Abstract
We report on the screening and development of haploidentical hematopoietic stem cell transplantation (HSCT) for adult patients with clinically aggressive sickle cell disease (SCD) at our institution. Of 50 adult SCD patients referred for HSCT between 1/2014–3/2017, 20% were denied by insurance. Of 41 patients initially screened, 10% lacked an available haploidentical donor, 29% had elevated donor specific antibodies (DSA) and 34% declined to proceed to HSCT. All 10 patients who were transplanted received peripheral blood stem cells (PBSC). The initial two were conditioned with alemtuzumab/total body irradiation (TBI) 3Gy followed by post-transplant cyclophosphamide and failed to engraft. The next 8 patients received the regimen developed at Johns Hopkins University (Bolaños-Meade J, Blood 2012) with TBI 3Gy. G-CSF was administered from day+12 in those with HbS <30%. All 8 patients engrafted with a median time to neutrophil >0.5 x109/L of 22 days (range, 18–23 days). One patient subsequently lost the graft and 7 (87.5%) maintained >95% donor cell chimerism at 1-year post-HSCT. Two patients developed acute GVHD ≥ grade 2. One had chronic GVHD and died >1 year after HSCT of unknown causes. With a median follow up of 16 months (range, 11–29 months), 7 patients (87.5%) are alive. Our findings suggest that limited insurance coverage, high rate of DSA and patient declining HSCT, may limit the availability of haploidentical HSCT in adult SCD patients. The modified Hopkins regimen used here demonstrates high engraftment and low morbidity rates and should be tested in larger, multicenter prospective clinical trials.
Keywords: Sickle cell disease, transplantation, haploidentical, donor specific antibody, G-CSF
Introduction
Allogeneic non-myeloablative hematopoietic stem cell transplantation (HSCT) from HLA-matched related donors results in event-free survival rates of 87–92%, overall survival rates of 97–100%, and 0% acute or chronic graft-versus host disease (GVHD) in adults with sickle cell disease (SCD).1,2 There is an unmet need for alternative donors because only 18% of SCD patients have an HLA-matched sibling.3 HSCT using HLA-matched unrelated donors is limited by the difficulty in finding HLA-compatible donors for this largely non-Caucasian patient population.4,5 Additionally, a recent study reported high rates of chronic GVHD (62%) and transplant-related mortality (21%) in SCD patients receiving transplants from HLA-matched unrelated donors.6
The discovery that post-transplant cyclophosphamide (PTCy) allows patients to engraft stem cells from haploidentical donors without an increased risk of GVHD has led to the rapid expansion of haploidentical HSCT in patients with hematologic malignancies.7 This strategy was utilized in 14 SCD patients transplanted with bone marrow cells after conditioning with fludarabine, cyclophosphamide, 2Gy total body irradiation (TBI), anti-thymocyte globulin (ATG), and GVHD prophylaxis with PTCy on days +3 and +4, mycophenolate mofetil for 30 days and tacrolimus or sirolimus for at least one year (the Hopkins protocol).8 No severe transplant-related complications were reported and 57% of the patients achieved a stable donor cell engraftment. In a subsequent study, the same regimen resulted in the engraftment of only 2 of 5 SCD patients.9 Modifications to this regimen, including the addition of azathioprine and hydroxyurea for 3 months pre-HSCT, hypertransfusion, and thiotepa on day-7, improved engraftment to 91% with 18% acute GVHD and 14% mortality in a pediatric series of SCD patients.9
Our institution has the largest adult sickle cell program in the Chicago area. Because only 20% of our SCD patients eligible for HSCT had a matched related donor, we initiated a haploidentical HSCT program.1 Here we report our center’s real-life experience of screening and treating adult SCD patients with haploidentical HSCT.
Material & Methods
Patients
Transplant eligibility requirements were similar to those for match related donor transplants1 and in accordance with the international expert panel for alternative donor transplantation in SCD,10 with the additional requirement that the recipient be negative for donor specific HLA antibodies (DSA). Positivity for DSA was considered moderate with a mean fluorescent intensity between 2000–5000, and high with a mean fluorescent intensity >5000.11 Institutional Review Board approval was obtained prior to collecting and analyzing the clinical data.
