Abstract
Platelet-activating factor (PAF) is a potent, bioactive phospholipid that acts on multiple cells and tissues through its G protein-coupled receptor (GPCR). PAF is not stored, but rapidly generated via enzymatic acetylation of the precursor, lysoPAF. The bioactivity of PAF is effectively and tightly regulated by PAF acetylhydrolases, which convert PAF back to lysoPAF. Previous studies report that lysoPAF is an inactive precursor and metabolite of PAF. However, lysoPAF has not been carefully studied in its own context. Here we report that lysoPAF has an opposing effect of PAF in the activation of neutrophils and platelets. Whereas PAF potentiates neutrophil NADPH oxidase activation, lysoPAF dose-dependently inhibits this function. Inhibition by lysoPAF is not affected by the use of a PAF receptor antagonist or genetic deletion of the PAF receptor gene. The mechanism of lysoPAF-mediated inhibition of neutrophils involves an elevation in the intracellular cAMP level, and pharmacological blockade of adenylyl cyclase completely reverses the inhibitory effect of lysoPAF. In addition, lysoPAF increases intracellular cAMP levels in platelets and inhibits thrombin-induced platelet aggregation, which can be reversed by inhibition of PKA. These findings identify lysoPAF as a bioactive lipid with opposing functions of PAF, and suggest a novel and intrinsic regulatory mechanism for balance of the potent activity of PAF.
Introduction
Platelet-activating factor (PAF, 1-O-hexadecyl-2-acetoyl-sn-glycero-3-phosphocholine) is one of the most active lipid species identified to date. It was first discovered in the early 1970's as a bioactive lipid generated by basophils capable of inducing platelet aggregation and anaphylaxis (Benveniste et al., 1972). Specifically, levels of PAF have been shown to directly correlate to the severity of anaphylaxis (Vadas et al., 2008). In addition, PAF plays important roles in inflammation. PAF is produced by a variety of cells, including neutrophils. These effects are thought to be mediated by a G protein-coupled PAF receptor (Honda et al., 1991; Hwang et al., 1983).
Because of the potent and sometimes deleterious biological effects, PAF synthesis is tightly regulated. PAF is not stored in large amounts in the cell, and its production is induced by proinflammatory factors (Szabo et al., 1993). The induced synthesis of PAF involves the de novo pathway and remodeling pathway, the latter being responsible for the majority of PAF induced by inflammatory stimuli (Reinhold et al., 1989). In this process, PLA2 cleaves membrane phospholipids at the sn-2 position and yields several lipid products, including lysoPAF (1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphocholine). The highly specific PAF-acetyltransferase (PAF-AT) then adds an acetyl group to the sn-2 position to yield PAF (Shindou et al., 2007). PAF is subsequently degraded through removal of the acetyl group by PAF-acetylhydrolases (PAF-AH) (Karasawa et al., 2003). The PAF-AH mediated conversion to lysoPAF is a primary pathway for PAF inactivation, and there have been efforts in the exploration of PAF-AH as a potential therapeutic agent (Arakawa et al., 2005; Gomes et al., 2006; Quarck et al., 2001). The levels of PAF-AH are inversely correlated with the severity of anaphylaxis(Vadas et al., 2008), and the administration of human recombinant PAF-AH can inhibit several mouse models of anaphylaxis (Fukuda et al., 2000).
Although lysoPAF is thought to lack bioactivity and is often used as a control in experiments exploring PAF function, it has not been carefully studied in its own context. Given that lysoPAF levels are inversely related to those of PAF, and that it is a metabolite as well a precursor of PAF, there seems to be a need to understand any potential activity that this lipid may have. Furthermore, the potential therapeutic use of recombinant PAF-AH in humans requires a comprehensive understanding of the cellular functions of the lipid produced by this enzyme. In the course of a study of neutrophil activation by bioactive lipids, we found that lysoPAF exhibits an inhibitory effect on neutrophil superoxide production. Further investigation has led to the discovery of lysoPAF-induced intracellular cAMP production as a potential mechanism for its inhibitory effect. Moreover, treatment of platelets with lysoPAF reduces their aggregation following low dose thrombin stimulation. These data suggest that lysoPAF balances some of the bioactivities of PAF, and the beneficial effects of PAF-AH may be attributed in part to this negative regulatory function of lysoPAF.
Materials and Methods
Materials
Synthetic lysoPAF (16:0, 18:0) and PAF (16:0) were obtained from Avanti Polar lipids (Alabaster, AL). The lipids were dissolved in 50% (v/v) EtOH/H2O. Percoll was purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). The PKA inhibitors H-89 and KT5720, and the adenylyl cyclase inhibitor SQ22536 were purchased from Calbiochem (San Diego, CA). The PAFR antagonist SR 27417 is a generous gift from Dr. J. M. Herbert (Sanofi-Aventis, France). Isoluminol, C5a, fMLF and cholera toxin were obtained from Sigma-Aldrich (St. Louis, MO). Horseradish peroxidase (HRP) was acquired from Invitrogen.
Preparation of neutrophils
Human peripheral blood was collected from healthy donors by venipuncture, using a protocol approved by the Institutional Review Board at University of Illinois at Chicago. Acid citrate dextran was used as anti-coagulant. Neutrophil isolation was carried out using discontinuous Percoll gradient (Lin et al., 2005). For preparation of mouse neutrophils, mice were anesthetized with CO2 and sacrificed by cervical dislocation. Femurs and tibias of wild type and PAFR-/- littermate mice, on a C57Bl6 background, were isolated and placed in HBSS-Prep (HBSS, 0.5% FBS, 10mM HEPES, and 1% Glucose). Bone marrow was then harvested, marrow was disaggregated, and then cells were separated by centrifugation using a discontinuous density gradient consisting of 3 ml NycoPrep 1.077 (Axis-Shield) underlayed with 72% Percoll (Amersham Biosciences). Neutrophils were collected at the NycoPrep-Percoll interface, and washed 2× with HBSS-Prep. Final resuspension was in 0.5% BSA in RPMI medium. Purity of neutrophils was consistently at 80% or above, as determined by FACS scatter, Wright staining, and Hemavet (Drew Scientific).
