Abstract
Previous developmental research suggests that motor experience supports the development of action perception across the lifespan. However, it is still unknown when the neural mechanisms underlying action-perception coupling emerge in infancy. The goal of this study was to examine the neural correlates of action perception during the emergence of grasping abilities in newborn rhesus macaques. Neural activity, recorded via electroencephalogram (EEG), while monkeys observed grasping actions, mimed actions and means-end movements during the first (W1) and second week (W2) of life was measured. Event-related desynchronization (ERD) during action observation was computed from the EEG in the alpha and beta bands, two components of the sensorimotor mu rhythm associated with activity of the mirror neuron system (MNS). Results revealed age-related changes in the beta band, but not the alpha band, over anterior electrodes, with greater desynchronization at W2 than W1 for the observation of grasping actions. Additionally, desynchronization to observed grasping actions at W2 was associated with infants’ motor skills – measured by a separate behavioral task – such that more grasping attempts were associated to greater beta ERD. These findings suggest the emergence of an early action-perception system, that relies on motor experience, shortly after birth.
Keywords: Development, Mu rhythm, ERD, Mirror neuron system, Grasping actions, Electroencephalogram
1. Introduction
The connection between motor development and the emergence of social and cognitive abilities has been widely investigated in both human and nonhuman primates (Ferrari et al., 2009; Kaburu et al., 2016; Marshall and Meltzoff, 2014, 2011; Woodward and Gerson, 2014). Human infants begin to show the capacity to infer others’ motor goals at the end of the first year of life, as developments in their self-produced actions contribute to improved perception of how others interact with their surrounding environment (Hunnius and Bekkering, 2014; Sommerville et al., 2005; Woodward and Gerson, 2014). The mirror neuron system (MNS), which activates during both the execution and the observation of goal directed actions, might represent an important neural correlate of this action-perception coupling. Since the initial discovery of mirror neurons in the premotor and parietal cortices of the adult macaque (Bonini et al., 2010; di Pellegrino et al., 1992; Fogassi et al., 2005; Gallese et al., 1996), it has been hypothesized that the MNS might mediate higher cognitive functions such as action understanding and imitation (Rizzolatti et al., 2001). The proposed mechanism through which the MNS operates relies on mapping the description of an observed action onto one’s own motor representation. Such sensory-motor mapping would support the observer’s embodied access to the meaning of the observed action, thus making the motor system central to both controlling the movement of the body in space and supporting cognitive functions related to the decoding of others’ actions (Gallese et al., 1996; Rizzolatti et al., 2001).
The electroencephalogram (EEG) is a non-invasive approach to measure brain activity in developmental populations, and is widely used to investigate infant motor and cognitive development, in particular through the investigation of a specific sensorimotor rhythm, called mu rhythm (Marshall and Meltzoff, 2011; Vanderwert et al., 2013). In human adults, this EEG oscillation falls within the alpha (8–13 Hz) and beta (15–30 Hz) frequency bands, and is recorded over central scalp locations, corresponding to sensorimotor areas (Fox et al., 2016; Hari and Salmelin, 1997; Pineda, 2005). The mu rhythm typically desynchronizes (i.e., decreases in spectral power) during both the execution of intentional motor acts and the observation of actions performed by others (Fox et al., 2016; Neuper and Pfurtscheller, 2001; Pineda, 2005). For this reason, desynchronization of mu rhythm (of both its components: alpha and beta) has been suggested to reflect the activation of the motor system and, indirectly, of the MNS, in both humans (Avanzini et al., 2012; Babiloni et al., 2002; Fox et al., 2016; Muthukumaraswamy and Johnson, 2004; Pineda, 2005) and monkeys (Coudé et al., 2014; Ferrari et al., 2012; Vanderwert et al., 2015).
An analogue of the adult mu rhythm has been described in human infants, starting from about the sixth month of life (Marshall et al., 2002). The infant mu rhythm peaks at lower frequencies than the adult mu rhythm (around 6–9 Hz for alpha and 15–17 Hz for beta), has a more diffuse scalp distribution (Marshall and Meltzoff, 2011; Thorpe et al., 2016) and desynchronizes during both executed and observed actions (Nyström et al., 2011; Southgate et al., 2010, 2009). Critically, infant mu suppression is strongly affected by the infant’s own motor competences. For example, Cannon et al. (2016) found that 9-month-old infants’ motor proficiency correlated with the strength of mu desynchronization during action observation and that mu desynchronization during action execution was directly associated with maturity of infants’ grasping skills. van Elk et al. (2008) studied the effects of spontaneous EEG variations in 14- to 16-month-old infants while observing movies of other infants crawling or walking. They found greater desynchronization in both sensorimotor alpha and beta bands while infants observed crawling, a more developed motor pattern in their repertoire, compared to walking. Moreover, they found a correlation between infants’ experience crawling and their mu reactivity. Further, Yoo et al. (2016) reported that 12- but not 9-month-old infants’ motor competences correlated with their mu desynchronization when they observed goal-directed actions performed with a tool. Finally, Gerson et al. (2015) experimentally manipulated motor experience by giving 10-month-old infants training observing a novel action and performing a separate novel action over the course of a week. They found that infants had greater mu desynchronization to observation of the executed actions compared to the only observed novel actions. Taken together, these studies show that motor experience, rather than visual experience, is the driving factor in the development of mu desynchronization.
To date, no study has reported EEG mu suppression to execution or observation of actions in newborn humans; however, there is evidence from recent EEG studies with newborn monkeys. Ferrari et al. (2012) and Vanderwert et al. (2015) recorded EEG data from newborn rhesus macaques during the first week of life and found desynchronization of the 5–7 Hz frequency band during both imitation and observation of lipsmacking and tongue protrusion gestures, in electrodes placed approximately over the motor cortex. These findings suggest that, at least in newborn monkeys, an action-perception coupling system for facial gestures is already active shortly after birth.
