Abstract
Importance
Modern prevention guidelines dramatically increase statin prescription. Efforts to refine specificity of statin eligibility via coronary calcification are studied in Caucasians, but not in large African American populations at high cardiovascular risk. Effective race-specific prevention efforts that rely on reconciling the interplay between statin eligibility, long-term estimated risk, and prognostic measures of subclinical cardiovascular disease (CVD) are warranted.
Objective
To compare effectiveness of contemporary guidelines (ACC/AHA) relative to older mandates (ATP-III) in identifying African Americans with subclinical and clinical CVD. To understand the prevalence of subclinical CVD across estimated risk categories.
Design
Prospective evaluation with median 10-year follow-up
Setting
Community-based
Participants
2893 African Americans (age 40–75 years) without prevalent CVD who completed computed tomography (n=1790).
Main Outcomes and Measures
Non-zero coronary artery calcium (CAC), abdominal aortic calcium (AAC) and incident CVD (myocardial infarction, ischemic stroke, or CVD death).
Results
Mean age at baseline was 55.4 years (65% female). Statin eligibility by modern ACC/AHA guidelines identified 70% of African Americans with non-zero CAC (vs. 40% by ATP-III, P<0.0001) with improved discrimination and reclassification; we observed similar results for aortic calcification. Presence of CAC (HR=2.28, 95% CI, 1.2–4.2, P=0.008) was associated with incident CVD independent of ACC/AHA statin eligibility status. African Americans who were not ACC/AHA-statin eligible experienced a CVD event rate of 0.9/1000 person-years regardless of CAC. A 10-year CVD risk estimate greater than 12.5% by pooled cohort equations captured >70% of African Americans with detectable CAC in the community.
Conclusions and Relevance
In African American adults, statin eligibility by newer guidelines had a high sensitivity (with lower specificity) for the identification of high-risk vascular phenotypes relative to older guidelines. While statin ineligible African Americans are at low risk regardless of CAC status, the presence of CAC appears to recapture specificity for CVD events in those who are statin eligible. Finally, pooled cohort 10-year risk estimate thresholds can provide bedside identification of a majority of African Americans with CAC in the community. These results suggest the utility of a complementary approach using directed imaging within current guidelines to refine therapeutic decision-making.
Keywords: JHS, African Americans, cardiovascular disease, statins, coronary calcium
INTRODUCTION
African Americans are at disproportionately high risk for cardiovascular disease (CVD)1,2, with a more adverse response to cardiovascular risk and subclinical CVD relative to other racial backgrounds3. Several recent reports suggest that while 2013 ACC/AHA guidelines utilizing race-specific risk estimators4 discriminate CVD risk well in African Americans, they also call for a dramatic increase in statin eligibility (relative to older 2004 Adult Treatment Panel ATP-III guidelines5), raising concerns over the appropriateness of widespread statin prescription6. In response, this increase in statin eligibility (with concurrent potential for increased cost and side effects) has led to the investigation of measures of subclinical CVD that would help to prioritize statin therapy7,8. Recent data from the Framingham Heart Study suggest that statin eligibility by ACC/AHA guidelines more effectively identify Caucasians with coronary artery calcification and long-term CVD events7. Despite a lower prevalence of vascular calcification in African Americans9, the presence of coronary calcium appears to identify Blacks at higher risk for CVD, incremental to older risk estimates (Framingham risk score10). Therefore, a critical next step is to determine whether newer clinical guidelines that more broadly advocate statin therapy for primary prevention accurately identify African Americans with high-risk vascular phenotypes and high downstream risk and whether measurement of vascular calcium provides additional information.
Here, we examine whether statin eligibility by contemporary 2013 ACC/AHA guidelines (relative to 2004 ATP-III) identifies African Americans with high-risk vascular phenotypes and higher CVD risk in the Jackson Heart Study (JHS), a large, established cohort of African Americans with coronary and aortic calcium quantification. Our goals were two-fold: (1) to understand whether statin eligibility by contemporary ACC/AHA guidelines identifies African American individuals with prognostic coronary or aortic vascular phenotypes and high downstream CVD risk better than older (ATP-III) clinical estimators and (2) to examine whether the presence of coronary calcification provides incremental value to ACC/AHA guidelines in determining CVD risk.
METHODS
Study population
The Jackson Heart Study (JHS) is a population-based prospective study of 5306 African-Americans 21 years or older from the Jackson, Mississippi, tricounty area (Hinds, Madison, and Rankin). The study was designed to identify causes of CVD among African-Americans11,12. Study subjects were examined at a baseline clinic visit (2000–2004) and during two additional visits: Visit 2 (2005–2008), Visit 3 (2009–2013). We excluded participants (1) with a history of CVD (n=572; defined as a history of myocardial infarction [MI] by self-report or 12-lead electrocardiogram [ECG], or self-reported stroke or carotid angioplasty); (2) participants already on statin treatment at the baseline clinic visit (n=414); (3) <40 and >75 years old (age range specified in ACC/AHA guidelines; n=827); (4) participants in whom data on statin treatment was not collected (n=278); and (5) who were missing data on variables included in risk scores (e.g., lipids, smoking, blood pressure; n=322). These exclusions left 2893 participants in our final analytic sample, of which 1790 had available computed tomography (CT) at visit 2.