Donors
Haploidentical donors were either HbAA or HbAS. The donors received granulocyte colony stimulating factor (G-CSF) subcutaneously at a dose of 5μg/kg/twice daily for five days followed by peripheral blood stem cell (PBSC) collection.
Conditioning regimens
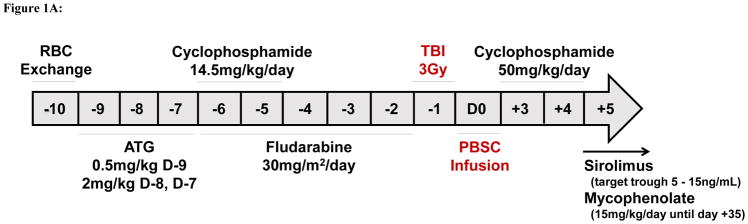
The conditioning protocol in the first two patients was as follows: alemtuzumab (0.03mg/kg on day −7, 0.1mg/kg on day −6, 0.3mg/kg on day −5 to −3), single dose TBI 3Gy on day −2, and cyclophosphamide (50mg/kg on day +3 and +4). Because both patients failed to engraft donor cells, we adopted the Hopkins protocol8 for the next eight patients with two modifications aimed at improving engraftment: a) increasing the dose of TBI from 2Gy to 3Gy and b) infusing growth factor mobilized PBSC instead of bone marrow cells (Figure 1A). The conditioning was as follows: rabbit ATG (Sanofi Genzyme, Cambridge, MA, USA) (0.5mg/kg on day −9, 2mg/kg on day −8 and −7), cyclophosphamide (14.5mg/kg on day −6 and −5), fludarabine (30mg/m2 on day −6 to −2), and single dose TBI 3Gy on day −1. GVHD prophylaxis consisted of cyclophosphamide (50mg/kg i.v. on day +3 and +4), oral mycophenolate mofetil (15mg/kg three times daily from day +5 to day +35), and sirolimus from day +5 dosed for a target trough of 5–15ng/mL. In patients with T-cell chimerism > 50% at 1 year post-HSCT and without signs of GVHD, treatment with sirolimus was tapered off over 3 months.
Figure 1.
Figure 1A: Regimen for haploidentical transplantation in adult patients with sickle cell disease.
Figure 1B: Screening process for haploidentical transplantation in adult patients with sickle cell disease (SCD). Of 50 patients referred to the SCD transplant clinic, 41 proceeded with HLA typing. 25 (61%) had a suitable HLA-haploidentical donor with 4 (10%) lacking an available HLA-haploidentical relative and 12 (29%) having donor-specific HLA antibodies. In addition, 10 (20%) were denied by insurance and 14 (34%) declined or deferred transplantation.
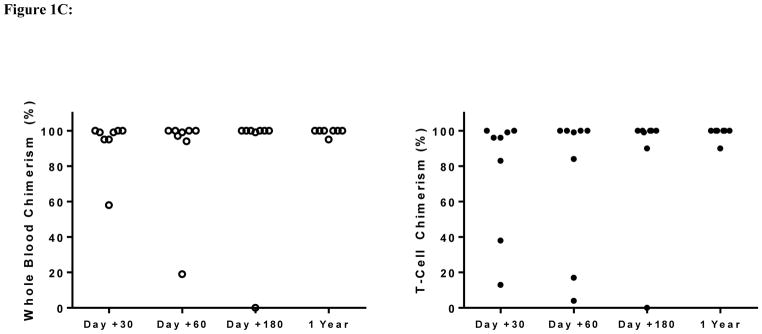
Figure 1C: Stable whole blood and CD3+ T-cell engraftment after haploidentical transplantation in adult patients with sickle cell disease.