Preparation of human platelets
Blood was taken as described above with approval. Acid citrate dextran was used as an anti-coagulant. Platelet isolation was performed as described previously (Li et al., 2006).
Superoxide generation
Human PMN superoxide generation was measured by isoluminol-enhanced chemiluminescence as described previously (Lin et al., 2005). Chemiluminescence was measured every minute using a Wallac Multilabel Counter plate reader (Perkin Elmer Life Sciences, Boston, MA). Unstimulated controls were recorded simultaneously. Alternatively, some samples were pre-incubated with lipid or inhibitors prior to stimulation.
Endothelial Cell Culture and transendothelial electrical resistance measurement
Human pulmonary microvessel endothelial cells (HPMECs) were purchased from Sciencell (Carlsbad, CA), and were cultured in endothelial basal medium (EBM-2) supplemented with EGM-2MV from Lonza (Walkersville, MD). HPMECs were plated on gelatin-coated Electric cell-substrate impedence sensing (ECIS) 8 well 10 electrode arrays from Applied Biophysics (Piscataway, NJ) until confluency prior to experiment. Isolated human neutrophils (4×104 per well) were pre-incubated with vehicle, lysoPAF (1μM) or PAF (1μM) for ten min prior to addition to endothelial cell monolayers. The electrode was then connected and the resistance was monitored as described previously (Tiruppathi et al., 1992). Then, fMLF (1 μM) was added to indicated samples and the recording continued for a further 5 hours.
Calcium mobilization
Increase in intracellular calcium was detected using Indo-1/AM-labeling of human PMNs kept in a 0.5% BSA/HBSS buffer as described previously (He et al., 2003).
cAMP assay
cAMP was measured using a competitive enzyme-linked immunosorbent assay (Biomol) as described previously (Lin et al., 2005).
Platelet aggregation
Platelet aggregation was measured as described previously (Li et al., 2006). Briefly, washed human platelets, at a concentration of 3×108/mL, were pre-treated with lysoPAF (10 nM to 1 μM) or control for 2 minutes, then stimulated with α-thrombin from Enzyme research laboratories (South Bend, IN).
Statistical analysis
Data were analyzed by unpaired student t test using Prism (ver.4.0) software from GraphPad, San Diego, CA.
Results
LysoPAF inhibits neutrophil NADPH oxidase activation and neutrophil-induced vascular damage
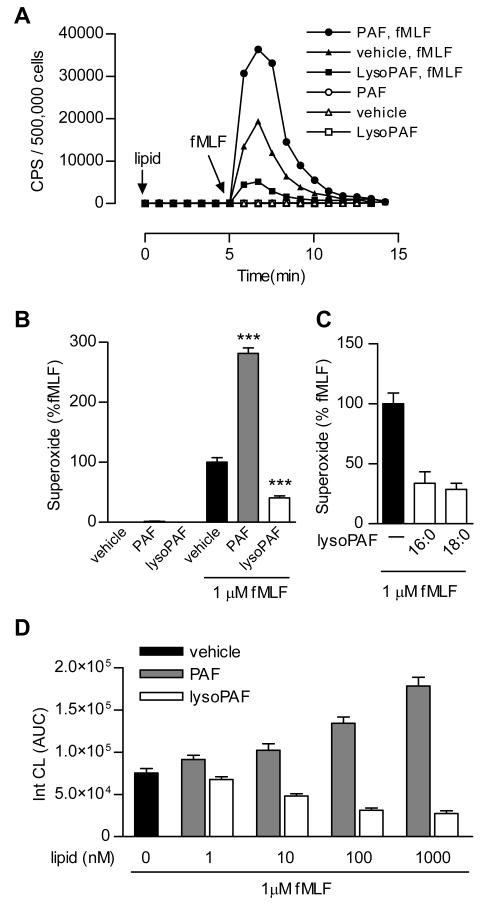
Treatment of human neutrophils with PAF does not lead to significant superoxide production. Instead, the cells are sensitized and respond more potently to subsequent stimulation with another agonist (Dewald and Baggiolini, 1985; Vercellotti et al., 1988). This “priming” effect is evident in human neutrophils first incubated with PAF and then stimulated with fMLF, a full agonist of neutrophils (Figure 1A). In PAF-treated neutrophils, fMLF (1 μM)-induced superoxide production was 2.85-fold higher than the level in buffer-treated cells (p < 0.01), based on isoluminol-enhanced chemiluminescence (ECL) (Figure 1A, 1B). LysoPAF, widely considered a non-active PAF metabolite and precursor, is often used as a negative control in studies of the bioactivity of PAF. When superoxide production in lysoPAF-treated neutrophils was set as baseline (open bar in Figure 1B), the “priming” effect of PAF became more evident (a 6.92-fold increase) following fMLF stimulation (p < 0.01). LysoPAF (1 μM) caused a 57% reduction of the fMLF-induced superoxide production when compared to buffer-treated neutrophils under the same experimental conditions (Figure 1A, 1B). These results suggest that lysoPAF is bioactive and produces an inhibitory effect on fMLF-induced neutrophil superoxide production.
