Introduction
Cardiorespiratory fitness refers to the ability of the body to transport and use oxygen, and greatly affects activities of daily living (ADLs), independence, quality of life, and mortality in older adults1, 2. It is well established that cardiorespiratory fitness, traditionally measured as peak or maximal oxygen consumption (VO2peak or max), declines at an accelerated rate of 15%–22% per decade after 50 years of age in sedentary individuals3. However, little is known about the status of cardiorespiratory fitness and its effect on health outcomes in the 25 million people with Alzheimer’s disease (AD) and other dementias worldwide4. One cross-sectional study defined Clinical Dementia Rating score 0.5 (very mild dementia) and 1 (mild dementia) as early AD and showed that people with early AD had a VO2peak of 19.8 mL/kg/min which is comparable to 21.2 mL/kg/min in their age peers without early AD (p = 0.26)5. A case studies analysis found that VO2peak ranged from 18.7 to 26.8 mL/kg/min in four men with moderate to severe AD6. However, older adults with early AD showed significant reductions in physical function and habitual physical activity levels, as compared to those without early AD5.
Aerobic exercise training increases VO2peak by 11%–27% in older adults without AD including the oldest old7, 8. However, its effect on cardiorespiratory fitness in older adults with AD is less clear. A meta-analysis of experimental studies showed that aerobic exercise training improved cardiorespiratory fitness in older adults 55 years old and older who had cognitive impairment; however, the etiologies of cognitive impairment in the original studies vary greatly from psychiatric conditions to dementias9. A later meta-analysis of randomized controlled trials (RCTs) of physical activity in older adults with AD and dementia found only four studies and showed that exercise had no effect on cognition, ADLs, and psychological and behavioral symptoms of dementia10. On the other hand, aerobic exercise training is increasingly suggested as a therapeutic intervention for AD because of its potential effect on cognition11, 12. Though still controversial, findings animal studies indicate that aerobic exercise training might positively affect cerebral structure and function and mitigate AD neuropathology by improving neuronal survivability and function, up-regulating growth factors important for neural survival and function, increasing cerebral vascularization and neuroendocrine response to stress, decreasing neuroinflammation, and reducing AD neuropathologic β–amyloid load13–15. Human studies further linked lower cardiorespiratory fitness to more age-related loss of gray matter16 and cognitive decline17 in nondemented individuals. Epidemiological studies associated increased physical activity and exercise with reduced AD risk and delayed AD onset18–20. Additionally, five meta-analyses of RCTs in adults without AD reported modest21–24 to moderate gains11 in cognition from aerobic exercise training. Cognitive improvement was also observed in older adults with mild cognitive impairment who participated in aerobic exercise training25.
The evidence above collectively suggests that aerobic exercise training could probably benefit older adults with AD via both the physiologic cardiorespiratory fitness and the neuropathologic cognitive pathways. Hence, the purpose of this paper was to develop a conceptual model to guide future aerobic exercise research and practice by synthesizing the current state of the science on aerobic exercise training in older adults with AD.
Methods of Literature Search and Review
Existing studies were searched using three steps. First, terms, e.g., AD, dementias, cognitive impairment, aerobic exercise, were mapped to the database-specific subject headings that were then used to separately search each database: Ovid MEDLINE(R) 1950 to December 2010, PsycINFO 1806 to December 2010, and EBSCO CINAHL to December 2010. Second, the abstracts of all generated articles were reviewed to select those that met the eligibility criteria. The inclusion criteria were: 1) experimental or quasi-experimental design; 2) inclusion of ≥ 2 weeks of any intensity of aerobic exercise as the sole intervention or part of a comprehensive exercise program; 3) study sample with a diagnosis of AD; and 4) publication in English. The exclusion criteria included: 1) doctoral dissertation; 2) studies of < 5 older adults; and 3) the study variable was examined in only one study. Last, the references of articles obtained from step 2 were cross-referenced and included if they met the eligibility criteria. Of the 391 articles generated, 12 articles met the eligibility criteria and were included in this review (see Table 1).
Table 1.
Summary of Aerobic Exercise Training Studies in Older Adults with AD (n = 12)
| Author & Year |
Design | Sample | Intervention | Main Findings |
|---|---|---|---|---|
| Arkin, 2001 | Two parallel groups | 11 community-dwelling subjects with AD (aged 59–86; MMSE 15–29): 7 subjects in the experimental group; 3 subjects in the control group | 10 weeks of exercise per semester: warm-up walk and stretches, a goal of 20 minutes aerobics divided on a treadmill and a stationary bicycle, two sets of 10–12 repetitions on five different weight training machines, twice weekly (one led by students and one led by family members), 20 minutes of brisk walk weekly, 10 times of biographical memory training, language stimulation activities during student-led exercises for 1 year. | The experimental group significantly outperformed the control group on MMSE, GDS, project-specific biographical memory test, on the ratio of topic comments to total utterances, and on the ratio of different nouns to total nouns. The two groups performed equally and showed no change from pre- to post-test on WMS-R Logical Memory I, WAIS-R Comprehension, Similarities, CERAD Boston Naming Test and Verbal Fluency Test, ABCD language battery, the ratio of vague nouns to total nouns. Exercisers significantly increased the duration of aerobic session as measured by a paired t-test: t(13) = 6.516, p < .01 and the distance walked in the six minute walk test: t(13) = 2.79, p < .01 at the end of one year. |
