Abstract
Objective
To evaluate a brief, clinic-based, safer sex program administered by a lay health advisor for young, heterosexual, African American men newly diagnosed with an STD.
Methods
Subsequent to STD diagnosis, eligible men (N=266) between the ages of 18–29 years, were randomized to either a personalized, single session intervention (delivered by a lay health advisor) or standard-of-care. Behavioral assessments were conduced at baseline and 3 months post-intervention (retention was 74.1%). A 6-month clinic record review was also conducted.
Results
Intervention men were significantly less likely to acquire subsequent STDs (50.4% vs. 31.9%, P=.002) and more likely to report using condoms during last sex (72.4% vs. 53.9%, P=.008). Intervention men reported fewer sex partners (mean of 2.06 vs. 4.15, P=.0003) and fewer acts of unprotected sex (mean of 12.3 vs. 29.4, P=.045). Based on a 9-point rating scale, intervention men had higher proficiency scores for condom application skills (mean difference = 3.17, P<.0001).
Conclusion
A brief, clinic-based intervention, delivered by a lay health advisor, may be an efficacious strategy to reduce incident STDs among young, heterosexual, African American men.
Introduction
In the United States AIDS case rates are approximately 8 times greater among African American men compared to white men;1,2 with African American men having the highest prevalence and incidence rates of AIDS relative to any other demographic classification of US residents, particularly in the South3–5 African American men are also disproportionately affected by sexually transmitted diseases (STDs).6 Given these multiple disparities, an important public health imperative is to develop and test interventions designed to reduce the risk of HIV/STD acquisition among African American men; especially young African American men who are at greatest risk of infection.7,8 The imperative applies to both African American men who have sex with men and those who have sex with women. In general, however, heterosexual men of all racial/ethnic origins have been largely neglected with respect to the development and evaluation of HIV prevention interventions.9–11
Few studies have specifically investigated clinic-based approaches to reducing HIV/STDs among young African American men having sex with women. For example, a recent review of effective behavioral interventions for HIV infection, Lyles and colleagues identified 18 programs that met established methodological criteria.12 Of these 18 programs 14 were designed for persons who were not knowingly HIV positive and of these 14, none were designed for heterosexual African American men. The Centers for Disease Control and Prevention (CDC) currently endorses a brief (60 minutes) clinic-based program, delivered in a small group format, designed to promote safer sex among African American and Hispanic men of all ages.13 To evaluate program efficacy, investigators used a clinic record review (mean of 17 months) to monitor subsequent STDs. Men randomized to the intervention were less likely to acquire a subsequent STD (22.5%) than men in the routine care condition (26.8%).14 Although demonstrating efficacy, from an operational perspective, organizing groups of 3 to 8 men may be problematic in many STD clinics. In a multicenter randomized controlled trial, a one-to-one tailored counseling intervention was evaluated among STD clinic patients.15 Patients randomized to the enhanced counseling and the brief counseling conditions were less likely to acquire subsequent STDs over a six-month follow-up (estimated OR = .69 and .71, respectively). The trial had a low participation rate (44%) and high attrition (49%). Although demonstrating a treatment advantage, there was a marginal treatment effect for the primary behavioral outcome, unprotected vaginal sex. Subsequent reanalysis of the data indicated intervention effects were not uniform across age groups. For example, among adolescents < 20 years old, the 12-month STD incidence was 17.2% in the enhanced condition versus 26.6% in the control condition. However, among young adults, 20–25 years, intervention effects were markedly smaller (13.1% versus 14.8%).16 In another study, a single session, clinic-based intervention observed significant effects in a subset analysis of young men (20–30 years of age) and African American men over a 6–9 month follow-up period while17 a 6-session, video-based, intervention observed reductions in self-reported unprotected vaginal sex among African American men recruited from an urban STD clinic.18 Unfortunately, the former study was not designed specifically for African American men and was evaluated using a non-randomized design while the latter study used a small-group intervention format that limits its utility for clinic-based implementation and did not assess subsequent STD acquisition. Finally, a clinic-based study using a 1 month (3 session) intervention format failed to observe significant differences in STD acquisition.19
The purpose of this study was to test the efficacy of a clinic-based, safer sex program specifically designed for young, heterosexual, African American men newly diagnosed with an STD and residing in the Southern U.S. The trial tested the hypotheses that men randomized to the intervention condition would be significantly less likely than controls to acquire a subsequent STD and to engage in unprotected sex. Hypotheses pertaining to fewer sex partners and greater condom application skills for men receiving the intervention were also tested.
Methods
Participants
The study was conducted from September 2004 – May, 2006. Study procedures were approved by the Office of Research Integrity at the University of Kentucky. The trial was registered with the ClinicalTrials.gov protocol registration system (#NCT00314028) and monitored by a DSMB.