The study was Institutional Review Board approved, and all participants provided written informed consent.
Risk factor measurement
Prevalent diabetes was defined according to the American Diabetes Association (ADA) criteria as fasting glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5% or use of medications for diabetes13. Current cigarette smoking was assessed at baseline. Blood pressure was calculated in the seated position as the average of two resting blood pressure recordings. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications. Lipid profile was measured as previously described14. Family history of premature heart disease was defined as participant reported history of any “heart disease” in a male parent at less than 55 years old, in a female parent at less than 65 years old or in siblings or children less than 60 years old.
Statin eligibility
Statin eligibility by 2013 ACC/AHA guidelines was defined as follows4: (1) low density lipoprotein cholesterol (LDL) ≥190 mg/dl; (2) diabetes, age 40–75 years, and LDL 70–189 mg/dl; or (3) no diabetes, LDL 70–189 mg/dl, and estimated 10-year atherosclerotic cardiovascular disease risk ≥7.5%, as determined by the pooled cohort equations (PCE)15.
Statin eligibility by 2004 ATP-III guidelines was defined using risk factors and the Framingham risk score (FRS) calculator16 as follows: (1) LDL level ≥100 mg/dl and either diabetes or 10-year FRS for coronary heart disease ≥20%; (2) LDL level ≥130 mg/dl and FRS of 10–20% and two or more risk factors; (3) LDL level ≥160 mg/dl and FRS <10% and two or more risk factors; (4) LDL ≥190 mg/dl and fewer than two risk factors. Risk factors included current cigarette smoking, hypertension, high density lipoprotein cholesterol (HDL) < 40 mg/dl, family history of premature heart disease (as defined above) and age (men ≥45 years; women ≥55 years).
Coronary and aortic vascular calcification
Cardiovascular imaging for coronary and aortic calcification was not performed at the baseline study visit in JHS. Therefore, we relied on detection of vascular calcification at visit 2 (median interval between visit 1 and 2: 4.5 years; interquartile range 4.3–5.0 years). Heart and abdominal imaging was obtained with a 16-channel multi-detector CT using a prospective ECG-triggered protocol (Lightspeed 16 Pro; GE Healthcare, Milwaukee, WI), as published10,17. Quality control and image analysis were performed by a central core laboratory. Technical settings included prospective ECG gating at 75% of the R-R interval, 120 KVp, 2.5 mm slice thickness, 35 cm display field of view, gantry speed of 0.40 seconds and a segmented reconstruction resulting in an effective temporal resolution of 0.24 seconds. Tube current was 400 mA and increased by 25% for participants weighing ≥220 lbs (100 Kg) to compensate for body size and maintain a more constant signal-to-noise ratio across participants. Coronary artery calcium score (CAC) and abdominal aortic calcium score (AAC) were quantified using the Agatston score (TeraRecon Aquarius Workstation, San Mateo, CA)18.
Incident cardiovascular disease
Incident CVD was defined as occurrence of first myocardial infarction [MI] or fatal CVD or ischemic stroke. Incident MI, fatal CVD, and ischemic stroke were determined through adjudication of relevant medical records as previously described19. The final date of follow-up was December 31, 2012.
Statistical analysis
Baseline characteristics are displayed as mean and standard deviation, median and interquartile range or number (percent). In order to address potential for a selection bias, we compared individuals in our analytic cohort with excluded participants (N=2413), with comparisons made by appropriate parametric or non-parametric testing (continuous; based on data normality) or chi square test (categorical). Of note, we performed several exclusions for high-risk features (statin use, CVD history) to arrive at a lower-risk, statin-naïve subset of participants.
We first used logistic regression models to estimate the probability of presence of CAC or AAC (zero vs. non-zero) based on statin eligibility by 2013 ACC/AHA guidelines vs. 2004 ATP-III guidelines. “Not statin eligible” was treated as the referent category. We assessed model discrimination (C-statistic) for vascular calcification. Differences in C-statistics between 2013 ACC/AHA and 2004 ATP-III statin eligibility and reclassification for CAC or AAC were calculated as described by Pencina20,21. As sensitivity analyses, we examined (1) the association of statin eligibility at study enrollment with a CAC score over 100 (a marker of more severe atherosclerosis) and (2) statin eligibility in statin-naïve individuals from visit 2 with zero or >100 CAC score to ensure the generalizability of our results. We applied the same exclusions at visit 2 for visit 1 and carried forward visit 1 smoking and family history (which were not assessed at visit 2).