| Arkin, 2003 | One group longitudinal design | 24 community-dwelling subjects with AD (aged 54 to 88; MMSE 15–29). | Same as Arkin’s 2001 except program duration was 1–4 years. | Significant increase in duration of aerobic exercise per session, distance walked in the 6 minute walk test, and amount of weights lifted in pounds using chest press and leg press from baseline to best performance. |
| Kemoun, 2010 | RCT | 38 nursing home residents with AD (aged 81.8 ± 5.3; MMSE < 23). | 15 weeks of physical activity based on walking, equilibrium, and stamina: 2 weeks of preparation activities & 13 weeks of physical activity, 3 sessions weekly. Each session had 10 minutes of warm-up and cool down respectively plus 40 minutes of exercise: 1 session on motor route walking, 1 session of moderate intensity ergocycle with arms and legs at 60–70% of cardiac reserve (unable to measure efforts); and 1 session of dance and stepping. Control: usual care. | Exercisers improved global cognition measured by the French ERFC from 26.81 to 30.38 while control subjects dropped from 28.33 to 23.23 (p < 0.01), walking speed (meters/second) from 0.74 to 1.02 while control subjects dropped from 0.91 to 0.75 (, and stride length (meters) from 0.93 to 1.02 while control subjects dropped from 1.00 to 0.86 (p < 0.01), and decreased double limb support time from 0.16 to 0.11 while control subjects increased from 0.13 to 0.14 (p < 0.01). Cognition was correlated to walking speed (p < 0.01). Changes in the ERFC were significantly correlated to walking speed (ρ = 0.76, p < 0.01), stride length (ρ = 0.61, p < 0.01), and double limb support (ρ = −0.74, p < 0.01). Dropout rate was 19.4% for both groups. Participation in the physical activity averaged 40.6 ± 9.0 of 45 sessions. |
| McCurry, 2010 | Two parallel groups | 66 dyads of community-dwelling subjects with AD (aged 59–94 years; MMSE 0–30) and family caregivers (aged 43–93 years). | Walking group: caregiver-supervised daily walking to gradually increase to a duration goal of 30 minutes a day for 8 weeks (n = 33). Combined group: individualized behavioral sleep program and light box in addition to walking. | At 1 week, 8 weeks and 6 months, 85%, 76%, and 63% of participants respectively walked ≥ 1 day per week. The number of days walked and the minutes walked per day declined from 4.6 days/week and 15.1 minutes/day at week 1 to 3.8 days/week and 15.8 minutes/day at 8 week to 3.3 days/week and 13.6 minutes/day at 6 months, which is mainly due to the increasing number of participants who stopped walking altogether. Participants who walked ≥ 1 day per week slightly increased their walking duration from 17.7 minutes at week 1, to 20.8 minutes a week 8 and 21.5 minutes at 6 months, while their days per week walked increased from 1.5 to 2.5 and 2.7 days /week actually. Less depression and cognitive impairment and having spouse caregiver at baseline was related to better exercise adherence. |
| Palleschi, 1996 | Pre post test design | 15 men with AD (aged 74.0 ± 1.50, MMSE 18–21). | After 3-week drug washout: 30 minutes of cycling with 20 minutes at 70% of maximal pulse frequency (monitored by ECG), 3 times a week for 3 months. | Subjects increased from 35.9 to 43.9 on test of attentional matrix, from 2.9 to 3.8 on verbal span test, and from 7.4 to 12.6 on supraverbal span test, and from 19.4 to 21.7 on the MMSE. The p values for all tests were significant at < 0.0001. |
| Rolland, 2000 | Pre- post-test design | 35 hospitalized subjects with AD (aged 71–92; MMSE 1–25). | 5–12 weeks of walking (for 20 subjects) or cycling advised over the phone with main daily duration of 35 minutes (10–80 minutes) (intensity not described). | Of the 35 pre-selected subjects, 23 exercise (20 walking, 1 running, and 2 cycling). ADL (4.9 to 4.9) and instrumental ADL limitations (1.7 to 2) did not change. Subjects showed improved nutrition (the Mini-Nutritional Assessment score increased from 22.3 to 24.5, p < 0.001), global cognition (MMSE score increased from 16.3 to 19.8, p < 0.001), walking speeds (Dynamic Tinetti test score increased from 7.2 to 8.1 and Static Tinetti test score from 11.2 to 11.5, p < 0.01) and decreased behavioral symptoms (NPI score decreased from 43 to 35.7, p < 0.05). Age, gender, and anti-cholinersterase medictions, initial MMSE score, and exercise intensity did not affect those results. Exercise did not increase caregiver burden (total Zarit scale remained unchanged). |
| Rolland, 2007 | RCT | 134 nursing homes residents with AD (aged 82.8 ± 7.8; mean MMSE 8.8 ± 6.6) | 12 months of individualized, group exercises (2–7 subjects per group), 1 hour a session, twice weekly, including aerobics (walking for at least 30 minutes, moderate intensity), low extremity strength, balance, and flexibility training. Routine medical care for the control group. | Exercisers showed slower decline in Katz ADL (p = .02) at 12 months and 6-meter walking speed at 6 months (p = .01) and 12 months (p = .002) than nonexercisers. No differences were observed for behavioral symptoms measured by the NPI, get up and go test, one leg balance test, depression measured by the MADRS, or nutrition measured by the MNA. The dropout rate was 17.9%. Reasons for dropouts were financial concern, dissatisfaction of the patient’s relatives, relocation of relatives, and no apparent reasons. Adherence ranged from 10.4% to 41.8% with a mean of 33.2%. |
| Steinberg, 2009 | RCT | 27 home-dwelling subjects with AD (aged 74.0 ± 8.1; MMSE 15.5 ± 5.4 for the control group, MMSE 20.1 ± 5.1 for the exercise group) | Caregivers delivered daily aerobics, strength, balance and flexibility for 12 weeks. Exercise duration and intensity was not described. The control group received home safety assessment. | Of the 59% of the exercise diaries received, exercisers achieved 79% (3.6 times a week), 74% (2.9 times a week), and 72% (2.7 times a week) of aerobic, strength, and balance goals, respectively. Exercisers showed better hand function as measured by the JTT (p = .04); however, no differences were found between the two groups on ADQRL, BNT, HVLT, NPI, and SCB. |
