Abstract
BACKGROUND AND PURPOSE
Cavitary plaques have been reported as a manifestation of otospongiosis. They have been related to third window manifestations, complications during cochlear implantation and sensorineural hearing loss. However, their etiology and clinical implications are not entirely understood. Our purpose was to determine the prevalence, imaging findings and clinical implications of cavitary plaques in the setting of otospongiosis.
MATERIAL AND METHODS
We identified patients with otospongiosis at the University of North Carolina from January 2012 to April 2017. Cross-sectional CT images and clinical records of 47 patients (89 temporal bones) were evaluated for presence, location, and imaging features of cavitary and noncavitary otospongiotic plaques, as well as clinical symptoms and complications in those who underwent cochlear implantation.
RESULTS
Noncavitary otospongiotic plaques were present in 86 (97%) temporal bones and cavitary plaques in 30 (35%). Cavitary plaques predominated with increasing age (mean 59 years, p=0.058), mostly involving the anteroinferior wall of the internal auditory canal (p=0.003), and their presence was not associated with a greater grade of otospongiosis by imaging (p=0.664) nor with a specific type of hearing loss (p=0.365). No patients with cavitary plaques had third window manifestations, and those with a history of cochlear implantation (n=6) did not have complications during the procedure.
CONCLUSIONS
Cavitary plaques occurred in one-third of patients with otospongiosis. Typically, occurred in the anteroinferior wall of the internal auditory canal. There was no correlation with the degree of otospongiosis, type of hearing loss or surgical complications. Cavitary plaques tended to present in older patients.
Introduction
Otospongiosis is an osteodystrophic disorder of the otic capsule that results in acquired hearing loss with a peak onset in the third decade.1–3 It is believed to originate in cartilaginous remnants within the endochondral layer of the otic capsule which are replaced by foci of more vascular bone (otospongiosis) that ultimately becomes highly calcified and sclerotic (otosclerosis).1,2,4–7
Otospongiosis manifests clinically when the lesion enlarges and encroaches upon the stapedial annular ligament, causing fixation of the stapes with resultant conductive hearing loss. If the lesion progresses to involve the cochlea, the result is irreversible sensorineural hearing loss or mixed hearing loss.
The formation of cavitary plaques in otospongiosis has been reported as a focal low-attenuation notch or diverticulum most commonly located along the anteroinferior wall of the internal auditory canal (IAC).1,7–11 Recently, isolated IAC diverticula have been associated with a different pattern of hearing loss than that seen in classic otospongiosis.8 However, the prevalence of such diverticula or cavitary changes and their clinical implications in the setting of lesion grade or extent are not completely understood. Cavitary plaques are also thought to be a possible cause of “third window lesions” secondary to involvement of the endosteal layer of the bony labyrinth and previous reports have also suggested that they may lead to CSF gushing or electrode misplacement during cochlear implantation.1,4,7,12
Therefore, the purpose of this study was to: 1) determine the prevalence of cavitary plaques in the setting of otospongiosis and correlate with lesion grade, 2) describe the imaging findings and locations within the temporal bone, and 3) determine the clinical significance in terms of pattern of hearing loss, third window manifestations and complications after cochlear implantation.
Materials and Methods
The radiology data base of the University of North Carolina was searched for all patients with a clinical diagnosis of otospongiosis who underwent a CT study from January 2012 to April 2017. The study was approved by our institutional review board, and because of its retrospective nature informed patient consent was waived. A total of 47 patients were included.
Patient Selection
Inclusion criteria were adult patients with imaging and/or clinical findings consistent with unilateral or bilateral otospongiosis. Clinical criteria were: history of progressive hearing loss with pure-tone audiometry showing conductive hearing loss with an air-bone gap > 20 dB above normal adult hearing level and with a perceptive hearing loss < 35 dB above normal adult hearing level in the range of 0.5, 1, 2, and 4 kHz. Imaging findings included: areas of demineralization appearing as radiolucency on CT-scans (otospongiotic plaques) involving the otic capsule, with or without complete or partial obliteration of the oval or round windows.