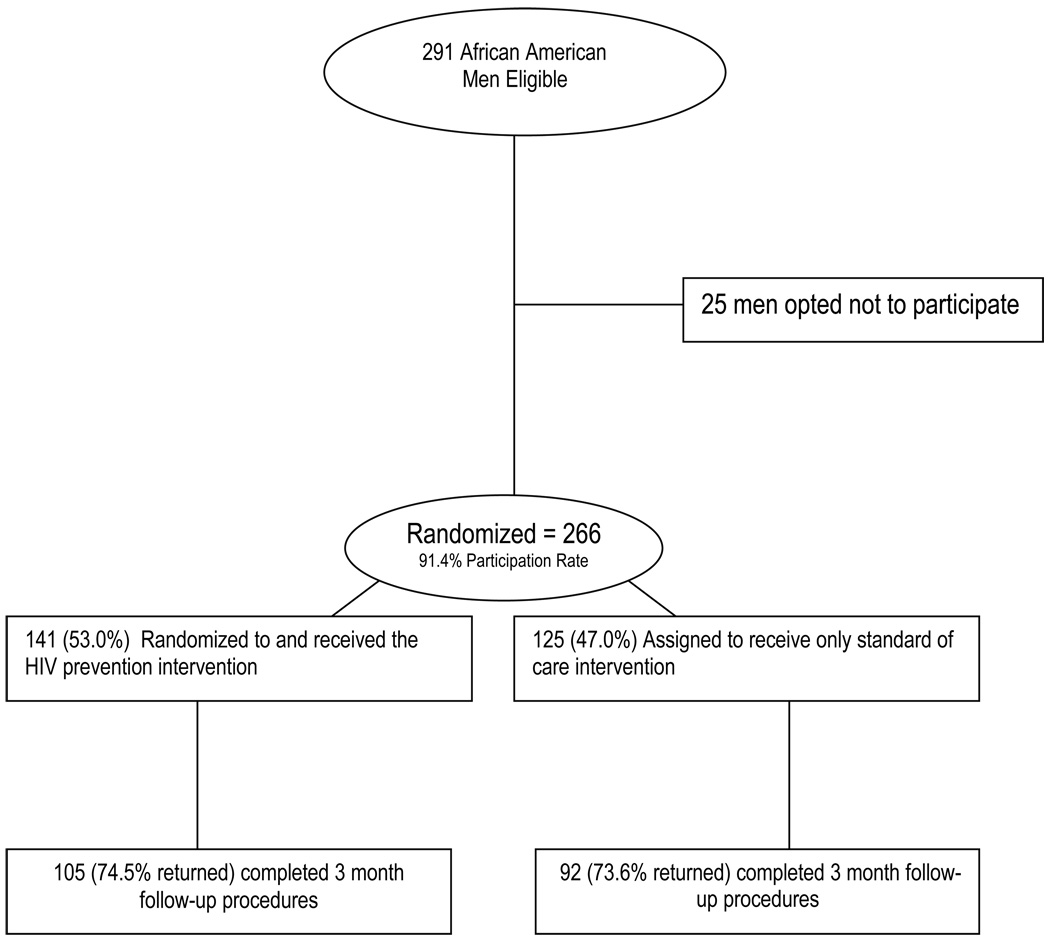
Men were recruited from a public STD clinic located in a Southern U.S. city. Recruitment occurred following diagnosis and treatment for STDs. Nurses assessed potential eligibility by determining whether men: 1) were newly diagnosed with an STD, 2) self-identified as African American, and 3) were 18 to 29 years of age. Potentially eligible men were asked if they would be interested in volunteering for a study. Those indicating any level interest (N = 306) were escorted to the project’s lay health advisor (in an adjacent office) who further screened men for eligibility by determining whether men were English speaking and by asking two questions: 1) are you knowingly HIV positive? and 2) have you used a male condom at least once in the past 3 months for sex (defined as “sexual intercourse,” or “penis in the vagina”) with a woman. Of the 306 potentially eligible men screened by the lay health advisor, 15 were deemed ineligible based on their responses to the second set of eligibility criteria. These inclusion criteria were important because the brief nature of the intervention was designed specifically to improve the quality and consistency of condom use among men reporting recent experience with condom use. Of the 291 men deemed eligible men, 266 (91.4%) were randomized to the two trial conditions (Figure 1).”
Figure 1.
Allocation of Study Participants
Study Design
A two-arm randomized control trial, using concealment of allocation techniques to minimize allocation bias.20 Prior to implementing the trial, a random sequence was determined and envelopes containing allocation cards (coded for intervention or control) were sealed in envelopes, randomly sequenced, and piled; the top envelop on the pile was always used to determine assignment to condition. The trial was conducted using a 3-month follow-up assessment and a 6-month medical records review to assess intervention efficacy.
Intervention Methods
Based on recent evidence suggesting that young African American men experience multiple difficulties with condoms,21,22 the intervention was designed to promote men’s quality, correctness, and consistency of condom use. A one-year formative phase was used to develop the intervention. An initial elicitation study (using the same inclusion criteria as the trial) collected qualitative data pertaining to men’s barriers in achieving correct and consistent condom use.21 Findings were used to develop the brief intervention (approximately 45 to 50 minutes in length) which was then tested and revised based on identified gaps.
The program (“Focus on the Future”) was based on a lay health advisor model. Evidence suggests that lay health advisors have been instrumental in achieving intervention success among various populations of African Americans across a broad range of health behaviors.23 The essence of the model is that the most effective change agents are people who come from the community for which the intervention program is intended. This goes beyond the concept of “matching” by race, age, and gender.24 A young African American male who had grown up and resided in the main catchment area served by the clinic was selected, hired, and trained to implement the intervention. His everyday experiences and way of communicating were indeed no different from that of the men participating in the intervention. The lay health advisor was selected based on his ability to relate effectively to men about sex and condom use, in a non-judgmental manner. Part of this ability included being adept in the process of quickly establishing rapport with men by finding common ground between them. Once selected, he was provided with a three-day training seminar designed to provide him the skills and information needed to deliver the single session intervention.