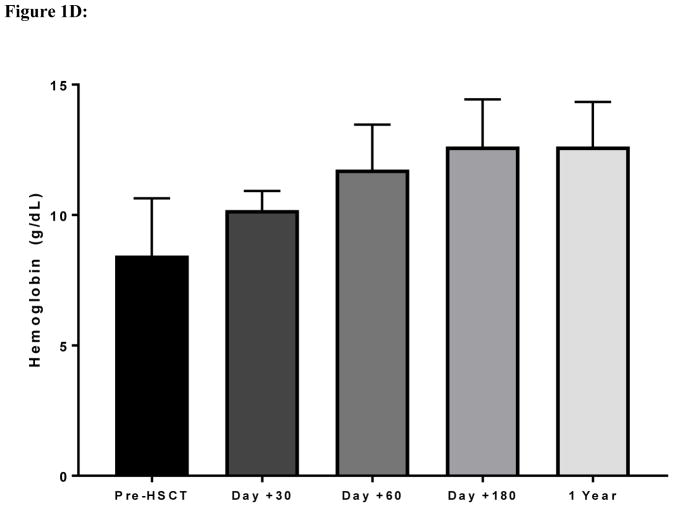
Figure 1D: Improvements in hemoglobin concentration after haploidentical transplantation in adult patients with sickle cell disease.
Recipients underwent a red blood cell exchange transfusion on day −10 (goal hemoglobin S <30%) and hydroxyurea was permanently discontinued on day −9. Platelet transfusions were administered to maintain platelet counts >50 x109 cells/L and penicillin V 250mg p.o. was administered twice daily, in addition to standard antimicrobial prophylaxis. Donor cell engraftment was assessed by chimerism analysis on circulating mononuclear cells and CD3+ T cells on days + 30, 60, 180, and 365.
Graft-versus host disease
Patients were monitored in the University of Illinois at Chicago bone barrow transplant clinic at least weekly until day +60, monthly until day +180, and bi-monthly until 1 year post-HSCT. Acute and chronic GVHD were graded according to standard consensus diagnostic criteria12,13
Results
Between January 2014 and March 2017, 50 adult SCD patients meeting haploidentical HSCT eligibility requirements10 were referred to our program (Figure 1B). Nine patients were initially denied by insurance and therefore only 41 could be screened for donor availability. Of these, four (10%) lacked an available haploidentical donor and 12 (29%) had moderate (n=7) or high (n=5) levels of DSA, a strong predictor of graft rejection in haploidentical HSCT.11 Of the 25 (61%) patients with an identified haploidentical donor, one additional patient was denied by insurance prior to transplant and 14 (34%) ultimately declined or deferred HSCT, resulting in a total of 10 patients being transplanted. All recipients received PBSC from their haploidentical donors. In seven Hb AS donors, mobilization with G-CSF did not lead to increased side effects and produced similar stem cell collections as in Hb AA donors.
The first two patients (Table 1A) were conditioned with alemtuzumab/3Gy TBI + PTCy but failed to engraft donor cells and recovered autologous neutrophils on day +39 and day +34, respectively. The following eight patients (Table 1B) were conditioned with the modified Hopkins regimen (TBI 3Gy and PBSC as the graft source) (Figure 1A). All eight patients engrafted >0.5 x109 neutrophils/L at a median of 22 days (range, 18–23 days). One patient had low donor T-cell chimerism levels at day +30 and +60 that spontaneously improved by day +180 without any changes in immunosuppression. Another patient had a progressive decline of donor whole blood and T-cell chimerisms and experienced secondary graft failure on day +90 with autologous hematopoietic recovery. At one year post-HSCT, seven patients maintained an average donor mononuclear chimerism >95% and T-cell chimerism ≥90% (Figure 1C).
Table 1A.