During the first month of life, newborn macaques exhibit fundamental developmental changes in their motor system. Improvements in hand reaching-grasping movements occur from the second to the fourth week of life, as the precision of grasping actions matures and infants more accurately move their body in relation to the surrounding space (Sclafani et al., 2015). This suggests that the first weeks of life are a critical period for infant macaques’ sensorimotor development and also marks a crucial period for the investigation of emerging neural mechanisms underlying the action-perception coupling. In the current study, we acquired EEG activity from newborn monkeys during the first and second weeks of life, while they observed grasping actions, mimed grasps and means-end movements. Based on previous EEG studies investigating the mu rhythm on infant and adult monkeys (Coudé et al., 2014; Ferrari et al., 2012), we focused on two frequency bands: 1) alpha (5–7 Hz) and 2) beta (15–17 Hz). We also compared, amongst a subset of infants, the relation between infants’ motor experience and their EEG cortical activity during action observation. We hypothesized that improvements in goal-directed reaching-grasping behaviors over the first two weeks of life would coincide with the emergence of the MNS, indexed by desynchronization in the sensorimotor alpha and beta rhythms for observed grasping actions.
2. Materials and methods
2.1. Subjects
We tested a sample of 56 infant rhesus macaques (Macaca mulatta), 36 males and 20 females, born and reared at the Laboratory of Comparative Ethology at the National Institutes of Health. All infants were separated from their mothers on the first day postpartum and reared in a nursery facility for unrelated research studies. Infants were individually housed in incubators (51 cm × 38 cm × 43 cm) containing a cloth surrogate mother and various toys (for further details about rearing procedures, see Simpson et al. (2016)).
EEG recordings were performed at two time points: during the first week of life (W1), between day 3 and day 6 postpartum, and again during the second week of life (W2), between day 7 and day 12 postpartum. Eleven infants were tested twice during W1, thus, the mean EEG value between the two recording days was calculated for each electrode and each experimental condition. The remaining 45 infants were tested only once during W1 and once during W2. On average 5.8 days (SD = 1.6) elapsed between the first and the second EEG recording session.
Our final sample included a total of 32 infants (16 males). Twenty infants were excluded from the initial sample due to insufficient epochs of clean EEG or technical difficulties at the time of testing (N = 12 at W1 and N = 8 at W2) and 4 infants were excluded because they were statistical outliers (i.e., exceeded ± 2.5 SD from the mean in one or more conditions).
All animal care and testing were conducted in accordance with regulations governing the care and use of laboratory animals and had prior approval from the Institutional Animal Care and Use Committees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the University of Maryland.
2.2. Behavioral procedures for EEG acquisition
At the beginning of each recording session, infant monkeys were removed from their incubators and brought to a testing room for the EEG procedures. During EEG data acquisition, one experimenter held the monkey, while a second experimenter served as model and presented the stimuli in front of the monkey at a distance of approximately 35–45 cm. The experimental paradigm included three conditions (see Fig. 1A): a) Grasping Condition, GC – the model grasped a red ball (6.5 cm diameter) at the end of a rod; b) Mimicking Condition, MC – the model mimicked a grasping action, in absence of the target ball; and c) Ball Condition, BC – the model moved a second red ball at the end of a rod toward the target ball. The MC was introduced to assess possible EEG oscillations related to simple biological movements rather than to the final goal of the action; the BC was designed to investigate the possible EEG modulation to the detection of non-biological but means-end motions. The order of presentation of the experimental conditions was pseudo-randomized between subjects.
Fig. 1.
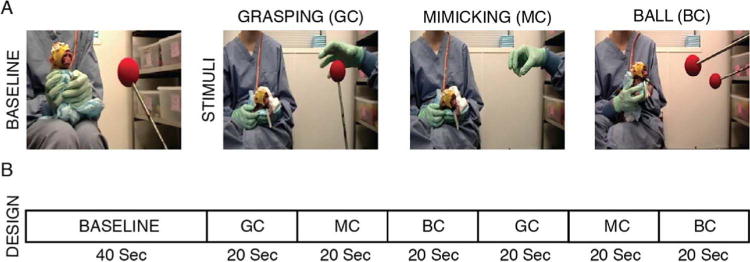
Experimental task and design. A: representation of baseline and experimental conditions. B: description of the experimental design.
Each EEG recording session started with a 40-s static baseline, where a red target ball was statically presented, followed by the presentation of the three experimental stimuli. Each stimulus was repeatedly presented over a period of 20 s, at a pace of 0.5 Hz; and the sequence GC-MC-BC was repeated twice over the recording session, for a total duration of 40 s for each stimulus. The experimental design is illustrated in Fig. 1B.
An experimenter wearing a surgical mask, cap and goggles presented stimuli live. The two red balls were presented at the end of a long (35 cm) rod. Considering the attire and distance, we believe the monkeys had limited perception of the model’s body/face and that it did not disturb stimuli presentation. Moreover, we assume that none of the experimental stimuli were perceived as threatening by the monkeys as, besides being attentive to them during the presentation, they sporadically attempted to grasp the red ball/s presented during the baseline and experimental conditions (GC/BC).
Each EEG recording session lasted about 10 min, which included the time required to cap the infant and short breaks between each stimulus presentation. Each infant participated in no more than one EEG recording session per day and, if not successfully completed, testing was, if possible, repeated once within the same week.
2.3. Behavioral coding for EEG processing
All testing sessions were video recorded. The video signal was recorded using a 30 Hz video camera (Sony Digital Video Camcorder ZR600, USA), positioned 0.5 m behind the model, and time-stamped with a vertical integrated time code that was synchronized online with the EEG acquisition software.