The single session was predicated on the Information, Motivation, and Behavioral Skills model.25 Information directly relevant to the quality of condom use was provided. For example, men learned that condoms come in a variety of sizes and shapes and they learned about the value of periodically adding water-based lubricants to condoms during sex. Men learned, by demonstration from the lay health advisor, that oil-based lubricants can quickly erode latex condoms. Enhancing men’s motivation to use condoms was an integral component of the session. Throughout the session, men were encouraged men to feel good about using condoms, to experience condoms as being compatible with sexual pleasure, and to actively protect themselves from future STD acquisition. The lay health advisor made constant attempts to equate condom use with an investment in men’s future. Using large posters illustrating the disproportionate HIV/AIDS burden experienced by African American men, they were also motivated to personally respond to the AIDS epidemic. An equally important, but implicit objective, was to be responsive to men’s questions, problems, and concerns regarding safer sex with their female partners. Men were prompted to think about ways they could initiate condom use with existing partners. Skill acquisition was also emphasized. Correct condom and lubrication use were demonstrated and practiced by men until they expressed a sense of mastery. Men were encouraged to use condoms they felt fit them comfortably and provided them with a sense of security. Based on formative work,21 it was determined that men would be provided with pocket size vials of water-based lubricants as well as 12 or more condoms of their choice from a broad selection of brands and sizes
All men enrolled in the study received nurse-delivered messages regarding condom use as per CDC guidelines.26 These messages were typically delivered in only a few minutes and essentially informed men that condoms are an effective means of preventing subsequent STD acquisition when used consistently. As patients of the clinic, all men were allowed to take up to 12 condoms –with only one size and brand available—from the clinic as they exited. In addition to the disease-specific diagnosis and treatment received by men, these procedures comprised clinical standard-of-care. Men randomized to the control condition received only this standard-of-care whereas men randomized to the intervention condition received this standard-of-care plus the Focus on the Future program.
Data Collection
Immediately following diagnosis and study enrollment, men completed a self-administered questionnaire provided to them by the lay health advisor. To avoid problems associated with low literacy, questions were recorded to a CD that men could choose to play using a portable headset. Next, men completed a directly observed condom application skills assessment. The same procedures were repeated at the follow-up assessment. Men were compensated $40 for the first assessment and $60 for the second.
Primary Outcome Measure
Subsequent diagnosis of an STD constituted the primary outcome. Because this publicly-funded clinic was the only low-cost option for men in the entire urban catchment area, a medical records review was used to assess this outcome.
Other Outcome Measures
Four behavioral outcomes were assessed: 1) number of female sex partners in the past 3 months; 2) condom use during the last act of penetrative (penile-vaginal or penile-anal) sex with a female partner; 3) frequency of unprotected penetrative sex with a female partner in the past 3 months, and 4) proficiency in using condoms as determined through direct observation of men’s ability to apply condoms to a stationary, life-size, rubber penile model. For the fourth behavioral outcome, a 9-item checklist was refined based on previous research conducted by the second author.27 This checklist comprised “yes vs. no” indicators completed by the lay health advisor as men demonstrated the task of condom application.
Statistical Analyses
Demographic and baseline attributes among intervention and control participants were compared using two-sample t-tests with unrestricted variances for quantitative variables and chi-square tests for dichotomous variables. Comparisons of demographic and baseline attributes among participants who dropped out of the study and participants who remained in the study were made similarly.
The outcomes of reinfection at any time within the six months and condom use at the last sexual act preceding 3-month follow-up were analyzed using logistic regression. Remaining outcomes were analyzed using linear regression. Univariate analyses used only intervention/control status as a predictor, while multivariable analyses used intervention/control status and several covariates. First, a dichotomized version of monthly income was used as a covariate. Monthly income served as a proxy indicator of socio-economic status (SES). Despite the relatively low-income of the sample we suspected that SES may nonetheless be an important determinant of safer sex practices and reinfection. Second, whether men were diagnosed as having one versus multiple STDs at baseline served as a covariate; this provided an objective marker of past sexual risk behavior among this sample of high-risk men. Given the strong predictive power of past behavior to predict future behavior, we determined this measure was an important covariate. Next, the corresponding baseline measure of the outcome variable (except for reinfection) was always included as a covariate. Finally, the outcome of reinfection was considered to be confounded by condom use and condom use skills thus follow-up values for these two variables were included as covariates.
Because outcome variables (except reinfection) had missing values due to attrition, the primary data analyses (described in the previous paragraph) were performed twice: first with only complete cases (participants for whom there were no missing values), and then using multiple imputation28 as implemented in the MI and MIANALYZE procedures of SAS, Version 9.1.
Finally, since there were some extreme outlying observations with respect to the first and third behavioral outcomes, sensitivity analyses were performed to complement the primary analyses. One set of sensitivity analyses entailed removal of records with extreme outlying values, while the other involved logarithmic transformations to mitigate the outliers’ influence. All analyses were conducted in Version 9.1 of SAS (SAS Institute, Cary, NC).
Results
Baseline Comparability of Groups
Differences between men randomized to the intervention and control conditions were assessed for demographic and other key variables at baseline (Table 1). The only significant difference observed was demonstrated condom application skills; with men in the control condition scoring lower than men in the intervention condition.
Table 1.