Exclusion criteria included: co-morbid middle or inner ear pathology based on clinical history and imaging findings, including cholesteatoma, tympanic membrane perforation, ossicular dislocation, osteogenesis imperfecta, Paget’s disease, otosyphilis, postsurgical changes, and patients with inconclusive clinical and/or imaging findings of otospongiosis. To analyze the type of hearing loss and third window manifestations, patients who presented with concomitant history of Meniere’s disease, semicircular canal dehiscence, enlarged vestibular aqueduct, and perilabyrinthine fistula were excluded.
Clinical findings
Medical charts were reviewed and the following data for each patient were recorded: 1) age, gender, type of hearing loss classified as: conductive hearing loss, sensorineural hearing loss, and mixed hearing loss. 2) presence of third window abnormalities defined as sound-induced vertigo, dizziness, nausea or eye movements (Tullio phenomenon). 3) Cochlear implantation and its possible complications such as CSF gusher and electrode misplacement in cavitary formations.
CT Studies
High-resolution scans of the temporal bones were performed on 128 or 64-slice multidetector CT scanners with 0.6 mm collimation, 0.55 pitch, 320 mAs and 120 kVp or “cone beam” CT with 0.6 mm collimation, 140 mAs, and 90 kVp. Axial images parallel to the lateral semicircular canal were obtained. Coronal reformatted images were created perpendicular to the axial images. Images with extensive motion or implant artifacts were excluded from the study. All studies were performed without intravenous contrast administration.
Image Evaluation
A total of 89 temporal bones from 47 patients were analyzed by 1 neuroradiology fellow (P.P.) and verified by 1 neuroradiologist with 3 years of experience reading temporal bone CT images and Certificate of Added Qualification in neuroradiology (C.Z.), both blinded to clinical findings. Findings on CT scans were classified into two groups: 1) otospongiotic plaques (noncavitary plaques), and 2) cavitary plaques. Otospongiotic plaques (areas of demineralization appearing as radiolucency on CT-scans) were classified according to the Symons/Fanning classification into: grade 0, no findings; grade 1, solely fenestral (fissula ante fenestram), evidence of a thickened stapes footplate, and/or decalcified, narrowed, or enlarged round or oval windows; grade 2, patchy localized cochlear disease (with or without fenestral involvement); and grade 3, diffuse confluent cochlear involvement of the otic capsule (with or without fenestral involvement).11
Cavitary plaques were defined as focal, well-delineated, low attenuating foci similar to CSF (Fig. 1). Their location was classified as: Zone 1 (region anterior to the oval window), zone 2 (pericochlear region), zone 3 (anteroinferior wall of the IAC), zone 4 (posterior wall of the IAC), and zone 5 (round window) (supplemental online figure). Endosteal involvement was defined as invasion of the cavitary plaque into the endosteal layer of the labyrinth. Communication between IAC cavitary plaques and CSF was determined by lack of normal bone between the cavity and the IAC (Fig. 2). Hounsfield units from the center of otospongiotic and cavitary plaques were measured by placing ROI according to the size of the lesion. Studies that were acquired with cone beam CT were excluded for this analysis. (n=5).
Fig 1.
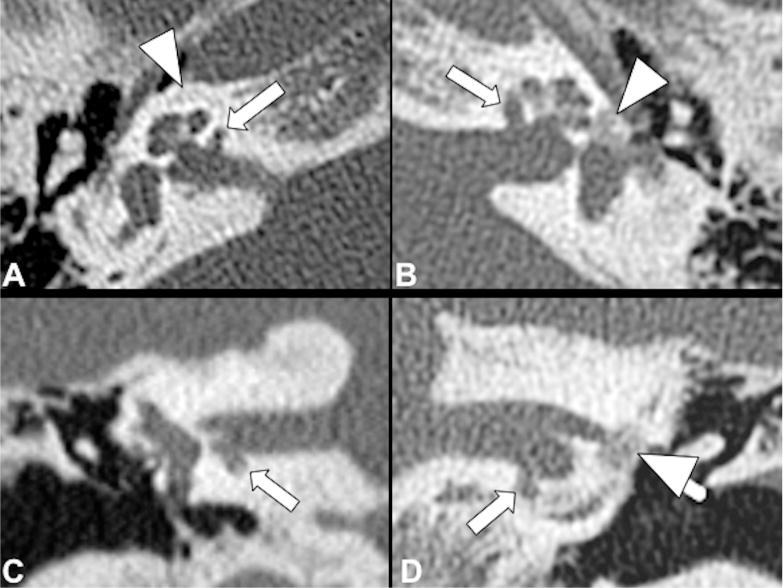
Bilateral cavitary plaques. Axial (top row) and coronal (bottom row) CT show presence of abnormal CSF-attenuating focal lesions (arrows) involving the anterior and inferior wall of the IAC next to the basal turn of the cochlea. Additionally, there are noncavitary plaques (arrowheads) around the cochlea on the right (A, C) and at the fissula ante fenestram on the left (B, D).
