Abstract
Background
A database analysis was conducted to assess the effectiveness of sucroferric oxyhydroxide (SO) on lowering serum phosphorus and phosphate binder (PB) pill burden among adult peritoneal dialysis (PD) patients prescribed SO as part of routine care.
Methods
Adult PD patients (n=258) prescribed SO through a renal pharmacy service were analyzed. Baseline was 3 months before SO prescription. SO-treated follow-up was 6 months or until either a new PB was prescribed, SO was not refilled PD modality changed or patient was discharged. In-range serum phosphorus was defined as ≤5.5 mg/dL.
Results
At baseline, mean serum phosphorus was 6.59 mg/dL with 10 prescribed PB pills/day. The proportion of patients achieving in-range serum phosphorus increased by 72% from baseline to month 6. Prescribed PB pills/day decreased by 57% (10 at baseline to 4.3 at SO follow-up, p<0.0001)). Mean length of SO follow-up was 5.1 months; SO follow-up ended for 38, 27, and 50 patients at months 4, 5, and 6 due to no further PB fills, and for 10, 11, and 4 patients at months 4, 5, and 6 due to another PB prescribed. In patients with baseline serum phosphorus >5.5 mg/dL who achieved in-range serum phosphorus during SO follow-up for ≥1 quarter, a notable improvement in serum phosphorus (6.54 to 5.10 mg/dL, p<0.0001) was observed, and a 53% reduction in PB pill burden (9.9 to 4.7, p<0.0001).
Conclusion
Among PD patients prescribed SO as part of routine care, improvements in serum phosphorus control and >50% reduction in PB pills/day were observed.
Keywords: Chronic kidney disease-mineral bone disorders, Phosphate binders, Peritoneal Dialysis, Phosphorus
Background
Peritoneal dialysis (PD) represents a safe and cost-effective treatment modality for patients with end-stage renal disease (ESRD) [1,2,3,4]. In the United States, by the end of 2014, 6.9% of prevalent ESRD cases were treated with PD [5]. Disorders of phosphorus metabolism among ESRD patients are common, with serum phosphorus levels in PD patients generally lower than in hemodialysis (HD) patients [6,7,8], though treatment with oral phosphate binders are usually indicated [9]. Favorable properties of phosphate binders would include low pill burden, low cost, minimal systemic absorption, and minimal side effects [6,10]. Potential risks include toxicity, gastrointestinal side effects, and contribution to hypercalcemia [11,12,13]. Adherence to phosphate binder therapy is essential for phosphorus control and low pill burden may be a key factor in adherence [14,15,16]. PD patients are prescribed, on average, 16–19 pills/day [17,18] and half are phosphate binders [18].
Sucroferric oxyhydroxide (SO) is an iron-based, chewable phosphate binder with a low pill burden indicated for treatment of hyperphosphatemia in patients with chronic kidney disease on dialysis. Coyne et al. reported significantly decreased pill burden and improved serum phosphorus levels among HD patients prescribed SO as part of routine clinical care [19]. Floege et al. reported results from a phase 3 randomized controlled trial and found SO to be non-inferior to sevelamer carbonate for the control of serum phosphorus in PD patients, with a lower pill burden [20]. The objective of this retrospective database analysis was to assess the effectiveness of SO on lowering serum phosphorus levels and phosphate binder pill burden among adult PD patients prescribed SO as part of routine clinical care.
Methods
Sucroferric oxyhydroxide (SO; Velphoro®, Fresenius Medical Care Renal Therapies Group, Waltham, MA, USA) is usually prescribed with a starting dose of 3 pills/day, administered as 1 tablet with each meal and titrated in increments or decrements of 1 tablet per day.
Adult (age > 18 years) peritoneal dialysis patients who were prescribed SO as part of routine clinical care at Fresenius Kidney Care (FKC) facilities, and received their first SO prescription through FreseniusRx (a specialty renal pharmacy service) between 4/1/2014 and 10/30/2015 were included in this retrospective analysis. Patients were required to have ≥ 3 months of SO prescription fills recorded, uninterrupted by another phosphate binder. De-identified demographic and clinical data were retrieved from the FKC data warehouse; phosphate binder prescription information originated from the FreseniusRx database. To supplement baseline phosphate binder information, we used phosphate binder orders from the FKC Electronic Health Record (EHR). However, we cannot assume that the orders recorded in this database are as precise or accurate as documented prescription fills, thus only documented prescriptions were used for calculations of phosphate binder pills per day. Treatment periods for assessment and comparison were defined as baseline (BL; three months before SO prescription), and SO-treated follow-up (SO; up to 6 months of SO prescription). SO-treated follow-up was 6 months or until either a new PB was prescribed, SO was not refilled, PD modality changed, or patient was discharged from FKC.
Basic demographic and treatment characteristics were evaluated at baseline. Clinical parameters included phosphate binder pills/day, mineral and bone disease markers (serum phosphorus, serum calcium, intact parathyroid hormone (iPTH)), nutritional and clearance parameters (weight, serum albumin, normalized protein catabolic rate (nPCR), serum creatinine, total Kt/V (PD + residual renal function), PD Kt/V, and Kru (residual urea clearance); and anemia and iron indices (ferritin, transferrin saturation (TSAT), hemoglobin, and iron). Laboratory tests were measured monthly, except for ferritin and iPTH which were measured quarterly. Calcium was albumin-corrected using the Payne’s formula [21]. Serum albumin and nPCR were each divided by serum phosphorus to calculate phosphorus-attuned albumin and nPCR. Baseline Kru was categorized into 3 groups: unavailable; ≤3 mL/min/1.73 m2; and > 3.0 mL/min/1.73 m2. Spectra Laboratories (Rockleigh, NJ, USA) performed all laboratory tests.