Characteristics and outcomes of 2 patients with sickle cell disease that underwent HSCT from a haploidentical, related donor with the alemtuzumab, TBI, and PTCy.
| Transplantation Characteristics | Transplantation Outcomes | Transplant-Related Toxicity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age at HSCT (years) | Sex | Hemoglobin Genotype | Indications* | Prior Therapy | Donor | CD34+ Dose (×106/kg) | Duration Follow-up (months) | Living Status | Neutrophil Engraftment | Infectious Complications | CMV Reactivation | aGVHD | cGVHD |
| A | 24 | F | SS | 20 VOC/year 3 ACS/lifetime |
Hydroxyurea | Mother (Hb AS) | 5.9 | 37 | Alive | Autologous, Day +39 | None | None | None | None |
| B | 52 | M | SC | 10 VOC/year | Hydroxyurea | Sister (Hb AA) | 5.4 | 36 | Alive | Autologous, Day +34 | None | Day +11 | None | None |
Table 1B.
Characteristics and outcomes of 8 patients with sickle cell disease that underwent HSCT from a haploidentical, related donor with the modified Hopkins regimen.
| Transplantation Characteristics | Transplantation Outcomes | Transplant-Related Toxicity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age at HSCT (years) | Sex | Hemoglobin Genotype | Indications* | Prior Therapy | Donor | CD34+ Dose (×106/kg) | Duration Follow-up (months)/Current Donor T-cell Chimerism | Living Status | Neutrophil Engraftment | Infectious Complications | CMV Reactivation | aGVHD | cGVHD |
| 1 | 38 | M | SS | 8 VOC/year 3ACS/lifetime |
Hydroxyurea | Daughter (Hb AS) | 14.2 | 30 / 100% | Alive | Day +23 | E. Coli UTI | Day +19 | None | None |
| 2 | 20 | M | Sβ+-thal | 10 VOC/year | Hydroxyurea | Sister (Hb AA) | 6.0 | 23 / 90% | Alive | Day +20 | None | None | None | None |
| 3 | 21 | M | SS | 10 VOC/year TRJV = 2.7 |
Hydroxyurea | Mother (Hb AS) | 5.3 | 15 | Deceased | Day +18 | Oral HSV1 Coronavirus Influenza | Day +20 | Grade II skin Grade IV liver | Moderate - eye - liver |
| 4 | 27 | M | SS | 4 VOC/year 2 ACS/lifetime |
Hydroxyurea | Father (Hb AS) | 8.2 | 17 / 0% | Alive | Day +23 | None | None | None | None |
| 5 | 31 | M | SS | Stroke 11 VOC/year 2 ACS/last 2 years |
Hydroxyurea | Brother (Hb AA) | 4.2 | 17 / 100% | Alive | Day +22 | None | None | None | None |
| 6 | 27 | F | SS | 19 VOC/year 2 ACS/last 2 years |
Hydroxyurea | Mother (Hb AS) | 10.8 | 13 / 100% | Alive | Day +22 | Enterococcus UTI | None | None | None |
| 7 | 37 | M | SS | Stroke 3 VOC/year 2 ACS/last 2 years |
Chronic Transfusion | Mother (Hb AS) | 12.2 | 13 / 100% | Alive | Day +19 | None | None | None | None |
| 8 | 29 | F | SS | 7 VOC/year 2 ACS/lifetime |
Hydroxyurea | Mother (Hb AS) | 6.1 | 12 / 100% | Alive | Day +18 | None | None | Grade II GI | None |
The VOC rate is an average of the rate over the two years preceding the date of consultation for transplantation.