Subsequently, two coders independently scored both the models and the monkeys’ behaviors in each video. Videos were coded frame-by-frame off-line with the Video Coding System (James Long Company, NY, USA). The following infants’ behaviors, during both baseline and stimuli presentation, were coded: (a) visual gaze (i.e., looking at the stimulus); (b) arm and hand movements, and (c) gross body movements. In addition, the following model’s behaviors, during each stimulus presentation, were coded: (a) action begin, corresponding to the first frame in which the experimenter’s hand started moving toward the target ball (GC), started the mimed action (MC) or started moving the second red ball toward the static target ball (BC), and (b) action completion, corresponding to the first frame in which the model’s whole hand was in contact with the target ball before moving back to the starting position (GC) or the hand was completely still before moving back to the starting position (MC) or the two balls were touching and still before moving back to the starting position (BC). Inter-rater agreement, within three frames (about 100 ms), was achieved on a minimum 85% of each video. The start and the end times of epochs of interest in which the infant was still and looking at the still target ball (during baseline) or at the presented stimuli were identified within the EEG signal for data analysis.
2.4. EEG acquisition and data processing
EEG recordings were performed as previously described in Vanderwert et al. (2015) and Ferrari et al. (2012). A custom lycra cap (Electro-Cap International, OH, USA) fitted with six tin electrodes was used. Two anterior electrodes were placed approximately over the motor cortex (A3: anterior left; A4: anterior right) and two posterior electrodes were placed approximately over the parietal/occipital cortex (P3: posterior left; P4: posterior right). The vertex served as reference, while an electrode located on the forehead served as ground. The choice of referencing the EEG signal to the vertex was mainly due to newborn monkeys’ head size resulting in a very limited area for electrodes placement and thereby in a low density of electrodes.
At the beginning of the recording session, the monkey’s head was shaved, and a mild abrading gel was applied to clean the scalp and improve impedances. Impedances were measured and kept below 20 kΩ.
The EEG signal was band-pass filtered online from 0.1 to 100 Hz, digitized with a 16-bit A/D converter (± 5 V input range) at 1 KHz and recorded on a separate acquisition computer. All data acquisition was performed with the James Long recording system (James Long Company, NY, USA).
The EEG signal was filtered, off-line, using a low pass filter with a cut off of 40 Hz. As in previous infant monkey EEG studies (Ferrari et al., 2012; Vanderwert et al., 2015) artifacts were automatically removed using a threshold of ± 250 μV, in order to capture gross movement artifacts while preserving good EEG signal, and then the signal was visually inspected to remove additional artifacts not identified in the automatic artifact process. Artifact-free EEG epochs included in both the baseline and the stimulus presentation period, specifically from the onset to the completion of the action (for all experimental conditions), were submitted to a fast Fourier transform using a 1000 ms Hanning window, with 50% overlap. Spectral power (μV2) was computed for 1-Hz bins from 2 to 25 Hz. Single hertz bins were then summed to compute two frequency bands: 5–7 Hz, alpha and 15–17 Hz, beta. All data processing was performed using the EEG Analysis System software (James Long Company, NY, USA).
Computation of event-related desynchronization (ERD) and/or event-related synchronization (ERS) was based on previous studies (Cannon et al., 2014; Pineda and Oberman, 2006) that used the natural log of the ratio of event to baseline activity [i.e. ln (“Event”/“Baseline”)], where “Event” is the absolute power in a particular frequency band while the monkey was still and observing the presented stimulus, and “Baseline” is the absolute power in a particular frequency band in which the monkey was still and observing the static target ball. Negative values indicate desynchronization (i.e., decrease in band power relative to the baseline) and positive values indicate synchronization (i.e., increase in band power relative to the baseline).
2.5. Data analysis and statistical approach
Our analyses focused on two different EEG frequency bands: 5–7 Hz and 15–17 Hz, hereafter referred as alpha and beta, respectively. While the choice of focusing on frequencies falling in the alpha band was driven by previous investigations in newborn monkeys, showing EEG desynchronization in this frequency band, during imitation and observation of facial gestures (Ferrari et al., 2012), the analysis of the beta band was based on more recent EEG findings showing that in adult monkeys the beta band desynchronizes during the observation and execution of grasping actions (Coudé et al., 2014; Bimbi et al., under revision).
EEG data were analyzed by means of within-subjects repeated measures ANOVAs. Significant main effects and interactions were followed up using 2-tailed paired t-tests. The regions analyzed were as follows: anterior (A3 and A4) and posterior (P3 and P4).
A preliminary analysis was run by means of an omnibus ANOVA with Band (Alpha, Beta), Condition (GC, MC and BC), Region (Anterior, Posterior), Hemisphere (Left, Right) and Week (W1, W2) as factors. This analysis revealed a main effect of Band (F (1, 31) = 7.576, p = 0.010, ηp2 = 0.196), with desynchronization in the beta band (M = −0.125, SE = 0.040) but not in the alpha band (M = 0.042, SE = 0.049). One sample t-tests compared to zero confirmed the absence of desynchronization in the alpha band in all conditions (GC, MC, BC), regions (Anterior and Posterior) and at both W1 and W2 (all ps > 0.05) except for MC at W2 which instead showed a significant synchronization over posterior scalp locations (p = 0.038). Therefore, we focused our remaining analyses on the beta band.
