 (group 3C.3a), the A(H3N2) reference virus for 2015–2016 Northern Hemisphere vaccines.
Vaccine effectiveness
Among all patients enrolled in the Flu VE network study, we compared characteristics of patients with genetically characterized A(H3N2) virus infection and all A(H3N2)-positive patients to influenza-negative patients, excluding patients infected with other influenza viruses. We tested statistical significance of differences with the χ2 test for categorical, and the Wilcoxon Mann-Whitney test for continuous variables. Influenza VE was estimated using a test-negative design,[16, 17] which compares the odds of 2014–2015 influenza vaccine receipt among A(H3N2)-positive patients (for any A(H3N2) virus and by A(H3N2) virus genetic group) with influenza test-negative patients. VE was estimated as 100% × [1 - adjusted odds ratios (OR)] from logistic regression models by including network site, patient age, presence of high-risk medical condition and calendar time as previously described;[9] addition of sex, race/Hispanic ethnicity and days from illness onset to specimen collection did not change the adjusted OR by ≥5%, the pre-determined threshold for inclusion in the model.[9] Logistic regression models for patients aged ≥6 months included patient age (in years, modeled using linear tail-restricted cubic spline functions with 5 knots based on percentiles); models for specific age categories included patient’s age in years. We assessed potential effect modification by prior season (2013–2014) vaccination status by including main effects and an interaction term for current and prior season vaccination in logistic regression models.[10] Additional analyses were conducted restricting patients to those enrolled within 4 days of symptom onset and using an expanded group of patients that included partially vaccinated children. All reported tests were 2-sided and a p-value <0.05 was considered statistically significant; VE was considered statistically significant if 95% confidence intervals (CI) excluded zero. Statistical analyses were conducted using SAS for Windows (version 9.3, Cary, NC).
RESULTS
At US Flu VE network sites, 9710 patients aged ≥ 6 months with ARI were enrolled during periods of influenza virus circulation from November 10, 2014 through April 10, 2015; 1868 (19%) patients tested positive for A(H3N2), while 7390 (75%) patients tested negative for influenza. In addition, 4 (<1%) patients were infected with influenza A(H1N1)pdm09 virus and 399 (4%) with influenza B viruses, including two patients co-infected with A(H3N2) and B viruses. VE against influenza B viruses will be published separately.
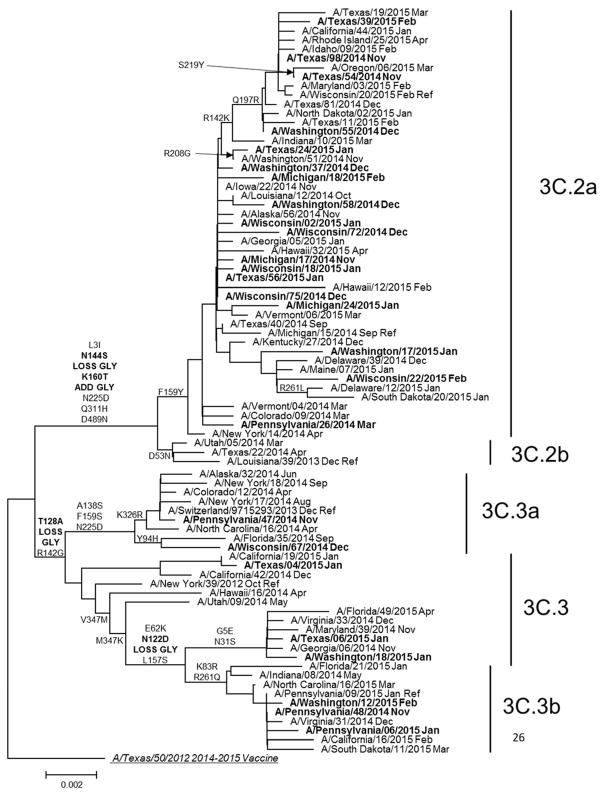
Genetic group was determined by pyrosequencing assay for 1397 (95%) of 1464 A(H3N2)-positive US Flu VE patient specimens selected for genetic characterization at CDC (78% of all A(H3N2)-positive cases). Proportions of patients for whom A(H3N2) genetic group was determined differed by study site, age, race/Hispanic ethnicity and self-rated illness severity (p<0.05), but were similar by vaccination status (Supplementary eTable 1). For 138 specimens characterized by complete HA sequencing and pyrosequencing, results were 100% concordant. A(H3N2) genetic group 3C.2a viruses predominated, accounting for 1134 (81%) of 1397 genetically characterized A(H3N2) viruses from Flu VE network sites. Compared to the 2014–2015 vaccine reference virus A/Texas/50/2012 (group 3C.1; Figure 1), HA sequences from group 3C.2a viruses shared an amino acid change from phenylalanine to tyrosine at position 159 (F159Y), which has been associated with reduced inhibition by ferret antisera raised against the vaccine virus. Of 35 A(H3N2) group 3C.2a viruses from the Flu VE network study characterized by HI, 31 (89%) were poorly inhibited by ferret antisera against the A/Texas/50/2012 vaccine reference virus, in agreement with data from US virologic surveillance (Supplementary eTable 2). Among other A(H3N2) viruses from Flu VE patients, 56 (4%) A(H3N2) viruses belonged to the antigenically drifted genetic group 3C.3a, 48 (3%) to vaccine-like group 3C.3 and 159 (11%) to group 3C.3b. Group 3C.3a viruses, including the 2015–16 A(H3N2) vaccine reference virus A/Switzerland/9715293/2013, had a parallel amino acid substitution to group 3C.2a viruses at position 159 (phenylalanine to serine [F159S]), but were considered antigenically similar to group 3C.2a viruses by HI.[18] Group 3C.3b viruses included an amino acid change from lysine to serine at position 157 (L157S) that was not associated with antigenic drift: 10 (63%) of 16 A(H3N2) group 3C.3b viruses from Flu VE patients were considered antigenically similar to A/Texas/50/2012 by HI, similar to proportions observed among US surveillance isolates (Supplementary eTable 2).