| Tappen, 2001 | Repeated measures three-group design | 71 nursing homes residents with AD (aged 70–105 years, MMSE 0–23) | 16 weeks of 30-minute treatment. Group 1 (n = 23): self-paced assisted walking, 3 times a week. Group 2: conversation (n = 22). Group 3: walking plus conversation (n = 20). | The walking, conversation, and the combined group received 57%, 90%, and 75% of the intended treatment. The walking group declined 20.9% in the distance walked in the 6-minute walk, while the conversation group declined 18.8% and the combined treatment group declined 2.5%. Controlling for treatment fidelity and pretest 6-minutes walk scores, the walking and the combined treatment group walked farther than the conversation group (284.8 vs. 322.6 vs. 238.5 feet, p = 0.01). The difference between the walking and combined treatment groups was not significant. The dropout rate was 8.5%. |
| Teri, 2003 | RCT | 153 community-dwelling subjects with mild to moderate AD (aged 55–93 years, mean MMSE 16.8) | Intervention: 12-hour session over 11 weeks plus phone follow-up to teach caregivers to implement exercise with a goal of ≥ 30-min aerobics, strength, balance, and flexibility daily over 2 years and learn behavioral management. Control: routine care. | At 3 months, more intervention subjects exercised > 60 min/week (p = 0.01). Intervention subjects had better physical function as measured by the SF-36 at 3 months (p < 0.001) and 24 months (p < .01), less depression as measured by the CSDD (p = .02) and less restricted activity (p < .001) at 3 months, and better physical function (p < .01) and mobility as measured by the SIP (p = .02) at 24 months, as compared to control subjects. The dropout rate was 8%. 91% of the intervention subjects attempted some to all their exercise homework (79% completed >75% of assigned homework). |
| Williams, 2007 | 3 randomized group, pre- post-test design | 90 nursing home subjects with AD (aged 71–101; MMSE 0–28). | 16 weeks of comprehensive exercise (progressed to 10-min strength, balance, flexibility and 20-min walking) vs. supervised walking progressed to 30 minutes vs. attention control (social conversation), 5 days a week. Walking was individualized, but appeared low in intensity. Treatment intensity was measured by the total recorded minutes of treatment actually provided: an average of 1484 minutes for the control group, 735 minutes for the comprehensive exercise group, and 807 minutes for the walking group. | Controlled for baseline mood scores, baseline MMSE, distance walked in 6 minutes, and treatment intensity, the comprehensive exercise group had a considerably better scores posttest on the 10-minute OAS negative (P = .0508), AMS negative subscale (p = .014), and DMAS (p = .022) than the other two groups. The walking group had similar scores on OAS and DMAS as the control group except that its score is similar to the comprehensive exercise group on AMS (p = .038). The dropout rate was 33.3%. |
| Williams, 2008 | 3-group, repeated-measures, randomized quasi-experimental design | 45 nursing home subjects with AD (age 71–101, MMSE 0–21) | 16 weeks of comprehensive exercise (n = 16: progressed to 10-min strength, balance, flexibility and 20-min walking over 4 weeks) vs. supervised walking progressed to 30 minutes over 4 weeks (n = 17) vs. attention control (n = 12, social conversation), 5 days a week. Walking was individualized, but appeared low in intensity. Treatment intensity was measured by the total recorded minutes of treatment actually provided: an average of 1537 minutes for the control group, 403 minutes for the comprehensive exercise group, and 577 minutes for the walking group. | Depression measured by the CSDD was reduced in all three groups from (p = 0.0001). As a whole, depression was significantly reduced because only 65% of the subjects were depressed (CSDD scores above 7) compared to 100% at baseline, and mood as measured by the DMAS and affect as measured by the OAS and AMS improved except for OAS Negative Affect from pre- to post-test. There were significant differences in OAS Subjects in the comprehensive exercise group had more improvement in OAS 2-Week Positive Affect subscale compared to the walking group (p = 0.0314) and control group (p = 0.0451). Subjects in both the comprehensive exercise (p = 0.0518) and the walking (p = 0.0161) groups had better and similar mood as measured by the AMS Negative Mood subscale. The dropout rate was 20%. |
Notes: ABCD: the Arizona Battery for Communication Disorders of Dementia; ADL: activities of daily living; AMS: Alzheimer’s Mood Scale; ADQRL: the Alzheimer’s Disease Quality Related Life Scale; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; BNT: Boston Naming Test; CSDD: the Cornell Scale for Depression in Dementia; DMAS: Dementia Mood Assessment Scale ERFC: Rapid Evaluation of Cognitive Function; HDRS: Hamilton Depression Rating Scale; HVLT: Hopkins Verbal Learning Test; JTT: Jebsen Total Time; MADRS: Montgomery-Asberg Depression Rating Scale; MMSE: Mini-Mental State Examination; NPI: Neuropsychiatric Investory; OAS: Observed Affect Scale; RCT: randomized controlled trial; SCB: Screen for Caregiver Burden; SF-36: 36-item Short-Form Health Survey; SIP: Sickness Impact Profile; WAIS-R: Wechsler Adult Intelligence Scale – Revised; WMS-R: Wechsler Memory Scale.
The study variables used by the 12 studies were first abstracted. Similar variables were then clustered together. A construct that represent each cluster of variables was then selected based on the exercise and dementia literature. The relationships among the constructs were graphed based on the current state of the science.