HSCT, hematopoietic stem cell transplant; TBI, total body irradiation; PTCy, post-transplant cyclophosphamide; CMV, cytomegalovirus; aGVHD, acute graft versus host disease; cGVHD, chronic graft versus host disease; M, male; F, female; VOC, vaso-occlusive crisis; ACS, acute chest syndrome; UTI, urinary tract infection; TRJV, tricuscpid regurgitant jet velocity; GI, gastrointestinal
Transplant-related toxicities included ≥ grade II mucositis in three patients and CMV reactivation in two patients without occurrence of CMV infection. Seven neutropenic patients with hemoglobin S <30% received a median of seven doses (range, 3–14 doses) of G-CSF at 5 μg/kg starting at day +12 post-HSCT. Only one patient experienced mild bone pain in the lower extremities. Small subarachnoid hemorrhages occurred in two patients. The first patient had a history of multiple red blood cell antibodies, became refractory to platelet transfusions and developed multifocal small subarachnoid hemorrhages in the left parietal lobe on day +10. Symptoms and brain imaging resolved four days later after platelet counts were maintained at >50 x109 cells/L with cross-matched platelets. The second patient, who had a prior stroke history, developed a seizure when the platelet count was 68 x109 cells/L. Brain MRI demonstrated a right frontal subarachnoid hemorrhage on day +12. Symptoms and imaging results improved two days later, after initiating levetiracetam and maintaining platelets >100 x109 cells/L. Acute GVHD was observed in two patients and chronic GVHD in one patient. One patient developed acute on chronic GVHD involving the skin, liver and eyes on day +83. Treatment with steroids and strict adherence to sirolimus improved eye symptoms and bilirubin levels, but the patient died unexpectedly at home on day +407. Another patient developed grade II acute gut GVHD that completely resolved after a short course of steroid therapy. With a median follow up of 17 months (range, 12 – 30 months), 7 patients are alive and 6 maintain >95% stable donor engraftment (Figure 1C) with improvements in their hemoglobin concentrations (Figure 1D). Three patients have stopped immunosuppression and the other three are being tapered off immunosuppression.
Discussion
In this single center experience of a haploidentical HSCT program for adults with SCD, we demonstrate several real-life barriers for access to haploidentical transplantation, the safety of G-CSF post-HSCT, and a high rate of long-term engraftment using PBSC with a modified Hopkins regimen. Following our positive results in match related donor HSCT,1 we initially attempted to apply the same alemtuzumab-based regimen with the addition of PTCy in two patients undergoing haploidentical HSCT. Both experienced graft failure, consistent with the experience reported using the same approach at the National Institute of Health.14 Because the risk of transplant-related mortality is higher in adults with SCD using standard myeloablative regimens,15 we transplanted the next eight patients with a non-myeloablative regimen developed at Johns Hopkins University.8 In order to decrease the high rate of rejection reported in the original study (43%), we modified the Hopkins protocol by increasing the dose of TBI from 2Gy to 3Gy and using PBSC instead of marrow cells. This led to improvements in stable donor cell engraftment from 40 – 57% previously reported8,9 to 87.5%, while maintaining manageable toxicities. The findings we report here should be considered in the context of the few small series of haploidentical HSCT in SCD patients that have been published to date (Table 2). Our engraftment results are comparable to those obtained in a pediatric series where azathioprine, HU and thiotepa were added to the preparative regimen.8 Use of PBSC has been associated with a greater risk for acute and chronic GVHD compared to unstimulated bone marrow in patients with hematologic malignancies undergoing a T-cell replete haploidentical HSCT.16 Although we observed chronic GVHD in only one of eight patients, this risk should be carefully considered in future studies testing the benefits of PBSC for reducing rejection in patients with non-malignant diseases conditioned with low intensity regimens.
Table 2.
Current published experience for haploidentical transplantation in sickle cell disease.