Figure 1. Phylogenetic tree based on hemagglutinin (HA) genes of influenza A(H3N2) viruses collected during March, 2014–April, 2015 and analyzed at CDC.
Phylogenetic tree is based on genetic differences compared to A/Texas/50/2012 consensus sequence. Amino acid substitutions delineating major branches are shown. Date of collection follows names of viruses from U.S. public health laboratories participating in U.S. virologic surveillance (normal font) and from patients enrolled at US Flu Vaccine Effectiveness network sites (bold font).
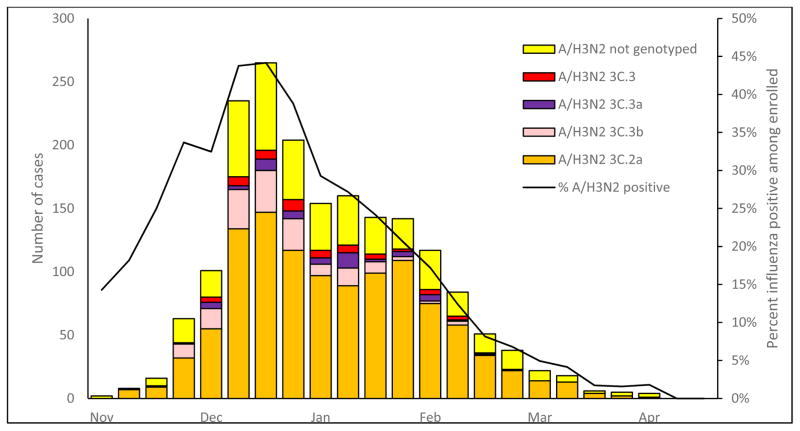
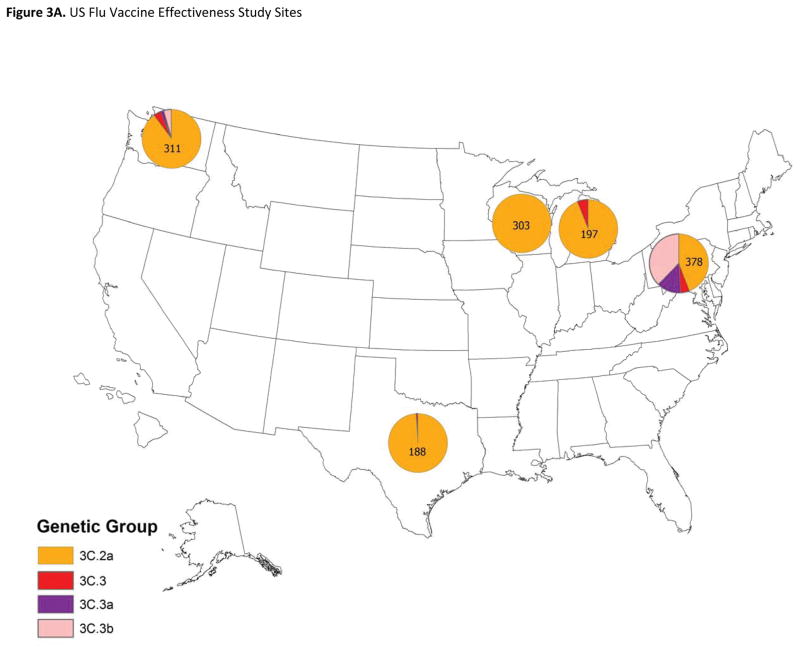
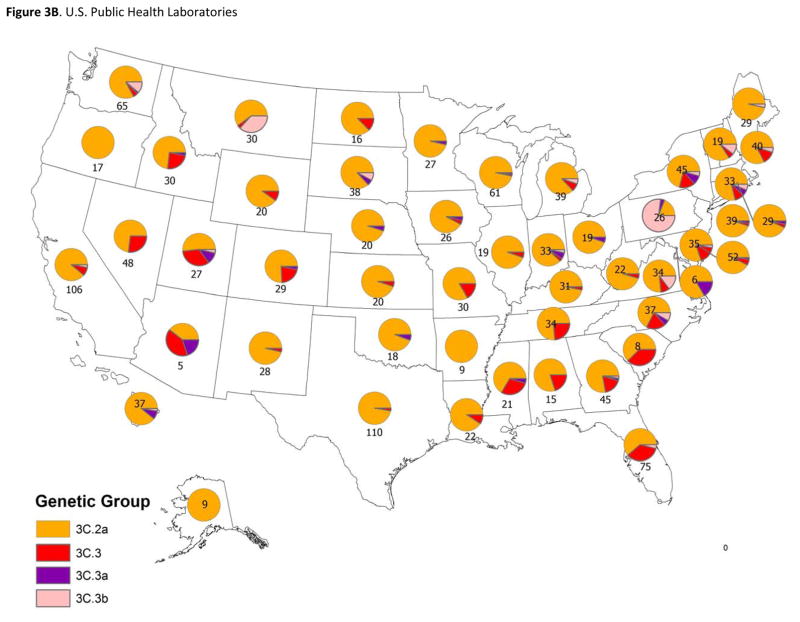
Prior to the enrollment period at Flu VE network sites, the proportion of influenza A(H3N2) viruses submitted to CDC from US surveillance laboratories that belonged to HA group 3C.2a increased from 38% (of 92 viruses characterized) in March 2014 to 73% (of 327) in November, 2014. At Flu VE sites, the proportion of group 3C.2a viruses increased during the 2014–15 season; peak numbers of group 3C.2a-positive patients were enrolled in December, corresponding to peak case enrollment (Figure 2). While group 3C.2a viruses predominated, distribution of genetic group varied by Flu VE network site (Figure 3A), with greater circulation of vaccine-like strains at the Pennsylvania site. Similar variability in the geographic distribution of HA genetic group was observed among influenza A(H3N2) viruses collected by US public health laboratories during November, 2014—April, 2015 (Figure 3B).
Figure 2.
Numbers of PCR-confirmed infections with influenza A(H3N2) viruses by genetic group and percent influenza positivity among patients with medically-attended acute respiratory illness, by week of symptom onset, US Flu Vaccine Effectiveness Network, United States, November 10, 2014—April 10, 2015.