Fig 2.
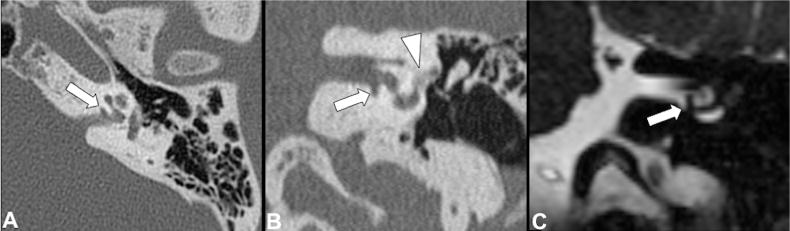
Axial (A) and coronal (B) CT images show the presence of a cavitary plaque (arrows) involving the anterior and inferior wall of the IAC next to the basal turn of the cochlea. Additionally, there is an otospongiotic plaque (arrowhead) at the fissula ante fenestram. Coronal CISS MR (C) demonstrates a clear communication between the cavity and CSF of the IAC.
Statistical Analysis
Descriptive statistics were used to determine the prevalence of cavitary plaques in otospongiosis. To account for multiple outcomes from a patient, a generalized linear (logistic) mixed effects model with a random intercept was used to determine the association of cavitary plaques with the degree of otospongiosis, type of hearing loss, third window manifestations, and complications during cochlear implantation, as well as relationships between cavitary plaques and patient’s age, gender and Hounsfield units. The aforementioned independent variables were coded according to their data type. Type III tests for fixed effects were used to determine the overall statistical significance of the variable. P values less than 0.05 were considered significant. SAS 9.4 (Cary, NC) was used to generate descriptive statistics as well generalized linear mixed models using PROC GLIMMIX.
Results
Patient Profile
We identified 47 patients with otospongiosis. Mean patient age was 55 years (range 28 to 83 years; SD=14). 25 patients (53%) were females and 22 (47%) were males. Of the 47 patients, 42 (89%) had otospongiosis bilaterally and 5 (11 %) unilaterally. Of the 5 patients with unilateral otospongiosis, their contralateral temporal bones were excluded because they had normal audiometry and no findings on CT.
Imaging Findings
A total of 89 temporal bones were analyzed. 3 had normal CT findings (otospongiosis grade 0), though history and clinical parameters were consistent with otospongiosis. 86 temporal bones had classic imaging findings of otospongiosis (noncavitary plaques) and 30 (35%) of them also presented cavitary plaques. Cavitary plaques were therefore never seen in isolation.
Otospongiotic plaques
Grade I otospongiosis was the most common presentation in 49.4% (44) of the temporal bones followed by grade III 29.2% (26). Hounsfield units from the center of the otospongiotic plaques were measured in 81 out of 86 temporal bones (5 temporal bones with cone beam CT were excluded) resulting in Hounsfield units of 953 (SD=278).
Otospongiotic and cavitary plaques
From the 30 temporal bones with cavitary plaques, 18 temporal bones (60%) showed bilateral and 12 (40%) unilateral cavitary changes (p=0.273) (Table 1). Regarding the number of cavitary plaques per temporal bone, 96.7% (29) of temporal bones had a single cavitary plaque and only 1 (3.3%) presented two cavitary lesions. Of 31 cavitary plaques, 93.5% (29) were located in the IACs and 6.5% (2) within the otic capsule (p=0.003) (Table 2). The anteroinferior wall of the IAC was the most common location for presence of cavitary plaques (Fig. 1).
Table 1.