Fig. 1. PAF and lysoPAF differentially regulate fMLF-induced neutrophil NADPH oxidase activation.
A, Detection of fMLF-induced superoxide generation as a function of time. CPS, counts per second of chemiluminescent light emitted. Isolated human neutrophils (5 × 105 per sample) were pre-incubated with indicated lipid (1 μM) or vehicle for 5 min prior to stimulation with 1 μM fMLF. Shown is a set of representative tracings from one of the 3 experiments that produced similar results. B, Data from all 3 experiments were quantified based on integrated areas under curve (AUC), and then expressed as percent of fMLF-induced response using the condition without lipid pretreatment as 100%. C, Comparison of the effects of lysoPAF pretreatment, using two lysoPAF species with different chain lengths (16:0, 18:0), on fMLF-induced superoxide production in neutrophils from different donors (n=3). D, Dose response of lysoPAF and PAF in fMLF-induced superoxide generation. Shown is a representative set of data, as mean ± SEM, from 1 of the 3 experiments that produced similar results.
The alkyl group in PAF and lysoPAF is connected through an ether linkage at the C1 position to a carbon chain of variable lengths. In the above study, 16:0 PAF and lysoPAF was used. To determine whether the length of the carbon chain affects the bioactivity of lysoPAF, we examined 18:0 lysoPAF from the same source and found it to be equally effective in the inhibition of fMLF-induced superoxide production (Figure 1C). Next, we determined the potency of PAF and lysoPAF in superoxide production assays. Both PAF and lysoPAF exhibited bioactivity at concentrations as low as 10 nM. The opposing effects of PAF and lysoPAF on superoxide production continue to increase up to the concentration of 1 μM (Figure 1D).
Neutrophil activation can lead to microendothelial injury, manifested as increased endothelial permeability and loss of barrier function. These changes are seen during Gram-negative bacterial infection and in numerous inflammatory disorders such as adult respiratory distress syndrome (Lee and Downey, 2001). In addition, pulmonary edema is one of the hallmarks of anaphylaxis. We determined the effects of PAF and lysoPAF on neutrophil-dependent changes of endothelial permeability by measuring transendothelial electrical resistance (TER), a well established in vitro assay of endothelial barrier function (Furie et al., 1984; Tiruppathi et al., 1992). In this assay, changes in TER reflect the integrity of the endothelial monolayer, which is compromised in the presence of reactive oxygen species from activated neutrophils. As shown in Figure 2A, fMLF stimulation of neutrophils caused a decrease in TER over time, and this change was further enhanced by pretreatment of neutrophils with PAF (1 μM). In contrast, lysoPAF used at the same concentration significantly reduced the fMLF-stimulated decrease in TER while having no significant effect on TER when applied alone (Figure 2B). These results suggest a potential function of lysoPAF in preventing neutrophil-mediated endothelial injury.
Figure 2. PAF- and lysoPAF-treated neutrophils affect endothelial barrier function.
A, HPMEC were grown to confluence on a gold electrode. Human neutrophils (4 × 104) were pre-incubated with 1 μM of the indicated lipid or vehicle control for 10 min, added to the electrode well, and stimulated with 1 μM fMLF. The changes in transendothelial electrical resistance (TER) were recorded over time. Data shown are from one representative experiment, chosen from a total of four similar experiments. No significant changes in TER were observed with PAF or lysoPAF in the absence of PMN (data not shown). B, The peak values at the 4 h time point of the above experiments are shown as mean ± SEM, based on 4 separate experiments. ns, not significant, *, p < 0.05, ***, p< 0.001.
LysoPAF does not block the fMLF receptor
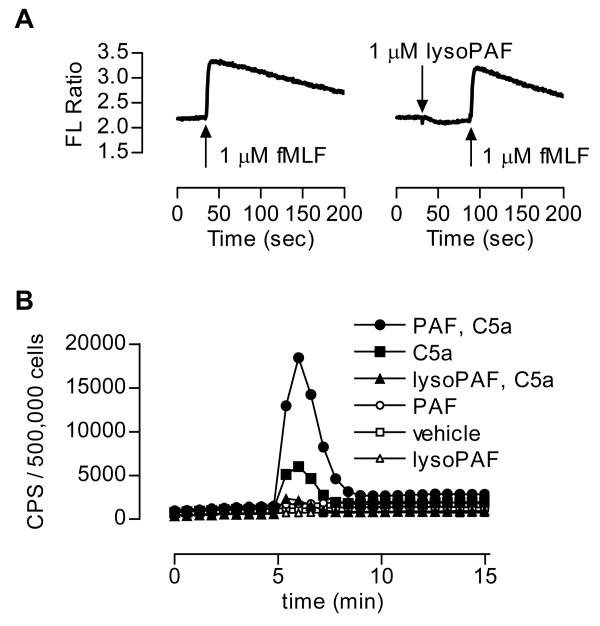
Inhibition of fMLF-stimulated superoxide generation could occur at multiple steps. To identify the related mechanisms, we first examined whether lysoPAF could block fMLF interaction with the formyl peptide receptor (FPR). One of the proximal signaling events downstream of the activated FPR is mobilization of intracellular Ca2+. Neutrophils were loaded with Indo-1/AM, then either treated with lysoPAF or with vehicle control before stimulation with fMLF (Figure 3A). The results indicated that lysoPAF did not induce Ca2+ mobilization in neutrophils, and treatment of neutrophils with lysoPAF had no effect on the fMLF-induced Ca2+ mobilization. These results preclude that lysoPAF blocks the fMLF interaction to FPR.