Figure 3. Geographic distribution of genetic groups of influenza A(H3N2) viruses in the United States and from patients enrolled in the US Flu Vaccine Effectiveness study.
3A: Viruses from influenza A(H3N2)-positive patients enrolled from November 10, 2014 through April 10, 2015 (n=1397). Pie charts present the proportional distribution of HA genetic group, based on the number of genetically characterized influenza A(H3N2) viruses from patients enrolled at each study site.
3B: Viruses identified by U.S. public health laboratories from November 10, 2014 through April 10, 2015 and submitted to CDC for genetic characterization (n=1633). Pie charts present the proportional distribution of HA genetic group, based on the number of genetically characterized influenza A(H3N2) viruses from each state or territory.
Vaccine effectiveness
A total of 1817 A(H3N2)-positive patients and 7079 influenza test-negative patients from the Flu VE network study contributed to VE estimates (Table 1), after excluding influenza B positive patients (n=399), incompletely vaccinated patients aged 6 months to 8 years (n=175), patients vaccinated 0–13 days before illness onset (n=149), and influenza test-negative patients (n=40) with illness onset outside the period when influenza cases were identified at each study site. Compared with influenza test-negative patients, higher percentages of A(H3N2)-positive patients were aged ≥65 years, White non-Hispanic, reported excellent or very good general health, enrolled within two days of illness onset and reported feverishness during current illness. Influenza test-negative patients were more likely to report cigarette smoking or household exposure to smoke, and to live in households with ≥1 child aged <12 years.
Table 1.