Findings from the Literature Synthesis
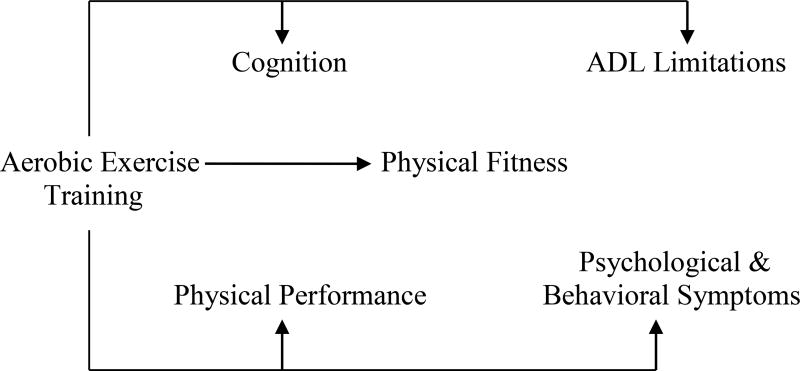
Except for two RCTs that had sufficient power to detect outcome differences26, 27, the remaining 10 studies had small sample sizes. Six key constructs emerged from this literature review, including aerobic exercise training, physical fitness, physical performance, cognition, ADL limitations, and psychological and behavioral symptoms. Figure 1 depicts the Functional Impact of Aerobic Exercise Training in AD (FIT-AD) model that describes the relationships among the six constructs based on literature synthesis described below.
Figure 1.
The Functional Impact of Aerobic Exercise Training in Alzheimer’s Disease (FIT-AD) Model. Aerobic exercise training is defined as repetitive and rhythmic movement of large muscle groups to improve the efficiency of energy producing systems that use oxygen; physical fitness is defined as an attained set of attributes including cardiorespiratory fitness, flexibility, agility, reaction time, body composition, and skeletal muscle strength, endurance, and power; physical performance is defined as physical functional abilities for a person to perform discrete tasks, e.g., walking, climbing stairs; cognition is defined as the information processing of one’s psychological function, including global cognition, executive function, memory, language, visuo-spatial function, and attention; ADL limitations is defined as the abilities to complete social, instrumental, and basic ADL; psychological and behavioral symptoms are defined as symptoms of disturbed perception, thought contents, mood, affect, and behaviors that frequently occurring in older adults with AD.
Aerobic Exercise Training
Definition
Aerobic exercise training is defined as repetitive and rhythmic movement of large muscle groups to improve the efficiency of energy producing systems that use oxygen28.
Literature review
Older adults with AD and family caregivers preferred aerobics and found resistance, balance, and flexibility exercises less appealing when they were advised to do different exercises at home in a feasibility study29. Of the 12 eligible studies, only one study of 15 men with AD used aerobic exercise training as the sole intervention and adequately monitored the intensity of aerobic exercise training as 70% of maximal heart rate for 20 minutes a session using electrocardiogram30. Sixteen weeks of 30-minute walking were used as one arm of the three comparison groups in three other studies31–33. Those walking programs were individualized, but appeared to be very low intensity. The seven other studies used aerobic exercise training as a component of a comprehensive exercise program that includes aerobics plus: 1) memory and verbal stimulation activities34, 35, 2) equilibrium and stepping36, 3) strength, flexibility, and balance26, 32, 37, or 4) strength, flexibility, balance, and education for managing dementia symptoms27. Among the 12 studies, the prescribed doses of aerobic exercise training vary greatly in terms of frequency: 136, 226, 34, 35, 330, 33, or 7 times per week29, 37, 38,29; session duration: 20–30 minutes30, 33–35, 39, 30– 40 minutes29, 29, 36, 1 hour26, or unreported37; intensity: unclear26, 29, 33, 37, 38, low to moderate intensity26, 34, 35, or moderate intensity30, 36; and program duration: 5–12 weeks29, 30, 37, 38, 15–16 weeks31–33, 36, 1 year26, 35, or four years34.
Older adults with AD increased the duration of aerobic exercise from 12.1 minutes at baseline to 29.0 minutes after four years34 and ten of the 11 participants met or exceeded the aerobic goal of 20 minutes per session at the end of one year with four doing 30 minutes or more35. When caregivers were taught o engage older adults with AD in a comprehensive exercise program, older adults with AD exercised > 60 minutes/week by three months (p = 0.01)27. Sixty-three percent of the 24 older adults with AD achieved 20 minutes or more of aerobic exercise per session after one semester, 21% after two semesters, and 8% after three semesters34.
The exercise dropout rates were 8%27 and 41%37 for community-dwelling samples, and 8.5%33, 20%32, 36, or 33.2%26, 31 for nursing home samples. The exercise compliance rates among community-dwelling older adults with AD ranged from 59% to 80% for caregiver-supervised sessions27, 34, 37 to 100% for student-supervised sessions34. In contrast, the compliance rates in nursing home residents with AD were 33.2% (10.4% to 41.8%)26, 57% – 75%33, or 90.2%36,37. Other studies did not report the compliance rate. For example, two studies prescribed 30 minutes of walking, 5 days a week for 16 weeks, meaning a total 2400 minutes of walking; however, the delivered walking duration averaged 807 minutes (33.6% of the 2400 minutes)31 and 403 minutes (16.8% of the 2400 minutes)32. Another study prescribed daily exercise assisted by caregivers, but older adults with AD completed an average 3.6 times a week of aerobics (51.4% of prescribed frequency)37. Factors affecting exercise adherence included lack of caregivers to accompany older adults, hospitalization, fall, aggressive behavioral, and refusal of family members29, acute disease, disagreement or unwillingness to continue, behavior disorders, increased disability in ADLs, and others26.
Summary
There is a wide variation in the dose (intensity, duration, and frequency) of aerobic exercise training across studies. Five of the 12 studies used aerobic exercise training alone or as one arm of the comparison groups. It appears that older adults with AD can progress and reach moderate intensity aerobic exercise training over time. Only one study objectively recorded the actually delivered exercise intensity using electrocardiogram. The dropout rates range from 8% to 41%, while the compliance rates vary from 10.4% to 90.2%.