Figure 3. The effect of lysoPAF is not mediated through inhibition of the fMLF receptor or confined to fMLF stimulation.
A, Real-time measurement of Ca2+ mobilization in human neutrophils stimulated with 1 μM fMLF, in the presence or absence of 1 μM lysoPAF. B, Detection of the effects of lysoPAF and PAF on C5a-induced superoxide generation. CPS, counts per second of chemiluminescent light emitted. Isolated human neutrophils (5 × 105 per sample) were pre-incubated with the indicated lipid (1 μM) or vehicle (same concentration of ethanol, 0.025% v/v) for 5 min prior to stimulation with 100 nM C5a. Shown is a representative set of tracings from one of the three independent experiments, each produced similar results.
To rule out the possibility that the inhibitory effect of lysoPAF is specifically targeted at FPR signaling pathways, we investigated whether lysoPAF affects C5a signaling. Stimulation of neutrophils with C5a caused superoxide production (Figure 3B, filled squares). In cells treated with PAF, the C5a-induced superoxide production was markedly increased (filled circles). In contrast, lysoPAF treatment markedly decreased the C5a-induced superoxide production (filled triangles). Therefore, the inhibitory effect of lysoPAF is not confined to one chemoattractant receptor.
The inhibitory effect of lysoPAF is independent of the PAF receptor
Because of the structural similarity between PAF and lysoPAF, we investigated whether the inhibitory effects of lysoPAF are mediated through the PAF receptor. When neutrophils were exposed to both PAF and lysoPAF at equal molar concentrations, the effect of the individual lipids was negated (Figure 4A). To determine whether lysoPAF directly affects PAF receptor (PAFR)-mediated signaling, we conducted a Ca2+ mobilization assay, in which neutrophils were treated with lysoPAF before measurement of PAF-induced increase in intracellular Ca2+ concentration. As shown in Figure 4B, lysoPAF did not induce Ca2+ mobilization, and it produced no effect on PAF-induced Ca2+ mobilization. We also used a PAF receptor antagonist, SR27417 (Herbert et al., 1991), to determine whether blocking PAFR could alter the effects of PAF and lysoPAF on fMLF-induced superoxide production. Neutrophils were incubated in the presence or absence of SR27417 (10 nM) and either PAF or lysoPAF, and then stimulated with fMLF. As shown in Figure 3C, SR27417 abrogated PAF priming in the neutrophils, but did not change the inhibitory effect of lysoPAF. This result argues against the notion that lysoPAF competes with PAF in utilizing PAFR, although it does not rule out the possibility that lysoPAF may interact with the receptor at a site different from the PAF binding site. This latter possibility was tested using neutrophils derived from PAFR knockout mice (Ishii et al., 1998). As shown in Figure 4D, genetic deletion of the PAFR gene abolished the priming effect of PAF, but did not alter the inhibitory effect of lysoPAF, on fMLF-induced superoxide production. Taken together, results from these experiments indicate that inhibition by lysoPAF is independent of PAFR.
Figure 4. The inhibitory effect of lysoPAF is not mediated through the PAF receptor.
A, human neutrophils (5 × 105 per sample) were incubated with the indicated lipid(s) (1 μM each) for 5 min prior to stimulation with 1 μM of fMLF. The changes in superoxide generation, expressed as integrated area under curve (AUC) are shown, with fMLF-induced response in the absence of lipid set as 100%. Data shown are mean ± SEM from 3 independent experiments. B, Real-time measurement of calcium mobilization in human neutrophils loaded with Indo-1/AM and stimulated with 1 μM PAF, or with 1 μM lysoPAF and then PAF. A representative set of tracings, from a total of 3 experiments, is shown. C, Superoxide generation was assessed in neutrophils treated for 5 min with the PAFR antagonist SR27417 (10 nM) prior to addition of PAF or lysoPAF (1 μM) or vehicle. Cells were then stimulated with fMLF (1 μM) and production of superoxide was measured over time. Data procession and presentation are similar to A. D, mouse neutrophils (1×106 per sample) isolated from PAFR+/+ or PAFR-/- mice were incubated with indicated lipid for 5 min prior to stimulation with fMLF (1 μM). Integrated AUC was calculated for each mouse and data (mean ± SEM) expressed as percent of fMLF stimulation for each experiment (n=3).
LysoPAF activates adenylyl cyclase and increases intracellular cAMP concentration in neutrophils
Molecular characterization of PAFR has led to the identification of its signaling pathways that include functional coupling to the Gq class of G proteins, activation of PLCβ, and induction of second messengers inositol (1,4,5)-trisphosphate and diacyl glycerol (Honda et al., 2002). PAFR also couples to the Gi class of G proteins that mediate the chemotactic and cross-regulatory signals and also contribute to the activation of PLCβ and exocytosis in transfected RBL-2H3 cells with the G proteins fused to the PAFR (Brown et al., 2006). As a result, PAF stimulation leads to Ca2+ mobilization in neutrophils. We have shown that stimulation of neutrophils with lysoPAF does not cause increase in intracellular Ca2+ concentration (Figure 4B), suggesting differences between PAF and lysoPAF in the activation mechanism and the utilization of downstream effectors. Because several anti-inflammatory molecules stimulate the adenylyl cyclase - cAMP- signaling pathway (Fantone et al., 1983; Fantone et al., 1984; Rivkin and Becker, 1976), we tested whether lysoPAF could activate this pathway and increase cAMP concentration in human neutrophils. As shown in Figure 5A, stimulation of neutrophils with lysoPAF resulted in dose-dependent increases in intracellular cAMP concentrations that reached up to 2-fold above baseline. It is possible that lysoPAF activates a Gαs-coupled receptor, leading to cAMP production. This is suggested by the observation that cholera toxin, which ADP ribosylates and activates Gαs, increased cAMP level to the same extent as in lysoPAF-stimulated cells (Figure 5B). Likewise, cholera toxin dose-dependently inhibited fMLF-stimulated superoxide production (Figure 5C). Pertussis toxin, which ADP ribosylates the Gαi proteins and interferes with GαI – receptor interaction, had no effect on lysoPAF-induced elevation of cAMP concentration.
