Abstract
Background and Purpose
Intraplaque haemorrhage (IPH) within intracranial atherosclerotic plaques identified by high resolution MRI (hrMRI) has been studied as a potential marker of stroke risk. However, previous studies only examined intracranial arteries with significant stenosis (degree of stenosis >50%). This study aimed to ascertain the clinical relevance of IPH in patients with low and high grade stenotic basilar artery plaques.
Methods
Patients with basilar artery stenosis (n= 126; mean age 62±10; 66 symptomatic and 60 asymptomatic) underwent hrMRI. The relationship between imaging findings (IPH, contrast enhancement, degree of stenosis, minimal lumen area [MLA] and plaque burden [PB]) and symptoms were analysed.
Results
IPH was identified in 22 patients (22%), including 21 (31.8%) symptomatic patients, and 1 (1.7%) asymptomatic patient. Multivariate analysis showed IPH was the strongest independent marker of symptomatic status (odds ratio [95% confidence interval]: 27.5 [3.4, 221.5]; p=0.002). Contrast enhancement was also independently associated with symptomatic status (odds ratio: 9.9 [1.5, 23.6]; p=0.016). Stenosis, MLA and PB were not correlated with symptoms (p>0.05). IPH was present in both low and high grade stenotic basilar arteries (11.3% vs. 16.3%, p=0.63). Diagnostic performance values of IPH for acute/subacute symptomatic stroke patients were: specificity 98.3%, sensitivity 31.8%, positive predictive value 95.5% and negative predictive value 56.7%.
Conclusions
IPH is present in both low and high grade stenotic basilar artery plaque and independently associated with symptomatic stroke status. IPH may identify high-risk plaque and provide new insight into the management of stroke patients without significant stenosis.
Introduction
Intracranial atherosclerotic disease (ICAD) is a major cause of stroke that has likely been underappreciated, in part due to challenges in detecting intracranial atherosclerotic plaque 1. ICAD may produce ischemia through multiple mechanisms including: thrombotic occlusion; occlusion of small perforating arteries; plaque rupture leading to artery-to-artery embolization; and vessel luminal narrowing leading to hypoperfusion 2. Current practice guidelines rely solely on the degree of stenosis (often ≥ 50%) of major intracranial arteries in determining management strategies. However, many authors have questioned this practice, especially given the high prevalence at autopsy of mild and moderate intracranial arterial stenosis in fatal stroke 2–6. Branch occlusive disease, in particular, has been underestimated and appears to constitute a more common cause of stroke 5. Other factors including plaque composition, arterial hemodynamic features, and collateral status have been proposed as alternative variables to better predict recurrent stroke risk 4.
ICAD with ischemia caused by artery-to-artery embolization from plaque rupture may require more aggressive antiplatelet therapy to mitigate clot progression whereas stenotic disease resulting in hypoperfusion might benefit more from angioplasty 2. Given these important distinctions in treatment selection for patients with ICAD based on stroke mechanism, the assessment of culprit plaque location and morphology is clinically relevant 2. In the past decade, many advances have been made in visualizing large vessel intracranial plaque morphology and composition using high-resolution MRI (hrMRI) 7, 8. Expanded use of hrMRI could result in the reclassification of many strokes previously termed cryptogenic and could improve clinical decision-making. A few studies have examined atherosclerotic plaque of the posterior circulation using hrMRI with improved characterization of basilar ICAD 9–13.
Intraplaque hemorrhage (IPH) occurs in atherosclerotic plaque and is attributed to fragile neovascularity with endothelial disruption that increases plaque wall stress, making plaque more vulnerable 14. IPH was first identified as T1-weighted hyperintense signal in extracranial carotid plaque imaging 15, and subsequent papers have supported carotid IPH as a risk factor for recurrent stroke independent of stenosis 16, 17. Preliminary work using intracranial hrMRI showed similar appearance of IPH within intracranial arteries in symptomatic ICAD 18. Xu et al. observed T1-weighted hyperintense signal more often in symptomatic middle cerebral artery plaque than in asymptomatic plaques 19. More recently, in patients with basilar artery stenosis, hrMRI has found IPH to be more frequent in symptomatic patients than in asymptomatic patients (42.3% versus 21.4%), suggesting a risk for IPH with a relative risk of 1.64 12.