Repeated-measures data were analyzed using linear mixed-effects regression. Mean clinical measures were summarized using least-squared means and compared between baseline and SO follow-up. In-range serum phosphorus was defined as mean serum phosphorus ≤ 5.5 mg/dL. To determine the impact of intermittent HD on changes in serum phosphorus, an analysis was conducted where all serum phosphorus levels were deleted for months when patients had intermittent HD (n=23). A sub-group analysis was conducted for all patients achieving serum phosphorus ≤ 5.5 mg/dL during at least one quarter of SO follow-up and a subset of these patients with baseline serum phosphorus > 5.5 mg/dL. Sub-analysis by Kru group was conducted to assess effect measure modification by residual renal function. For this sub-analysis, missing baseline Kru were imputed for 33 patients with follow-up Kru measured. Serum phosphorus levels were compared for subgroups who did and did not complete 6 months of SO treatment. P-values < 0.05 were considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). This database analysis was approved by the New England Institutional Review Board.
Results
Analysis of study cohort (n=258)
In total, 258 patients met the requirements for analysis. Patients were, on average, 50.6 ± 13.9 years old, with a mean dialysis vintage of 29.3 ± 27.6 months (table 1). The majority of patients (76%) received continuous cycling peritoneal dialysis (CCPD), 13.2% received continuous ambulatory peritoneal dialysis (CAPD) and the remaining patients (10.8%) switched from CAPD to CCPD during baseline. On average, patients were prescribed 4.9 ± 1 exchanges per day and 6.8 ± 0.6 days per week. Mean length of SO follow-up was 5.1 months; 151 patients did not complete all 6 months of SO monotherapy. SO follow-up ended for 38, 27, and 50 patients at months 4, 5, and 6 due to no further PB fills, and for 10, 11, and 4 patients at months 4, 5, and 6 due to another PB prescribed. The other 11 patients who did not complete the 6 months, were discharged from the clinic or switched to hemodialysis. [See monthly patient disposition in supplementary table S1.] The majority of patients with phosphate binder fills from FreseniusRx at baseline (n=92) were prescribed sevelamer (63.0%), followed by calcium acetate (20.7%), more than one phosphate binder (10.8%) and lanthanum carbonate (5.4%). The remainder of patients (n=166) had no recorded baseline phosphate binder fills in the FreseniusRx database. Among the 166 patients who did not receive a FreseniusRx phosphate binder fill during baseline, 110 patients had PB prescriptions available through the FKC EHR database. Figure 1 shows that the distribution of phosphate binder type was similar at baseline for patients with and without phosphate binder prescriptions through FreseniusRx.
TABLE 1.
Baseline Characteristics at SO Therapy Initiation
| Baseline Characteristic | Study Cohort (n=258) |
|---|---|
| Age, years | 50.6 ± 13.9 |
| Dialysis vintage, months | 29.3 ± 27.5 |
| BMI, kg/m2 | 30.4 ± 8 |
| Male, n(%) | 143 (55.4) |
| Race, n(%) | |
| White | 160 (62.0) |
| African American | 64 (24.8) |
| Other | 34 (13.2) |
| Diabetes, n(%) | 121 (46.9) |
| Primary cause of ESRD, n (%) | |
| Diabetes mellitus | 90 (34.9) |
| Hypertension | 69 (26.7) |
| Glomerulonephritis | 47 (18.2) |
| Polycystic kidney disease | 10 (3.9) |
| Other | 34 (13.2) |
| Unknown | 8 (3.1) |
| Baseline PB dispensed through FreseniusRx | |
| No PB recorded | 166 (64.3) |
| PB recorded in the FKC EHR database | 110 (66.3) |
| PB recorded | 92 (35.7) |
| Calcium acetate | 19 (20.7) |
| Lanthanum carbonate | 5 (5.4) |
| Sevelamer | 58 (63.0) |
| >1 PB recorded | 10 (10.8) |
| PD modality | |
| Manual (CAPD) | 34 (13.2) |
| Automated (CCPD) | 196 (76) |
| Switched (CAPD to CCPD) | 28 (10.8) |
| Clinical parameters | |
| Serum phosphorus (mg/dL) | 6.5 ± 1.4 |
| Albumin-corrected calcium (mg/dL) | 9.4 ± 0.7 |
| Intact PTH (pg/mL) | 459 ± 317 |
| Serum albumin (g/dL) | 3.7 ± 0.4 |
| Kru (mL/min/1.73 m2) | 2.72 ± 2.45 |
| PD Kt/V | 1.7 ± 0.4 |
| Total Kt/V | 2.2 ± 0.5 |
Values are expressed as mean ± standard deviation, or n (%)
Abbreviations: BMI - body mass index (kg/m2); CAPD - continuous ambulatory PD; CCPD - continuous cycling PD; Kru - residual urea clearance; FKC EHR – Fresenius Kidney Care electronic health records; PB – phosphate binder; PD – peritoneal dialysis; PTH – parathyroid hormone
FIGURE 1. Baseline Phosphate Binder Prescriptions Prior to SO Initiation.