2.6. Correlation between infants’ ERD and motor proficiency
Some infants showed attempts to reach and grasp during EEG testing, but the presence of excessive motor artifacts, which contaminated EEG data during action execution, together with the short-term duration of each recording session and the low number of grasping actions exhibited by each infant made it impossible to acquire the minimum number of trials required to analyze the EEG data. However, a subset of monkeys (N = 14) from our sample completed a separate behavioral Reaching-grasping task, originally designed to investigate infants’ space perception in relation to their grasping behavior maturation over the first month of life (for details on infants’ behavioral grasping capacities, see Sclafani et al. (2015)). Briefly: monkeys were tested twice a week from the second to the fourth week postpartum. Each monkey was presented with a series of small and large balls, either at a reachable distance (infant’s peripersonal space) or at a non-reachable distance (infant’s extrapersonal space). If the infant made no grasping attempt with the ball within 20 s, the ball was removed and the trial was terminated. Infants were presented with one ball at a time, and completed up to 8 trials (2 trials for each ball size and distance). Infants could attempt to grasp with different effectors, hand or mouth, and different motor strategies, stepping toward the target ball before attempting to grasp it or simply extending their arm/mouth to approach the ball. In the present study, we considered the number of hand grasping attempts—both successful and unsuccessful—exhibited by each infant at two weeks of age as an index of infants’ grasping propensity. We chose to include both successful and unsuccessful attempts in our analyses because, at this age, infants make a high proportion of unsuccessful attempts as they initially develop their manual reach-grasping skills. There were, however, significant individual differences in the frequencies of attempts overall, so we focused on this measure. The choice of investigating hand grasping skills in relation to the EEG activity only in the second week of life and not in the first week was motivated by the fact that in the first week of life almost no infants attempted to reach the ball. Only in the second week of life did infants start to develop a rudimentary form of grasping, even though there were significant individual differences (Sclafani et al., 2015), with some individuals making attempts and others not yet displaying any attempt to reach the object. We therefore considered this transition phase, from week 1 to week 2, as an ideal time to capture emerging neural activity during action observation. While empirically interesting, further longitudinal measurements at week 3 and 4 post partum were not feasible as the monkeys became too active by these ages.
Since data were not normally distributed we used Spearman’s correlation to explore the possible association between the number of hand grasping attempts exhibited by each infant at W2, during the Reaching-grasping task, and the EEG activity recorded at the same age during the observation of grasping, mimed actions and means-end movements. Bonferroni corrections were applied to account for multiple comparisons.
3. Results
Preliminary analyses revealed the presence of desynchronization in the beta band but not in the alpha band. Therefore, results reported in this section will be focused on beta band only.
To examine whether there was EEG desynchronization during GC, MC, and BC, one-sample t-tests compared with zero were run. Analyses revealed significant ERD over anterior electrodes during W2 (GC: t(31) = −4.141, p < 0.001, d = 0.73; MC: t(31) = −2.084, p = 0.045, d = 0.37; BC: t(31) = −2.967, p = 0.006, d = 0.52) but not during W1 (GC: t(31) = −1.085, p = 0.286; MC: t(31) = −1.850, p = 0.074; BC: t(31) = −1.940, p = 0.062), and no significant ERD either at W1 (GC: t(31) = −0.483, p = 0.632; MC: t(31) = −1.888, p = 0.068; BC: t(31) = −0.994, p = 0.328) or at W2 (GC: t(31) = −0.829, p = 0.413; MC: t(31) = 0.811, p = 0.424; BC: t(31) = −0.411, p = 0.684) for posterior electrodes. Fig. 2 shows mean ERDs in the beta band over anterior and posterior electrodes.
Fig. 2.
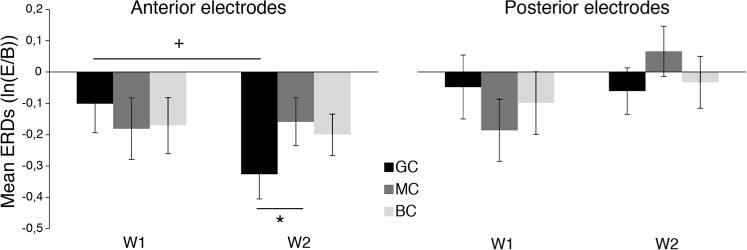
Beta event-related desynchronization. Means and standard errors of EEG beta event-related desynchronizations (ERDs) for anterior and posterior electrodes in each condition (GC, MC, BC) and week (W1, W2). E: Event, B: Baseline. *p < 0.05, +p = 0.071.
To explore the possible presence of any longitudinal effects, from W1 to W2, we implemented further analyses. Because the effect of hemisphere in preliminary analyses was not significant, we averaged ERD and ERS values across the left and right hemisphere. We implemented a 3 Condition (GC, MC and BL) × 2 Region (Anterior and posterior) × 2 Week (W1, W2) within-subject ANOVA, which revealed a main effect of Region (F (1, 31) = 5.881, p = 0.020, ηp2 = 0.159), with greater desynchronization in anterior electrodes (M = −0.189, SE = 0.42) than posterior electrodes (M = −0.061, SE = 0.053), and a Condition × Week interaction (F (2, 62) = 4.021, p = 0.026, ηp2 = 0.156). Follow-up paired comparisons focused only on anterior electrodes and revealed no differences between W1 and W2 in MC (t(31) = −0.183, p = 0.856; MCW1: M = −0.181, SE = 0.098; MCW2: M = −0.159, SE = 0.076) and BC (t(31) = −0.253, p = 0.802; BCW1: M = −0.170, SE = 0.088; BCW2: M = −0.198, SE = 0.067), while a trend was found in GC (t(31) = −1.86, p = 0.071, d = 0.33), with more ERD in W2 (M = −0.329, SE = 0.079) than W1(M = −0.101, SE = 0.093) (Fig. 2). Follow-up paired comparisons also showed a significant difference between GC and MC at W2 (t(31) = −2.06, p = 0.048, d = 0.36) (Fig. 2), but not at W1 (t(31) = 0.79, p = 0.435). No difference between GC and BC were found at both W1 and W2 (W1:t(31) = 0.63, p = 0.535; W2:t(31) = −1.46, p = 0.158). MC and BC did not differ at either time point (W1: t (31) = 0.14, p = 0.891; W2: t(31) = −0.494, p = 0.624).