These prior basilar artery hrMRI studies have only imaged basilar artery plaque with high grade stenosis. The primary aim of this study is to ascertain the presentation and clinical relevance of basilar artery IPH in both low and high grade stenotic basilar artery plaques. Given the frequency of non-stenotic ICAD in stroke and preliminary evidence supporting IPH as a risk factor, we hypothesize that IPH status might be useful in distinguishing acute/subacute symptomatic from chronic/asymptomatic basilar artery plaque regardless of degree of stenosis.
Methods
The authors declare that all supporting data are available within the article.
Study Population
This study was approved by the local Institutional Review Board with all patients providing written informed consent.
This is a prospective study. Patients with basilar artery atherosclerotic disease were recruited to this study between September 2013 and October 2016. The inclusion criteria were: 1) ischemic stroke or transient ischemic attacks (TIA) in the basilar artery territory, and/or basilar artery stenosis >30% on DSA, CTA or MRA; and 2) more than one atherosclerotic risk factors, including hypercholesterolemia, hypertension, smoking, and diabetes mellitus. Exclusion criteria included: 1) coexistent unilateral or bilateral vertebral artery stenosis >50% on MRA; 2) complete basilar artery occlusion; 3) dissection; 4) intracranial dolichoectasia; 5) non-atherosclerotic intracranial arterial disease, e.g. inflammatory arteritis and congenital agenesis; 6) presence of atrial fibrillation on 24-hour monitoring; (7) clinical contraindications to MRI.
Symptomatic plaque was defined if conventional neuroimaging (FLAIR and DWI images) demonstrated infarct within the basilar artery territory. Patients were classified into two groups based on their symptom presentation: 1) symptomatic ischemic stroke/TIA symptoms presented less than 12 weeks before imaging; and 2) patients with asymptomatic basilar plaque without neuroimaging evidence of infarct.
Patients’ clinical information including age, sex, diabetes, hypertension, smoking, hyperlipidemia, pre-admission statin and aspirin use, ischemic coronary heart disease and National Institute of Health Stroke Scale (NIHSS) score were collected.
MRI protocol
MRI was performed on a 3 Tesla whole body MRI scanner (Skyra; Siemens Healthcare, Erlangen, Germany) with a 20-channel phased array head and neck coil. Three-dimensional time-of-flight images were acquired in the axial plane (TR/TE = 21/3.43ms, FOV = 181×200mm, thickness = 0.7mm, and matrix = 331×384). 3D TOF images were reformatted using multi-planar reconstruction. HrMRI scanning was then performed in planes perpendicular to the basilar artery. The hrMRI protocol included three sequences with one sequence repeated post-contrast (12 slices with 2mm slice thickness; in-plane resolution of 0.4mm × 0.3mm; FOV 100mm100mm; matrix 256× 320): pre-contrast T1-weighted fast-spin-echo (TR/TE=581/18ms; echo train length (ETL)=4; number of excitations (NEX)=4), T2-weighted FSE (TR/TE = 2890ms/46ms; ETL=20; NEX=3), and post-contrast T1-weighted FSE. In-flow saturation bands was placed below the imaging slab for blood suppression. In addition, 12 coronal slices were scanned with similar scan parameters, to help exclude the potential IPH-mimicking flow artefacts. Clinical DWI and FLAIR imaging were used for the identification of infarct.
Image Analysis
Stenosis value was measured independently on hrMRI images by two experienced radiologists (XT and QL, 7 and 15 years’ experience in neuroradiology), who were blinded to the patients’ clinical information 20. The stenosis value was calculated as (1−DStenosis/DNormal) × 100%, while DStenosis is the minimal lumen diameter at the site of at the site maximal stenosis, and DNormal is the lumen diameter at the site of normal basilar artery (either distal or proximal to the stenosis site). Presence of fresh IPH was identified as >150% signal relative to nearby medial pterygoid muscles on pre-contrast T1-weighted images by the two radiologists independently, blinded to the patient’s clinical information 12. Because intraluminal thrombus/hematoma in intracranial dolichoectasia or dissection also exhibits hyperintense signal on pre-contrast T1-weighted images, we carefully excluded these conditions based on their imaging features 21,22. Basilar dolichoectasia was identified if the basilar diameter was enlarged greater than 4 mm and demonstrated a tortuous appearance on MRA 21. Dissection might appear with a dissection flap, double lumen or as a tapering vessel; intraluminal thrombus in dissection also usually involves a long segment 22. Acute or subacute thrombus demonstrates different morphology compared with IPH. Thrombus is long and close to the lumen, while IPH is focal within plaque and is often eccentric to the lumen.