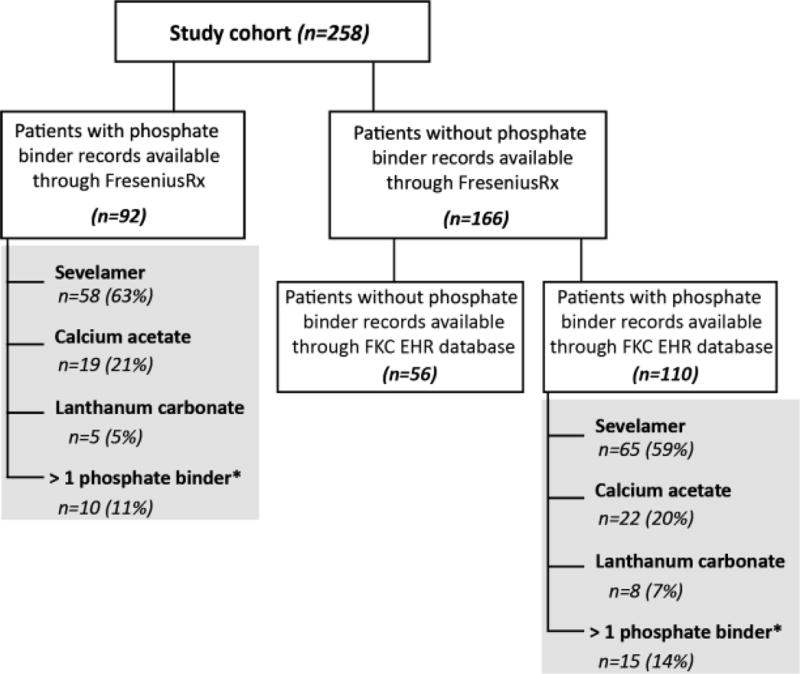
*Combination of sevelamer, calcium acetate, and/or lanthanum carbonate.
Abbreviations: FKC EHR – Fresenius Kidney Care Electronic Heath Record database
Mean changes between baseline and SO follow-up are compared in Table 2. A significant improvement was observed in mean serum phosphorus (6.59 mg/dL at BL to 6.26 mg/dL at SO, p<0.0001). Prescribed phosphate binder pills/day decreased by 57% from 10 pills/day at baseline to 4.3 pills/day at SO follow-up (p<0.0001). There was a slight decrease in corrected calcium (9.36 to 9.31 mg/dL, p=0.018) and a slight increase in intact PTH from 468 to 497 pg/mL (p=0.03). There were no changes observed in unadjusted nPCR (1.0 g/kg/day at BL and SO, p=0.9) and weight (86.8 to 86.9 kg, p=0.8), but a slight decrease was observed in unadjusted serum albumin from 3.70 to 3.62 g/dL (p<0.0001). When adjusted for serum phosphorus, increases were observed in phosphorus-attuned albumin from 0.6 ×103 at BL to 0.63 ×103 at SO follow-up (p<0.0001), and phosphorus-attuned nPCR (0.17 ×103 to 0.18 ×103 dL/kg/day, p=0.04). Although there was a slight decrease in residual urea clearance Kru (2.85 to 2.47 mL/min/1.73 m2, p=0.008) and increase in serum creatinine (12.0 to 12.2 mg/dL, p=0.001), there were no changes in total Kt/V (2.1 at BL and SO, p=0.7) and a numeric increase in PD Kt/V (1.65 to 1.70, p=0.5).
TABLE 2.
Comparison of Clinical Parameters between Baseline and SO Therapy (258 PD Patients)
| Parameter | Baseline | SO follow-up | P-value |
|---|---|---|---|
| Phosphate binder pills/day* | 10.0 (0.2) | 4.3 (0.2) | <0.0001 |
| Mineral and bone disease markers | |||
| Serum phosphorus (mg/dL) | 6.59 (0.09) | 6.26 (0.09) | <0.0001 |
| Albumin-corrected calcium (mg/dL) | 9.36 (0.04) | 9.31 (0.04) | 0.018 |
| Intact PTH (pg/mL) | 468 (23) | 497 (22) | 0.032 |
| Nutritional and clearance parameters | |||
| Weight (kg) | 86.8 (1.5) | 86.9 (1.5) | 0.8 |
| Serum albumin (g/dL) | 3.70 (0.02) | 3.62 (0.02) | <0.0001 |
| Phosphorus-attuned albumin (×103) | 0.6 (0.01) | 0.63 (0.01) | <0.0001 |
| nPCR (g/kg/day) | 1.0 (0.03) | 1.0 (0.02) | 0.9 |
| Phosphorus-attuned nPCR (×103 dL/kg/day) | 0.17 (0.005) | 0.18 (0.004) | 0.044 |
| Serum creatinine (mg/dL) | 12.0 (0.3) | 12.2 (0.3) | 0.001 |
| Kru (mL/min/1.73 m2) | 2.85 (0.20) | 2.47 (0.19) | 0.008 |
| PD Kt/V | 1.65 (0.05) | 1.70 (0.04) | 0.5 |
| Total Kt/V | 2.1 (0.06) | 2.1 (0.05) | 0.7 |
| Anemia and iron indices | |||
| Patients not receiving IV iron therapy (n=93) | |||
| Ferritin (ng/mL) | 801 (66) | 876 (65) | 0.018 |
| Transferrin saturation (%) | 38.5 (1.4) | 38.5 (1.3) | 0.9 |
| Hemoglobin (g/dL) | 11.2 (0.2) | 11.3 (0.2) | 0.039 |
| Iron (mcg/dL) | 98.1 (3.5) | 93.2 (3.2) | 0.077 |
| Patients receiving IV iron therapy (n=165) | |||
| Ferritin (ng/mL) | 748 (36) | 940 (34) | <0.0001 |
| Transferrin saturation (%) | 34.0 (0.9) | 38.4 (0.8) | <0.0001 |
| Hemoglobin (g/dL) | 10.6 (0.1) | 10.7 (0.1) | 0.078 |
| Iron (mcg/dL) | 85.8 (2.4) | 92.3 (2.1) | 0.005 |
Values are expressed as least-squared means (standard error), p-values compare least-squared means between treatment periods.