Characteristics of patients enrolled in the US Flu Vaccine Effectiveness network study, by influenza RT-PCR resulta
| Genetically characterized influenza A(H3N2)-positive cases (N=1397) | All influenza A(H3N2)-positive cases (N=1817) | Influenza-negative controls (N=7078) | P-valueb | |
|---|---|---|---|---|
|
|
||||
| n (col %) | n (col %) | n (col %) | ||
| Site | <.001 | |||
| Michigan | 201 (14.4) | 307 (16.9) | 1152 (16.3) | |
| Pennsylvania | 382 (27.3) | 424 (23.3) | 1024 (14.5) | |
| Texas | 191 (13.7) | 280 (15.4) | 1318 (18.6) | |
| Washington | 317 (22.7) | 416 (22.9) | 2272 (32.1) | |
| Wisconsin | 306 (21.9) | 390 (21.5) | 1312 (18.5) | |
| Age | <.001 | |||
| 6 mo–8 y | 327 (23.4) | 396 (21.8) | 1946 (27.5) | |
| 9–17 y | 229 (16.4) | 306 (16.8) | 950 (13.4) | |
| 18–49 y | 389 (27.9) | 531 (29.2) | 2206 (31.2) | |
| 50–64 y | 205 (14.7) | 281 (15.5) | 1118 (15.8) | |
| ≥ 65 y | 247 (17.7) | 303 (16.7) | 858 (12.1) | |
| Male sex | 601 (43.0) | 767 (42.2) | 2969 (42.0) | 0.84 |
| Race/ethnicity | <.001 | |||
| White, non-Hispanic | 1081 (77.7) | 1397 (77.1) | 5182 (73.4) | |
| Black, non-Hispanic | 108 (7.8) | 133 (7.3) | 552 (7.4) | |
| Hispanic | 82 (5.9) | 119 (6.6) | 685 (9.7) | |
| Other, non-Hispanic | 120 (8.6) | 162 (9.0) | 673 (9.5) | |
| Any high-risk condition | 521 (37.3) | 671 (36.9) | 2636 (37.2) | 0.81 |
| Interval from onset to specimen collection | <.001 | |||
| 0–2 d | 698 (50.0) | 839 (46.2) | 1904 (26.9) | |
| 3–4 d | 488 (35.0) | 638 (35.1) | 2771 (39.1) | |
| 5–7 d | 211 (15.1) | 340 (18.7) | 2403 (34.0) | |
| Reported general health statusc | 0.01 | |||
| Excellent/very good | 1026 (76.1) | 1342 (74.2) | 4991 (70.6) | |
| Good | 282 (20.3) | 368 (20.3) | 1639 (23.2) | |
| Fair/poor | 83 (6.0) | 99 (5.5) | 442 (6.3) | |
| Self/household exposure to smokec | 176 (12.6) | 229 (12.6) | 1248 (17.7) | <.001 |
| ≥1 child <12 years of age in householdc | 522(37.6) | 708 (39.0) | 2828 (40.0) | 0.30 |
| Reported fever | 1132 (81.2) | 1459 (80.4) | 3876 (54.9) | <.001 |
| Reported current health assessment score, median (IQR)d | 50 (40–70) | 50 (40–70) | 60 (45–75) | <.001 |
| Vaccination status 2014–2015 | 0.03 | |||
| ≥1 doses | 749 (53.6) | 939 (51.7) | 3866 (54.6) | |
| 0 doses | 648 (46.4) | 878 (48.3) | 3212 (45.4) | |
Abbreviation: IQR, interquartile range.
Exclusions from total enrollment: influenza A H1N1pdm09-positive (n=4), influenza B-positive (n=397), co-infections with influenza A(H3N2) and B viruses (n=2), influenza A-positive of undetermined subtype (n=19), indeterminate influenza rRT-PCR result (n=7).
The χ2 statistic was used to assess differences between the numbers of persons with influenza A(H3N2)-positive and influenza-negative test results with respect to the distributions of site, age group, sex, race/ethnicity, presence of any high risk condition, interval from illness onset to specimen collection, general health status, smoke exposure in self/household, presence of children ages <12 years in the household and vaccination status. The Wilcoxon Mann-Whitney test was used to assess differences with respect to the distribution of the current health assessment. A P value <.05 is statistically significant.
Data were missing for 14 enrollees.
Possible values range from 1 (the worst) to 100 (the best). Data were missing for 16 enrollees.
In all, 4811 patients (54%) included in the analysis had received 2014–2015 influenza vaccine, including 939 (52%) of 1817 A(H3N2)-positive patients and 3866 (55%) of 7078 influenza-negative patients; VE against A(H3N2)-associated illness was 7% (95% CI, −5% to 17%) (Table 2). Estimated VE against A(H3N2)-associated illness was 14% (95% CI, −3% to 28%) among patients enrolled prior to January 2, 2015 and −2% (95% CI, −20% to 14%) among those enrolled after January 2, 2015.
Table 2.
Adjusted vaccination odds ratios and vaccine effectiveness against influenza A(H3N2)-associated illness among patients aged ≥6 months enrolled at US Influenza Vaccine Effectiveness Network sites, 2014–2015.