Lumen and outer wall boundary was manually segmented using CMRTools software (Cardiovascular Imaging Solutions Ltd, UK) on T2-weighted images. Reproducibility of this area measurement method was previously reported (measurement error for plaque area: 7.5%) 23.
The contrast enhancement percentage was measured at the slice of greatest enhancement, using adjacent gray matter (in a region of ~15 mm2) to normalize signal intensity. The contrast enhancement percentage was calculated as ((signal of plaque [post-contrast]/signal of gray matter [post-contrast])/(signal of plaque [pre-contrast]/signal of gray matter[pre-contrast]) − 1) × 100%. Plaque burden was measured on the maximal stenosis site, and was defined as (1 − Lumen Area/Outer Area) × 100%.
Statistical Analysis
Normality assumptions were formally assessed using a Shapiro–Wilk’s test. Distributions were summarized using the mean ± standard deviation or median [inter-quartile range]. Categorical data were expressed as counts or percentages. Continuous data were compared using either a Mann-Whitney U test or Student’s t-test. Categorical variables were analyzed using Fisher’s exact test. Multivariate logistic regression analysis was used to determine the independent factors associated with acute/sub-acute symptom. Intraclass correlation coefficient (ICC) and Cohen’s kappa coefficient were used to evaluate the agreement between two reviewers for the measurement of degree of stenosis and the identification of IPH. A p-value of less than 0.05 was considered statistically significant. All p-values were two-sided. GraphPad prism 5 (GraphPad Software Inc., CA, USA) and R Statistics (version 3.1.3, www.r-project.org) were used for data analysis.
Results
Patients demographics and imaging findings
A total of 175 patients met the inclusion criteria. 49 patients were excluded due to intracranial aneurysm (n=28), moyamoya disease (n=3), vasculitis (n=1), dissection (n=6), coexistent vertebral artery stenosis >50% (n=8) and bad image quality (n=3). As a result, 126 patients were included in the final analysis (age 61.2±10.4, 71 male, mean degree of stenosis of 51.6±15.5%). A total of 66 patients were symptomatic and 60 were asymptomatic. 46 patients had a degree of stenosis ≤50% (low grade stenosis, range 13.4% to 48.7%), while 80 patients had a degree of stenosis >50% (high grade stenosis, range 50.1% to 80.3%). Demographic information and imaging findings are summarized in Table 1 and Table 2 based on patients’ degree of stenosis and symptom status.
Table 1.
Clinical Characteristics and Imaging Findings of Patients with low and high grade stenotic basilar artery plaque.
| ALL (n=126) | Low grade stenosis (<50%, n=46) | High grade stenosis (≥50%, n=80) | p value | |
|---|---|---|---|---|
| Age | 61.5±10.0 | 63.70±9.4 | 60.3±10.1 | 0.07 |
| Sex (male) | 82 (65.1%) | 33(71.7%) | 49(61.3%) | 0.23 |
| Diabetes | 42 (33.3%) | 15 (32.6%) | 27 (33.8%) | 0.90 |
| Smoking | 35 (27.8%) | 13 (28.3%) | 22 (27.5%) | 0.93 |
| Hypertension | 101(80.2%) | 37 (80.4%) | 64 (80.0%) | 0.96 |
| Hyperlipidemia | 65 (51.6%) | 18 (39.1%) | 47 (58.8%) | 0.03* |
| Coronary Artery Disease | 5 (4.0%) | 3 (6.5%) | 2 (2.5%) | 0.27 |
| Pre-admission Aspirin use | 31 (24.6%) | 9 (19.6%) | 22 (27.5%) | 0.32 |
| Pre-admission Statin use | 17 (13.5%) | 4 (8.7%) | 13 (16.3%) | 0.23 |
| NIHSS score (median and range) | 1 (0,11) | 2 (0,11) | 0(0,6) | <0.001* |
| Degree of Stenosis (%) | 52.6±15.8 | 35.2±9.7 | 62.6±7.9 | <0.001* |
| Enhancement Percentage (%) | 17.4±28.4 | 21.1 ±33.1 | 15.3 ±25.3 | 0.27 |
| Intraplaque Haemorrhage | 22 (17.5%) | 9 (19.6%) | 13(16.3%) | 0.63 |
| Minimum Lumen Area | 3.2±2.97 | 5.1±3.4 | 2.1±1.3 | <0.001* |
| Plaque Burden (%) | 84.0±9.1 | 75.9±8.2 | 88.6±5.7 | <0.001* |
| Symptomatic | 66 (52.4%) | 28(60.9%) | 38 (47.5%) | 0.15 |
NIHSS: National Institute of Health Stroke Scale
Statistically significant
Table 2.