To address our second aim, we examined the impact of statin eligibility by newer ACC/AHA guidelines (vs. ATP-III) on clinical outcomes, independent of CAC. First, we sought to extend prior work in Caucasians7 by fitting Cox proportional hazards regression models and estimating annualized event rates for incident CVD as a function of statin eligibility by newer ACC/AHA guidelines versus ATP-III. Event rates were calculated per 1000 patient-years of follow-up, analogous to a recently published study22. In the subgroup of 1790 individuals with CT imaging, we examined whether the presence of CAC by CT imaging would be associated with CVD risk independent of statin eligibility by newer ACC/AHA guidelines. The assumption of proportional hazards was confirmed via Kolmogorov’s supremum test. Kaplan Meier survival curves were used to visualize cumulative incidence over time.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A two-tailed P value of 0.05 was considered significant.
RESULTS
Baseline characteristics
Demographic, clinical and biochemical characteristics (at the baseline visit) and imaging characteristics (at Visit 2) stratified by statin eligibility by 2013 ACC/AHA guidelines are presented in Table 1. We included 2893 JHS participants (54.5%) in our analytic cohort. A CONSORT diagram of the exclusions performed for all analyses is shown in Supplemental Figure 1. Mean age in our analytic sample was 55.4 ± 9.4 years (65.1% female) and mean body mass index (BMI) was 31.6 ± 7.0. The calculated median 10-year PCE risk was 7.0% (interquartile range IQR 3.2–13.2%) and median 10-year FRS score was 3.1% (IQR 1.1–7.2). Greater than 40% of our study cohort had detectable coronary calcification (756, 42.2%), and more than 60% had detectable abdominal aortic calcification (1145, 64.0%). By design, no individual in our analytic cohort had CVD or statin use at baseline study visit. Nearly 1 in 4 individuals in the excluded sample had CVD and 28.6% used statins. Excluded subjects had an overall higher prevalence of cardiovascular risk factors (Supplemental Table 1).
Table 1.
Baseline characteristics of JHS participants stratified by statin eligibility by 2013 ACC/AHA guidelines. Values are mean (standard deviation), median (interquartile range) or number (%). Abbreviations: body mass index, BMI; cardiovascular disease, CVD; high density lipoprotein, HDL; interquartile range, IQR; low density lipoprotein, LDL; number, N; standard deviation, SD
| Statin Eligible by ACC/AHA | Statin ineligible by ACC/AHA | ||
|---|---|---|---|
| n = 1455 | n = 1438 | P value | |
| Age, mean (SD), years | 60.9 ± 8.3 | 49.9 ± 6.9 | <0.0001 |
| Female, N (%) | 839 (57.7) | 1045 (72.7) | <0.0001 |
| BMI, mean (SD), kg/m2 | 31.7 ± 6.7 | 31.5 ± 7.3 | 0.59 |
| Current smoking, N (%) | 230 (15.8) | 143 (9.9) | <0.0001 |
| Hypertension, N (%) | 1047 (72.0) | 499 (34.7) | <0.0001 |
| Diabetes, N (%) | 431 (29.6) | 20 (1.4) | <0.0001 |
| Family history of premature heart disease, N (%) | 341 (23.6) | 363 (25.4) | 0.25 |
| Antihypertensive therapy, N (%) | 923 (63.4) | 439 (30.5) | <0.0001 |
| Total cholesterol, mean (SD), mg/dl | 213.0 ± 41.5 | 191.5 ± 33.7 | <0.0001 |
| LDL-C, mean (SD), mg/dl | 139.6 ± 38.0 | 119.4 ± 33.7 | <0.0001 |
| HDL-C, mean (SD), mg/dl | 50.7 ± 14.1 | 53.7 ± 14.9 | <0.0001 |
| Pooled cohort equation risk score, median (IQR), % | 12.9 (9.3–19.2) | 3.4 (1.3–5.2) | <0.0001 |
| Framingham risk score, median (IQR), % | 6.5 (3.6–11.4) | 1.2 (0.5–2.4) | <0.0001 |
| Coronary artery calcium score | |||
| Mean (SD) | 186.3 ± 427.5 | 32.7 ± 147.8 | |
| Median (IQR) | 23.9 (0–142.3) | 0 (0–0) | <0.0001 |
| N (%) with score >0 | 526 (62.3) | 230 (24.3) | <0.0001 |
| N (%) with score >100 | 261 (30.9) | 66 (7.0) | <0.0001 |
| Abdominal aortic calcium score | |||
| Mean (SD) | 1109.3 ± 1566.0 | 243.3 ± 758.7 | |
| Median (IQR) | 431.9 (43.6–1574.8) | 0 (0–109.0) | <0.0001 |
| N (%) with score >0 | 707 (83.9) | 438 (46.3) | <0.0001 |
Distribution of statin eligibility in African Americans
Of 2893 participants not on statin therapy at baseline, 50% were statin eligible by 2013 ACC/AHA guidelines relative to 28% by 2004 ATP-III guidelines (1455/2893 [50%] vs. 822/2893 [28.4%], P<0.0001). Criteria for meeting statin eligibility by both guidelines are shown in Figure 1. Statin eligibility by ACC/AHA guidelines was primarily due to 10-year estimated atherosclerotic CVD risk greater than 7.5% by PCE. On the other hand, a diagnosis of diabetes or 10-year FRS risk more than 20% were responsible for the greatest frequency (42.6%) of statin eligibility by ATP-III guidelines.