| Influenza A(H3N2) Positive (Cases) | Influenza Negative (Controls) | Vaccine Effectivenessa | ||||
|---|---|---|---|---|---|---|
| No. vaccinated/ | % Vaccinated | No. vaccinated/ | % Vaccinated | VE % | (95% CI) | |
| Total | Total | |||||
| All A(H3N2) virusesb | ||||||
| All ages | 939/1817 | (51.7) | 3866/7078 | (54.6) | 7% | (−5, 17) |
| 6 months–8 years | 160/396 | (40.4) | 1013/1946 | (52.1) | 20% | (−3, 37) |
| 9–49 years | 355/837 | (42.4) | 1387/3156 | (44.0) | −5% | (−24, 12) |
| ≥50 years | 424/584 | (72.6) | 1466/1976 | (74.2) | 9% | (−14, 28) |
| A(H3N2) genetic group 3C.2a | ||||||
| All ages | 597/1101 | (54.2) | 3866/7078 | (54.6) | 1% | (−14, 14) |
| 6 months–8 years | 103/243 | (42.4) | 1013/1946 | (52.1) | 16% | (−13, 37) |
| 9–49 years | 217/486 | (44.7) | 1387/3156 | (44.0) | −15% | (−41, 7) |
| ≥50 years | 277/372 | (74.5) | 1466/1976 | (74.2) | 8% | (−21, 30) |
| A(H3N2) genetic group 3C.3b | ||||||
| All ages | 56/156 | (35.9) | 3866/7078 | (54.6) | 44% | (16, 63) |
| 6 months–8 years | 4/36 | (11.1) | 1013/1946 | (52.1) | NR | |
| 9–49 years | 25/78 | (32.1) | 1387/3156 | (44.0) | 35% | (−13, 63) |
| ≥50 years | 27/42 | (64.3) | 1466/1976 | (74.2) | NR | |
| A(H3N2) genetic group 3C.3a | ||||||
| All ages | 31/55 | (56.4) | 3866/7078 | (54.6) | −48% | (−169, 19) |
| A(H3N2) genetic group 3C.3 | ||||||
| All ages | 27/47 | (57.5) | 3866/7078 | (54.6) | 1% | (−87, 48) |
Abbreviations: CI, confidence interval; NR—not reported.
Vaccine effectiveness was estimated as 100 × (1-odds ratio [ratio of odds being vaccinated among subjects with influenza A(H3N2)-associated illness to the odds of being vaccinated among influenza-negative subjects]). Odds ratios were estimated using logistic regression. If the 95% CI excludes 0, the results are considered statistically significant. Models adjusted for site, age, any high-risk condition and calendar time (biweekly intervals)
Includes influenza A(H3N2) viruses for which genetic group was not determined.
VE estimates were similar when restricted to 1359 infections with genetically characterized A(H3N2) viruses (6% [95% CI, −7% to 18%]) or 458 infections with uncharacterized A(H3N2) viruses (11% [95% CI, −10 to 27]), but differed by HA genetic group. Among all patients aged ≥6 months, estimated VE against illness due to infection with HA genetic group 3C.2a viruses was 1% (95% CI, −14% to 14%]) versus 44% (95% CI, 16% to 63%) against illness due to group 3C.3b viruses (Table 2). Among patients infected with viruses from HA genetic groups 3C.3 or 3C.3a, VE estimates were not statistically significant. No statistically significant interaction was observed between current (2014–2015) and prior (2013–14) season vaccination status, for any A(H3N2)-related illness or for genetic groups of A(H3N2) viruses. Inclusion of partially vaccinated children and restriction of analysis to patients enrolled within four days of symptom onset resulted in similar VE estimates (data not shown).
DISCUSSION
The 2014–2015 influenza season in the United States was notable for the early and widespread circulation of A(H3N2) viruses, the majority of which belonged to an emergent HA genetic group 3C.2a and showed substantial antigenic drift from the A(H3N2) component of 2014–2015 Northern Hemisphere seasonal influenza vaccines.[1] Expanded genetic characterization of A(H3N2) viruses from surveillance sites in the United States identified variability in geographic and temporal distribution of genetic groups of A(H3N2) viruses. Development of a pyrosequencing assay, with high throughput, provided genetic group information on a large number of influenza A(H3N2) viruses from US Flu VE network sites, permitting strain-specific estimates that revealed differences in VE caused by specific HA genetic groups of A(H3N2) viruses. Low VE against genetic group 3C.2a viruses was consistent with antigenic drift. Low or null effectiveness for 2014–2015 influenza vaccines against influenza A(H3N2)-associated illness was also reported in mid-season estimates from other surveillance networks in which A(H3N2) group 3C.2a viruses predominated, including networks in Canada[19] and the United Kingdom.[20] In contrast, we found evidence of modest (44%) VE against A(H3N2) viruses in group 3C.3b, which were characterized as antigenically similar to the 2014–2015 vaccine reference virus A/Texas/50/2012. Viruses from the 3C.3b genetic group appeared to be more common in some regions of the country, including the Pennsylvania Flu VE network site. Although vaccination provided some protection against A(H3N2) viruses belonging to group 3C.3b, the predominance of the drifted 3C.2a viruses in the United States resulted in low overall VE against medically attended influenza-associated illness during the 2014–2015 season.[21]
Differences in VE have been observed during seasons with mixed circulation of viruses antigenically matched and mismatched to vaccine components,[22, 23] with the 2014–2015 season having a very high proportion of circulating A(H3N2) viruses that were antigenically mismatched to the vaccine. During seasons in which antigenically drifted viruses circulate, VE has not consistently been associated with the degree of antigenic similarity as measured by the HI assay.[23] The addition of genetic characterization of a large number of A(H3N2) viruses from patients enrolled in the Flu VE network study during the 2014–2015 season provided the opportunity to examine differences in VE by genetic group. This analysis suggests that protection against A(H3N2) viruses may be related to vaccine protection against specific virus genetic groups and the distribution of those viruses in the community. In addition, genetic characterization of A(H3N2) viruses from US laboratories provided insight into the rapid spread of antigenically mismatched viruses from a single genetic group, as well as local variability in the distribution of HA genetic groups antigenically matched to vaccine. Genetic data also provided better representation of antigenic mismatch between vaccine and circulating A(H3N2) viruses, many of which could not be characterized using the HI assay. In the future, studies to assess VE against distinct groups of influenza viruses may augment data from traditional methods to identify potential vaccine candidates and ultimately lead to improved vaccines.[24]