3.1. Motor competence and ERD during observation of grasping actions
To investigate the relation between infants’ motor competence at 2 weeks of age and beta ERD recorded during action observation at W2, Spearman correlations were run for a subset of 14 monkeys.
For anterior electrodes, this analysis revealed a trend-significant negative correlation between the total number of hand grasping attempts exhibited by each infant at W2, during the behavioral Reaching-grasping task, and EEG desynchronization to observed grasping actions (Spearman correlation: rs (14) = −0.616, pcorr = 0.057). Specifically, greater ERD in GC were associated with more grasping attempts, suggesting that infants with more mature manual motor abilities may also be exhibiting stronger sensorimotor neural activation during observation of grasping actions. No significant correlations were found between the total number of hand grasping attempts exhibited by each infant at W2 and EEG desynchronization to observed mimed actions (Spearman correlation: rs (14) = −0.242, p = 0.404) or observed means-end movements (Spearman correlation: rs (14) = 0.174, p = 0.560).
Similarly, no significant correlations were found between the total number of grasping attempts exhibited by each monkey during the Reaching-grasping task and EEG values over posterior electrodes in GC (Spearman correlation: rs (14) = 0.104, p = 0.723), MC (Spearman correlation: rs (14) = 0.586, pcorr = 0.102) and BC (Spearman correlation: rs (14) = 0.202, p = 0.508).
We further explored the possible relations between EEG activity recorded during grasping action observation (GC) and the number of grasping attempts exhibited by each infant, during the behavioral Reaching-grasping task, when the object was presented either in the peripersonal or in the extrapersonal space. In fact, compared to the peripersonal space trials, the extrapersonal space trials required infants to engage in not only grasping, but also locomotion, moving toward the ball, which involve a more elaborate encoding of the surrounding space and evaluation of their body representation in it. The number of grasping attempts made when the target ball was presented in the infant’s peripersonal space was correlated with EEG desynchronization recorded during grasping action observation (Spearman correlation: rs (14) = −0.735, pcorr = 0.009) (Fig. 3). Specifically, greater desynchronization was associated with more grasping attempts. No relation was found between EEG activity and the number of grasping attempts executed in the extrapersonal space (Spearman correlation: rs (14) = −0.210, p = 0.471).
Fig. 3.
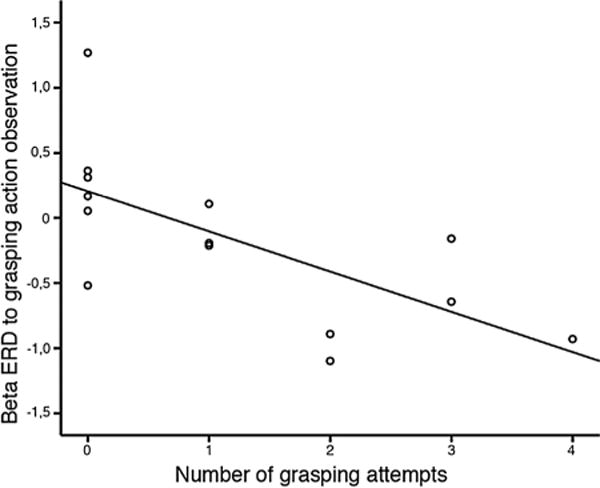
Correlation between EEG beta desynchronization over the anterior scalp region and infants’ propensity to grasp: Negative correlation between ERD values and the number of hand grasping attempts exhibited in the peripersonal space at W2 (p < 0.05). The x-axis corresponds to the number of grasping attempts exhibited by each monkey-during a separate behavioral task- when the object was presented in the infant’s peri-personal space. The y-axis is the beta ERD recorded over anterior electrodes at W2.
Together, these results suggest that grasping motor abilities, particularly when objects are available within reaching distance, are associated with EEG reactivity while observing others’ grasping actions.
4. Discussion
In the current study, we acquired EEG data on newborn macaque monkeys while they observed grasping actions, mimed actions and means-end motions. In a subset of monkeys, we also examined the relations between EEG reactivity and the emergence of infants’ manual motor skills. To track any possible longitudinal development, we performed EEG recordings at two different time points: during the first and second week postpartum (W1 and W2) and, following previous EEG studies in human infants (Cannon et al., 2016; Southgate et al., 2010; van Elk et al., 2008; Yoo et al., 2016) and, in infant (Ferrari et al., 2012) and adult (Coudé et al., 2014) monkeys, we focused analyses on two specific frequency bands, representing the main components of the mu rhythm: alpha, 5–7 Hz, and beta, 15–17 Hz.
In newborn monkeys, the first month of life represents a crucial transitional stage for developing reaching-grasping movements, with the greatest motor changes occurring between the second and the third week. During this period, infants start showing successful motor strategies to reach and grasp objects in the surrounding environment (Sclafani et al., 2015). Therefore, we hypothesized that rudimentary cortical mechanisms underlying the perception of others’ manual actions may emerge and operate in parallel with improvements in infants’ hand motor skills. In particular, our goal was to observe the possible emergence of neural modulations to action observation from the first week of life, when there is almost total absence of grasping abilities (Sclafani et al., 2015), to the second week of life, when a rough propensity to approach objects emerges.
Our results confirmed our hypothesis identifying desynchronization in the beta band, in anterior electrodes, during the observation of grasping actions, that is enhanced between the first and second week of life. Importantly, this desynchronization coincided with the emergence of the propensity to engage with objects, between the first and the second week, and was greatest for observation of grasping actions compared to the observation of mimed action or means-end motions.