Abbreviations: Kru- residual urea clearance; nPCR - normalized protein catabolic rate; PD – peritoneal dialysis; PTH - parathyroid hormone; SO – sucroferric oxyhydroxide
Pill burden at baseline was calculated only for patients who received phosphate binder prescriptions through FreseniusRx (n=92). Follow-up PB pill burden for patients with no baseline binder available through FreseniusRx (n=166) was found to be 4.3 SO pills/day
As a sensitivity analysis, several subgroup analyses on serum phosphorus changes were conducted. There were 21 patients who received intermittent HD during the study periods. Changes in serum phosphorus levels after removing these measurements showed a similar improvement in serum phosphorus (6.60 to 6.25 mg/dL, p<0.0001). Patients who completed the full 6 months of SO follow-up (n=107) had serum phosphorus reduced from 6.56 mg/dL at BL to 6.16 mg/dL at SO follow-up (p<0.0001). Phosphate binder pill burden reduced from 9.9 to 4.3 pills/day (p<0.0001) for the patients prescribed PB at baseline (92 patients out of the 107). Patients with phosphate binder prescriptions recorded through FreseniusRx at baseline and who completed the 6-month follow-up (n=30) had a mean serum phosphorus decrease from 6.33 mg/dL at baseline month -1 (month before switch to SO) to 5.37 mg/dL at month 6 of SO follow-up (p<0.001) and percentage of patients achieving in-range serum phosphorus levels increased from 11 (29.7%) patients at baseline month -1 to 18 (48.6%) patients at SO follow-up month 6. Patients with phosphate binder prescriptions recorded through FreseniusRx or FKC EHR database at baseline and who completed the 6-month follow-up (n=84) had a mean serum phosphorus decrease from 6.90 mg/dL at baseline month -1 to 5.94 mg/dL at SO follow-up month 6 (p<0.0001) and the percentage of patients achieving in-range serum phosphorus levels increased from 21 (25%) patients at baseline month -1 to 34 (40.5%) at SO follow-up month 6. An additional analysis of patients who did not complete the full 6 months of SO follow-up, was conducted using all serum phosphorus measurements available, regardless of end of SO monotherapy prescription, and they had a mean serum phosphorus of 6.62 at BL and 6.34 mg/dL at follow-up (p<0.001).
At baseline, 127 (49.2%) patients received IV iron therapy and 195 (75.6%) patients received erythropoietin stimulating agent (ESA) therapy. At SO follow-up, 132 (51.1%) patients received IV iron therapy (iron sucrose) and 200 (77.5%) patients received ESA therapy. Mean iron sucrose dose was minimally changed from 202 mg/month at BL to 204 mg/month at SO follow-up (p=0.6). Similarly, mean epoetin alfa dose was minimally changed from 11,805 to 11,667 units/month (p=0.5) among patients treated exclusively with epoetin alfa (83%). Among patients who received, IV iron therapy (n=165), significant increases in ferritin (748 to 940 ng/dL, p<0.0001), TSAT (34.0 to 38.3%, p<0.0001) and hemoglobin (10.6 to 10.7 g/dL, p=0.08) were observed from baseline to SO follow-up. Among patients who did not receive any IV iron therapy (n=93), serum ferritin increased from 801 to 876 ng/mL (p=0.02), hemoglobin increased from 11.2 to 11.3 g/dL (p=0.03) and TSAT was unchanged (38.5%, p=0.9).
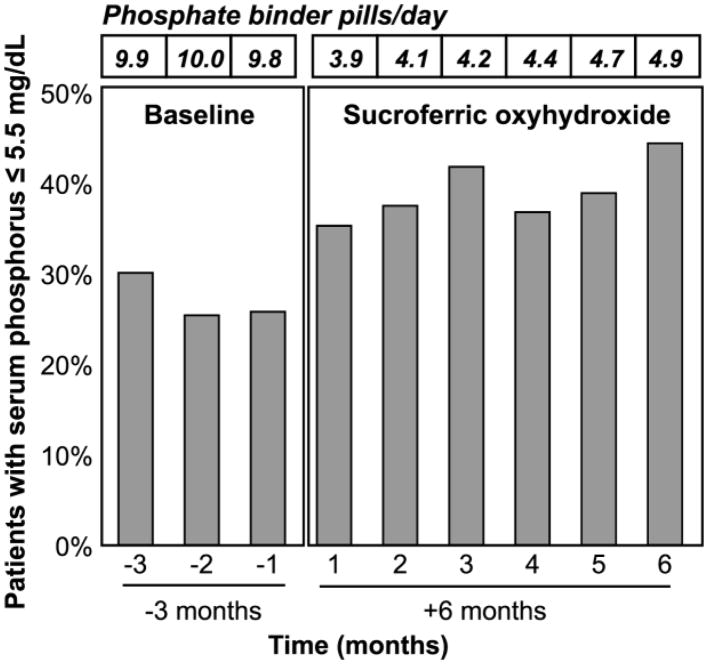
Percent of patients achieving in-range serum phosphorus (≤ 5.5 mg/dL) increased from baseline to SO follow-up (Figure 2). At baseline 30.1%, 25.4%, and 25.8% of patients were in-range for serum phosphorus at months -3, -2, and -1, respectively. During SO follow-up, the percent of patients who were in-range for serum phosphorus increased to 35.3%, 37.5%, 41.8%, 36.8%, 38.9%, and 44.4% at months 1–6, respectively. Comparing BL month -1 to SO follow-up month 6, there was a 72% increase in patients achieving in-range serum phosphorus along with >50% reduction in phosphate binder pills/day.