Fig. 5. LysoPAF activates the adenylyl cyclase–cAMP–PKA pathway.
A, human neutrophils were incubated with lysoPAF at different concentrations, or with PAF at 1 μM, for 5 min in the presence of the phsphodiesterase inhibitor IBMX. The cells were lysed, and cAMP was assayed by competitive immunoassay. Shown is a representative experiment of 3 that produced similar results. B, cAMP measurement of human neutrophils in the presence or absence of lysoPAF (1 μM, 5 min), CTx (1 μg/mL, 1 h with or without lysoPAF), or pertussis toxin (0.5 μg/mL, 1 h with or without lysoPAF (1 μM), in the presence of IBMX. cAMP was measured as described above. Shown are data from 2 experiments, normalized based on non-stimulated controls. C, Superoxide generation in the presence or absence of increasing concentrations of CTx, in the presence or absence of 1 μM lysoPAF. Human neutrophils were incubated with CTx for 1 h prior to addition of 1 μM lysoPAF for 5 min, then stimulated with 1 μM fMLF. Data are shown as % relative to fMLF-induced superoxide generation without lysoPAF, and are based on 2 different donors. D, Superoxide generation in the presence or absence of increasing concentrations of the adenylyl cyclase inhibitor SQ22536. Human neutrophils were incubated with indicated dose of SQ22536 or vehicle for 10 min prior to addition of lysoPAF (1 μM) for 5 min. Data are shown as % relative to fMLF-induced superoxide generation without lysoPAF or SQ22536, using neutrophils from 3 different donors. E, Superoxide generation was assessed in neutrophils treated with or without the PKA inhibitors H-89 (5 μM) or KT5720 (5 μM) prior to addition of lysoPAF (1 μM) or vehicle. The fMLF (1 μM) induced superoxide generation was measured, and expressed as percent change relative to superoxide generation in the absence of inhibitor and lysoPAF. Data shown are mean ± SEM based on 3 independent experiments.
The involvement of an adenylyl cyclase is suggested by the results from experiments using the adenylyl cyclase inhibitor SQ22536 (9-(tetrahydro-2-furyl)adenine) (Graber and Hawiger, 1982), which dose-dependently reversed the inhibitory effect of lysoPAF on the production of syperoxide (Figure 5D). Protein kinase A (PKA), a key kinase activated by cAMP, also plays a role in the inhibition by lysoPAF. Using the PKA inhibitor H-89, we observed a partial restoration (p < 0.05) of superoxide production in fMLF-stimulated neutrophils that were pretreated with lysoPAF (Figure 5E). This result is confirmed in a parallel experiment using another PKA inhibitor, KT5720, which produced similar effect in reversal of the effect of lysoPAF. These results indicate that lysoPAF signaling involves adenylyl cyclase-mediated generation of cAMP, and that PKA contributes to the inhibitory effect of lysoPAF.
LysoPAF induces cAMP elevation in platelets and inhibits platelet aggregation
In addition to neutrophils, other blood cells including platelets are involved in inflammatory responses. PAF was originally named for its activating effect on platelets (Benveniste et al., 1972). However, a bioactivity of lysoPAF on platelets has not been reported. We conducted experiments to determine whether lysoPAF could affect the intracellular cAMP levels in platelets, and if treatment of platelets with lysoPAF would affect its activation. In washed human platelets, lysoPAF induced a significant increase in cAMP levels (p < 0.01) (Figure 6A), whereas PAF does not have an effect on the intracellular cAMP levels in platelets under the same experimental conditions. Similar to neutrophils, cAMP inhibits many activation processes in platelets, including platelet aggregation (Salzman et al., 1972). We therefore tested whether lysoPAF-treated platelets display changes in agonist-induced platelet aggregation. When washed platelets were pre-incubated with lysoPAF and then stimulated with α-thrombin (0.03U/ml), a lysoPAF dose-dependent inhibition in platelet aggregation was observed (Figure 6B). Also similar to neutrophils, PKA is known to be involved in downstream signaling of cAMP. The inhibitory effect of lysoPAF on thrombin-induced platelet aggregation is partially reversed by the PKA inhibitors H-89 and KT5720 (Figure 6C).
Figure 6. LysoPAF induces cAMP elevation in platelets and suppresses thrombin-induces platelet aggregation.
A, human platelets (3 × 108 /ml) were incubated with the indicated lipid (1 μM) for 2 min. Cells were lysed in IBMX-containing buffer, and cAMP was assayed by competitive immunoassay. Data shown are mean ± SEM and are representative of 3 experiments with similar results. B, Thrombin-induced platelet aggregation in the absence or presence of lysoPAF. Human platelets were incubated with the indicated concentrations of lysoPAF for 2 min prior to stimulation with α-thrombin (0.03 U/mL) at 37°C under stirring conditions. Platelet aggregation, measured by light transmission, was determined over time. C, Thrombin-induced platelet aggregation in the absence or presence of the PKA inhibitors H-89 (5 μM) or KT5720 (5 μM), prior to stimulation with vehicle or lysoPAF (1 μM). Platelet aggregation was measured as in B. Data shown are representative of 2 independent experiments with similar results.