Clinical characteristics and imaging findings of patients with different symptom stages.
| ALL (n=126) | Symptomatic (n=66) | Asymptomatic (n=60) | p value | |
|---|---|---|---|---|
| Age | 61.5±10.0 | 61.7±10.5 | 61.4±9.4 | 0.86 |
| Sex (male) | 82 (65.1%) | 50 (75.8%) | 32 (53.3%) | <0.001* |
| Diabetes | 42 (33.3%) | 23 (34.8%) | 19 (31.7%) | 0.71 |
| Smoking | 35 (27.8%) | 25 (37.9%) | 10 (16.7%) | <0.001* |
| Hypertension | 101(80.2%) | 52 (78.8%) | 49 (81.7%) | 0.69 |
| Hyperlipidemia | 65 (51.6%) | 38 (57.6%) | 27 (45.0%) | 0.16 |
| Coronary Artery Disease | 5 (4.0%) | 3 (4.5%) | 2 (3.3%) | 0.73 |
| Pre-admission Aspirin use | 31 (24.6%) | 21 (31.8%) | 10 (16.7%) | 0.06 |
| Pre-admission Statin use | 17 (13.5%) | 10 (15.2%) | 7 (11.7%) | 0.57 |
| NIHSS score (median and range) | 1 (0,11) | 2 (0,11) | 0 (0,4) | <0.001* |
| Degree of Stenosis (%) | 52.6±15.8 | 52.4±15.5 | 52.8±16.1 | 0.88 |
| Enhancement Percentage (%) | 17.4± 28.4 | 25.5±26.1 | 8.5±28.5 | <0.001* |
| Intraplaque Haemorrhage | 22 (17.5%) | 21 (31.8%) | 1 (1.7%) | <0.001* |
| Minimum Lumen Area (mm2) | 3.2±2.7 | 3.7±2.8 | 2.7±2.6 | 0.04* |
| Plaque Burden (%) | 84.0±9.1 | 83.6±9.8 | 84.4±8.2 | 0.62 |
NIHSS: National Institute of Health Stroke Scale
Statistically significant
IPH was identified in 22 patients (17.5%), including 21 symptomatic patients (31.8%), and 1 (1.7%) asymptomatic patient (Table 2). IPH appeared over a range of stenosis values (Table 1), and there was no difference in the presentation rate in low and high grade stenosis basilar arteries (19.6% vs. 16.3%, p=0.63). The IPH to muscle signal ratio was 2.10±0.54 (range 1.50 to 3.19). In these 21 stroke patients with IPH, 15 were paramedian pontine infarct stroke, and 6 were lacunar infarction stroke. Univariate and multivariate analysis of the parameters associated with symptoms is shown in Table 3. IPH was the strongest independent indicator of symptomatic status (odds ratio [95% confidence interval]: 27.5 [3.4, 221.5]; p=0.002). Contrast enhancement was also independently associated with symptomatic status (odds ratio: 9.9 [1.5, 23.6]; p=0.016). Degree of stenosis, minimal lumen area and plaque burden were not significantly associated with symptoms (p>0.05). Presence of IPH was not associate with stenosis (r = 0.16, p=0.10) or plaque burden (r = 0.16, p=0.11). Representative basilar artery plaque images with and without IPH were shown in Figure 1 to 3.
Table 3.
Univariate and multivariate analysis of the parameters associated with symptomatic status.