FIGURE 2. Percent of Patients with Monthly Serum Phosphorus ≤ 5.5 mg/dL at Baseline and SO Follow-up.
Phosphate binder pills/day for patients with in-range serum phosphorus decreased from (9.7, 8.8, 9.0) at baseline to (3.9, 4.4, 4.2, 4.4, 4.5, 4.8) at SO follow-up
Analysis of patients with in-range serum phosphorus recorded during SO follow-up
Patients (n=110) who achieved in-range serum phosphorus during ≥ 1 quarter of SO follow-up were analyzed to determine the associated effects of being in-range on other mineral bone disease and nutrition markers [Table 3]. Among these patients, 75 patients (68%) had a recorded phosphate binder at baseline (67% sevelamer, 27% calcium acetate, 4% lanthanum carbonate, and 3% PB combination therapy), and 35 patients (32%) did not have a recorded PB at baseline (for prescriptions by month, see online supplementary table S2). Comparing baseline and SO follow-up, a >50% reduction in phosphate binder pill burden (9.1 to 4.2 pills/day, p<0.0001) and improvement in serum phosphorus (5.81 to 4.97 mg/dL, p<0.0001) were observed. Intact PTH was unchanged (406 pg/mL to 425 pg/ml, p=0.4). There was a slight decrease in corrected calcium (9.43 to 9.36 mg/dL, p=0.08) and serum albumin (3.70 to 3.64 g/dL, p<0.001), and no changes observed in nPCR (1.0 g/kg/day, p=0.2). Comparing baseline and SO follow-up, there were significant improvements in phosphorus-attuned albumin (0.68 × 103 to 0.77 × 103, p<0.0001) and phosphorus-attuned nPCR (0.18 ×103 to 0.21 ×103 dL/kg/day), p<0.001).
TABLE 3.
Comparison of Clinical Parameters for Patients with In-Range Phosphorus during SO Follow-up
| Parameter | Baseline | SO follow-up | P-value |
|---|---|---|---|
| Patients with serum phosphorus ≤ 5.5 mg/dL for ≥ 3 months of SO follow-up (n=110) | |||
| Phosphate binder pills/day* | 9.3 (0.2) | 4.4 (0.2) | <0.0001 |
| Serum phosphorus (mg/dL) | 5.81 (0.08) | 4.97 (0.07) | <0.0001 |
| Albumin-corrected calcium (mg/dL) | 9.43 (0.06) | 9.36 (0.06) | 0.08 |
| Intact PTH (pg/mL) | 406 (33) | 425 (32) | 0.4 |
| Weight (kg) | 88.1 (2.3) | 87.5 (2.3) | 0.3 |
| Serum albumin (g/dL) | 3.70 (0.03) | 3.64 (0.03) | <0.001 |
| Phosphorus-attuned albumin (×103) | 0.68 (0.01) | 0.77 (0.01) | <0.0001 |
| Phosphorus-attuned nPCR (×103 dL/kg/day) | 0.18 (0.01) | 0.21 (0.01) | <0.001 |
| Serum creatinine (mg/dL) | 10.3 (0.4) | 10.2 (0.4) | 0.12 |
| Kru (mL/min/1.73 m2) | 3.58 (0.38) | 3.25 (0.34) | 0.3 |
| PD Kt/V | 1.57 (0.12) | 1.73 (0.09) | 0.3 |
| Total Kt/V | 2.2 (0.1) | 2.3 (0.1) | 0.3 |
| Subset of patients with serum phosphorus > 5.5 mg/dL at baseline (n=63) | |||
| Phosphate binder pills/day** | 9.9 (0.3) | 4.7 (0.3) | <0.0001 |
| Serum phosphorus (mg/dL) | 6.54 (0.10) | 5.10 (0.08) | <0.0001 |
| Albumin-corrected calcium (mg/dL) | 9.31 (0.09) | 9.36 (0.08) | 0.3 |
| Intact PTH (pg/mL) | 430 (32) | 391 (30) | 0.087 |
| Weight (kg) | 85.9 (2.7) | 87.1 (2.7) | 0.007 |
| Serum albumin (g/dL) | 3.71 (0.04) | 3.63 (0.04) | 0.001 |
| Phosphorus-attuned albumin (×103) | 0.59 (0.01) | 0.75 (0.01) | <0.0001 |
| Phosphorus-attuned nPCR (×103 dL/kg/day) | 0.17 (0.01) | 0.21 (0.01) | <0.0001 |
| Serum creatinine (mg/dL) | 11.7 (0.5) | 11.3 (0.5) | 0.002 |
| Kru (mL/min/1.73 m2) | 2.71 (0.44) | 2.40 (0.37) | 0.5 |
| PD Kt/V | 1.59 (0.17) | 1.80 (0.13) | 0.3 |
| Total Kt/V | 2.0 (0.2) | 2.2 (0.1) | 0.3 |
Values are expressed as least-squared means (standard error), p-values compare least-squared means between treatment periods
Abbreviations: Kru - residual urea clearance; nPCR - normalized protein catabolic rate; PD – peritoneal dialysis; PTH - parathyroid hormone; SO – sucroferric oxyhydroxide
Pill burden at baseline was calculated only for patients who received phosphate binder prescriptions through FreseniusRx (n=68). Follow-up PB pill burden for patients with no baseline binder available through FreseniusRx (n=42) was found to be 4.1 SO pills/day.
Pill burden at baseline was calculated only for patients who received phosphate binder prescriptions through FreseniusRx (n=29). Follow-up PB pill burden for patients with no baseline binder available through FreseniusRx (n=34) was found to be 4.4 SO pills/day.