Figure 1.
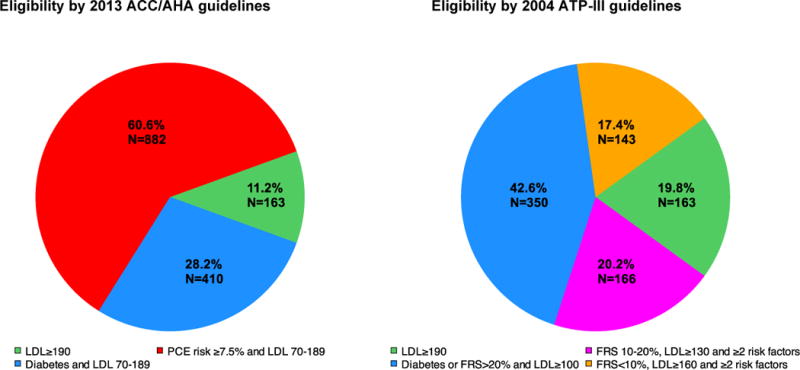
Criteria for statin eligibility by 2013 ACC/AHA guidelines and 2004 ATP-III guidelines
Statin eligibility and clinical risk in statin-naïve African Americans
Over a median 10-year follow-up (interquartile range 9.1–10.7 years), we observed 129 incident CVD events (63 CHD events and 66 ischemic strokes), corresponding to an overall event rate (4.6%; Supplemental Table 1). We observed a greater than 5-fold increased hazard of incident CVD events (hazard ratio HR 5.4, 95% CI 3.4–8.6, P<0.0001) among ACC/AHA statin eligible-participants (versus non-eligible), relative to a 2.3-fold increased hazard in ATP-III statin eligible participants (HR 2.3, 95% CI 1.6–3.3, P<0.0001; Supplementary Figure 2).
Statin eligibility and subclinical vascular calcification
Of our overall cohort, 1790 (62%) underwent cardiac and abdominal CT for CAC and AAC scoring at visit 2. Participants in the CT subgroup were at lower CVD risk (Supplemental Table 2). Nevertheless, statin eligibility by ACC/AHA captured nearly 70% of African Americans (526/756 [69.6%] relative to 301/756 [39.8%] by ATP-III, P<0.0001) who had coronary calcification and over 60% of participants with aortic calcification (707/1145 [61.8%] relative to 396/1145 [34.6%] by ATP-III, P<0.0001; Figure 2). We observed a near 5-fold greater odds of detectable CAC (odds ratio OR 5.1, 95% CI 4.2–6.3, P<0.0001) and a 6-fold greater odds of detectable AAC (OR 6.0, 95% CI 4.8–7.5, P<0.0001) among participants eligible for statins by ACC/AHA guidelines at baseline (vs. non-eligible; Table 2). In a sensitivity analysis assessing ACC/AHA statin eligibility at Visit 2, statin-eligible African Americans had greater odds of any CAC and more extensive CAC (vs. non-eligible; for any CAC: OR 4.7, 95% CI 3.7–6.1, P<0.0001; for CAC ≥100: OR 5.2, 95% CI 3.6–7.6, P<0.0001).
Figure 2.
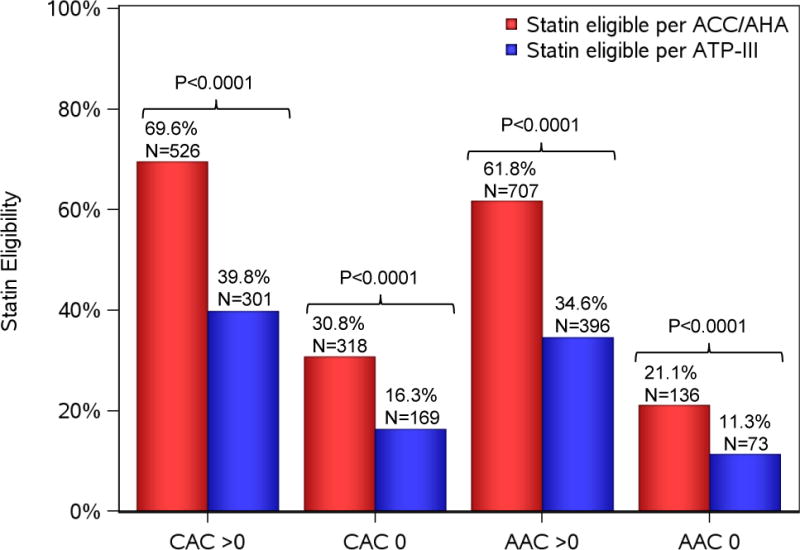
Prevalence of coronary and aortic vascular calcification by statin eligibility criteria.