The 2014–2015 influenza season highlighted challenges with formulation of influenza vaccines that require frequent updates due to rapid changes in circulating viruses, as well as limitations inherent in the current methods to select vaccine virus candidates. In February 2014 at the time of virus strain selection for 2014–2015 Northern Hemisphere vaccines, greater than 90% of characterized A(H3N2) viruses were found to be antigenically similar to the 2013–2014 vaccine reference virus, A/Texas/50/2012.[15] WHO recommended no change in the A(H3N2) vaccine strain for the 2014–2015 season.[15] In March, 2014, after the 2014–15 vaccine strain had been selected, antigenic drift variants belonging to HA genetic groups 3C.3a and 3C.2a were detected. During May–September, 2014, the proportion of these drift variants increased rapidly with a predominance of HA genetic group 3C.2a viruses in the United States, and fewer than 50% of A(H3N2) viruses characterized by HI were inhibited by ferret reference antisera against A/Texas/50/2012.[25] These virologic findings together with epidemiologic and human serology data resulted in a change in recommended A(H3N2) vaccine component to an A/Switzerland/9715293/2013-like virus from HA group 3C.3a for the 2015 Southern Hemisphere season.[18]
Results from the US Flu VE network indicated that differences in vaccine effectiveness may have resulted from a small degree of genetic change associated with substantial antigenic differences within related HA genetic groups of A(H3N2) viruses. Amino acid substitutions at specific HA antigenic sites have been associated with major antigenic changes (referred to as cluster transitions) in A(H3N2) viruses since their emergence in humans in 1968.[26] A(H3N2) genetic group 3C.2a viruses possessed multiple amino acids changes in HA relative to the A/Texas/50/2012 reference vaccine virus, including the F159Y change near the receptor binding site.[27] In addition, the majority of viruses in HA genetic group 3C.2a contain an amino acid substitution at residue 160 that adds a glycosylation site at residues 158–160, which may shield antigenic sites from neutralizing antibodies.[28] Interestingly, A(H3N2) genetic group 3C.3a viruses, including the 2015–2016 Northern Hemisphere vaccine reference virus A/Switzerland/9715293/2013, contain a different amino acid at residue 159 that nonetheless may have resulted in similar antigenic properties to 3C.2a viruses.[18] In contrast, HA genetic group 3C.3b viruses, which do not have antigenically important amino acid substitutions like that at residue 159 were characterized as antigenically similar to A/Texas/50/2012, consistent with the observation of modest VE. Small numbers of infections with HA group 3C.3 and 3C.3a viruses limited our ability to estimate VE against illness due to these genetic groups. From October 4—November 28, 2015, the majority of 3C.2a viruses characterized were well inhibited by ferret reference antisera raised against A/Switzerland/9715293/2013, indicating better antigenic match between 2015–2016 vaccine and circulating A(H3N2) viruses.[29] However, the effectiveness of influenza vaccines against circulating A(H3N2) viruses will need to be monitored closely because of the likelihood of continuing drift among circulating strains.
Our results are subject to several limitations. Influenza A(H3N2)-positive specimens from study participants submitted for characterization had lower rRT-PCR cycle threshold values possibly corresponding with higher viral load [30]. Antigenic properties were inferred from a subset of viruses characterized by HI. However, antigenic characterization of viruses from the US Flu VE network agreed with virologic surveillance data. Secondly, the validity of the test-negative design depends upon accurate classification of influenza status.[31] In addition to the use of the highly specific rRT-PCR assay, patients in the US Flu VE network study were enrolled within 7 days of illness onset when viral shedding was highest, decreasing the likelihood of false negative results. Lastly, patients at higher risk of influenza-related illness have higher rates of influenza vaccination; while estimates adjust for potential confounding variables, residual confounding may bias estimates. However, the test-negative design, by testing patients presenting for care, reduces potential selection bias related to care-seeking behavior.[16, 17]
During 2014–15, several genetically related influenza A(H3N2) viruses circulated in the US but one group predominated over time. We found that influenza vaccination in 2014–2015 did not reduce the likelihood of influenza illness caused by the predominant genetic group of A(H3N2) viruses, but prevented some disease due to A(H3N2) viruses in less prevalent genetic groups.[21] Overall low effectiveness of influenza vaccines may have contributed to high rates of influenza-associated hospitalizations observed during the season[1] despite vaccination of approximately two-thirds of the US population aged ≥65 years.[32] Expanded availability of high-throughput platforms for nucleotide sequencing and genetic characterization of larger numbers of influenza viruses may provide more complete and timely information on emergent influenza viruses to inform vaccine strain selection and help evaluate protection provided by vaccination.