Demographic and Other Baseline Attributes of 266 Young African American Men Enrolled in the Intervention Trial, Stratified by Assignment to Condition
| Intervention (n = 141) |
Control (n = 125) |
p-value | |
|---|---|---|---|
| Age | 23.1 ± 3.4 | 23.4 ± 3.1 | 0.49 |
| Net monthly income > $1,000 | 38 (27.0%) | 42 (33.9%) [124] |
0.22 |
| Current relationship is monogamous | 68 (48.6%) [140] |
60 (48.0%) | 0.92 |
| Current relationship is not monogamous | 59 (42.1%) [140] |
54 (43.2%) | 0.86 |
| Previously taught how to use condoms | 127 (90.1%) | 110 (88.7%) [124] |
0.72 |
| Multiple STDs diagnosed at baseline | 41 (29.3%) [140] |
27 (22.1%) [122] |
0.19 |
| Baseline diagnosis included Chlamydia | 55 (39.0%) | 50 (40.3%) [124] |
0.83 |
| Baseline diagnosis included gonorrhea | 87 (61.7%) | 76 (61.3%) [124] |
0.94 |
| Demonstrated condom use skills | 3.83 ± 2.24 [131] |
2.60 ± 1.67 [112] |
< 0.0001 |
| Number of female sex partners, last 3 months1 | 2.91 ± 2.73 | 3.08 ± 2.43 | 0.60 |
| Unprotected acts of sex, last 3 months2 | 16.0 ± 47.3 [123] |
14.3 ± 21.0 [114] |
0.72 |
| Used condoms the last time sex occurred | 74 (52.5%) | 53 (42.4%) | 0.10 |
Entries in the “Intervention” and “Control” columns are Mean ± SD for quantitative variables and Number (Percent) for dichotomous variables. All results pertain to men who self-identified as heterosexual. For variables on which not all subjects had data, the numbers in square brackets identify how many subjects did have data.
Median and interquartile range are 3.0, 1.0 for control group and 2.0, 1.0 for intervention group. Excluding four subjects in control group and three in intervention group who claimed more than 100 unprotected acts at baseline or follow-up or who claimed more than 25 partners at baseline or follow-up, mean and standard deviation are 3.02, 2.29 for control group and 2.70, 1.71 for intervention group.
Median and interquartile range are 6.5, 18.0 for control group and 4.0, 13.0 for intervention group. Excluding four subjects in control group and three in intervention group who claimed more than 100 unprotected acts at baseline or follow-up or who claimed more than 25 partners at baseline or follow-up, mean and standard deviation are 13.51, 18.08 for control group and 11.75, 19.44 for intervention group.
Attrition
Among the 266 participants, 69 (25.9%) did not return to complete the 3-month follow-up assessment (Figure 1). However, we were still able to determine if these men acquired a subsequent STD. Comparing the 197 participants who remained in the study to the 69 who dropped out, there were no significant differences in sociodemographics or baseline attributes (Table 2). Further, the proportions of men dropping out were not significantly different between the two groups (intervention/control). Finally, men dropping out were not significantly different from men completing the study with respect to STD reinfection rates.
Table 2.
Differences Between African American Men Completing Follow-up Assessments and Those Not Completing Follow-up Assessments
| Stayed in (n = 197) |
Dropped out (n = 69) |
p-value | |
|---|---|---|---|
| Age | 23.4 ± 3.3 | 23.0 ± 3.3 | 0.47 |
| Net monthly income > $1,000 | 61 (31.1%) [196] |
19 (27.5%) | 0.58 |
| Current relationship is monogamous | 88 (44.9%) [196] |
40 (58.0%) | 0.06 |
| Current relationship is not monogamous | 88 (44.9%) [196] |
25 (36.2%) | 0.21 |
| Previously taught how to use a condom | 179 (91.3%) [196] |
58 (84.1%) | 0.09 |
| Multiple STDs diagnosed at baseline | 52 (26.7%) {195} |
16 (23.9%) [67] |
0.65 |
| Baseline diagnosis included Chlamydia | 73 (37.2%) [196] |
32 (46.4%) | 0.18 |
| Baseline diagnosis included gonorrhea | 122 (62.2%) [196] |
41 (59.4%) | 0.68 |
| Demonstrated condom use skills | 3.39 ± 2.16 [181] |
2.90 ± 1.84 [62] |
0.09 |
| Number of female sex partners, last 3 months | 3.13 ± 2.81 | 2.61 ± 1.78 | 0.08 |
| Unprotected acts of sex, last 3 months | 16.6 ± 42.0 [171] |
11.6 ± 17.9 [66] |
0.21 |
| Used condoms last time sex occurred | 90 (45.7%) | 37 (53.6%) | 0.26 |
| Assigned to intervention group | 105 (53.3%) | 36 (52.2%) | 0.87 |
| Reinfection | 78 (39.6%) | 30 (43.5%) | 0.57 |
Entries in the “Stayed in” and “Dropped out” columns are Mean ± SD for quantitative variables and Number (Percent) for dichotomous variables. All results pertain to men who self-identified as heterosexual. For variables on which not all subjects had data, the numbers in square brackets identify how many subjects did have data.
Effects of the Intervention
With one exception, the five outcome measures achieved univariate significance in both the complete case and multiple imputation analyses (Table 3). Using the complete case analysis, compared to men in the control condition, those in the intervention were significantly less likely to acquire a subsequent STD within the 6-month follow-up interval (50.4% vs. 31.9%; univariate odds ratio estimate = .46, 95% CI=.28, .76). Men in the intervention scored higher on the condom application skills assessment (mean difference estimate=3.17, 95% CI= 2.81, 3.53, relative difference= +145%). Also, men in the intervention reported significantly fewer sex partners (2.06 vs. 4.15, mean difference estimate= −2.10, 95% CI=−3.22, −.98, relative difference= −51%), significantly fewer acts of unprotected sex (12.3 vs. 29.4, mean difference estimate= −17.1, 95% CI=−33.6, −.5, relative difference=−58%), and were significantly more likely to report using condoms during their last sex episode (72.4% vs. 53.9%, univariate odds ratio estimate = 2.25, 95% CI = 1.24, 4.07). Of note, these results remained relatively unchanged using multiple imputation, with the exception of unprotected sex, which narrowly missed significance.