Among patients who achieved in-range serum phosphorus during SO follow-up, ninety-seven patients did not change PD prescription modality (86% CCPD, 14% CAPD, mean serum phosphorus 5.69 mg/dL at baseline and 4.94 mg/dL at SO follow-up, p<0.0001), and 13 patients were prescribed a switch between both PD modalities (mean serum phosphorus was 6.42 mg/dL at baseline and 4.92 mg/dL at SO follow-up, p<0.001). Fifty-nine patients did not change the prescribed number of PD exchanges/day (mean number of PD exchanges= 4.7/day, mean serum phosphorus 5.57 mg/dL at baseline and 5.02 mg/dL at SO follow-up, p<0.0001). Fifty-one patients had an increase in prescribed number of PD exchanges/day (mean =4.8/day at baseline and 5.2/day at follow-up) and had a mean serum phosphorus of 6.03 mg/dL at baseline and 4.83 mg/dL at SO follow-up (p<0.0001). Eighty-three patients received 7 days/week of PD treatment throughout baseline and follow-up (mean serum phosphorus=5.81mg/dL at baseline and 4.91 mg/dL at SO follow-up, p<0.0001), and 27 patients had an increase in prescribed number of days of PD treatment (5.6 PD treatment days/week at baseline and 6.8 PD treatment days/week at SO follow-up) and serum phosphorus decrease of 0.7 mg/dL (5.69 mg/dL at baseline to 4.98 mg/dL at SO follow-up, p=0.008). A subset of the patients (n=63) who achieved in-range serum phosphorus during SO follow-up, had serum phosphorus levels >5.5 mg/dL during baseline [Table 3]. Comparing baseline and SO follow-up, a notable improvement in serum phosphorus (6.54 to 5.10 mg/dL, p<0.0001) was observed along with a 53% reduction in phosphate binder pill burden (9.7 to 4.6 pills/day, p<0.0001). There were non-significant changes in intact PTH (430 to 391 pg/mL, p=0.09), corrected calcium (9.31 to 9.36 mg/dL, p=0.3) and total Kt/V (2.0 to 2.2, p=0.3). Although a slight decrease in serum albumin (3.71 to 3.63 g/dL, p=0.001) was observed, phosphorus-attuned albumin (0.59 ×103 to 0.75 ×103, p<0.0001) and phosphorus-attuned nPCR (0.17 × 103 to 0.21 × 103 dL/kg/day, p<0.0001) increased.
Assessing impact of residual renal function on phosphate binder pill burden and serum phosphorus levels
In Table 4, patients are stratified by their baseline residual urea clearance (Kru). The Kru >3 group had more patients with baseline and SO follow-up serum phosphorus levels < 5.5 mg/dL (40.2% and 52.8%, respectively) when compared to patients with no Kru measured (15.6% and 31.3%) and patients with Kru ≤ 3 (14.8% and 25.4%). Patients with Kru >3 also had lower baseline and SO follow-up phosphate binder pills/day (6.9 and 3.9 pills, respectively) when compared to patients with no Kru measured (10.9 and 4.3 pills) and patients with Kru ≤ 3 (6.9 and 3.9 pills).
TABLE 4.
Comparison of Pill Burden and Serum Phosphorus by Residual Urea Clearance (Kru) at Baseline
| Parameter | No Kru Recorded (N=64) | Kru ≤ 3 mL/min/1.73 m2 (N=122) | Kru > 3 mL/min/1.73 m2 (N=72) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| BL | SO | P-value | BL | SO | P-value | BL | SO | P-value | |||
| Phosphate binder pills/day* | 10.7 (0.3) | 4.4 (0.3) | <0.0001 | 10.6 (0.3) | 4.4 (0.3) | <0.0001 | 6.7 (0.3) | 3.7 (0.3) | <0.0001 | ||
| Serum phosphorus (mg/dL) | 6.57 (0.16) | 6.21 (0.15) | 0.001 | 6.96 (0.14) | 6.52 (0.13) | <0.0001 | 5.97 (0.16) | 5.85 (0.15) | 0.22 | ||
| Patients with serum phosphorus ≤ 5.5 mg/dL | 10/64 (15.6%) | 20/64 (31.3%) | 0.008 | 18/122 (14.8%) | 31/122 (25.4%) | 0.012 | 29/72 (40.2%) | 38/72 (52.8%) | 0.049 | ||
BL refers to baseline, SO refers to SO follow-up
Values are expressed as least-squared means (standard error) for continuous variables, and n/N (%) for categorical variables.
P-values compare summary estimates between treatment periods
Abbreviations: Kru= residual urea clearance;
Discussion
The current retrospective analysis included patients treated under routine care and the analysis had no impact on the prescription or treatment of the patients. The patients did not go through a wash-out period and the current analysis relied on physician judgement and preference in phosphate binder choice. Compared to a representative national sample of dialysis patients, with 34% of patients with serum phosphorus >5.5 mg/dL [22], the patients in our analysis could be considered difficult to treat, as 74% had serum phosphorus >5.5 mg/dl. However, a 72% increase of patients in-range for serum phosphorus (≤5.5 mg/dl) was achieved with SO, with an absolute level of 44.4% of patients in-range at 6 months and a 57% reduction in phosphate binder pills/day.
Non-adherence to prescribed phosphate binder regimen has been reported in 62% of dialysis patients [18]. Phosphate binder pill burden is a cited determinant of phosphate binder adherence [6]. Thus, the observed average pill burden decreased from 10 to 5 pills/day is favorable. The increase in adherence to phosphate binder therapy may be a factor in the improvements in serum phosphorus control in patients prescribed SO.