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
| ||||
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Sex (male) | 2.7 (1.2,5.8) | <0.001 | ||
| Smoking | 3.0 (1.3, 7.1) | <0.001 | ||
| Enhancement Percentage (%) | 13.0 (2.6, 25.0) | <0.001 | 9.9 (1.5, 23.6) | 0.016 |
| Intraplaque Haemorrhage | 27.5 (3.6, 212.4) | 0.002 | 27.5 (3.4, 221.5) | 0.002 |
| Minimum Lumen Area | 1.2 (1.0, 1.4) | 0.04 | ||
Figure 1.
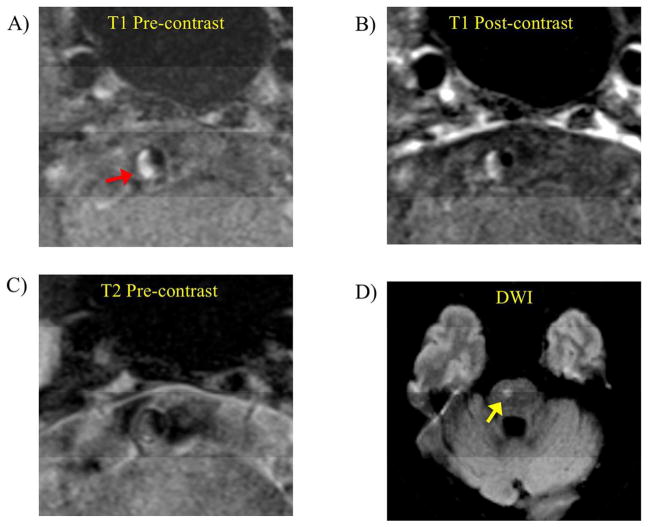
Intraplaque hemorrhage presented in a low grade stenotic basilar artery plaque (43% degree of stenosis) in an acute symptomatic patient (age 65, female). A) T1 weighted black blood MRI showed high signal (fresh IPH, red arrow) in the plaque. B) Post contrast T1 weighted images showed slight enhancement of the plaque. C) T2 weighted images showed iso-intense signal of the plaque. D) DWI showed infarct in the brain stem (yellow arrow).
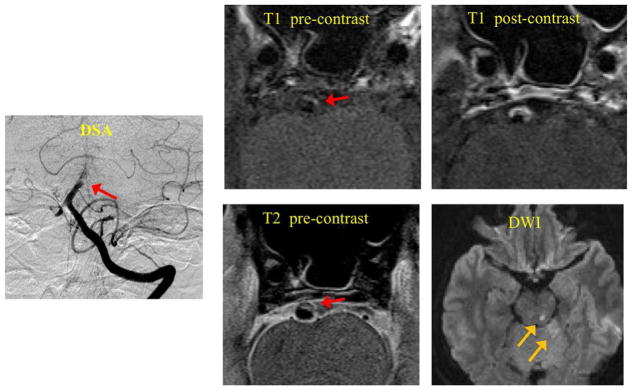
Figure 3.
A symptomatic patient with infarctions distal to the location of basilar artery plaque. Red arrows show the location of the plaque. Yellow arrows show the location of infarctions on DWI. T1 weighted black blood MRI showed high signal in the plaque (IPH). Post contrast T1 weighted images showed strong enhancement of the plaque.
Diagnostic performance of IPH for acute/sub-acute symptomatic stroke patients is summarized in Table 4. IPH had a high specificity of 98.3% and a high positive predictive value of 95.5%, however the sensitivity (31.8%) and negative predictive value (56.7%) were lower.
Table 4.
Diagnostic performance of intraplaque hemorrhage in identifying symptomatic/asymptomatic basilar artery plaque.
| IPH-positive (n=22) | IPH-Negative (n=104) | |
|---|---|---|
| Symptomatic | 21 | 45 |
| Asymptomatic | 1 | 59 |
| Diagnostic Performance | ||
| Specificity (%, 95% CI) | 98.3 (91.1,100.0) | |
| Sensitivity (%) | 31.8 (20.9, 44.4) | |
| PPV (%) | 95.5 (74.4, 99.3) | |
| NPV (%) | 56.7 (52.6, 60.8) | |
IPH: Intraplaque haemorrhage
PPV: Positive predict value
NPV: Negative predict value
MRI measurements reproducibility
There was excellent inter-reader agreement for measuring degree of stenosis (ICC = 0.97, 95% CI: [0.94, 0.98]) and for identification of IPH (96% agreement, kappa = 0.88, 95% CI: [0.76,1.00]).