| Study | N | Age Range (years) | Conditioning Regimen | Stem Cell Source | aGVHD | cGVHD | Stable Engraftment | Overall Survival |
|---|---|---|---|---|---|---|---|---|
| Bolanos-Meade et al, 20128 | 14 | 15–42 | Flu 30mg/m2/day, Cy 14.5mg/kg/day, ATG, TBI 2Gy, 50mg/kg/day PT-Cy | Bone marrow | 0 | 0 | 8 | 14 |
| Dallas et al, 201328 | 8 | 4 – 17 | 1) Flu 150–200mg/m2, Thiotepa 10mg/kg, Bu (target 900ng/mL), ATG (10mg/kg), muromonab-CD3 (0.1mg/kg) 2) HU/azathioprine 3 months pre-transplant; Bu (target 900ng/mL), thiotepa, Cy (200mg/kg), muomonab-CD3 (0.1mg/kg) |
Bone marrow | 4 | 3 | 5 | 6 |
| Dhedin et al, 20169 | 5 | 12 – 50 | 1) Flu 30mg/m2/day, Cy 14.5mg/kg/day, ATG, TBI 2Gy, PT-Cy 50mg/kg/day | Bone marrow | 0 | 0 | 2 | 5 |
| 8 | 7 – 26 | 2) Thiotepa 10mg/kg/day, Flu 30mg/m2/day, Cy 14.5mg/kg/day, ATG, TBI 2Gy, PT-Cy 50mg/kg/day | 1 | 0 | 7 | 8 | ||
| 23 | 3 – 18 | 3) Pre-conditioning for 3 months with azathioprine 3mg/kg/day and HU 30mg/kg/day; Thiotepa 10mg/kg/day, Flu 30mg/m2/day, Cy 14.5mg/kg/day, ATG, TBI 2Gy, PT-Cy 50mg/kg/day | 4 | 0 | 21 | 18 | ||
| Fitzhugh et al, 201714 | 12 | 20 – 56 | Alemtuzumab 1mg/kg, TBI 4 Gy, PT-Cy 50mg/kg/day | PBSC | 1 | 1 | 6 | 11 |
| Pawlowska et al, 201829 | 4 | 13 – 23 | Flu 40mg/m2/day, dexamethasone 25mg/m2/day × 2 cycles pre-HSCT Rabbit ATG 1.5mg/kg/day, Flu 35mg/m2/day, Bu 130mg/m2/day, PT-Cy 50mg/kg/day | 3 Bone Marrow, 1 PBSC | 1 | 3 | 4 | 4 |
| Current Study | 8 | 20 – 38 | Flu 30mg/m2/day, Cy 14.5mg/kg/day, ATG, TBI 3Gy, PT-Cy 50mg/kg/day | PBSC | 2 | 1 | 7 | 7 |
| Summary | 82 | 3 – 51 | — | — | 13 (16%) | 8 (10%) | 60 (73%) | 73 (89%) |
aGVHD, acute graft versus host disease; cGVHD, chronic graft versus host disease; Flu, fludarabine, Cy, cyclophosphamide, ATG, antithymocyte globulin; TBI, total body irradiation; PT-Cy, post-transplantation cyclophosphamide; Bu, busulfan; PBSC, peripheral blood stem cell
In our experience of 50 adult SCD patients referred for HSCT, only 20% ended up receiving a haploidentical HSCT. Medical insurance denial accounted for 20% of the lack of access for HSCT. Other factors, such as high rates of DSA in frequently transfused SCD patients and personal decisions to decline HSCT, also played significant roles. Our rate of available haploidentical donors is lower than was previously observed at Johns Hopkins University, where 90% of SCD patients referred had haploidentical donors.8 This difference may be because many of the SCD patients reported on here were already being followed in our clinics and had not been pre-screened or selected by referring physicians. The presence of DSA is a major barrier to haploidentical HSCT in SCD that should be addressed when discussing treatment options with patients. A possible strategy to increase the donor pool could be to select patients with low DSA titers and a negative cross-match result. Desensitization protocols used in hematologic malignancies11 should also be tested in clinical trials for SCD patients with clinically aggressive disease. Our findings are consistent with a previous report showing that a substantial proportion of eligible SCD patients do not proceed to HSCT due to fear of toxicity and satisfaction with the current quality of life.17 The two main reasons that patients reported for declining HSCT were: a) the risk of GVHD in an HLA mismatched HSCT, and b) the toxicity associated with chemotherapy in the conditioning regimen. Interestingly, in previous studies the severity of SCD was not associated with the degree of risk that a parent or a patient is willing to accept for cure.18–21 Only 35% of adolescents and 46% of parents would accept HSCT if recommended by their hematologist.22 Thirty-two percent of adolescents believe that SCD will shorten their life-span and only 26% believe that SCD will prevent the achievement of life goals, which is in contrast to 86% of adults who perceived that employment opportunities are affected by SCD.19,22 There is a need for better education of patients about the course of SCD and for large, prospective haploidentical HSCT studies to guide the decisions of patients and their families for the risk-benefit assessment of HSCT. The risks of curative treatment with alternative donors will need to be carefully considered as new therapies, such as gene therapy23 and non-transplant therapies,24 are being developed for SCD.