Table 3.
Comparison of Outcomes, Assessed Three Months Post Intervention, Between 266 Young African American Men Randomly Assigned to Intervention and Control Conditions
| Intervention (n = 141) |
Control (n = 125) |
Univariate Measure of Effect Estimate; [95% CI] p-value |
Multivariable Measure of Effect Estimate; [95% CI] p-value |
|
|---|---|---|---|---|
| Reinfection1 | 45 (31.9%) |
63 (50.4%) |
0.46; [0.28, 0.76] 0.002 |
0.32; [0.12, 0.86] 0.02 |
| Condom use skills2 complete case analysis |
5.35 ± 1.21 [104] |
2.18 ± 1.30 [84] |
3.17; [2.81, 3.53] < 0.0001 |
3.21; [2.80, 3.63] < 0.0001 |
| Condom use skills2 multiple imputation |
3.17; [2.79, 3.54] < 0.0001 |
3.19; [2.81, 3.56] < 0.0001 |
||
| Partners in last 3 months3, 4 complete case analysis |
2.06 ± 1.65 [105] |
4.15 ± 5.59 [91] |
−2.10; [−3.22, −0.98] 0.0003 |
−2.09; [−3.18, −0.99] 0.0002 |
| Partners in last 3 months4 multiple imputation |
−1.85; [−2.97, −0.74] 0.002 |
−1.87; [−2.96, −0.79] 0.001 |
||
| Unprotected acts, last 3 months5,6 complete case analysis |
12.3 ± 25.8 [99] |
29.4 ± 79.3 [84] |
−17.1; [−33.6, −0.5] 0.045 |
−13.4; [−35.6, 8.8] 0.23 |
| Unprotected acts, last 3 months6 multiple imputation |
−14.9; [−31.0, 1.3] 0.07 |
−11.9; [−31.3, 7.5] 0.21 |
||
| Condom used at last act7 complete case analysis |
76 (72.4%) [105] |
49 (53.9%) [91] |
2.25; [1.24, 4.07] 0.008 |
2.20; [1.08, 4.48] 0.03 |
| Condom used at last act7 multiple imputation |
2.27; [1.23,4.19] 0.009 |
2.06; [1.07,3.96] 0.03 |
Entries in the “Intervention” and “Control” columns are Mean ± SD for quantitative variables and Number (Percent) for dichotomous variables.
For quantitative variables, the measure of effect is a mean difference (expected score for intervention participant minus expected score for control participant, adjusted in the multivariable analyses for covariates specified below) and is estimated by linear regression.
For dichotomous variables, the measure of effect is an odds ratio (odds in favor for intervention participant divided by odds in favor for control participant, adjusted in the multivariable analyses for covariates specified below) and is estimated by logistic regression.
Complete case analyses use only those subjects for whom there are no missing values on variables in the regression model. Multiple imputation analyses use all subjects. All results pertain to men who self-identified as heterosexual. For variables on which not all subjects had data, the numbers in square brackets identify how many subjects did have data.
Multivariable analysis controls for monthly income level, having one versus two or more STDs diagnosed at study enrollment (“mixed STDs”), follow-up values for condom skills, and follow-up values for condom use at last sex
Multivariable analysis controls for income, mixed STDs, and the baseline value of condom skills
Median and interquartile range are 2.0, 3.0 for control group and 2.0, 2.0 for intervention group. Excluding four subjects in control group and three in intervention group who claimed more than 100 unprotected acts at baseline or follow-up or who claimed more than 25 partners at baseline or follow-up, mean and standard deviation are 3.52, 4.04 for control group and 2.00, 1.47 for intervention group. See also Table 3.
Multivariable analysis controls for income, mixed STDs, and the baseline value for number of female sex partners in the past 3 months
Median and interquartile range are 4.5, 21.0 for control group and 1.0, 11.0 for intervention group. Excluding four subjects in control group and three in intervention group who claimed more than 100 unprotected acts at baseline or follow-up or who claimed more than 25 partners at baseline or follow-up, mean and standard deviation are 17.24, 28.77 for control group and 11.12, 21.96 for intervention group. See also Table 3.
Multivariable analysis controls for income, mixed STDs, and baseline values for skill, unprotected sex, and condom use at last sex
Multivariable analysis controls for income, mixed STDs, and the baseline values for skills and condom use at last sex
Multivariable analysis yielded more robust intervention effects on subsequent STD acquisition (Table 3). Men randomized to the intervention had about 68% lower odds of acquiring a subsequent STD (AOR estimate=.32, 95% CI=.12, .86). Furthermore, findings from the multiple imputation analyses indicated that men in the intervention condition had a higher score on the condom application assessment (mean difference estimate=3.19; 95% CI=2.81, 3.56), fewer female sex partners (mean difference estimate=−1.87, 95% CI=−2.96,−.79), and were more likely to report condom use at last sexual episode (AOR estimate=2.06, 95% CI=1.07, 3.96). One outcome did not achieve statistical significance in multivariable analyses, namely, number of episodes of unprotected sex in the past 90 days (mean difference estimate = −11.9, 95% CI = −31.3, 7.5).