Supplementary Material
Acknowledgments
Financial support. This study was supported by the Centers for Disease Control and Prevention [cooperative agreements U01IP000466-U01IP000474].
We thank the study participants at each of the 5 Centers for Disease Control and Prevention (CDC) US Flu VE Network sites: Joyce Benoit, Erika Kiniry, Lawrence Madziwa, Matt Nguyen, and C. Hallie Phillips, Group Health Research Institute; Heather Eng, G.K. Balasubramani, PhD, Terri Sax, Stephen Wisniewski, PhD, Krissy K. Moehling, MPH, Jonathan M. Raviotta, MPH, Michael Susick, MPH, Sean Saul, Samantha Ford, Edmund M. Ricci, PhD, Jennifer Gray, Leonard Urbanski, MD, Christopher Olbrich, MD, Donald B. Middleton, MD, Barbara Kevish, MD, Evelyn C. Reis, MD, Edmund Garofolo, MD, Sandra Sauereisen, MD, Rina Chabra, MD, Robert Hickey, MD, Philip Iozzi, MD, University of Pittsburgh, Pittsburgh, PA; Joshua Petrie, Caroline Cheng, Casey Martens, EJ McSpadden, Anne Kaniclides, Ryan Malosh, Emily Martin, Samantha Harrison, Kajal Magal, Brian Nixon, Jessica Obidike, Mallory Theisen, Emily Valice, Kevin Zhang, University of Michgian School of Public Health, Ann Arbor, MI; Lois Lamerato and Heather Lipkovich, Henry Ford Health System, Detroit, MI; Donald Wesson, Michael Reis, Madhava Beeram, Jessica Pruszynski, Lydia Clipper, Archana Nangrani, Kempapura Murthy, Anne Robertson, Patricia Sleeth, Virginia Gandy, Teresa Ponder, Mary Kylberg, Hope Gonzales, Martha Zayed, Deborah Furze, Baylor Scott and White Health, Temple, TX; Vasanthi Avadhanula, Alan Jewell, Kirtida Patel and Sneha Thaker, Baylor College of Medicine, Houston, Texas.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest: R. K. Z. has received grants from Sanofi Pasteur, Pfizer and Merck. M. P. N. received grants from Pfizer and Merck. A. S. M. has received grants from the CDC and Sanofi Pasteur and has served as a consultant for GSK, Sanofi Pasteur, Novavax, Novartis and Protein Sciences. S. E. O. and H. Q. M. have received grants from CDC. E. A. B. has received grants from CDC and MedImmune. M. G. received CDC, during the conduct of the study; grants from MedImmune/AstraZeneca, outside the submitted work. P. A. P. served on the speaker bureau at Medimmune, and within the past year, he served as a scientific advisor on influenza for AstraZeneca. All other authors report no potential conflicts.
Presented in part at the Meeting of the Advisory Committee on Immunization Practices (ACIP), Atlanta, Georgia, June 24–25, 2015.
References
- 1.Appiah GD, Blanton L, D’Mello T, et al. Influenza activity - United States, 2014–15 season and composition of the 2015–16 influenza vaccine. MMWR Morbidity and mortality weekly report. 2015;64:583–90. [PMC free article] [PubMed] [Google Scholar]
- 2.D’Mello T, Brammer L, Blanton L, et al. Update: Influenza activity--United States, September 28, 2014–February 21, 2015. MMWR Morbidity and mortality weekly report. 2015;64:206–12. [PMC free article] [PubMed] [Google Scholar]
- 3.Hensley SE. Challenges of selecting seasonal influenza vaccine strains for humans with diverse pre-exposure histories. Current opinion in virology. 2014;8:85–9. doi: 10.1016/j.coviro.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimov AI, Garten R, Russell C, et al. WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011. Vaccine. 2012;30:6461–71. doi: 10.1016/j.vaccine.2012.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowronski DM, Chambers C, Sabaiduc S, et al. Integrated Sentinel Surveillance Linking Genetic, Antigenic, and Epidemiologic Monitoring of Influenza Vaccine-Virus Relatedness and Effectiveness During the 2013–2014 Influenza Season. The Journal of infectious diseases. 2015;212:726–39. doi: 10.1093/infdis/jiv177. [DOI] [PubMed] [Google Scholar]
- 6.Skowronski DM, Chambers C, Sabaiduc S, et al. A Perfect Storm: Impact of Genomic Variation and Serial Vaccination on Low Influenza Vaccine Effectiveness During the 2014–2015 Season. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 doi: 10.1093/cid/ciw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaglani M, Pruszynski J, Murthy K, et al. Influenza Vaccine Effectiveness Against 2009 Pandemic Influenza A(H1N1) Virus Differed by Vaccine Type During 2013–2014 in the United States. The Journal of infectious diseases. 2016;213:1546–56. doi: 10.1093/infdis/jiv577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaglani M, Pruszynski J, Murthy K, et al. Influenza Vaccine Effectiveness Against 2009 Pandemic Influenza A(H1N1) Virus Differed by Vaccine Type During 2013–2014 in the United States. The Journal of infectious diseases. 2016 doi: 10.1093/infdis/jiv577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. The Journal of infectious diseases. 