Discussion
Results from this study demonstrate that lysoPAF is bioactive and produces an effect opposite of that of PAF in neutrophil superoxide production. Whereas PAF potentiates NADPH oxidase activation and superoxide production, lysoPAF inhibits this function. The inhibitory effect of lysoPAF does not depend on PAFR, and is not limited to a particular agonist such as fMLF or one cell type such as neutrophils. In our experiments, the C5a-stimulated neutrophil superoxide production and thrombin-induced platelet aggregation are also inhibited by lysoPAF. These observations are novel, and provide evidence for a possible function of lysoPAF in balancing the proinflammatory activities of PAF. While PAF plays important roles in regulating cellular functions, its proinflammatory activities require temporal regulatory mechanisms including induced synthesis and efficient degradation. Therefore, enzymes responsible for PAF synthesis and degeneration, mainly the PAF-ATs and PAF-AHs, are potential targets of therapeutic intervention. Results from the current study suggest that PAF-AHs, in addition to removing PAF from circulation and tissues, may at the same time create a bioactive molecule with opposing functions, thereby effectively negating the bioactivity of PAF. It is likely that the inhibitory effects of lysoPAF on neutrophils and platelets contribute to the anti-inflammatory functions of PAF-AHs (Henderson et al., 2000).
Previous studies of the bioactivity of PAF often included lysoPAF as a negative control. Other reports showed that lysoPAF displayed PAF-like activities in stimulating DNA synthesis in smooth muscle cells (Chai et al., 2000) and microvascular leakage when administered to guinea pigs by inhalation (Sakamoto et al., 1993). These PAF-like activities, including the ability to induce Ca2+ mobilization, were blocked by PAFR antagonists, indicating that they are mediated by the identified PAFR (Sakamoto et al., 1993). Marathe and colleagues have shown that treatment of the bioactive lysoPAF and lysoPC with PAF-AH or with saponification abolishes the activities of these lysophospholipids, indicating that these activities come from contaminating phospholipids (Marathe et al., 2001). Before chemically synthesized PAF became widely available, many studies were conducted using PAF isolated from crude methanol extracts of cells or egg yolk, and those studies were prone to contamination with PAF-like phospholipids. The same methods apparently contributed to the identification of lipids migrating with a similar Rf as PAF on thin-layer chromatography and containing both platelet-activating and neutrophil-inhibiting properties (O'Donnell et al., 1981). To eliminate contaminations with other phospholipids, in this study we used synthetic lysoPAF and PAF from the same source, and prepared stock solutions for both lipids using exactly the same method. Experiments that compared lysoPAF and PAF were strictly carried out in parallel. Our study has shown dose-dependent inhibition of neutrophil NADPH oxidase activation by lysoPAF, with minimal active concentrations as low as 10 nM. Under the same experimental conditions, the potentiation effect of PAF was detectable.
The exact mechanism by which lysoPAF exerts its bioactivity is still unknown. As the first step towards an answer to this question, we determined whether elevation of intracellular cAMP level contributes to the inhibitory effects of lysoPAF. Our results confirmed that lysoPAF, but not PAF, was able to stimulate an increase in cAMP levels in both neutrophils and platelets. Moreover, pharmacological inhibition of PKA, an effector of cAMP, partially reversed the inhibitory effect of lysoPAF, indicating that the cAMP-PKA pathway is involved, but PKA-independent mechanism may also exist. We also found that treating neutrophils with SQ22536, an inhibitor of adenylyl cyclase, dose-dependently reversed the inhibitory effect of lysoPAF on superoxide generation. These results confirm that adenylyl cyclase-mediated production of cAMP is key to the inhibitory activity of lysoPAF. These effects are not likely the results of non-specific actions of the inhibitors on other kinases, most of which are important for neutrophil NADPH oxidase activation and platelet aggregation.
We hypothesize that lysoPAF activates adenylyl cyclase through either one or both of the following pathways. First, lysoPAF may bind to a receptor that couples to the Gs class of G proteins. Because Gαs activates adenylyl cyclase, lysoPAF binding to this receptor leads to increased cAMP production. This is likely given the emergence of an increasing number of GPCRs for bioactive lipids (Im, 2004). Our study using cholera toxin, which activates Gαs, produced similar effect in the elevation of cAMP levels and inhibition of superoxide generation as seen in lysoPAF-stimulated cells. To determine which receptor(s) is activated by lysoPAF, we have conducted an exhaustive search of the existing GPCR database and identified 28 receptors that are related in sequence to known lipid receptors. These receptors were individually analyzed in transfected cells for their abilities to mediate lysoPAF-induced increase in intracellular cAMP, CREB-driven luciferase reporter expression, and calcium mobilization. G2A has been shown to mediate the bioactivity of lysoPC (Lin et al., 2005; Wang et al., 2005), although it lacks certain pharmacological properties of a receptor such as direct binding of lysoPC (Kabarowski et al., 2001; Witte et al., 2005). When tested in our assays, G2A and structurally similar receptors such as GPR4, TDAG8 and OGR1, did not respond significantly to lysoPAF stimulation (Supplemental Table I). Other GPCRs tested also failed to respond to lysoPAF in these functional assays. Therefore, screening of additional GPCRs will be necessary to further test the hypothesis. We noticed that fMLF, which activates a Gi-coupled receptor FPR, is reported to enhance intracellular cAMP levels through a pertussis toxin-sensitive mechanism (Ali et al., 1998). However, fMLF also stimulates Ca2+ flux whereas lysoPAF does not, indicating that lysoPAF does not activate the same signaling pathway that is induced by fMLF. Nevertheless, expanding the search to other GPCRs may be beneficial to understanding of how lysoPAF induces cAMP elevation. The second possible mechanism for the inhibitory effect of lysoPAF involves direct activation of adenylyl cyclase. To date, ten isoforms of transmembrane adenylyl cyclase have been identified (Hurley, 1999). Adenylyl cyclase isoform 1 expression is restricted to the brain. Isoforms 6 and 7 are ubiquitously expressed in cells and tissues. We have tested several other cell types, including HPMECs, which were unable to respond to lysoPAF with increased cAMP levels (data not shown). Therefore, it is unlikely that the candidate is a ubiquitously expressed isoform of adenylyl cyclase. Store operated calcium channels are known to activate adenylyl cyclase isoforms 3 and 8; however we observed no changes in calcium levels in cells treated with lysoPAF. A further analysis is required to more carefully examine these cyclase isoforms.