Table 2.
Identification of vascular calcification by statin eligibility. “Not statin eligible” was considered the referent category in logistic regression. Comparison of eligibility status was performed such that reclassification metrics and C-statistics represent the improvement of 2013 ACC/AHA guidelines over 2004 ATP-III guidelines. Detailed methods described in text. Abbreviations: AAC, abdominal aortic calcium score; CAC, coronary artery calcium score; NRI, net reclassification index; Ref, referent category.
| Eligibility | CAC score >0 (N=756/1790 participants) |
CAC score ≥100 (N=327/1790 participants) |
AAC score >0 (N=1145/1789 participants) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number with CAC (%) | Statistic (95% CI) | p value | Number with CAC≥100 (%) | Statistic (95% CI) | p value | Number with AAC (%) | Statistic (95% CI) | p value | |
| 2013 ACC/AHA guidelines | |||||||||
| Statin eligible | 526/844 (62.3) | 5.1 (4.2–6.3) | <0.0001 | 261/844 (30.9) | 6.0 (4.5–8.0) | <0.0001 | 707/843 (83.9) | 6.0 (4.8–7.5) | <0.0001 |
| Not statin eligible | 230/946 (24.3) | 1 (Ref) | 66/946 (7.0) | 1 (Ref) | 438/946 (46.3) | 1 (Ref) | |||
| C-statistic | 0.69 (0.67–0.72) | 0.70 (0.67–0.73) | 0.70 (0.68–0.72) | ||||||
| 2004 ATP-III guidelines | |||||||||
| Statin eligible | 301/470 (64.0) | 3.4 (2.7–4.2) | <0.0001 | 160/470 (34.0) | 3.6 (2.8–4.6) | <0.0001 | 396/469 (84.4) | 4.1 (3.1–5.4) | <0.0001 |
| Not statin eligible | 455/1320 (34.5) | 1 (Ref) | 167/1320 (12.7) | 1 (Ref) | 749/1320 (56.7) | 1 (Ref) | |||
| C-statistic | 0.62 (0.60–0.64) | 0.64 (0.61–0.67) | 0.62 (0.60–0.63) | ||||||
| Statin eligibility comparison (2013 ACC/AHA vs. 2004 ATP-III) | |||||||||
| ΔC-statistic | 0.08 (0.06–0.10) | <0.0001 | 0.06 (0.03–0.09) | <0.0001 | 0.09 (0.07–0.11) | <0.0001 | |||
| NRI | 0.15 (0.11–0.20) | <0.0001 | 0.12 (0.06–0.18) | 0.0006 | 0.17 (0.14–0.21) | <0.0001 | |||
| Individuals with CAC correctly reclassified by ACC/AHA | 30% | <0.0001 | 31% | <0.0001 | 27% | <0.0001 | |||
| Individuals without CAC incorrectly reclassified by ACC/AHA | −14% | <0.0001 | −19% | <0.0001 | −10% | <0.0001 | |||
Of note, we observed that statin eligibility by ATP-III was associated with greater probability of calcification. However, statin eligibility by contemporary ACC/AHA guidelines provided better risk discrimination and net reclassification for the presence and extent of calcification across both aortic and coronary vascular beds (Table 2; Supplemental Table 3). The impact of ACC/AHA statin eligibility on reclassification of calcification risk (vs. ATP-III) resided in correct “upward” reclassification of individuals with CAC. Individuals without CAC were more often incorrectly reclassified by newer statin eligibility guidelines. These results correspond to an overall greater recommendation for statin prescription in individuals with CAC, with a smaller cohort without vascular calcification recommended for statins, suggesting improved sensitivity (with reduced specificity) for 2013 ACC/AHA guidelines for calcification (relative to ATP-III) in African Americans.
Calcium and Incident CVD
To understand whether the presence of CAC might influence contemporary clinical recommendations for statin use we next investigated the impact of adding CAC to statin eligibility by ACC/AHA guidelines on incident CVD. In the subgroup of 1790 participants with CAC imaging, over a median 10-year follow-up, we observed 58 events (33 CHD events and 25 ischemic strokes). Participants in this subgroup who were statin eligible by ACC/AHA guidelines experienced a higher 10-year CVD event rate per 1000 person years of 8.3 (95% CI 6.1–11.2) in the presence of CAC relative to 3.0 (95% CI 1.6–5.8), without CAC, P=0.01 (Figure 3). Those who were not statin eligible had a low 10-year CVD event rate regardless of presence or absence of CAC (0.9 per 1000 person-years; Figure 3). Finally, after adjustment for statin eligibility status in Cox models, presence of any CAC was associated with incident CVD (HR=2.28, 95% CI, 1.2–4.2, P=0.008), suggesting that statin-eligible African Americans with CAC experienced the greatest long-term CVD risk.
Figure 3.