2015;211:1529–40. doi: 10.1093/infdis/jiu647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) -- United States, 2014–15 influenza season. MMWR Morbidity and mortality weekly report. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. The Journal of infectious diseases. 2009;199:159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 13.Levine M, Sheu TG, Gubareva LV, Mishin VP. Detection of hemagglutinin variants of the pandemic influenza A (H1N1) 2009 virus by pyrosequencing. Journal of clinical microbiology. 2011;49:1307–12. doi: 10.1128/JCM.02424-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B, Donnelly ME, Scholes DT, et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza A viruses. Journal of virology. 2009;83:10309–13. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Recommended composition of influenza virus vaccines for use in the 2014–2015 northern hemisphere influenza season. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2014;89:93–104. [PubMed] [Google Scholar]
- 16.Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31:3104–9. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–8. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 18.Recommended composition of influenza virus vaccines for use in the 2015 southern hemisphere influenza season. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2014;89:441–52. [PubMed] [Google Scholar]
- 19.Skowronski DM, Chambers C, Sabaiduc S, et al. Interim estimates of 2014/15 vaccine effectiveness against influenza A(H3N2) from Canada’s Sentinel Physician Surveillance Network, January 2015. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2015:20. doi: 10.2807/1560-7917.es2015.20.4.21022. [DOI] [PubMed] [Google Scholar]
- 20.Pebody RG, Warburton F, Ellis J, et al. Low effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 mid-season results. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2015;20:21025. [PubMed] [Google Scholar]
- 21.Flannery B, Clippard J for the U.S. Flu VE Network. End-of-season influenza vaccine effectiveness estimates for the 2014–15 season: US Influenza Vaccine Effectiveness (Flu VE) Network. Available at: http://www.cdc.gov/vaccines/acip/meetings/meetings-info.html.
- 22.Darvishian M, Bijlsma MJ, Hak E, van den Heuvel ER. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. The Lancet Infectious diseases. 2014;14:1228–39. doi: 10.1016/S1473-3099(14)70960-0. [DOI] [PubMed] [Google Scholar]
- 23.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. The Lancet Infectious diseases. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 24.Skowronski DM, Chambers C, Sabaiduc S, et al. Integrated Sentinel Surveillance Linking Genetic, Antigenic, and Epidemiologic Monitoring of Influenza Vaccine-Virus Relatedness and Effectiveness During the 2013–2014 Influenza Season. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv177. [DOI] [PubMed] [Google Scholar]
- 25.Blanton L, Brammer L, Smith S, et al. Update: influenza activity -- United States and worldwide, May 18–September 20, 2014. MMWR Morbidity and mortality weekly report. 2014;63:861–4. [PMC free article] [PubMed] [Google Scholar]
- 26.Koel BF, Burke DF, Bestebroer TM, et al. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science. 2013;342:976–9. doi: 10.1126/science.1244730. [DOI] [PubMed] [Google Scholar]
- 27.Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of Hemagglutinin Residues Responsible for H3N2 Antigenic Drift during the 2014–2015 Influenza Season. Cell reports. 2015;12:1–6. doi: 10.1016/j.celrep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tate MD, Job ER, Deng YM, Gunalan V, Maurer-Stroh S, Reading PC. Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses. 2014;6:1294–316. doi: 10.3390/v6031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith S, Blanton L, Kniss K, et al. Update: Influenza Activity - United States. MMWR Morbidity and mortality weekly report. 2015;64:1342–8. doi: 10.15585/mmwr.mm6448a4. [DOI] [PubMed] [Google Scholar]
- 30.Spencer S, Chung J, Thompson M, et al. Factors associated with real-time RT-PCR cycle threshold values among medically attended influenza episodes. Journal of medical virology. 2016;88:719–23. doi: 10.1002/jmv.24373. [DOI] [PubMed] [Google Scholar]
- 31.Jackson ML, Rothman KJ. Effects of imperfect test sensitivity and specificity on observational studies of influenza vaccine effectiveness. Vaccine. 2015;33:1313–6. doi: 10.1016/j.vaccine.2015.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. Flu Vaccination Coverage, United States, 2014–15 Influenza Season. Available at: http://www.cdc.gov/flu/fluvaxview/coverage-1415estimates.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.