Both sets of sensitivity analyses preserved the conclusions from the primary analyses that intervention men had significantly fewer female sex partners than control men (Table 4). The sensitivity analyses involving logarithmically transformed number of unprotected acts preserved the mixed conclusions from the primary analyses, in particular statistical significance with univariate complete cases but lack thereof with multivariable multiple imputation; the sensitivity analyses entailing removal of records disagreed with the primary analyses only in that statistical significance was not achieved with univariate complete cases.
Table 4.
Results of Sensitivity Analyses for Selected Outcomes Measures Used to Compare Men Randomized to the Intervention Versus Control Conditions
| Observations with extreme outlying values excluded1 |
Response variable transformed logarithmically to reduce the impact of outlying values2 |
|||
|---|---|---|---|---|
| Univariate Measure of Effect Estimate; [95% CI] p-value |
Multivariable Measure of Effect Estimate; [95% CI] p-value |
Univariate Measure of Effect Estimate; [95% CI] p-value |
Multivariable Measure of Effect Estimate; [95% CI] p-value |
|
| Partners in last 3 months3 complete case analysis |
−1.52; [−2.37, −0.67] 0.0005 |
−1.37; [−2.18, −0.55] 0.001 |
−0.33; [−0.49, −0.17] < 0.0001 |
−0.32; [−0.47, −0.17] < 0.0001 |
| Partners in last 3 months3 multiple imputation |
−1.28; [−2.14, −0.43] 0.004 |
−1.19; [−2.01, −0.36] 0.006 |
−0.29; [−0.48, −0.10] 0.004 |
−0.29; [−0.48, −0.11] 0.004 |
| Unprotected acts, last 3 months4 complete case analysis |
−6.1; [−13.7, 1.4] 0.11 |
−1.0; [−9.8, 7.9] 0.83 |
−0.53; [−1.00, −0.07] 0.03 |
−0.32; [−0.88, 0.24] 0.26 |
| Unprotected acts, last 3 months4 multiple imputation |
−4.6; [−12.0, 2.8] 0.21 |
−3.3; [−11.9, 5.4] 0.43 |
−0.50; [−0.99, 0.00] 0.051 |
−0.42; [−1.00, 0.17] 0.15 |
Four subjects in the control group and three subjects in the intervention group were excluded who claimed more than 100 unprotected acts at baseline or follow-up or who claimed more than 25 partners at baseline or follow-up.
The transformed value is the natural logarithm of one plus the original value. Point and 95% interval estimates for measures of effect are not directly comparable to those obtained in the absence of a logarithmic transformation; the main feature of interest is whether the p-value is in qualitative agreement with the corresponding p-value in Table2(i.e.,both less than 0.05 or both greater than 0.05).
Multivariable analysis controls for income, mixed STDs, and the baseline value for number of female sex partners in the past 3 months
Multivariable analysis controls for income, mixed STDs, and baseline values for skill, unprotected sex, and condom use at last sex.
Comment
Findings provide support for this brief, clinic-based intervention for young, heterosexual, African American men at-risk of STD/HIV acquisition. The lower rate of subsequent STD acquisition, reduction in STD/HIV-associated sexual behaviors, and improvement in condom application skills clearly support intervention efficacy. The practical value of the findings is paramount as they demonstrate marked reductions in STD incidence without the use of lengthy, resource-intensive programs. Moreover, the reduction in incidence over the 6-month post-intervention period produced a larger effect size than those observed in previous trials of brief, clinic-based interventions for African American men.14–19,29 The observed protective value relative to subsequent infection was also greater than that derived from a recent meta-analysis of clinic-based STD prevention programs (.32 vs. .85).29 The treatment advantage may be attributable to multiple factors such as tailoring to a relatively homogenous population of men, intervening only with men who reported prior experience in using condoms, and the use of a lay health advisor model. The effect may also be partially explained by the observation that men randomized to the intervention group reported significantly fewer sex partners at follow-up (an unexpected finding).
In an era when CDC has stated, “In the United States, the HIV/AIDS epidemic is a health crisis for African Americans,”30 the findings offer one approach to address this marked racial disparity. The findings also suggest a protective benefit for men’s female sex partners, typically African American women. Because power imbalances in heterosexual relationships may favor males, intervention with African American men may also protect African American women against HIV/STD acquisition.31 Indirect effects may also occur by lowering the prevalence of STDs within African American women’s sexual networks.32 In turn, reductions in STD prevalence and incidence among African American men and women may mitigate the racial disparity in HIV/AIDS by removing STDs as a co-factor.33–36
The brief nature of the intervention also warrants comment. Implementation of small-group interventions or multi-session interventions may not be optimally efficient in STD clinics. Because clinics are designed to provide patients with a series of one-to-one interactions with clinical staff, the concept of triaging young African American men, newly diagnosed with an STD, into an additional one-to-one session with a lay health advisor represents a relatively simple expansion of the existing clinical paradigm. In addition, the use of a lay health advisor to implement the intervention may be a cost-effective strategy. The relative ease of implementation and higher cost-effectiveness may address the problem of effectively translating evidence-based research into practice.37–40
There are a number of limitations to the study. First, as is true for all sexuality research, findings are limited by the validity of retrospective self-report, although this limitation is somewhat mitigated by the medical records review findings pertaining to STD reinfection. Further, as is typically true for STD/HIV behavioral randomized trials, the use of a non-probability sample limits the ability to generalize the findings to young, heterosexual, African American men newly diagnosed with an STD in other clinics of the United States. Another concern was the attrition rate. That 26% of the enrolled men did not return for the follow-up assessment (despite potential compensation of $60 and lack of employment) suggests that these men may experience instability in their daily lives, perhaps as a consequence of poverty and discrimination. However, differences between drop-outs and men completing the study were not observed and attrition was not a problem relative to the primary study outcome given that these data were collected by medical record review. Although urine-based PCR testing for subsequent STD acquisition may have been a more rigorous approach, the use of archival data is not uncommon, even in large-scale trials that employ PCR testing.41 Although this study design could not ascertain whether men were diagnosed with subsequent STD infections elsewhere, options for alternative sources of clinical care were limited and most likely would be comparably distributed between study conditions. The relatively short duration of the follow-up period pertaining to behavioral outcomes is also a limitation given that maintenance of intervention effects could not be assessed over longer time periods. Also noteworthy is that the program was specifically designed to improve the quality, correctness, and frequency of use among men recently using condoms thereby excluding those entirely rejecting condom use. This planning decision was made based on our awareness that a 40-minute intervention is unlikely to change behaviors of men who never use condoms. However, a complete lack of condom use (“never use”) among young, African American men is not be the norm, as nationally representative data indicate that less than one of every 6 young African American men reported never using condoms during a 12-month recall period.42 It must also be acknowledged that the use of multiple raters would have allowed us to establish intra-rater reliability for the measure of demonstrated condom application skills; this limitation should be considered in the larger context of the study findings (that is, the “skills variable” was only one of several supporting outcomes). Finally, the study design cannot determine what portion of the observed effect was attributable to the provision of condoms to men in a variety of sizes and brands. This is less of a limitation than a product of the intervention’s intent to increase men’s pleasure in using condoms by providing a range of options.