Physical Fitness
Definition
Physical fitness is defined as an attained set of attributes including cardiorespiratory fitness, flexibility, agility, reaction time, body composition, and skeletal muscle strength, endurance, and power28.
Literature review
Despite the use of aerobic exercise training, none of the available studies measured cardiorespiratory fitness using VO2peak. The 6-minute walk test was the field test of choice which can give some indications of cardiorespiratory capacity. Older adults with AD who received 20 – 30 minutes of aerobics twice a week and 20 minutes of brisk walk weekly significantly increased the distance walked from 1073 feet at baseline to 1346 feet at their best performance in six minutes34. Controlling for treatment fidelity and pretest 6-minute walk distances, nursing home residents who walked without or with conversations walked farther in the 6-minute walk test than the control group (284.8 vs. 322.6 vs. 238.5 feet, p = 0.01). The difference between those who walked without conversation and those with conversation was not significant33. Comprehensive exercise was found to improve muscle strength, showing that older adults with AD significantly improved the amount of weight they could lift from 48.9 pounds at baseline to 65.0 pounds in chest press and from 268.4 to 355.3 pounds in leg press over 1–4 years34.
Summary
Older adults with AD improved the distance they walked in the 6-minute walk test from aerobic exercise training or comprehensive exercise that includes aerobics. Although the VO2peak is the gold-standard cardiorespiratory fitness measure, it was not used in any studies. Improved muscle strength was observed in older adults with AD who received comprehensive exercise.
Physical Performance
Definition
Physical performance is defined as physical functional abilities for a person to perform discrete tasks, e.g., gait speed, climbing stairs40.
Literature review
Twenty-three older adults with AD improved their walking speeds (Dynamic Tinetti test score increased from 7.2 at baseline to 8.1 at posttest and Static Tinetti test score from 11.2 at baseline to 11.5 at posttest, p < 0.01) from participating in 5–12 weeks of walking, running, or cycling daily for 35 minutes. Age, gender, anti-cholinesterase medications, baseline cognition, and exercise intensity did not affect those results29. Similar improvement was observed in a RCT of 38 older adults with AD who improved their walking speed (meters/second) from 0.74 to 1.02, stride length (meters) from 0.93 to 1.02, and decreased double limb support time from 0.16 to 0.11 seconds after participating in 15 weeks of comprehensive exercise, while control individuals declined in all three measures (p < 0.01)36. Additionally, older adults with AD who received caregiver-administered comprehensive exercise for 12 weeks at home improved their hand function as measured by the Jebsen Total Time, compared to those who did not exercise37. A RCT further reported that community-dwelling older adults with AD whose caregivers learned to implement comprehensive exercise and manage behavioral symptoms had better physical function as measured by the 36-item Short Form Health Survey at 3 months (p < 0.001) and 24 months (p < .01), less restricted activity (p < .001) at 3 months, and better physical function (p < .01) and mobility as measured by the Sickness Impact Profile (p = .02) at 24 months, as compared to control individuals27. In contrast, another RCT of 134 nursing home residents with AD did not find any differences in the get up and go test and the one leg balance test between exercisers and nonexercisers after one year of individualized, group exercise26,
Summary
Older adults with AD improved physical performance in four of the five studies which used comprehensive exercise that included aerobics, strength, and balance. Although all those measures fall into the physical performance construct, none of the studies evaluated the same operationalized variable.
Cognition
Definition
Cognition is defined as the information processing of one’s psychological function, including global cognition, executive function, memory, language, visuo-spatial function, and attention41.
Literature review
Older adults with AD improved global cognition measured by the Mini-Mental State Examination (MMSE) from 16.3 at baseline to 19.8 post-training (p < 0.001) from participating in 5–12 weeks of aerobic exercise, controlling for age, gender, anti-cholinersterase medictions, initial MMSE score, and exercise intensity (n = 35)29. Three months of cycling increased the scores on the test of attentional matrix from 35.9 to 43, the verbal span test from 2.9 to 3.8, the supraverbal span test from 7.4 to 12.6, and the MMSE from 19.4 to 21.7 (all p < 0.001) from pre- to post-test in 15 men with AD30. A RCT (n = 38) further showed that nursing home residents with AD improved their global cognition that was measured by the French Rapid Evaluation of Cognitive Function from 26.8 to 30.4 (p < 0.01) while the control group dropped from 28.3 to 23.2 (p < 0.01)36. A significant correlation between changes in global cognition and walking speed was reported from aerobic exercise training36.
Although the seven exercisers also outperformed the four control individuals on global cognition, all 11 older adults performed equally and showed no change from pre- to post-test on other cognitive tests such as Wechsler Memory Scale -Revised Logical Memory I, Boston Naming Test, and Verbal Fluency Test35. No differences in the Boston Naming Test and Hopkins Verbal Learning Test scores were found between exercisers whose caregivers provided comprehensive exercises daily for 12 weeks at home and nonexercisers (n = 27)37.
Summary
There is emerging evidence that older adults with AD showed improved cognition from aerobic exercise training alone and comprehensive exercise. One study suggested that the change in cognition was associated with the change in physical performance. Despite the small sample sizes in those studies, they often measured multiple outcome variables within a study, which makes it impossible to detect a significant effect even if there is one.
ADL Limitations
Definition
ADL limitations are defined as the abilities to complete social, instrumental, and basic ADL41.