We also observed that G-CSF could be safely administered to SCD adults to shorten the duration of post-HSCT neutropenia. Concern for the safety of G-CSF was highlighted in a previous case series of 11 SCD patients receiving G-CSF for reducing the duration of neutropenia post-chemotherapy or to mobilize autologous stem cells.25 In that case series, 7 of 11 patients had serious adverse events with G-CSF use and a lower hemoglobin S level did not reduce the rate of adverse events. In contrast, in a pediatric cohort of children with SCD undergoing matched related donor HSCT, G-CSF 5 μg/kg/day was safely given starting on day +7 until full neutrophil recovery.26 Only one patient in our study had mild lower extremity pain while receiving G-CSF consistent with tolerability of G-CSF post-HSCT in SCD. Although sickle cell trait donors did not experience any severe side effects with G-CSF mobilization, an alternative strategy to mobilize stem cells in sickle cell trait donors and to mobilize autologous stem cells from SCD patients may be the use of plerixafor, as safely demonstrated in gene therapy studies.27
In conclusion, our findings suggest that a non-myeloablative haploidentical PBSC transplant using TBI 3Gy and PTCy could cure many adult patients with advanced SCD and larger clinical studies are warranted. Based on our findings, barriers limiting the access to haploidentical HSCT for SCD patients should also be addressed by the transplant community with multilevel interventions.
Highlights.
Moderate or high titer donor specific antibodies were present in 12 of 41 sickle cell adults.
G-CSF can be safely administered post-transplantation in sickle cell adults with HbS<30%.
Modified Hopkins haplo-protocol led to long-term engraftment in 7 of 8 sickle cell adults.
Acknowledgments
S.L.S. receives research support from the National Institutes of Health through grant K23HL125984. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study has been partially supported by a Michael Reese Research & Education Foundation endowment to D.R.
Footnotes
Financial Disclosures: The authors declare no competing financial interests.
Authorship:
S.L.S and D.R. designed the study and wrote the manuscript. S.L.S, A.L.O, P.R.P., K.S., M.K., S.C.L., J.G.Q., R.E.M., N.M., V.R.G., and D.R. performed the research, collected, analyzed and interpreted the data. M.G., S.J., D.P., and I.K. performed the research and contributed to the data analysis. All the authors critically reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saraf SL, Oh AL, Patel PR, et al. Nonmyeloablative Stem Cell Transplantation with Alemtuzumab/Low-Dose Irradiation to Cure and Improve the Quality of Life of Adults with Sickle Cell Disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016 Mar;22(3):441–448. doi: 10.1016/j.bbmt.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. Jama. 2014 Jul 2;312(1):48–56. doi: 10.1001/jama.2014.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mentzer WC, Heller S, Pearle PR, Hackney E, Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. The American journal of pediatric hematology/oncology. 1994 Feb;16(1):27–29. [PubMed] [Google Scholar]
- 4.Eapen M, Horowitz MM. Alternative donor transplantation for aplastic anemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2010;2010:43–46. doi: 10.1182/asheducation-2010.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. The New England journal of medicine. 2014 Jul 24;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy S, Eapen M, Panepinto JA, et al. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. 2016 Nov 24;128(21):2561–2567. doi: 10.1182/blood-2016-05-715870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008 Jun;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolanos-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012 Nov 22;120(22):4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhedin N, Fuente J, Bernaudin F, et al. Haploidentical bone marrow transplant with post-transplant cytoxan plus thiotepa improves donor engraftment in patients with sickle cell anemia: results of an international multicenter learning collaborative. Blood (abstract #1233) 2016 doi: 10.1016/j.bbmt.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014 May;99(5):811–820. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009 Oct 27;88(8):1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005 Dec;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995 Jun;15(6):825–828. [PubMed] [Google Scholar]