Floege et al, a sub-analysis of a phase III study, focused on PD patients over 1 year of follow-up and found SO effective for the lowering of serum phosphorus levels [20]. Although patients in the SO arm had higher baseline serum phosphorus levels when compared to the sevelamer arm (7.5 mg/dL compared to 6.7 mg/dL), there were similar proportion of patients achieve within-range serum phosphorus at 24 and 52 weeks in the SO (57.1% and 62.5%) and sevelamer (60.7% and 64.3%) arms. This was accomplished with 58% lower pill burden when using SO compared to sevelamer, which is comparable to the findings of our analysis.
In this analysis, we also used two novel metrics to account for the role of nutritional status in the management of phosphorus, i.e., phosphorus attuned albumin and nPCR, in that serum albumin and nPCR were each divided by serum phosphorus, respectively. These two metrics are meant to address the concern that lowering serum phosphorus by restricting dietary protein intake (represented by nPCR) may cause more harm [23,24], and that lower protein intake is associated with lower serum albumin in dialysis patients [25]. Indeed, both low nPCR and low serum albumin levels are associated with worse outcomes including higher mortality in dialysis patients. Hence, controlling serum phosphorus without restricting dietary protein intake or without lowering serum albumin is the preferred goal.
Patients with serum phosphorus ≤5.5 mg/dL during SO follow up had a mean reduction in serum phosphorus from 5.81 to 4.97 mg/dL (p<0.0001) and a 54% reduction in phosphate binder pills/day. Significant improvements in phosphorus-attuned albumin (0.68 ×103 to 0.77 ×103, p<0.0001) and phosphorus-attuned nPCR (0.18 ×103 to 0.21 ×103 dL/kg/day), p<0.001) were also observed. In patients who achieved in-range serum phosphorus during SO follow-up but had serum phosphorus levels >5.5 mg/dL during baseline, a notable improvement in serum phosphorus (6.54 to 5.1 mg/dL, p<0.0001) and iPTH (430 to 391 pg/mL, p=0.09) were observed, along with a 53% reduction in phosphate binder pills/day (9.7 to 4.6, p<0.0001) Treatment with calcimimetics and active vitamin D for all patients and patients with in-range serum phosphorus is shown in supplementary tables S3 and S4. Phosphorus-attuned albumin (0.59 ×103 to 0.75 ×103, p<0.0001) and phosphorus-attuned nPCR (0.17 ×103 to 0.21 ×103 dL/kg/day, p<0.0001) also increased from baseline. These changes may show the benefits of achieving in-range serum phosphorus levels.
Residual renal function is a contributor to phosphate clearance in PD patients [26], thus we stratified patients based on residual urea clearance (Kru) at baseline. Patients with Kru >3 had a higher percentage with in-range serum phosphorus levels at baseline and SO follow-up when compared to patients without baseline Kru measured or Kru ≤3. There was also a lower number of phosphate binder pills/day in the patients with Kru >3 at both baseline (6.9) and SO follow-up (3.9) compared to baseline (10.8) and SO follow-up (4.5) for patients without recorded Kru and Kru <3.
SO is an iron-based phosphate binder thus anemia and iron indices were analyzed. The changes in ferritin and TSAT levels were consistent with prior reports of minimal iron absorption in phase III clinical trial results [20,27]. Patients not treated with IV iron during the phase III clinical trial, showed small mean increase in serum ferritin (+71.9) and TSAT (+3.9%) between weeks 24 to 52 [27].
Limitations of our study include utilization of existing clinical records. Although the results may be more generalizable, possible errors or missing data from the clinical database are possible. Our analysis relies on pharmacy fills, which allows for good accuracy for timing and pills/day, but ends follow-up if patient receives fills from a different pharmacy. Reasons for patient being switched from their baseline phosphate binder to SO or discontinuing SO during follow-up are not available, but may include non-adherence to filling prescriptions, adverse events, switch to another pharmacy, insurance coverage, and out of pocket costs. Although dietitians provide nutritional education to patients using approved educational materials, exact dietetic advice was not sufficiently recorded in the EHR. Reasons for discontinuation of baseline or follow-up phosphate binders were not available. Also, there is no independent unexposed group available for comparison with the SO group, who act as their own comparator group during baseline.
Conclusion
In conclusion, the number of prescribed phosphate binder pills/day was reduced by half among adult PD patients treated with SO, while the proportion of patients who achieved in-range serum phosphorus (serum phosphorus ≤5.5 mg/dL) increased.
Supplementary Material
Acknowledgments
Funding
LHF, VP, LA, NJO, CM, and RJK are employees of Fresenius Medical Care, North America. CM and RJK own stock in the company. RJK is on the Board of Directors of Advanced Renal Technologies. DWC is a consultant and speaker for Fresenius Medical Care. KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, ASN (American Society of Nephrology), Astra-Zeneca, Aveo, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, IFKF (International Federation of Kidney Foundations), ISH (International Society of Hemodialysis), International Society of Renal Nutrition & Metabolism (ISRNM), JSDT (Japanese Society of Dialysis Therapy), Hospira, Kabi, Keryx, Novartis, OPKO, NIH (National Institutes of Health), NKF (National Kidney Foundations), Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, ZS-Pharma. Fresenius Medical Care North America Renal Therapies Group provided funding for the study.