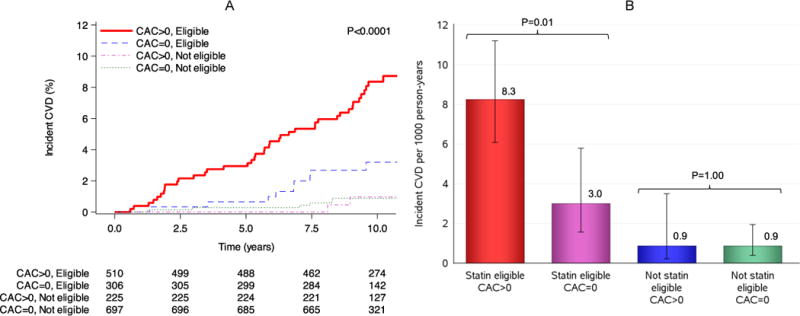
(A) Kaplan-Meier survival curves for incident CVD stratified by presence of coronary artery calcification among ACC/AHA statin eligibility groups. P value represents Bonferroni adjusted comparison among groups using the Log rank test. (B) Incident CVD rate stratified by presence of coronary artery calcium among ACC/AHA statin eligibility groups. Comparison in events rates was performed using Poisson regression.
Distribution of CAC in individuals across PCE risk
Given the proposed role for CAC to enhance the specificity of PCE-directed statin recommendations8, an important question is whether specific thresholds of 10-year risk by PCE might influence decisions to perform CAC screening in African Americans. To provide the broadest cohort for addressing this question, we included all JHS participants in our cohort who underwent CT imaging, but did not qualify for statins by diabetes or LDL ≥190 mg/dl and had an LDL between 70–189 and a measurable PCE risk score (N=1601). Figure 4 shows the distribution of prevalent (non-zero) CAC across 10-year PCE risk. We observed a continuous increase in CAC prevalence across PCE risk. In “grey-zone” risk (5–7.5% 10-year risk), 43% of African Americans had CAC. While above a 12.5% 10-year CVD risk, over 70% harbored CAC. These results support current guideline recommendations (class II) to further risk refinement for statin therapy in African Americans falling into the grey-zone PCE (5–7.5% 10-year risk), while providing PCE risk thresholds over which CAC screening may not be clinically expedient or cost-effective (e.g., >12.5%).
Figure 4. Coronary artery calcium by estimated PCE risk.
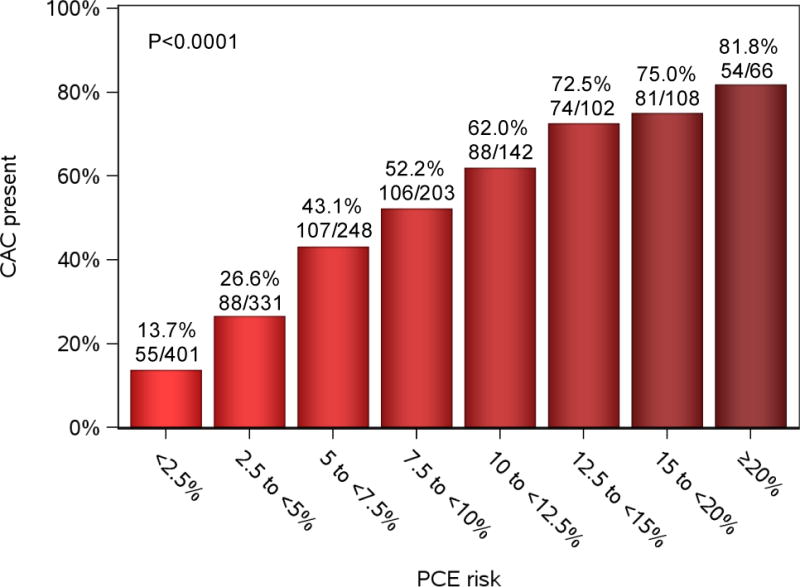
The frequency of non-zero coronary artery calcium by PCE risk score was measured in 1601 statin-naïve participants with available calcium score, without diabetes and LDL equal to or above 70 mg/dl and less than 190 mg/dl. P value for trend is shown.
DISCUSSION
In this large community-based cohort of African-American adults, we identified two major findings critical to prevention efforts in this at-risk population. First, contemporary ACC/AHA guidelines successfully identify African Americans with high-risk vascular phenotypes more sensitively than previous lipid prevention guidelines and more effectively reassign individuals with underlying vascular calcification who would otherwise not be statin eligible (by ATP-III). This refinement in delineating the at-risk African American population appears to come with increased statin prescription in individuals without vascular calcification (reduced specificity) who are at lower 10-year CVD risk. Second, in African Americans not on statins, statin eligibility and presence of coronary vascular calcification are associated with the greatest long-term CVD risk. Importantly, for study participants who were not eligible for statins by ACC/AHA, 10-year incident CVD rate did not differ by CAC status. On the other hand, the absence of CAC in statin-eligible African Americans was associated with an approximate 3% 10-year CVD rate (less than the 5% 10-year PCE-projected rate cited as the floor for statin recommendation)4. Finally, we detected CAC in 43% of individuals in the grey-zone of PCE risk (5–7.5%) and >70% of those with PCE risk over 12.5%, supporting current guideline recommendations for subclinical CVD testing in intermediate risk individuals, while providing additional thresholds for PCE risk above which CAC testing may not be clinically expedient. Collectively, these results not only provide support for the application of contemporary guidelines for statins in African Americans. They also suggest that targeted cardiovascular imaging (or other surrogates) may be useful to reinforce a personalized approach to statin use to maximize the benefits in those with lower cardiometabolic risk.