Conclusions
The weight of evidence suggests that a brief, clinic-based intervention may be efficacious in reducing subsequent acquisition of STDs among young, heterosexual, African American men newly diagnosed with an STD. By using a lay health advisor, these intervention effects can be achieved with minimal resources. As the United States40 and other countries43 implement clinic-based counseling in settings that provide STD screening, the option of post-diagnostic counseling conducted by a lay health advisor, may prove useful. Adaptation and application of the program in geographic areas (domestically and globally) experiencing epidemics of STD/HIV may be worth pursuing in future studies.
References
- 1.Centers for Disease Control and Prevention. Atlanta, GA: US Department of Health and Human Services; HIV/AIDS Surveillance. (year end edition) 2004
- 2.Centers for Disease Control and Prevention. Atlanta, GA: Department of Health and Human Services; African Americans and AIDS. 2006
- 3.Centers for Disease Control and Prevention. HIV/AIDS Among African Americans. Fact Sheet. [Accessed on March 5, 2006]; Available on-line at http://www.cdc.gov/hiv/pubs/facts/afam.htm.
- 4.Centers for Disease Control and Prevention. Health Disparities Experienced by Black or African Americans, United States. MMWR. 2005;54:1–3. [PubMed]
- 5.Southern States AIDS/STD Directors Work Group. Southern States Manifesto: HIV/AIDS & STDs in the South – A call to action. 2003 [Google Scholar]
- 6.Centers for Disease Control and Prevention. Atlanta, GA: U.S. Department of Health and Human Services; Sexually transmitted disease surveillance, 2003. 2004
- 7.Ruiz MS, Gable AR, Kaplan EH, Soto MA, Fienberg HV, Russell J. Institutes of Medicine. Washington, DC: National Academy Press; 2001. No time to lose: Getting more from HIV prevention. [PubMed] [Google Scholar]
- 8.National Institutes of Health. NIH Fiscal Year 2007 Plan for HIV-Related Research. [Accessed March 9. 2006]; Available online at: http://www.nih.gov/od/oar/public/pubs/fy2007/VIII_RacialEthnic.pdf.
- 9.Seal DW, Ehrhardt AA. HIV-prevention-related sexual health promotion for heterosexual men in the United States: pitfalls and recommendations. Arch Sex Behav. 2004;33:211–212. doi: 10.1023/B:ASEB.0000026621.21559.cf. [DOI] [PubMed] [Google Scholar]
- 10.Seal DW, Exner TM, Ehrhardt AA. HIV sexual risk reduction intervention with heterosexual men. Arch Intern Med. 2003;163:738–739. [PubMed] [Google Scholar]
- 11.Elway AR, Hart GJ, Hawkes S, Petticrew M. Effectiveness of interventions to prevent sexually transmitted infections and human immunodeficiency virus in heterosexual men: A systematic review. Arch Intern Med. 2002;162:1818–1830. doi: 10.1001/archinte.162.16.1818. [DOI] [PubMed] [Google Scholar]
- 12.Lyles CM, Kay LS, Crepaz N, et al. Best-evidence interventions: Findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Am J Public Health. 2007;97:133–143. doi: 10.2105/AJPH.2005.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Compendium of HIV prevention interventions with evidence of effectiveness. [Accessed October 17, 2006]; Available on-line at http://www.cdc.gov/hiv/pubs/HIVcompendium/HIVcompendium.htm.