These results are consistent in both their spectral and topographical characteristics with previous human EEG studies, involving infants or adults, in which EEG mu desynchronization to action observation has been described in electrodes placed over motor areas and in frequencies belonging to the beta band range (Avanzini et al., 2012; Babiloni et al., 2002; van Elk et al., 2008). Findings in the present study are also consistent with EEG investigations in adult monkeys showing that the observation of grasping actions produces EEG desynchronization over frontal and central scalp regions in the beta band more than in the alpha band (Coudé et al., 2014).
Importantly, our data represent the first evidence showing EEG reactivity to the observation of hand goal-directed actions as early as the second week of life. The only other neurophysiological evidence concerning the emergence of a neural system underlying action and perception right after birth comes from previous EEG investigations in neonate monkeys (Ferrari et al., 2012; Vanderwert et al., 2015). These studies showed a distinct EEG suppression of the 5–7 Hz band during imitation and execution of communicative facial gestures (i.e., lip smacking and tongue protrusion). Thus, it has been proposed that a rudimentary mirror mechanism underlying imitation may operate very early in development and may even be pre-formed in utero (Casile et al., 2011; Simpson et al., 2014; Vanderwert et al., 2013). However, it is important to note that, compared to oro-facial movements, the development of arm and hand movements requires a more complex and longer maturation processes after birth. For example, in both humans and monkeys, the myelination process of the corticospinal tract, which plays a primary role in the development of voluntary arm movements (Galea and Darian-Smith, 1995; Lemon, 1999), is completed only between the second and the third year of life (Olivier et al., 1997). Although newborn monkeys display only exploratory movements in the first week of life, such space exploration greatly contributes to the initial development of their visual-motor coordination, proprioception and internal representation of space (Sclafani et al., 2015; von Hofsten, 2004), and all these factors together may contribute to the later development of successful goal-directed reaching-grasping actions (Nelson et al., 2011; Sclafani et al., 2015). Further, these neurodevelopmental processes likely contribute to the emergence of desynchronization in the beta band during observation of actions in anterior scalp locations, along with the emerging cortical networks involved in action execution.
Our results also support the idea that the observation of others’ actions recruits mirror neuron populations hosted in the ventral pre-motor cortex, in the primary motor cortex and in the posterior parietal lobe (Bonini et al., 2010; Fogassi et al., 2005; Gallese et al., 1996; Vigneswaran et al., 2013), and therefore raise the possibility that, even at this early developmental stage, a mirror mechanism, probably still broadly tuned, may be emerging. This hypothesis remains speculative, however, two lines of evidence support this conclusion. First, beta desynchronization is greater for goal-directed actions than for observed mimed action and, although we did not find any significant differences in the beta desynchronization between grasping action observation and means-end movement observation, it is plausible that this latter condition relies on multiple factors, including the final goal of the action, the presence of multiple objects (two red balls versus one) and the movement of an interesting object rather than the model’s hand. Evidence at the single cell level in adult monkeys reveals that F5 and PFG mirror neurons respond predominantly to goal-directed action rather than mimed actions (Ferrari et al., 2005; Gallese et al., 1996; Rozzi et al., 2008). Data at the single cell level are not available in infant monkeys and therefore we cannot infer whether the neuronal activation to specific visual stimuli is similar to that of adults. Our EEG data, however, suggest that the cortical network recruited in infants during action observation shares similar stimulus-response properties in frequency and distribution of activity recorded from the scalp in adults.
Second, not surprisingly, we did not find any desynchronization during the first week of life, when reaching-grasping attempts are very sporadic and movements do not appear to be voluntarily controlled (Sclafani et al., 2015). Our data thus reflect the changes occurring at the behavioral and neural levels, suggesting that a rudimentary cortical system involved in action observation and execution starts operating along with significant improvements in motor skills as well as the development of specific cortical visual-motor integrations. As infant macaques start developing reach and grasp skills by the second week of life, the EEG brain responses become more tuned for goal-directed actions and this, according to our hypothesis, reflects a more mature organization of cortical motor areas which are capable not only of supporting hand actions, but also involved in the decoding of others’ actions in terms of goals.
Unfortunately, we were unable to analyze the EEG data during action execution. Limited testing time and the excessive artifact contamination that characterized the EEG signal during infants’ spontaneous reaching-grasping movements resulted in an inability to assess whether desynchronization occurred in the same frequencies for action execution and observation. However, the relation between EEG beta desynchronization, found during observation of grasping actions and infants’ motor skills (i.e., grasping attempts), in a subset of monkeys, provides some clues to a pairing of execution and observation of actions at the neural level. Our analysis showed that infants who made more reaching-grasping attempts to the target ball had greater beta desynchronization over motor-related brain regions. Moreover, this correlation was specific for grasping attempts made in the peripersonal but not the extrapersonal space, confirming what has been previously demonstrated in monkeys (Sclafani et al., 2015) and in human infant studies (Rochat and Goubet, 1995) showing that although infants progressively increase their body representation in the space along with their motor abilities, they might detect the distance at which an object can be reached concurrently with the emergence of the motor reaching-grasping skills. Attempts to grasp, thus, represent a marker of neurodevelopment in infants revealing the capacity to coordinate movements requiring complex visual processing and visuomotor coordination in space. This capacity is known to heavily rely on parietal-premotor circuits and on the maturation of the corticospinal tract (Lemon, 1999; Olivier et al., 1997; Rizzolatti and Luppino, 2001). It is possible that individuals who show reaching-grasping propensity have more mature cortical circuits compared to those who are unable or uninterested in attempting to grasp, consistently with an emerging body of evidence from human developmental EEG studies (Cannon et al., 2016; van Elk et al., 2008; Yoo et al., 2016). Our data suggest that parietal and premotor circuits start their maturation and refinement between the first and second week, as reflected in the emergence of EEG responses during grasping observation and their association with infants’ readiness to attempt to grasp, especially when objects are located in peripersonal space. It is in fact reasonable to think that at this developmental stage grasping attempts made within the peripersonal space would better correlate with ERD to grasping observation, as they would reflect the activation of more refined neural circuits also hypothetically activated during action observation. In contrast, attempts to grasp when an object is presented in the extrapersonal space are more sporadic at this age (Sclafani et al., 2015) and this might reflect the fact that some of the parietal-premotor circuits involved in reaching and in space coding are not yet fully developed and still require sensorimotor experience to be refined and become functional.