The arrival of contemporary lipid prevention guidelines in 2013 was heralded by significant controversy surrounding significant increases in statin prescription and potential implications on cost23, downstream cardiometabolic risk, and the potential for over-treatment6. As a result, significant efforts have been undertaken to test prognostic performance and calibration of risk estimates across race, geography, and against previous, more restrictive lipid mandates (e.g., ATP-III)8,24. Nevertheless, a call for personalization of statin therapy beyond clinical risk estimators has led to increasing investigation in the use of direct measures of atherosclerotic vascular disease to further target statin therapy to those individuals most apt to benefit. Given available data on vascular calcification in large epidemiologic studies with carefully adjudicated follow-up, a focus on coronary calcium score as one such measure to restructure ACC/AHA-recommended statin prescription has grown.
Recent work by Nasir and colleagues in the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated over 40% of statin-eligible individuals under new guidelines have a zero calcium score, with a 5.2% 10-year CVD event rate (near the 5% 10-year event rate floor where ancillary testing is guideline suggested)8. In this study, the majority (77%) of new statin recommendations stemmed from elevations in 10-year PCE risk, similar to participants in JHS (Figure 1). Our results suggest that while adjudication of CAC may not necessarily be paramount in African Americans not statin-eligible by ACC/AHA guidelines, absence of CAC among the statin-eligible is clearly associated with a more benign long-term cardiovascular prognosis, with a much lower CVD event rate (8.3 compared to 3.0 per 1000 person-years over 10-years). Importantly, other than differences in vascular calcification phenotypes, age, hypertension and 10-year PCE or FRS risk estimates, we did not find important differences in sex, obesity or other CVD risk factors between ACC/AHA statin-eligible participants with versus without CAC (Supplemental Table 4), suggesting a potential importance of biomarkers of atherosclerosis in therapeutic decision making. Of note, while event rates in MESA (5.2%) and the Jackson Heart Study (4.6%) were comparably low, the potential value of CAC to improve the specificity of ACC/AHA guidelines in African Americans even in this low risk selected cohort is striking, lending support to ancillary investigations to develop markers of atherosclerotic disease burden that are widely generalizable, track in vivo phenotypes (e.g., AAC or CAC), and apply across race.
In addition, we found a heightened sensitivity of the contemporary ACC/AHA guidelines for identifying African Americans with elevated coronary or abdominal vascular calcification relative to ATP-III. Nearly 1 in 3 African Americans with subclinical CVD (by CAC or AAC) who would otherwise not be statin eligible by older guidelines were reclassified to higher risk by 2013 ACC/AHA criteria. Importantly, as expected, this increased statin eligibility in the higher risk subgroups with calcification came at the cost of increased statin recommendation for all, including individuals without calcification presumably at lower risk. Indeed, we found that African Americans without CAC who were statin eligible by ACC/AHA had a 3 per 1000 person-year 10-year CVD event rate, significantly lower than those with calcification (8.3 per 1000 person-years), and that presence of CAC was associated with a near 2-fold hazard of CVD beyond being identified for statin therapy. These results extend findings presented by Pursnani and colleagues in the Framingham Heart Study7 by suggesting a PCE risk interval where detection of CAC might influence therapeutic decisions. Collectively, the findings in our work provide therapeutic context to recent work from Fox and colleagues in JHS. Fox et al. demonstrated good calibration and risk discrimination of CVD risk estimates in African Americans using PCE, not significantly improved with biomarker or ancillary imaging22. Several key differences between the work from Fox et al. and ours merit mention. We were concerned with implications of guideline application on therapeutic decisions, and its relationship to directly quantifiable measures of atherosclerosis burden (not on risk calibration or discrimination). We limited our analyses therefore to a smaller subgroup of JHS with available coronary or abdominal imaging. In addition, we did not include heart failure (given lack of evidence of effects of statin therapy on heart failure progression), focusing instead on hard cardiovascular events (stroke, MI, and CHD death). Nevertheless, our findings suggest that bedside application of ACC/AHA guidelines to direct statin therapy in African Americans captures the majority of individuals with subclinical CVD at higher risk for long-term events. Finally, the presence of CAC is associated with CVD events independent of statin eligibility, and may actually help refine the “therapeutic specificity” of ACC/AHA guidelines in a “grey-zone” PCE risk (e.g., 5–7.5%).