Review of the literature
Twenty-three individuals with AD were able to maintain their levels of basic ADLs (4.9 at pre-test and 4.9 at post-test on the ADL scale) and instrumental ADL levels (1.7 at pre-test and 2 at post-test on the IADL scale) from participating in 5–12 weeks of aerobic exercise29. A year of comprehensive exercise reduced ADL decline by 6.7% in older adults with AD compared to those in usual care and exploratory analysis indicated that exercise adherence appeared to moderate the change in ADLs26.
Summary
Emerging evidence suggested that comprehensive exercise reduces ADL limitations in older adults with AD. However, the doses of exercise for reducing decline in ADLs are drastically different between the two studies.
Psychological and Behavioral Symptoms
Definition
Psychological and behavioral symptoms are defined as symptoms of disturbed perception, thought contents, mood, affect, and behaviors that frequently occurring in older adults with AD41.
Literature review
The scores on the Neuropsychiatric Inventory decreased from 43 at baseline to 35.7 at post-test (p < 0.05) in 23 older adults who participated in 5–12 weeks of aerobic exercise, while age, gender, anti-cholinesterase medications, initial MMSE score, and exercise intensity did not affect this decrease29. Williams and colleagues (2007) randomly assigned 90 nursing home residents with AD to three groups (16 weeks of comprehensive exercise, walking, or attention control) and reported that controlling for baseline mood scores, MMSE, distance walked in 6 minutes, and treatment intensity, the comprehensive exercise group had a considerably better scores posttest on the 10-minute Observed Affect Scale negative (P = .051), Alzheimer’s Mood Scale negative subscale (p = .014), and Dementia Mood Assessment Scale (p = .022) than the other two groups31. Using the same interventions, the authors found later (n = 45) that nursing home residents with AD and depression who participated in the comprehensive exercise improved their positive affect as measured by the Observed Affect 2-Week Positive Affect subscale compared to those in the walking group (p = 0.031) and control group (p = 0.045)32. Caregivers education about comprehensive exercise and behavioral symptom management reduced depression as measured by the Cornell Scale of Depression in Dementia in those who exercised, as compared to control individuals (p = .02)27.
On the other hand, two RCTs did not find comprehensive exercise affects the change in the Neuropsychiatric Inventory scores in older adults with AD. In one study, the caregivers were instructed to provide aerobic, strength, and balance exercises daily for 12 weeks37, while the other study utilized an occupational therapist for leading group exercises with nursing homes residents for one year26.
Summary
Four studies found aerobic exercise or comprehensive exercise that includes aerobics improved psychological and behavioral symptoms in older adults with AD, while two studies did not. Except for one study of older adults with AD and depression, the others did not particularly sample individuals who manifested psychological and behavioral symptoms; hence, might not be able to detect the effect, if any, of aerobic exercise on those symptoms.
Discussions and Implications for Further Research and Practice
This state-of-the-science literature review indicated the feasibility for aerobic exercise training in older adults with AD and the emerging evidence of positive impacts of aerobic exercise training on physical fitness, physical performance, cognition, ADL limitations, and psychological and behavioral symptoms, which led to the conceptualization of the FIT-AD model. Of the 12 identified studies, only two RCTs were adequately powered to detect significant differences in outcomes between exercisers and nonexercisers26, 27. In addition to the limitation imposed by the small sample sizes in the remaining 10 studies29–38, multiple outcomes were measured within a study which further undermines the study’s power to detect significant changes, if any, in outcomes and reduces the number of studies available for comparing the effect of aerobic exercise training on the same outcome across studies.
Aerobic exercise training is the core construct for exercise studies in AD because its dose (intensity, frequency, and duration) and delivery (dropout, compliance, actually delivered dose, and/or treatment fidelity) likely affects its effect on the other five constructs. Lone aerobic exercise training was feasible in older adults with AD as shown by this review and two other aerobic exercise studies that did not meet the sample size criteria for inclusion in this review6, 30, 42. However, only one of the 12 studies clearly defined and monitored the aerobic exercise training dose30. Despite the finding that older adults with AD preferred aerobics over strength, flexibility, and balance exercises29, comprehensive exercise was often used, which limits our ability to understand and interpret the sole effect of aerobic exercise training on outcomes. Another drawback among the 12 studies is the lack of clear criteria for validating an actually delivered dose of aerobic exercise and determining the effective levels of aerobic exercise training needed for influencing physical fitness, physical performance, cognition, ADL limitations, and psychological and behavioral symptoms. It is particularly important for future studies to include indicators of exercise intensity, e.g., heart rate levels, perceived exertion levels, talking ability during exercise, and/or signs and symptoms of over-exertion43 and measure the actually delivered dose of aerobic exercise training to examine the presence/absence of a dose-response relationship. Future studies also need to address strategies for sustaining aerobic exercise participation in the context of AD, a progressive, neurodegenerative disease, determine the methods and doses for optimal aerobic exercise training prescription, and measure physiological markers that are sensitive to aerobic exercise training.
Adequately delivered aerobic exercise training should physiologically stimulate the cardiorespiratory system and generate a change in cardiovascular fitness. Existent studies showed that older adults with AD walked faster and farther in the 6-minute walk test, but it was yet unknown if a true improvement in cardiorespiratory fitness was achieved or necessary for affecting other constructs such as cognition. In contrast, we now know that older adults without cognitive impairment (including the oldest old) could increase VO2peak by 11%–27%7, 8 and decrease mortality1 from aerobic exercise training. Whether similar improvement is achievable in older adults with AD is yet to be explored. Additionally, many factors might have affected the potential for older adults with AD to improve their physical fitness from aerobic exercise training, but have not been examined, including intra-personal factors such as AD stage, medical frailty, physical fitness, medications, psychological and behavioral symptoms of dementia, premorbid personal exercise history and habits, and apolipoprotein genotype, and extra-personal factors such as caregiver willingness, availability, and perception of aerobic exercise training, living situation, access to gyms and exercise sites, and friendliness of gym environments.