In summary, results from the current study identify lysoPAF as a bioactive lipid with inhibitory functions in neutrophil NADPH oxidase activation and platelet aggregation. This novel finding may create an opportunity to investigate the biological functions of PAF-AHs in regulating the activities of PAF. Because lysoPAF is a precursor as well as metabolite of PAF, its tissue concentration is regulated by the presence of enzymes in the PAF remodeling pathway. Recent cloning and characterization of acetyl-CoA:lyso-PAF acetyltransferase (Shindou et al., 2007) and clinical correlation between lowered PAF-AH concentration and severity of anaphylaxis (Vadas et al., 2008) are expected to promote continued exploration of PAF-AH and related pathways. The discovery of lysoPAF as having opposite activities of PAF in the functional assays performed in this study may contribute to a better understanding of these phospholipids for their functions in human physiology.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Jean-Marc Herbert for the gift of SR27417, Dr. Guy Le Breton and Dr. Tohru Kozasa for suggestions on cAMP assays, Dr. Feng Qian for assistance with blood cell preparation, Fumie Hamano for technical assistance in receptor screening assays, and members of the Ye Laboratory for helpful discussions.
This work was supported in part by NIH grants AI033503 and HL077806. E.J.W. is a recipient of a Predoctoral Fellowship from American Heart Association, Midwest Chapter.
Abbreviations
- PLA2
phospholipase A2
- PAF
platelet-activating factor (1-O-hexadecyl-2-acetoyl-sn-glycero-3-phosphocholine)
- lysoPAF
1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphocholine
- GPCR
G-protein coupled receptor
- PKA
protein kinase A
- PAFR
platelet-activating factor receptor
- fMLF
N-Formyl-L-methionyl-L-leucyl-L-phenylalanine
- HPMECs
human pulmonary microvessel endothelial cells
- PAF-AH
platelet-activating factor acetylhydrolase
- PAF-AT
platelet-activating factor acetyltransferase
- TER
transendothelial Electrical Resistance
- SQ22536
9-(tetrahydro-2-furyl)adenine
- KT5720
(9S,10S,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid hexyl ester
- H-89
N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride CTx, Cholera Toxin
- PTx
pertussis toxin
- Rf
retardation factor
- IBMX
3-Isobutyl-1-methylxanthine
References
- Ali H, Sozzani S, Fisher I, Barr AJ, Richardson RM, Haribabu B, Snyderman R. Differential regulation of formyl peptide and platelet-activating factor receptors. Role of phospholipase Cbeta3 phosphorylation by protein kinase A. J Biol Chem. 1998;273:11012–11016. doi: 10.1074/jbc.273.18.11012. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Qian JY, Baatar D, Karasawa K, Asada Y, Sasaguri Y, Miller ER, Witztum JL, Ueno H. Local expression of platelet-activating factor-acetylhydrolase reduces accumulation of oxidized lipoproteins and inhibits inflammation, shear stress-induced thrombosis, and neointima formation in balloon-injured carotid arteries in nonhyperlipidemic rabbits. Circulation. 2005;111:3302–3309. doi: 10.1161/CIRCULATIONAHA.104.476242. [DOI] [PubMed] [Google Scholar]
- Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972;136:1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Jala VR, Raghuwanshi SK, Nasser MW, Haribabu B, Richardson RM. Activation and regulation of platelet-activating factor receptor: role of G(i) and G(q) in receptor-mediated chemotactic, cytotoxic, and cross-regulatory signals. J Immunol. 2006;177:3242–3249. doi: 10.4049/jimmunol.177.5.3242. [DOI] [PubMed] [Google Scholar]
- Chai YC, Binion DG, Chisolm GM. Relationship of molecular structure to the mechanism of lysophospholipid-induced smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol. 2000;279:H1830–1838. doi: 10.1152/ajpheart.2000.279.4.H1830. [DOI] [PubMed] [Google Scholar]
- Dewald B, Baggiolini M. Activation of NADPH oxidase in human neutrophils. Synergism between fMLP and the neutrophil products PAF and LTB4. Biochem Biophys Res Commun. 1985;128:297–304. doi: 10.1016/0006-291x(85)91678-x. [DOI] [PubMed] [Google Scholar]
- Fantone JC, Marasco WA, Elgas LJ, Ward PA. Anti-inflammatory effects of prostaglandin E1: in vivo modulation of the formyl peptide chemotactic receptor on the rat neutrophil. J Immunol. 1983;130:1495–1497. [PubMed] [Google Scholar]
- Fantone JC, Marasco WA, Elgas LJ, Ward PA. Stimulus specificity of prostaglandin inhibition of rabbit polymorphonuclear leukocyte lysosomal enzyme release and superoxide anion production. Am J Pathol. 1984;115:9–16. [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Kawashima H, Saito K, Inomata N, Matsui M, Nakanishi T. Effect of human plasma-type platelet-activating factor acetylhydrolase in two anaphylactic shock models. Eur J Pharmacol. 2000;390:203–207. doi: 10.1016/s0014-2999(99)00920-6. [DOI] [PubMed] [Google Scholar]