- 14.O'Donnell CR, O'Donnell L, San Doval A, Duran R, Labes K. Reductions in STD infections subsequent to an STD clinic visit: Using video-based patient education to supplement provider interactions. Sex Transm Dis. 1998;25:161–168. doi: 10.1097/00007435-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kamb ML, Fishbein M, Douglas JM, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. JAMA. 1998;280:1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 16.Bolu OO, Lindsey C, Kamb M, et al. Is HIV/Sexually transmitted disease prevention counseling effective among vulnerable populations? A subset analysis of data collected for a randomized controlled trail evaluating counseling efficacy (Project RESPECT) Sex Transm Dis. 2004;31:469–474. doi: 10.1097/01.olq.0000135987.12346.f2. [DOI] [PubMed] [Google Scholar]
- 17.Cohen DA, Dent C, MacKinnon D, Hahn G. Condoms for men, not women: Results of a brief promotion program. Sex Transm Dis. 1992;19:245–251. doi: 10.1097/00007435-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kalichman SC, Cherry C, Browne-Sperling F. Effectiveness of a video-based motivational skills-building HIV risk reduction intervention for inner-city African American men. J Consult Clin Psych. 1999;67:959–966. doi: 10.1037//0022-006x.67.6.959. [DOI] [PubMed] [Google Scholar]
- 19.Maher JE, Peterman TA, Osewe PL, et al. Evaluation of a community based organization’s intervention to reduce the incidence of sexually transmitted diseases: A randomized controlled trial. South Med J. 2003;96:248–253. doi: 10.1097/01.SMJ.0000054605.31081.07. [DOI] [PubMed] [Google Scholar]
- 20.Schulz KF. Subverting randomization in controlled trials. JAMA. 1995;274:1456–1458. [PubMed] [Google Scholar]
- 21.Crosby RA, Graham CA, Yarber WL, Sanders SA. If the condom fits, wear it: A qualitative study of young African American men. Sex Transm Infect. 2004;80:306–309. doi: 10.1136/sti.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crosby RA, Yarber WL, Sanders SA, Graham CA, et al. Men with Broken Condoms: Who and Why? Sex Transm Infect. 2007;83:71–75. doi: 10.1136/sti.2006.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eng E, Parker E. Natural helper models to enhance a community’s health and competence. In: DiClemente RJ, Crosby RA, Kegler M, editors. Emerging Theories in Health Promotion Practice and Research. San Francisco, CA: Jossey-Bass Wiley; 2002. pp. 126–156. [Google Scholar]
- 24.Jemmott JB, III, Jemmott LS, Fong GT. Abstinence and safer sex HIV risk- reduction interventions for African American adolescents: A randomized, controlled trial. JAMA. 1998;279:1529–1536. doi: 10.1001/jama.279.19.1529. [DOI] [PubMed] [Google Scholar]
- 25.Fisher J, Fisher W. The Information-Motivation-Behavioral Skills Model. In: DiClemente RJ, Crosby RA, Kegler M, editors. Emerging Theories in Health Promotion Practice and Research. San Francisco, CA: Jossey-Bass Wiley; 2002. pp. 40–70. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. Morb Mortal Wkly Rep. 2002;51:1–60. [PubMed] [Google Scholar]
- 27.DiClemente RJ, Wingood GM, Harrington KF, et al. Efficacy of an HIV prevention intervention for African American adolescent females: A randomized controlled trial. JAMA. 2004;292:171–179. doi: 10.1001/jama.292.2.171. [DOI] [PubMed] [Google Scholar]
- 28.Roderick JA, Little, Rubin Donald B. Statistical Analysis with Missing Data. 2nd Edition. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 29.Crepaz N, Horn AK, Rama SM, et al. The efficacy of behavioral interventions in reducing HIV risk sex behaviors and incident sexually transmitted diseases in black and Hispanic sexually transmitted disease clinic patients in the United States: A meta-analytic review. Sex Transm Dis. 2007;34:319–332. doi: 10.1097/01.olq.0000240342.12960.73. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. A heightened national response to the HIV/AIDS crisis among African Americans. Atlanta, GA: Department of Health and Human Services; 2007. Mar, [Google Scholar]
- 31.Puri CP, Balaiah D, Iyer KS. Increased male responsibility and participation: The key to improving reproductive health. ICMR Bull. 1999;29:59–70. [PubMed] [Google Scholar]
- 32.Pourbohloul B, Brunham RC. Network models and transmission of sexually transmitted diseases. Sex Transm Dis. 2004;31:388–390. doi: 10.1097/00007435-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Laga M, Monoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: Results from a cohort study. AIDS. 1993;7:95–101. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Sorvillo F, Kernott P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- 35.Wasserheit JN. Epidemiological synergy: Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–72. [PubMed] [Google Scholar]
- 36.Grosskurth H, Mosha F, Todd J, et al. Impact if improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: A randomized controlled trial. Lancet. 1995;346:53–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 37.Kerner J, Rimer B, Emmons K. Dissemination research and research dissemination: How can we close the gap? Health Psychol. 2005;24:443–446. doi: 10.1037/0278-6133.24.5.443. [DOI] [PubMed] [Google Scholar]
- 38.Glasgow RE, Lichtenstein E, Marcus AC. Why don't we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–1267. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasgow RE. The future of health behavior change research: What is needed to implement translation research into health promotion practice. Annals Behav Med. 2004;27:3. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- 40.Rietmeijer CA. Risk reduction counseling for prevention of sexually transmitted infections: how it works and how to make it work. Sex Transm Infect. 2007;83:2–9. doi: 10.1136/sti.2006.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The National Institutes of Mental Health (NIMH) Multisite HIV Prevention Trial Group. The NIMH multisite HIV prevention trial: Reducing HIV sexual risk behavior. Science. 1998;280:1889–1894. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- 42.Udry R, Mullen Harris K, Elder GH. [Accessed on July 9th, 2007];The National Longitudinal Study of Adolescent Health. Available online at: http://www.cpc.unc.edu/addhealth/data.
- 43.Ward H. One-to-one counseling for STI prevention: not so much whether as how. Sex Transm Infect. 2007;83:1. [PMC free article] [PubMed] [Google Scholar]