Improvement in physical performance such as better walk speed, stride length, double extremity use, and hand function was observed in older adults with AD who participated in comprehensive exercise that had an aerobic exercise component36, 37. Although those variables fall under physical performance, they were only examined in one study. Future studies need to elucidate the role of aerobic exercise training on physical performance and the mediating effects of intra-personal and extra-personal covariates on physical performance.
Older adults with AD showed improved cognition from participating in aerobic exercise training, but it is unlikely that the effects of 3 months of moderate intensity cycling and 1 year of comprehensive exercise are comparable in their physiological effects and impact on cognitive function. Future studies should address what level of aerobic exercise training is needed for achieving cognitive improvement, what cognitive domains and measures are more sensitive to aerobic exercise training in older adults with AD, and if intra- and extra-personal covariates such as cognitive status and AD stage mediate the potential for improvement in cognitive function. Emerging evidence now suggests that older adults with mild cognitive impairment showed greater improvement in memory than those without cognitive impairment, indicating that those with cognitive impairment have more room to improve to some degree24.
ADL limitations are a major contributor to poor health care outcomes in older adults with AD12. This review suggested that older adults with AD could maintain their basic and instrumental ADLs and reduce the amount of decline in ADL from comprehensive exercise that includes aerobic exercise26, 29. Future research need to address if aerobic exercise training improves a particular ADL such as social interaction, meal preparation, and dressing. It is also important to examine whether intra- and extra-personal factors such as baseline cognition, physical fitness, physical performance, and medical comorbidities mediate the effect of aerobic exercise training on ADL limitations.
Comprehensive exercise that includes aerobic exercise training improves psychological and behavioral symptoms in older adults with AD in some studies, but not others. Such a discrepancy might be attributable to the baseline prevalence of those symptoms and the inadequacy of small sample sizes for detecting any significant changes in those symptoms. Future studies could address the following questions: Who are likely to benefit from aerobic exercise training for reducing psychological and behavioral symptoms? What psychological and behavioral symptoms are more amenable to aerobic exercise training?
In summary, the FIT-AD model describes six constructs that have been investigated in older adults with AD, including aerobic exercise training, physical fitness, physical function, cognition, ADL limitations, and psychological and behavioral symptoms. The preliminary relationships among the six constructs need to be further examined in older adults with AD using well-designed and delivered aerobic exercise training to advance our understanding of the role of aerobic exercise training in managing AD. The emerging evidence suggests that engaging older adults with AD in exercise might be an important practice.
Conclusion
Until AD can be prevented or cured, promoting functional capacity and symptom management in this population remains both a practice and a research priority with significant social implications. The FIT-AD model identifies six constructs and provides a preliminary framework for advancing future research and practice on aerobic exercise training in older adults with AD.
Figure 2.
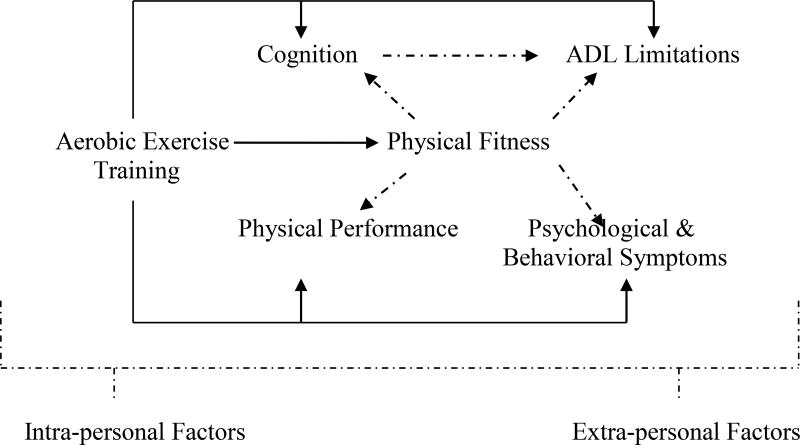
Implications for Future Research Based on the FIT-AD Model. a) Investigate the effect of aerobic exercise training on all other five constructs by exploring, quantifying, and operationalizing different components of each construct; b) Examine the dose-response relationships between aerobic exercise training dose and all five other constructs; c) Investigate the mediating/moderating effects of physical fitness on physical performance, cognition, ADL limitations, and psychological & behavioral symptoms from aerobic exercise training; d) Study the mediating/moderating effects of cognition on ADL limitations from aerobic exercise training since cognition explains 25%–50% of ADL variance; e) Evaluate the effects of individualized aerobic exercise training based on inter- and extra-personal factors on all other five constructs; and f) Identify the individuals who are most likely to benefit from aerobic exercise training based on the mediating/moderating effects of inter- and extra-personal factors on all six constructs.
Acknowledgments
This work was supported by a National Institute of Health K12 Career Advancement Award (RR023247-04).
Footnotes
Disclosure: The author has reported no conflicts of interest.