Demographic Characteristics
| Characteristic | Otospongiosis + Cavitary
plaques (n=30) |
Otospongiosis
only (n=56) |
P Value |
|---|---|---|---|
| Median age (yr) | 59 | 51 | 0.058 |
| Temporal bone involvement | 0.273 | ||
| Unilateral (%) | 12 (40) | 14 (25) | |
| Bilateral (%) | 18 (60) | 42 (75) | |
| HU | 115 | 953 | <0.001 |
Table 2.
Location of Cavitary Plaques in Otospongiosis
| Location | Cavitary
Plaques (n=31) |
|
|---|---|---|
| No. | % | |
| IAC | 29 | 93.54 |
| • Anteroinferior wall | 28 | 90.34 |
| • Posterior wall | 1 | 3.2 |
| NON-IAC | 2 | 6.46 |
| • Pericochlear | 1 | 3.2 |
| • Fenestral | 1 | 3.2 |
Presence of cavitary plaques was not associated with a greater grade of otospongiosis by imaging (p=0.664). Otospongiosis with cavitary changes tended to present in patients older (mean=59, SD=11) than those without cavities (mean=51, SD=15) although the difference was not statistically significant (p=0.058) (Table 1). There was no statistically significant association between the presence of cavitary plaques and gender (p= 0.667).
Of the 29 cavitary plaques located in the IACs, 97% (28) showed direct communication with the CSF space of the IAC (Fig. 2). Endosteal involvement was seen affecting the basal turn of the cochlea in 3 (10%) temporal bones with cavitary plaques.
The average length and width of the cavitary plaques were 4.44 (SD=2.32) and 1.19 (SD=0.45) mm respectively. There was a statistically significant difference in the mean Hounsfield units value between the noncavitary and cavitary plaques, 953 (SD=278) vs. 115 (SD=75) respectively, (p<0.001).
Clinical Findings
Type of hearing loss was analyzed in 83 of 89 temporal bones (6 temporal bones had an associated history of Meniere’s disease). In the group of patients with cavitary changes (n=29), sensorineural (41.4%) and mixed hearing loss (41.4%) were the most common. Mixed hearing loss (51.9%) was the most common type in the group without cavities (Table 3). Presence of cavitary plaques showed no significant association with a specific type of hearing loss (p=0.365).
Table 3.
Hearing Loss According to Type of Otospongiosis
| Characteristic | Otospongiosis + Cavitary Plaques | Otospongiosis only | P Value |
|---|---|---|---|
| Type of Hearing Loss | 0.365 | ||
| Conductive | 5 (6%) | 13 (15.7%) | |
| Sensorineural | 12 (14.5%) | 13 (15.7%) | |
| Mixed | 12 (14.5%) | 28 (33.7%) |
None of the temporal bones with otospongiosis, either with or without cavitary changes, had a clinical history of third window manifestations. There were 6 temporal bones with cavitary plaques who underwent cochlear implantation, none of which had procedural complications such as CSF gusher or misplacement of electrodes into the cavitary plaques.
Discussion
The first report of cavitary plaques in otospongiosis was published by Schuknecht in 1974 where he described a case showing a large cavity surrounding the middle and apical turns of the cochlea.13 After this initial case, cavitary changes were mainly described in reports that include an average of one to two cases.1,7,9,13 An abstract in 2012 reported 32 cases of cavitary changes from a series of 147 temporal bones with a history of hearing loss, in which lesions were referred to as diverticula.10 However, imaging features were not described and to the best of our knowledge, a full paper with the details of this work has not been published in the English literature.
In 2017, Pippin et al.8 reported a cavitary plaque prevalence of 18% among 66 temporal bones with otospongiosis. Our prevalence was higher at 35% which could be related to a larger sample of temporal bones with otospongiosis in our study (86) as well as possibly variations in referral bias at both institutions.
In our study, we identified 31 cavitary plaques among 30 temporal bones and none of the cavitary plaques were seen in isolation (i.e. they were associated with classic findings of otospongiosis in all cases). This observation differs from that of Pippin et al.8 who reported 57 temporal bones with cavitary plaques as an isolated finding among 807 patients. This difference could be explained by our smaller sample size and the fact that we focused on patients with clinical and/or imaging findings of otospongiosis, whereas in Pippin’s study they analyzed patients regardless of diagnosis. It is interesting to note that Hoeberigs et al.10 in 2012 reported only 2 temporal bones with isolated cavitary plaques among 222 temporal bones in patients with conductive or mixed hearing loss. However, as mentioned before, this was an abstract and the details of the study have not been published.