- 14.Fitzhugh CD, Hsieh M, Taylor T, et al. Cyclophosphamide improves engraftment in patients with SCD and severe organ damage who undergo haploidentical PBSCT. Blood advances. 2017;1(11):652–661. doi: 10.1182/bloodadvances.2016002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluckman E, Cappelli B, Bernaudin F, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2016 Dec 13; doi: 10.1182/blood-2016-10-745711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Sep 10;35(26):3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansbury EN, Schultz WH, Ware RE, Aygun B. Bone marrow transplant options and preferences in a sickle cell anemia cohort on chronic transfusions. Pediatric blood & cancer. 2012 Apr;58(4):611–615. doi: 10.1002/pbc.23304. [DOI] [PubMed] [Google Scholar]
- 18.Meier ER, Dioguardi JV, Kamani N. Current attitudes of parents and patients toward hematopoietic stem cell transplantation for sickle cell anemia. Pediatric blood & cancer. 2015 Jul;62(7):1277–1284. doi: 10.1002/pbc.25446. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarti S, Bareford D. A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease. Bone marrow transplantation. 2007 Apr;39(8):447–451. doi: 10.1038/sj.bmt.1705622. [DOI] [PubMed] [Google Scholar]
- 20.van Besien K, Koshy M, Anderson-Shaw L, et al. Allogeneic stem cell transplantation for sickle cell disease. A study of patients’ decisions. Bone marrow transplantation. 2001 Sep;28(6):545–549. doi: 10.1038/sj.bmt.1703208. [DOI] [PubMed] [Google Scholar]
- 21.Kodish E, Lantos J, Stocking C, Singer PA, Siegler M, Johnson FL. Bone marrow transplantation for sickle cell disease. A study of parents’ decisions. The New England journal of medicine. 1991 Nov 07;325(19):1349–1353. doi: 10.1056/NEJM199111073251905. [DOI] [PubMed] [Google Scholar]
- 22.Roth M, Krystal J, Manwani D, Driscoll C, Ricafort R. Stem cell transplant for children with sickle cell anemia: parent and patient interest. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012 Nov;18(11):1709–1715. doi: 10.1016/j.bbmt.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene Therapy in a Patient with Sickle Cell Disease. The New England journal of medicine. 2017 Mar 2;376(9):848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 24.de Montalembert M, Brousse V, Chakravorty S, et al. Are the risks of treatment to cure a child with severe sickle cell disease too high? Bmj. 2017 Nov 23;359:j5250. doi: 10.1136/bmj.j5250. [DOI] [PubMed] [Google Scholar]
- 25.Fitzhugh CD, Hsieh MM, Bolan CD, Saenz C, Tisdale JF. Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy. 2009;11(4):464–471. doi: 10.1080/14653240902849788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King AA, Kamani N, Bunin N, et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. American journal of hematology. 2015 Dec;90(12):1093–1098. doi: 10.1002/ajh.24183. [DOI] [PubMed] [Google Scholar]
- 27.Lagresle-Peyrou C, Lefrere F, Magrin E, et al. Plerixafor enables the safe, rapid, efficient mobilization of haematopoietic stem cells in sickle cell disease patients after exchange transfusion. Haematologica. 2018 Feb 22; doi: 10.3324/haematol.2017.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallas MH, Triplett B, Shook DR, et al. Long-term outcome and evaluation of organ function in pediatric patients undergoing haploidentical and matched related hematopoietic cell transplantation for sickle cell disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 May;19(5):820–830. doi: 10.1016/j.bbmt.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlowska AB, Cheng JC, Karras NA, et al. HLA Haploidentical Stem Cell Transplant with Pretransplant Immunosuppression for Patients with Sickle Cell Disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018 Jan;24(1):185–189. doi: 10.1016/j.bbmt.2017.08.039. [DOI] [PubMed] [Google Scholar]