References
- 1.Vogel T, Brechat PH, Lepretre PM, Kaltenbach G, Berthel M, Lonsdorfer J. Health benefits of physical activity in older patients: A review. Int J Clin Pract. 2009;63:303–320. doi: 10.1111/j.1742-1241.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 2.Sui X, Laditka JN, Hardin JW, Blair SN. Estimated functional capacity predicts mortality in older adults. J Am Geriatr Soc. 2007;55:1940–1947. doi: 10.1111/j.1532-5415.2007.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollenberg M, Yang J, Haight TJ, Tager IB. Longitudinal changes in aerobic capacity: Implications for concepts of aging. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2006;61:851–858. doi: 10.1093/gerona/61.8.851. [DOI] [PubMed] [Google Scholar]
- 4.Qiu C, Kivipelto M, von Strauss E. Epidemiology of alzheimer's disease: Occurrence, determinants, and strategies toward intervention. Dialogues in Clinical Neuroscience. 2009;11:111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE. Cardiorespiratory fitness in early-stage alzheimer disease. Alzheimer Disease & Associated Disorders. 2008;22:39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- 6.Yu F, Leon A, Bliss D, Dysken M, Savik K, Wyman J. Case studies of progressing older men with alzheimer's disease through aerobic training. Research in Gerontological Nursing. 2011 doi: 10.3928/19404921-20110303-01. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: Implications for exercise training. Sports Medicine. 2003;33:877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 8.Malbut KE, Dinan S, Young A. Aerobic training in the 'oldest old': The effect of 24 weeks of training. Age & Ageing. 2002;31:255–260. doi: 10.1093/ageing/31.4.255. [DOI] [PubMed] [Google Scholar]
- 9.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database of Systematic Reviews. 2008:CD006489. doi: 10.1002/14651858.CD006489.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 12.Yu F, Kolanowski AM, Strumpf NE, Eslinger PJ. Improving cognition and function through exercise intervention in alzheimer's disease. Journal of Nursing Scholarship. 2006;38:358–365. doi: 10.1111/j.1547-5069.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 13.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: Learning from animal models. Alzheimer's & Dementia. 2007;3 doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Radak Z, Hart N, Sarga L, et al. Exercise plays a preventive role against alzheimer's disease. J Alzheimer's Dis. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 15.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn Sci (Regul Ed) 2007;11 doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Colcombe SJ, Erickson KI, Raz N, Webb AG, et al. Aerobic fitness reduces brain tissue loss in aging humans. The Journals of Gerontology: Series A: Bilogical Sciences and medical Sciences. 2003;58A:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 17.Barnes D, Yaffe K, Satariano W, Tager I. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 18.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 19.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 20.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and alzheimer's disease. Lancet Neurology. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 21.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2008:005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Etnier J, Salazar W, Landers D, Petruzzello S, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. Journal of Sport and Exercise Psychology. 1997;19:249–77. [Google Scholar]
- 23.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with alzheimer's disease: A 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 27.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with alzheimer disease: A randomized controlled trial. JAMA. 2003;290:2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 28.American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. Vol. 5. Baltimore, MD: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 29.Rolland Y, Rival L, Pillard F, et al. Feasibily of regular physical exercise for patients with moderate to severe alzheimer disease. Journal of Nutrition, Health and Aging. 2000;4:109–113. [PubMed] [Google Scholar]
- 30.Palleschi L, Vetta F, De Gennaro E, Idone G, Scottosanti G, Gianni W, Marigliano Effect of aerobic training on the cognitive performance of elderly patients with senile dementia of alzheimer type. Arch Gerontol Geriatr. 1996;5:47–50. doi: 10.1016/0167-4943(96)86912-3. [DOI] [PubMed] [Google Scholar]
- 31.Williams CL, Tappen RM. Effect of exercise on mood in nursing home residents with alzheimer's disease. Am j Alzheimer's Dis Other Dementias. 2007;22:389–397. doi: 10.1177/1533317507305588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams CL, Tappen RM. Exercise training for depressed older adults with alzheimer's disease. Aging & Mental Health. 2008;12:72–80. doi: 10.1080/13607860701529932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tappen RM, Roach KE, Applegate EB, Stowell P. Effect of a combined walking and conversation intervention on functional mobility of nursing home residents with alzheimer disease. Alzheimer Disease & Associated Disorders. 2000;14:196–201. doi: 10.1097/00002093-200010000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arkin SM. Student-led exercise sessions yield significant fitness gains for alzheimer's patients. American Journal of Alzheimer's Disease & Other Dementias. 2003;18:159–170. doi: 10.1177/153331750301800302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arkin SM. Alzheimer rehabilitation by students: Interventions and outcomes. Neuropsychological Rehabilitation. 2001;11:273–317. [Google Scholar]
- 36.Kemoun G, Thibaud M, Roumagne N, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dementia & Geriatric Cognitive Disorders. 2010;29:109–114. doi: 10.1159/000272435. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg M, Leoutsakos JS, Podewils LJ, Lyketsos C. Evaluation of home-based exercise program in the treatment of alzheimer's disease: The maximizing independence in dementia (MIND) study. Int J Geriatr Psychiatry. 2009;24 doi: 10.1002/gps.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCurry SM, Pike KC, Logsdon RG, Vitiello MV, Larson EB, Teri L. Predictors of short- and long-term adherence to a daily walking program in persons with alzheimer's disease. American Journal of Alzheimer's Disease and Other Dementias. 2010;25:505–512. doi: 10.1177/1533317510376173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for alzheimer's disease: A randomized, controlled trial. J Am Geriatr Soc. 2005;53:793–802. doi: 10.1111/j.1532-5415.2005.53252.x. [DOI] [PubMed] [Google Scholar]
- 40.McAuley E, Morris KS, Doerksen SE, Motl RW, Liang H, White SM, Wójcicki TR, Rosengren K. Effects of change in physical activity on physical function limitations in older women: Mediating roles of physical function performance and self-efficacy. J Am Geriatr Soc. 2007;55:1967–1973. doi: 10.1111/j.1532-5415.2007.01469.x. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Ed.) Text Revision (DSM-IV-TRTM) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 42.Yu F, Kolanowski A. Facilitating aerobic exercise training in older adults with alzheimer's disease. Geriatr Nurs. 2009;30:250–259. doi: 10.1016/j.gerinurse.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Yu F, Bil K. Correlating heart rate and perceived exertion during aerobic exercise in alzheimer's disease. Nurs Health Sci. 2010;12:375–380. doi: 10.1111/j.1442-2018.2010.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]