- Furie MB, Cramer EB, Naprstek BL, Silverstein SC. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984;98:1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RN, Bozza FA, Amancio RT, Japiassu AM, Vianna RC, Larangeira AP, Gouvea JM, Bastos MS, Zimmerman GA, Stafforini DM, Prescott SM, Bozza PT, Castro-Faria-Neto HC. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 2006;26:41–49. doi: 10.1097/01.shk.0000209562.00070.1a. [DOI] [PubMed] [Google Scholar]
- Graber S, Hawiger J. Evidence that changes in platelet cyclic AMP levels regulate the fibrinogen receptor on human platelets. J Biol Chem. 1982;257:14606–14609. [PubMed] [Google Scholar]
- He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr, Lu J, Poole KM, Dietsch GN, Chi EY. Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J Immunol. 2000;164:3360–3367. doi: 10.4049/jimmunol.164.6.3360. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Bernat A, Valette G, Gigo V, Lale A, Laplace MC, Lespy L, Savi P, Maffrand JP, Le Fur G. Biochemical and pharmacological activities of SR 27417, a highly potent, long-acting platelet-activating factor receptor antagonist. J Pharmacol Exp Ther. 1991;259:44–51. [PubMed] [Google Scholar]
- Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem. 2002;131:773–779. doi: 10.1093/oxfordjournals.jbchem.a003164. [DOI] [PubMed] [Google Scholar]
- Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991;349:342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Hurley JH. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem. 1999;274:7599–7602. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- Hwang SB, Lee CS, Cheah MJ, Shen TY. Specific receptor sites for 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) on rabbit platelet and guinea pig smooth muscle membranes. Biochemistry. 1983;22:4756–4763. doi: 10.1021/bi00289a022. [DOI] [PubMed] [Google Scholar]
- Im DS. Discovery of new G protein-coupled receptors for lipid mediators. J Lipid Res. 2004;45:410–418. doi: 10.1194/jlr.R300006-JLR200. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- Karasawa K, Harada A, Satoh N, Inoue K, Setaka M. Plasma platelet activating factor-acetylhydrolase (PAF-AH) Prog Lipid Res. 2003;42:93–114. doi: 10.1016/s0163-7827(02)00049-8. [DOI] [PubMed] [Google Scholar]
- Lee WL, Downey GP. Neutrophil activation and acute lung injury. Cur Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin αIIbβ3. Blood. 2006;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174:2981–2989. doi: 10.4049/jimmunol.174.5.2981. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Silva AR, de Castro Faria Neto HC, Tjoelker LW, Prescott SM, Zimmerman GA, McIntyre TM. Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. J Lipid Res. 2001;42:1430–1437. [PubMed] [Google Scholar]
- O'Donnell MC, Siegel JN, Fiedel BA. Platelet activating factor: an inhibitor of neutrophil activation? Clin Exp Immunol. 1981;43:135–142. [PMC free article] [PubMed] [Google Scholar]
- Quarck R, De Geest B, Stengel D, Mertens A, Lox M, Theilmeier G, Michiels C, Raes M, Bult H, Collen D, Van Veldhoven P, Ninio E, Holvoet P. Adenovirus-mediated gene transfer of human platelet-activating factor-acetylhydrolase prevents injury-induced neointima formation and reduces spontaneous atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;103:2495–2500. doi: 10.1161/01.cir.103.20.2495. [DOI] [PubMed] [Google Scholar]
- Reinhold SL, Zimmerman GA, Prescott SM, McIntyre TM. Phospholipid remodeling in human neutrophils. Parallel activation of a deacylation/reacylation cycle and platelet-activating factor synthesis. J Biol Chem. 1989;264:21652–21659. [PubMed] [Google Scholar]
- Rivkin I, Becker EL. Effect of exogenous cyclic AMP and other adenine nucleotides on neutrophil chemotaxis and motility. Int Arch Allergy Appl Immunol. 1976;50:95–102. doi: 10.1159/000231485. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Elwood W, Barnes PJ, Chung KF. Effect of inhaled lyso-platelet-activating factor on airway microvascular leakage in the guinea pig. J Appl Physiol. 1993;74:1117–1122. doi: 10.1152/jappl.1993.74.3.1117. [DOI] [PubMed] [Google Scholar]
- Salzman EW, Kensler PC, Levine L. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Ann N Y Acad Sci. 1972;201:61–71. doi: 10.1111/j.1749-6632.1972.tb16287.x. [DOI] [PubMed] [Google Scholar]
- Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSOPAF acetyltransferase. J Biol Chem. 2007;282:6532–6539. doi: 10.1074/jbc.M609641200. [DOI] [PubMed] [Google Scholar]
- Szabo C, Wu CC, Mitchell JA, Gross SS, Thiemermann C, Vane JR. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993;73:991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- Vercellotti GM, Yin HQ, Gustafson KS, Nelson RD, Jacob HS. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988;71:1100–1107. [PubMed] [Google Scholar]
- Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, Witte ON. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell. 2005;16:2234–2247. doi: 10.1091/mbc.E04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte ON, Kabarowski JH, Xu Y, Le LQ, Zhu K. Retraction. Science. 2005;307:206. doi: 10.1126/science.307.5707.206b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.