Discussion
This study adds to the growing literature that emphasizes the importance of intracranial plaque properties compared to degree of luminal narrowing 2. In this study, IPH was the only finding associated with stroke symptom status whereas contrast enhancement, degree of stenosis, minimal lumen area and plaque burden were not associated with symptom status. As IPH was present in both low and high grade stenotic basilar artery plaque with a high positive predictive value (95.5%) for symptomatic stroke, it is evident that symptomatic ischemia may be explained by factors other than stenosis resulting in hypoperfusion. The presence of IPH likely indicates a high risk for artery-to-artery embolic occlusion following plaque rupture. In this conceptualization, future stroke risk for a smaller plaque with IPH is possibly greater than for a larger plaque with a stable fibrous cap.
A study of basilar artery ICAD in patients with at least 30% stenosis found T1-weighted hyperintense intraplaque signal in 8 of 38 cases (21%) that could indicate IPH, although the authors did not specifically term this finding IPH 9. The prevalence of basilar artery T1-weighted hyperintense signal reported by Huang et al. (21%) was similar to that reported in the current study (19.6%). On the other hand, a study of 74 patients with greater than 50% stenosis documented IPH in 42.3% of patients with an increased frequency in symptomatic patients 12. The reason for this higher incidence of IPH may be related to the larger plaque size in these patients with a mean stenosis of 72.9%, although we did not observe a significant difference between IPH frequency in stenotic versus non-stenotic plaque in our study 12. Alternatively, patients in our study were younger than those of Yu et al. with a difference in mean age of approximately ten years between the populations in the two studies. IPH may occur more frequently with age, although this hypothesis is untested. The diagnostic performance of IPH in the aforementioned study was similar to the current study with relatively high specificity (79%) but low sensitivity (53%) 12. Studies of IPH in other intracranial arteries have observed similar findings. IPH was significantly more frequent in symptomatic middle cerebral artery plaque than in asymptomatic plaque (19.6% versus 3.2%) 19.
While current management guidelines focus on stenosis, our work and that of others supports the use of identification of plaque properties to better indicate future stroke risk. Our study emphasizes the highly specific (98.3%) nature of IPH as an indicator of symptomatic stroke, implying that this represents unstable basilar artery atherosclerotic plaque. Unfortunately, IPH is not particularly sensitive (31.8%) in indicating the presence of a symptomatic basilar artery. These observations likely reflect the multiple stroke mechanisms encountered in ICAD, where IPH could predispose to artery-to-artery embolization with small vessel occlusion and could cause branch occlusive disease affecting perforating arteries. On the other hand, some symptomatic strokes in this study could also be caused by stenotic basilar artery plaque leading to hypoperfusion. IPH was the best overall marker of symptomatic plaque with an odds ratio of 27.5. This study’s findings support the use of IPH in basilar artery atherosclerotic plaque as a better indicator of symptomatic plaque rather than stenosis or plaque burden alone.
Other intracranial plaque characteristics including contrast enhancement and minimal lumen area have been associated with symptoms 24,25. We observed a similar relationship between contrast enhancement and symptom status in our study. A smaller study examining the basilar artery with contrast-enhanced hrMRI observed wall enhancement in patients with recent infarction and also those who would go on to have ischemic events 26. Another study of intracranial atherosclerotic disease also concluded that contrast enhancement was associated with culprit plaques with a substantial odds ratio of approximately 35 25. Research suggests that the vasa vasorum evolve with age and demonstrate different distributions with less vasa vasorum in the intracranial vasculature and differing distributions within cerebral vascular territories 27. Theoretically, enhancement of the basilar artery wall could reflect physiologic enhancement of the vasa vasorum rather than pathological plaque enhancement, which could reduce the specificity of vessel wall enhancement. An improved understanding of the vasa vasorum may enhance the specificity of basilar artery enhancement.
Further work is needed to histologically validate hrMRI assessment of IPH with only one case of histologically verified intracranial IPH demonstrated as T1-weighted hyperintense intraplaque signal on post-mortem hrMRI 28. Due to the reliance on post-mortem assessment of plaque, histological validation of intracranial plaque imaging characteristics is more challenging than in the extracranial carotid artery plaque, which may be ascertained following endarterectomy. While a study of intracranial plaque characterization using ex vivo 3T hrMRI in 53 post-mortem specimens determined relaxation times for many plaque components, no IPH was encountered in these plaque specimens 29. Therefore, IPH detection on hrMRI largely infers plaque characteristics from existing extracranial atherosclerosis imaging literature.