Increased prevalence of cavitary plaques has been reported in patients with greater degrees of otospongiosis by CT (grade 3) suggesting that they may be a manifestation of severe disease.10 However, in our study we found that cavitary plaques tended to be more common in grade 1 otospongiosis (16.9%), followed by grade 3 (10.1%) and were not significantly associated with the degree of otospongiosis (p=0.365). These results suggest that the formation of cavities represents an additional manifestation in the dynamic process of otospongiosis.
The mean age of patients with cavitary plaques (59 years, SD=11) tended to be higher than that of patients with noncavitary plaques (51 years, SD=15) (p=.058). A similar outcome was seen by Pippin et al8 who found that patients with cavitary plaques were significantly older (61 years) than those without cavities (52 years). Two growth patterns have been identified in otospongiotic plaques: one grows for a short period of time and then becomes inactive. The other pattern shows continued growth and progression throughout life.15 Since most cases of cavitary plaques were seen in patients with a long-standing diagnosis of otospongiosis, it is possible that cavitation may belong to the second growth pattern and present in older individuals, but this remains uncertain.
To assess the location of cavitary plaques, we evaluated the sites within the temporal bone that have been most commonly reported in the literature.1,7,9,10,16 Our analysis identified that the walls of the IAC were the most commonly affected sites by cavitary plaques (p=0.003); 90.3% involving the anteroinferior wall and 3.2% the posterior wall. Involvement of the anteroinferior IAC as the most common location is consistent with what has been reported in the literature8,10. In this location, cavitary plaques have been called cavitary formations, cavitations and also diverticula or indentations of the IAC.1,7–10 We found one temporal bone with a cavitary plaque involving the posterior wall of the IAC in a patient with advanced otospongiosis (grade 3) and sensorineural hearing loss. However, the preference for this site in this particular patient is uncertain. Cavitary plaques outside the IAC were seen in 2 instances (Fig. 3). These locations are rare and have been previously documented in 4 case reports, most of them identified on histological analysis.1,9,14,17
Fig 3.
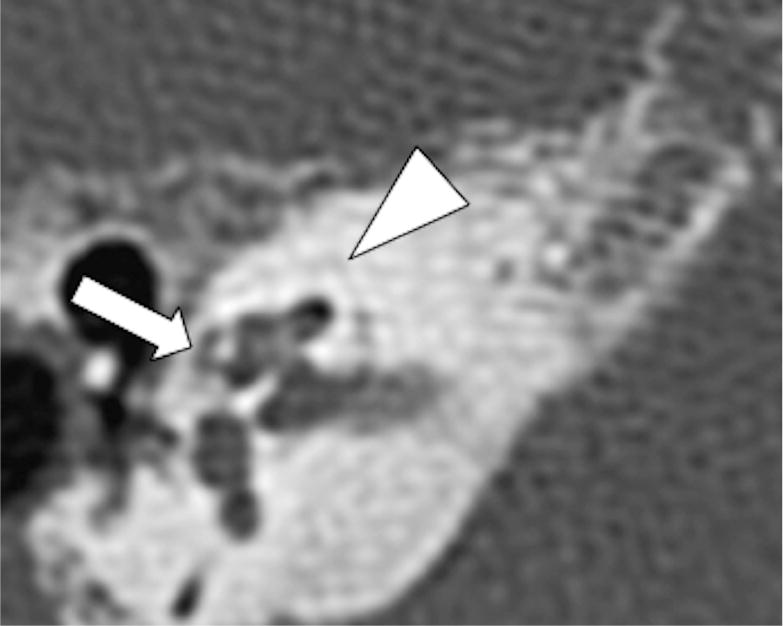
Axial CT shows presence of a cavitary plaque involving the pericochlear region (arrow), note the density of the cavitary plaque similar to the components in the IAC. Additionally, there are noncavitary otospongiotic plaques surrounding the otic capsule (arrowhead).