The strengths of our study are a large cohort of African-Americans with measurement of coronary and aortic calcification, detailed cardiovascular phenotyping, long follow-up and careful event adjudication. Nevertheless, the results of this study should be viewed in the context of the cohort design. While our event rates appeared low overall, they were consistent with prior work from several other multi-racial epidemiologic studies with long-term follow-up7,8. Furthermore, an inherent limitation of the JHS study design to address this question is that CT imaging was performed only in a (healthier) subgroup introducing the possibility of referral bias and the timing of CT imaging was separated from determination of statin eligibility. Nevertheless, we chose to use a “zero” versus “non-zero” CAC threshold, noting that individuals without CAC at visit 2 would necessarily be without CAC at visit 1. We recognize that this may exclude some individuals with interval development of calcification (itself a higher-risk scenario) or may focus on individuals who do not develop CAC over time (itself a lower-risk scenario). While we chose to evaluate statin eligibility at visit 1 to maximize follow-up time and power for CVD events, in sensitivity analyses using visit 2 statin eligibility, we observed directionally consistent associations. Although the selected population within JHS was necessarily at lower risk (by study design), we had sufficient power to observe similar associations as reported in previous work in Caucasian Americans7. We excluded individuals with statin use at study baseline to target the population in whom statin therapy should be considered based on atherosclerotic disease risk. While therapeutic use is carefully classified in JHS (only 7% with incomplete recording of medications), the prevalence of statin therapy at baseline was low (11.3%) raising concern regarding possible confounding by participants who should have been prescribed statins (but were not). However, the prevalence is similar to the 13% statin use observed in a large African-American cohort of the Southern Community Cohort study25, suggesting our estimates may resemble those of the general African-American population prior to the release of 2013 guideline recommendations. Further study in large, real-world African American populations at higher background risk is warranted. Finally, we focused on imaging-based measures of atherosclerosis in this study; examination of more accessible biomarkers of subclinical CVD (e.g., high-sensitivity troponin) may provide more widely generalizable methods for risk stratification at reduced cost.
In conclusion, in a large cohort of African Americans, we found that that clinical application of the ACC/AHA guidelines to determine statin eligibility identifies significantly more African Americans with prognostic indices of subclinical CVD, at the price of greater statin prescriptions to individuals without coronary calcification. Subclinical atherosclerosis remains associated with CVD events in statin eligible African Americans, but is not associated with altered CVD prognosis in the statin ineligible, suggesting that imaging may reclaim some specificity of broader, modern guidelines. Finally, presence of CAC increases across PCE-determined risk, suggesting that bedside application of PCE risk estimates may effectively identify African Americans with CAC for patient-centered, therapeutic decision-making. Despite debate over potential cost23 and metabolic health26 implications of increasing statin use, these results support guideline-based approach to statin recommendation, leveraging targeted imaging (or other surrogate atherosclerotic measures) in African Americans to further personalize statin-based prevention programs.
Supplementary Material
Acknowledgments
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Dr. Shah is funded by grants from the National Institutes of Health (K23HL127099) and the American Heart Association (16SFRN31740000). The authors thank the participants and data collection staff of the Jackson Heart Study for their tireless efforts in prevention of cardiovascular disease in African Americans.
Footnotes
Conflict of Interest: No authors have any relevant disclosures.
Disclosures
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
Aferdita Spahillari had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. Jama. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 7.Pursnani A, Massaro JM, D’Agostino RB, Sr, O’Donnell CJ, Hoffmann U. Guideline-Based Statin Eligibility, Coronary Artery Calcification, and Cardiovascular Events. JAMA. 2015;314(2):134–141. doi: 10.1001/jama.2015.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015;66(15):1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111(10):1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 10.Sung JH, Yeboah J, Lee JE, et al. Diagnostic Value of Coronary Artery Calcium Score for Cardiovascular Disease in African Americans: The Jackson Heart Study. Br J Med Med Res. 2016;11(2) doi: 10.9734/BJMMR/2016/21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-4–17. [PubMed] [Google Scholar]
- 12.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 Suppl 6):S6-18–29. [PubMed] [Google Scholar]
- 13.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 17.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Keku E, Rosamond W, Taylor HA, Jr, et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. 2005;15(4 Suppl 6):S6-62–70. [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 22.Fox ER, Samdarshi TE, Musani SK, et al. Development and validation of risk prediction models for cardiovascular events in black adults: The jackson heart study cohort. JAMA Cardiology. 2016;1(1):15–25. doi: 10.1001/jamacardio.2015.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. Jama. 2015;314(2):142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipworth L, Fazio S, Kabagambe EK, et al. A prospective study of statin use and mortality among 67,385 blacks and whites in the Southeastern United States. Clin Epidemiol. 2014;6:15–25. doi: 10.2147/CLEP.S53492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. Jama. 2011;305(24):2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