Our results cannot directly confer causality of IPH in the setting of symptomatic stroke and this cross-sectional study design cannot provide an assessment of future stroke risk. Prospective assessment of IPH and subsequent stroke risk would extend the clinical relevance of this study’s findings and such data could be utilized in risk assessment calculations. HrMRI can also determine IPH timing and duration. Potentially, signal characteristics of IPH could provide information on the onset of hemorrhage, and serial examination of patients with ICAD exhibiting IPH could provide insight into its natural history. Moreover, serial assessment of intracranial IPH evolution while on different pharmacologic therapies might potentially provide objective information regarding treatment efficacy.
In addition, there are technical limitations of our study to consider. Our study used a standard 2D T1-weighted black blood fast-spin-echo sequence. The use of 3D sequences with higher resolution 30, better T1-weighted contrast 31, and advanced blood suppression techniques (such as diffusion preparation 32 or variable flip angle train 30) can potentially improve the identification of IPH. However, the 3D high resolution sequences can increase scan time, and the use of diffusion preparation can reduce the signal to noise ratio and induce T2 contrast 32, and variable flip angle train can induce blurring with a wider point spread function 30. All of these limitations will need to be accounted for in future clinical studies.
Conclusion
In conclusion, evidence from this study suggests that IPH in the basilar artery found on hrMRI identifies atherosclerotic lesions that are more likely to be symptomatic regardless of the degree of stenosis. This plaque property appears to be substantially associated with symptom status whereas many other factors including plaque size, contrast enhancement and degree of stenosis do not. In the future, the use of hrMRI for the early detection of basilar artery IPH may allow clinicians to select individuals at greater risk of imminent stroke and help provide optimal therapeutic intervention.
Figure 2.
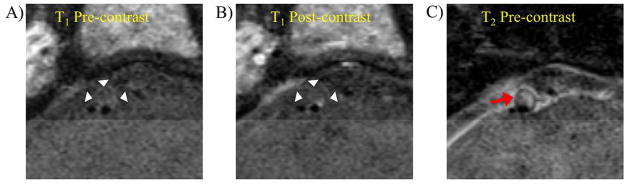
A high grade stenotic basilar artery plaque (degree of stenosis 73%) without intraplaque hemorrhage in an asymptomatic patient (age 56, female). A) T1-weighted black blood MRI showed isointense signal in the plaque. B) Post-contrast T1-weighted images showed enhancement of the plaque surface. C) T2-weighted images showed high signal of the plaque.
Acknowledgments
Grant Support: National Natural Science Foundation of China (31470910) and United States NIH grants R01HL114118, R01NS059944, and K99HL136883.
Abbreviations
- IPH
intraplaque haemorrhage
- MLA
minimal lumen area
- ICAD
Intracranial atherosclerotic disease
Footnotes
Previous Presentations: Presented in part at ISMRM 2016 annual meeting, Singapore
References
- 1.Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke; a journal of cerebral circulation. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 2.Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44:287–292. doi: 10.1161/STROKEAHA.112.664680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 4.Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke. 2014;45:645–651. doi: 10.1161/STROKEAHA.113.002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryoo S, Park JH, Kim SJ, et al. Branch occlusive disease: clinical and magnetic resonance angiography findings. Neurology. 2012;78:888–896. doi: 10.1212/WNL.0b013e31824c4699. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Degnan AJ, Gallagher G, Teng Z, et al. MR angiography and imaging for the evaluation of middle cerebral artery atherosclerotic disease. AJNR American journal of neuroradiology. 2012;33:1427–1435. doi: 10.3174/ajnr.A2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieleman N, van der Kolk AG, Zwanenburg JJ, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation. 2014;130:192–201. doi: 10.1161/CIRCULATIONAHA.113.006919. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Yang WQ, Liu XT, et al. Basilar artery atherosclerotic plaques distribution in symptomatic patients: a 3.0T high-resolution MRI study. Eur J Radiol. 2013;82:e199–203. doi: 10.1016/j.ejrad.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Klein IF, Lavallee PC, Mazighi M, et al. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010;41:1405–1409. doi: 10.1161/STROKEAHA.110.583534. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Lim SH, Oh KW, et al. The advantage of high-resolution MRI in evaluating basilar plaques: a comparison study with MRA. Atherosclerosis. 2012;224:411–416. doi: 10.1016/j.atherosclerosis.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Yu JH, Kwak HS, Chung GH, et al. Association of Intraplaque Hemorrhage and Acute Infarction in Patients With Basilar Artery Plaque. Stroke. 2015;46:2768–2772. doi: 10.1161/STROKEAHA.115.009412. [DOI] [PubMed] [Google Scholar]