Cavitary plaques showed low attenuation on CT similar to that of CSF in the IAC (Fig. 1). We found that there was a statistically significant difference in the mean Hounsfield units between the noncavitary plaques and cavitary lesions, 953 (SD=278) vs 115 (SD=75) respectively, (p<0.001). This difference in Hounsfield units was expected as 96% of cavitary plaques were in apparent communication with the IAC and presumably filled with CSF (Fig. 2). This was also demonstrated in a patient with cavitary otospongiosis who underwent MRI which serves as an illustrative example (Fig. 2c). Cavitary plaques may be difficult to evaluate on imaging due to their small size. Therefore, in patients with suspected otospongiosis it is important to carefully scrutinize the anteroinferior wall of the IAC next to the cochlea which is where cavitary changes are most commonly identified.
Some unrelated disorders affecting the labyrinth can produce third window lesions resulting in conductive or sensorineural hearing loss, vestibular manifestations (sound and/or pressure-induced vertigo), or a combination of them18,19. Cavitary plaques have been described as a cause of third window lesions when they reach the endosteal margin of the bony labyrinth.1,14,20 However, this complication is probably rare since 90% of cavitary plaques in our study did not show extension into the endosteal layer of the cochlea. Three cavitary plaques showed contact with the endosteal margin of the basal turn of the cochlea but none of them had clinical manifestations of third window phenomena. It is possible that involvement of the endosteal layer in these cases was too mild to result in third window abnormalities.
Pippin et al8 demonstrated a significant correlation between the presence of cavitary plaques and isolated sensorineural hearing loss. In our study, the presence of cavitary plaques was not statistically associated with a specific type of hearing loss (p=0.365), however, this could be related to our smaller sample size. Also, there was probably an effect of patient selection as their cohort included a large number of patients with cavitary changes but without classic findings of otospongiosis.
Complications of cochlear implantation in patients with otospongiosis are reported to occur in 10–20% of patients.21,22 Cavitary plaques as a potential cause of CSF gushing and misplacement of electrode arrays into the pericochlear cavities have been reported in around 4 cases in the literature. Otospongiosis leads to loss of part of the wall of the cochlea which can result in direct communication between the IAC and the basal turn.1,16,21,22
In our patients, we found 6 temporal bones with cavitary plaques that underwent cochlear implantation. These cases showed a discrete layer of intervening bone between the cavitary plaques and the basal turn of the cochlea and as expected none of them had any complications related to surgery. The absence of complications in our study, however, could also be explained by the limited number of temporal bones that underwent a cochlear implant.
The limitations of our study include its retrospective nature, relatively small sample size, and absence of pathological confirmation since biopsies are not routinely performed during stapedectomy. However, all patients met imaging and/or clinical criteria for otospongiosis. Measurement of Hounsfield units could have also been affected by the small size of the lesions and partial averaging with adjacent bone.
CONCLUSIONS
Cavitary plaques in otospongiosis were seen in one third of temporal bones and their most common location was the anteroinferior wall of the IAC next to the cochlea. Cavitary plaques were mostly seen in older patients and there was no association between them and greater degree of otospongiosis by imaging or third window manifestations. There were no procedural complications such as CFS gusher or misplacement of electrodes within cavitary plaques during cochlear implantation.
Supplementary Material
Acknowledgments
We acknowledge the editorial assistance of the NC Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111.