References
- 1.Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol. 2013;8(4):619–28. doi: 10.2215/CJN.04810512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klarenbach SW, Tonelli M, Chui B, Manns BJ. Economic evaluation of dialysis therapies. Nat Rev Nephrol. 2014;10(11):644–52. doi: 10.1038/nrneph.2014.145. [DOI] [PubMed] [Google Scholar]
- 3.Coentrão LA, Araújo CS, Ribeiro CA, Dias CC, Pestana MJ. Cost analysis of hemodialysis and peritoneal dialysis access in incident dialysis patients. Perit Dial Int. 2013;33:662–670. doi: 10.3747/pdi.2011.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin HR, Fink NE, Plantinga LC, Sadler JH, Kliger AS, Powe NR. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA. 2004;291(6):697–703. doi: 10.1001/jama.291.6.697. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System. Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2016. 2016 USRDS annual data report. [Google Scholar]
- 6.Tonelli M, Pannu N, Manns B. Oral Phosphate binders in patients with kidney failure. N Engl J Med. 2010;362(14):1312–24. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 7.Cupisti A, Gallieni M, Rizzo MA, Caria S, Meola M, Bolasco P. Phosphate control in dialysis. Int J Nephrol Renovasc Dis. 2013;6:193–205. doi: 10.2147/IJNRD.S35632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rroji M, Seferi S, Cafka M, Petrela E, Likaj E, Barbullushi M, Thereska N, Spasovski G. Is residual renal function and better phosphate control in peritoneal dialysis an answer for the lower prevalence of valve calcification compared to hemodialysis patients? Int Urol Nephrol. 2014;46:175–182. doi: 10.1007/s11255-013-0438-7. [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino M, Stucchi A, Rizzo MA, Brenna I, Elli F, Ciceri P, Bover J, Cusi D, Gallieni M. Phosphate control in peritoneal dialysis. Contrib Nephrol. 2012;178:116–23. doi: 10.1159/000337831. [DOI] [PubMed] [Google Scholar]
- 10.Galassi A, Cupisti A, Santoro A, Cozzolino M. Phosphate balance in ESRD: diet, dialysis and binders against the low evident masked pool. J Nephrol. 2015;28(4):415–429. doi: 10.1007/s40620-014-0142-4. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison AJ. Oral phosphate binders. Kidney Int. 2009;75(9):906–914. doi: 10.1038/ki.2009.60. [DOI] [PubMed] [Google Scholar]
- 12.Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin. 2007;23(12):3167–3175. doi: 10.1185/030079907X242719. [DOI] [PubMed] [Google Scholar]
- 13.Gutekunst L. An Update on Phosphate Binders: A Dietitian’s Perspective. J Ren Nutr. 2016;26(4):209–18. doi: 10.1053/j.jrn.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant. 2014;29(11):2092–9. doi: 10.1093/ndt/gft280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung KY, Liao SC, Chen TH, Chao MC, Chen JB. Adherence to phosphate binder therapy is the primary determinant of hyperphosphatemia incidence in patients receiving peritoneal dialysis. Ther Apher Dial. 2013;17(1):72–77. doi: 10.1111/j.1744-9987.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- 16.Parker K, Nikam M, Jayanti A, Mitra S. Medication burden in CKD-5D: impact of dialysis modality and setting. Clin Kidney J. 2014;7:557–561. doi: 10.1093/ckj/sfu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covic A, Rastogi A. Hyperphosphatemia in patients with ESRD: assessing the current evidence linking outcomes with treatment adherence. BMC Neph. 2013;14:153. doi: 10.1186/1471-2369-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–96. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coyne D, Ficociello LH, Parameswaran V, Anderson L, Vemula S, Ofsthun NJ, Mullon C, Maddux FW, Kossmann RJ, Sprague SM. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: A retrospective analysis of pharmacy data. Clin Nephrol. 2017;88(8):59–67. doi: 10.5414/CN109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floege J, Covic AC, Ketteler M, Mann J, Rastogi A, Spinowitz B, Rakov V, Lisk LJ, Sprague S. One-year efficacy and safety of the iron-based phosphate binder sucroferric oxyhydroxide in patients on peritoneal dialysis. Nephrol Dial Transplant. 2015;30(6):1037–1046. doi: 10.1093/ndt/gfw460. [DOI] [PubMed] [Google Scholar]
- 21.Payne RB, Carver ME, Morgan DB. Interpretation of serum total calcium: effects of adjustment for albumin concentration on frequent frequency of abnormal values and on detection of change in the individual. JCP. 1979;32:56–60. doi: 10.1136/jcp.32.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US-DOPPS Practice Monitor. 2017 Sep; http://www.dopps.org/dpm.
- 23.Lynch KE, Lynch R, Curhan GC, Brunelli SM. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(3):620–9. doi: 10.2215/CJN.04620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88(6):1511–8. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriguchi R, Obi Y, Streja E, Tortorici AR, Rhee CM, Soohoo M, Kim T, Kovesdy CP, Kalantar-Zadeh K. Longitudinal Associations among Renal Urea Clearance-Corrected Normalized Protein Catabolic Rate, Serum Albumin, and Mortality in Patients on Hemodialysis. Clin J Am Soc Nephrol. 2017;12(7):1109–1117. doi: 10.2215/CJN.13141216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evenepoel P, Meijers BK, Bammens B, Viaene L, Claes K, Sprangers B, Naesens M, Hoekstra T, Schlieper G, Vanderschueren D, Kuypers D. Phosphorus metabolism in peritoneal dialysis- and haemodialysis-treated patients. Nephrol Dial Transplant. 2016;31(9):1508–14. doi: 10.1093/ndt/gfv414. [DOI] [PubMed] [Google Scholar]
- 27.Covic AC, Floege J, Ketteler M, Sprague SM, Lisk L, Rakov V, Rastogi A. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant. 2017;32(8):1330–1338. doi: 10.1093/ndt/gfw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.