In contrast to previous newborn monkey EEG investigations (Ferrari et al., 2012; Vanderwert et al., 2015), we did not find any significant desynchronization in the sensorimotor alpha band. A possible explanation for this apparent discrepancy might be related to the type of effector used (hand versus mouth) and/or the value (social versus nonsocial) of the actions assessed in the two different studies. While Ferrari et al. (2012) recorded EEG during a facial neonatal imitation task, the current study included stimuli involving hand reaching-grasping actions. Thus, the desynchronization in the alpha band might reflect the recruitment of pre-formed circuits activated by oro-facial gestures with communicative values (i.e., lipsmacking and tongue protrusion); conversely the observation of goal-directed actions may reflect the activation of circuits still under development, involving premotor and motor hand cortical regions requiring a longer period of maturation before becoming adult-like. Electrophysiological studies in adult monkeys show that mouth and hand mirror neurons are distributed in different, yet partially overlapping, neuroanatomical sectors within the ventral premotor cortex, with the hand represented predominantly in the most medial part of F5 and the mouth neurons distributed in the lateral sector of the F5 convexity (Ferrari et al., 2003; Maranesi et al., 2012). Neuroimaging studies in human adults report that observing mouth actions activates a more lateral sector of the premotor cortex than observing hand actions (Buccino et al., 2001). Thus, although highly interconnected, mouth and hand mirror neurons could rely on different and only partially overlapped cortical networks, very early in development (Casile et al., 2011).
From a developmental perspective, it is possible that cortical mouth circuits are already present at birth and subsequently shaped by social experience (Casile et al., 2011; Tramacere et al., 2017; Vanderwert et al., 2015) while hand cortical circuits, although present at birth, may undergo finer and slower development relying more heavily on body maturation and motor experience (Gerson et al., 2015). This interpretation would also be partially in line with more recent views on the development of mirror neuron systems which support the idea that action observation might benefit from experience as a result of associative learning processes and maturational processes that are canalized during development (Cook et al., 2014; Del Giudice et al., 2009; Ferrari et al., 2013; Heyes, 2013, 2010; Tramacere et al., 2017). Therefore, ERD to grasping action observation emerging at the second week of life would be sustained by the emergence of grasping motor skills and modulated by grasping experience.
The functionality of the alpha and beta bands may further represent developmental markers for maturation of the mirror neuron system. The alpha band, therefore, may reflect the activation of broader parieto-frontal circuits integrating the activity of other areas besides the motor regions that might require a longer period of time for myelination processes. Thus, it is possible that the reactivity of alpha frequencies to others’ hand actions might emerge at later ages as a result of greater motor experience, compared to the beta band. In line with this hypothesis, it has been shown that the two frequency bands originate from different cortical sources, with beta band having its cortical source in the motor cortex and the alpha band originating from the post-central gyrus (Hari and Salmelin, 1997). This suggests that local circuits, restricted to the motor and premotor cortex, may develop and integrate information reflected in the EEG modulation earlier than later developing complex networks involving fronto-parietal connections. Moreover, a recent neurophysiological investigation using simultaneous EEG and single neurons recordings (Bimbi et al., under revision) in adult monkeys, demonstrated that F5 mirror neurons activity correlated with EEG desynchronization to grasping action observation in the beta band but not in the alpha band. Thus, it is possible that the major contribution of mirror neurons to the beta band desynchronization recorded over anterior and central scalp locations is already present at this early developmental stage.
In conclusion, our findings suggest that EEG beta suppression recorded over the scalp may represent a marker of the activation of cortical networks, probably including mirror neurons, underlying goal-directed hand action perception, starting from the second week of life and that develop along with the emergence of grasping motor skills.
Acknowledgments
This research was supported by the National Institutes of Health (NICHD and NIH P01HD064653).