Abbreviations
- IAC
internal auditory canal
References
- 1.Makarem AO, Hoang T-A, Lo WWM, et al. Cavitating otosclerosis: clinical, radiologic, and histopathologic correlations. Otol Neurotol. 2010;31:381–4. doi: 10.1097/MAO.0b013e3181d275e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton AE, Mikulec AA, Mikulec KH, et al. Association between osteoporosis and otosclerosis in women. J Laryngol Otol. 2004;118:617–21. doi: 10.1258/0022215041917790. [DOI] [PubMed] [Google Scholar]
- 3.Redfors YD, Möller C. Otosclerosis: Thirty-year follow-up after surgery. Ann Otol Rhinol Laryngol. 2011;120:608–14. doi: 10.1177/000348941112000909. [DOI] [PubMed] [Google Scholar]
- 4.Palacios E, Valvassori G. Cochlear otosclerosis. Ear, Nose Throat J. 2000;79:494. [PubMed] [Google Scholar]
- 5.Wycherly BJ, Berkowitz F, Noone AM, et al. Computed tomography and otosclerosis: A practical method to correlate the sites affected to hearing loss. Ann Otol Rhinol Laryngol. 2010;119:789–94. doi: 10.1177/000348941011901201. [DOI] [PubMed] [Google Scholar]
- 6.Sanverdi SE, Ozgen B, Dolgun A, et al. Incomplete endochondral ossification of the otic capsule, a variation in children: Evaluation of its prevalence and extent in children with and without sensorineural hearing loss. Am J Neuroradiol. 2015;36:171–5. doi: 10.3174/ajnr.A4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou-Assaly W, Mukherji S, Srinivasan A. Bilateral cavitary otosclerosis: A rare presentation of otosclerosis and cause of hearing loss. Clin Imaging. 2013;37:1116–8. doi: 10.1016/j.clinimag.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Pippin XKJ, Muelleman XTJ, Hill XJ, et al. Prevalence of Internal Auditory Canal Diverticulum and Its Association with Hearing Loss and Otosclerosis. doi: 10.3174/ajnr.A5399. https://doi.org/10.3174/ajnr.A5399. [DOI] [PMC free article] [PubMed]
- 9.Makarem AO, Linthicum FH. Cavitating otosclerosis. Otol Neurotol. 2008;29:730–1. doi: 10.1097/MAO.0b013e3181799763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeberigs M, Postma A, Waterval J, et al. Prevalence of Anterior Internal Auditory Canal “Diverticulum” on High Resolution CT in Patients with Otosclerosis; Radiological Society of North America 2012 Scientific Assembly and Annual Meeting; Chicago, IL. November 25–30, 2012. [Google Scholar]
- 11.Lee TC, Aviv RI, Chen JM, et al. CT grading of otosclerosis. Am J Neuroradiol. 2009;30:1435–9. doi: 10.3174/ajnr.A1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merkus P, Van Loon MC, Smit CF, et al. Decision making in advanced otosclerosis: An Evidence-Based Strategy. Laryngoscope. 2011;121:1935–41. doi: 10.1002/lary.21904. [DOI] [PubMed] [Google Scholar]
- 13.Schuknecht HF, Kirchner JC. Cochlear otosclerosis: Fact or fantasy. Laryngoscope. 2009;84:766–82. doi: 10.1288/00005537-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Richard CC, Linthicum FH., Jr An unexpected third window in a case of advanced cavitating otosclerosis. Otol Neurotol. 2012;33:e47–8. doi: 10.1097/MAO.0b013e318245cb3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brondbo K, Hawke M, Abel SM, et al. The natural history of otosclerosis. A correlation of the volume and activity of the otosclerotic lesion with age. J Otolaryngol. 1983;12:163–8. [PubMed] [Google Scholar]
- 16.Ramsden R, Bance M, Giles E, et al. Cochlear implantation in otosclerosis: a unique positioning and programming problem. J Laryngol Otol. 1997;111:262–5. doi: 10.1017/s0022215100137028. [DOI] [PubMed] [Google Scholar]
- 17.Hederstierna C, Cureoglu S, Paparella MM. Temporal Bone Histopathology Case of the Month Undiagnosed Severe Cochlear Otosclerosis as a Cause of Profound Hearing Loss. Otol Neurotol. 2013;34:14–5. doi: 10.1097/MAO.0b013e31826bf3bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29:282–9. doi: 10.1097/mao.0b013e318161ab24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho ML, Moonis G, Halpin CF, et al. Spectrum of third window abnormalities: Semicircular canal dehiscence and beyond. Am J Neuroradiol. 2017;38:2–9. doi: 10.3174/ajnr.A4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quesnel AM, Moonis G, Appel J, et al. Correlation of computed tomography with histopathology in otosclerosis. Otol Neurotol. 2013;34:22–8. doi: 10.1097/MAO.0b013e318277a1f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotteveel LJC, Proops DW, Ramsden RT, et al. Cochlear implantation in 53 patients with otosclerosis: Demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol. 2004;25:943–52. doi: 10.1097/00129492-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Ramsden R, Rotteveel L, Proops D, et al. Cochlear implantation in otosclerotic deafness. Adv Otorhinolaryngol. 2007;65:328–34. doi: 10.1159/000098855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