- 13.Chung JW, Kim BJ, Choi BS, et al. High-resolution magnetic resonance imaging reveals hidden etiologies of symptomatic vertebral arterial lesions. J Stroke Cerebrovasc Dis. 2014;23:293–302. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Teng Z, Canton G, et al. Intraplaque hemorrhage is associated with higher structural stresses in human atherosclerotic plaques: an in vivo MRI-based 3D fluid-structure interaction study. Biomed Eng Online. 2010;9:86. doi: 10.1186/1475-925X-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107:3047–3052. doi: 10.1161/01.CIR.0000074222.61572.44. [DOI] [PubMed] [Google Scholar]
- 16.McNally JS, McLaughlin MS, Hinckley PJ, et al. Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke. 2015;46:84–90. doi: 10.1161/STROKEAHA.114.006286. [DOI] [PubMed] [Google Scholar]
- 17.Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. 2007;38:1633–1635. doi: 10.1161/STROKEAHA.106.473066. [DOI] [PubMed] [Google Scholar]
- 18.Turan TN, Bonilha L, Morgan PS, et al. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging. 2011;21:e159–161. doi: 10.1111/j.1552-6569.2009.00442.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. 2012;71:195–198. doi: 10.1002/ana.22626. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Huang J, Degnan AJ, et al. Comparison of high-resolution MRI with CT angiography and digital subtraction angiography for the evaluation of middle cerebral artery atherosclerotic steno-occlusive disease. The international journal of cardiovascular imaging. 2013;29:1491–1498. doi: 10.1007/s10554-013-0237-3. [DOI] [PubMed] [Google Scholar]
- 21.Lou M, Caplan LR. Vertebrobasilar dilatative arteriopathy (dolichoectasia) Ann N Y Acad Sci. 2010;1184:121–133. doi: 10.1111/j.1749-6632.2009.05114.x. [DOI] [PubMed] [Google Scholar]
- 22.Choi YJ, Jung SC, Lee DH. Vessel Wall Imaging of the Intracranial and Cervical Carotid Arteries. J Stroke. 2015;17:238–255. doi: 10.5853/jos.2015.17.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zhu C, Peng W, et al. Scan-Rescan Reproducibility of High Resolution Magnetic Resonance Imaging of Atherosclerotic Plaque in the Middle Cerebral Artery. PloS one. 2015;10:e0134913. doi: 10.1371/journal.pone.0134913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng Z, Peng W, Zhan Q, et al. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol. 2015 doi: 10.1007/s00330-015-4008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271:534–542. doi: 10.1148/radiol.13122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lou X, Ma N, Ma L, et al. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol. 2013;34:513–517. doi: 10.3174/ajnr.A3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portanova A, Hakakian N, Mikulis DJ, et al. Intracranial vasa vasorum: insights and implications for imaging. Radiology. 2013;267:667–679. doi: 10.1148/radiol.13112310. [DOI] [PubMed] [Google Scholar]
- 28.Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke. 2014;9:E19. doi: 10.1111/ijs.12277. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y, Zhu C, Peng W, et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis. 2016;249:10–16. doi: 10.1016/j.atherosclerosis.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu C, Haraldsson H, Tian B, et al. High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI. Magma. 2016;29:559–570. doi: 10.1007/s10334-016-0531-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhu DC, Vu AT, Ota H, et al. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magnetic resonance in medicine. 2010;64:1341–1351. doi: 10.1002/mrm.22517. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C, Graves MJ, Yuan J, et al. Optimization of improved motion-sensitized driven-equilibrium (iMSDE) blood suppression for carotid artery wall imaging. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:61. doi: 10.1186/s12968-014-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]