References
- Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: an EEG study. PLoS One. 2012;7:e37534. doi: 10.1371/journal.pone.0037534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, Del Percio C, Moretti DV, Rossini PM. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. Neuroimage. 2002;17:559–572. [PubMed] [Google Scholar]
- Bimbi M, Festante F, Coudé G, Vanderwert RE, Fox NA, Ferrari PF. Simultaneous scalp recorded EEG and multiunit recording from monkey ventral premotor cortex during action observation and execution reveals the contribution of mirror and motor neurons to the mu-rhythm. Neuroimage. doi: 10.1016/j.neuroimage.2018.03.037. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex. 2010;20:1372–1385. doi: 10.1093/cercor/bhp200. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. doi: 10.1046/j.1460-9568.2001.01385.x. [DOI] [PubMed] [Google Scholar]
- Cannon EN, Yoo KH, Vanderwert RE, Ferrari PF, Woodward AL, Fox NA. Action experience, more than observation, influences mu rhythm desynchronization. PLoS One. 2014;9:e92002. doi: 10.1371/journal.pone.0092002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Simpson EA, Fox NA, Vanderwert RE, Woodward AL, Ferrari PF. Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Dev Sci. 2016;19:50–62. doi: 10.1111/desc.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casile A, Caggiano V, Ferrari PF. The mirror neuron system: a fresh view. Neuroscientist. 2011;17:524–538. doi: 10.1177/1073858410392239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: from origin to function. Behav Brain Sci. 2014;37:177–192. doi: 10.1017/s0140525x13000903. [DOI] [PubMed] [Google Scholar]
- Coudé G, Vanderwert RE, Thorpe S, Festante F, Bimbi M, Fox NA, Ferrari PF. Frequency and topography in monkey electroencephalogram during action observation: possible neural correlates of the mirror neuron system. Philos Trans R Soc Lond B: Biol Sci. 2014;369:20130415. doi: 10.1098/rstb.2013.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Manera V, Keysers C. Programmed to learn? The ontogeny of mirror neurons. Dev Sci. 2009;12:350–363. doi: 10.1111/j.1467-7687.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci. 2005;17:212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Paukner A, Ruggiero A, Darcey L, Unbehagen S, Suomi SJ. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Dev. 2009;80:1057–1068. doi: 10.1111/j.1467-8624.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Vanderwert RE, Paukner A, Bower S, Suomi SJ, Fox NA. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J Cogn Neurosci. 2012;24:1165–1172. doi: 10.1162/jocn_a_00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Tramacere A, Simpson EA, Iriki A. Mirror neurons through the lens of epigenetics. Trends Cogn Sci. 2013;17:450–457. doi: 10.1016/j.tics.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fox NA, Bakermans-Kranenburg MJ, Yoo KH, Bowman LC, Cannon EN, Vanderwert RE, Ferrari PF, Van Ijzendoorn MH. Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychol Bull. 2016;142:291–313. doi: 10.1037/bul0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cereb Cortex. 1995;5:518–540. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gerson SA, Bekkering H, Hunnius S. Short-term motor training, but not observational training, alters neurocognitive mechanisms of action processing in infancy. J Cogn Neurosci. 2015;27:1207–1214. doi: 10.1162/jocn_a_00774. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Heyes C. Where do mirror neurons come from? Neurosci Biobehav Rev. 2010;34:575–583. doi: 10.1016/j.neubiorev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Heyes C. A new approach to mirror neurons: developmental history, system-level theory and intervention experiments. Cortex. 2013;49:2946–2948. doi: 10.1016/j.cortex.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Hunnius S, Bekkering H. What are you doing? How active and observational experience shape infants’ action understanding Philos. Trans R Soc B: Biol Sci. 2014;369:20130490. doi: 10.1098/rstb.2013.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaburu SSK, Paukner A, Simpson EA, Suomi SJ, Ferrari PF. Neonatal imitation predicts infant rhesus macaque (Macaca mulatta) social and anxiety-related behaviours at one year. Sci Rep. 2016;6:34997. doi: 10.1038/srep34997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Neural control of dexterity: what has been achieved? Exp Brain Res. 1999;128:6–12. doi: 10.1007/s002210050811. [DOI] [PubMed] [Google Scholar]
- Maranesi M, Rodà F, Bonini L, Rozzi S, Ferrari PF, Fogassi L, Coudé G. Anatomo-functional organization of the ventral primary motor and premotor cortex in the macaque monkey. Eur J Neurosci. 2012;36:3376–3387. doi: 10.1111/j.1460-9568.2012.08252.x. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Dev Cogn Neurosci. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring mechanisms and imitation in human infants. Philos Trans R Soc Lond B: Biol Sci. 2014;369:20130620. doi: 10.1098/rstb.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin Neurophysiol. 2002;113:1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology. 2004;41:152–156. doi: 10.1046/j.1469-8986.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Nelson EL, Emery MS, Babcock SM, Novak MFSX, Suomi SJ, Novak MA. Head orientation and handedness trajectory in rhesus monkey infants (Macaca mulatta) Dev Psychobiol. 2011;53:246–255. doi: 10.1002/dev.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001:41–58. doi: 10.1016/S0167-8760(01)00178-7. [DOI] [PubMed]
- Nyström P, Ljunghammar T, Rosander K, Von Hofsten C. Using mu rhythm desynchronization to measure mirror neuron activity in infants. Dev Sci. 2011;14:327–335. doi: 10.1111/j.1467-7687.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. J Neurosci. 1997;17:267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA, Oberman LM. What goads cigarette smokers to smoke? Neural adaptation and the mirror neuron system. Brain Res. 2006;1121:128–135. doi: 10.1016/j.brainres.2006.08.128. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Rev. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/S0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rochat P, Goubet N. Development of sitting and reaching in 5- to 6-month-old infants. Infant Behav Dev. 1995;18:53–68. doi: 10.1016/0163-6383(95)90007-1. [DOI] [Google Scholar]
- Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci. 2008;28:1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- Sclafani V, Simpson EA, Suomi SJ, Ferrari PF. Development of space perception in relation to the maturation of the motor system in infant rhesus macaques (Macaca mulatta) Neuropsychologia. 2015;70:429–441. doi: 10.1016/j.neuropsychologia.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Murray L, Paukner A, Ferrari PF. The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Philos Trans R Soc B: Biol Sci. 2014;369:20130289. doi: 10.1098/rstb.2013.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EA, Miller GM, Ferrari PF, Suomi SJ, Paukner A. Neonatal imitation and early social experience predict gaze following abilities in infant macaques. Sci Rep. 2016:1–14. doi: 10.1038/srep20233. [DOI] [PMC free article] [PubMed]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biol Lett. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychol Sci. 2010;21:355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Thorpe SG, Cannon EN, Fox NA. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin Neurophysiol. 2016;127:254–269. doi: 10.1016/j.clinph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramacere A, Pievani T, Ferrari PF. Mirror neurons in the tree of life: mosaic evolution, plasticity and exaptation of sensorimotor matching responses. Biol Rev Camb Philos Soc. 2017;92:1819–1841. doi: 10.1111/brv.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: neurophysiological evidence for experience-dependent motor resonance in infancy. Neuroimage. 2008;43:808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- Vanderwert RE, Fox NA, Ferrari PF. The mirror mechanism and mu rhythm in social development. Neurosci Lett. 2013:1–6. doi: 10.1016/j.neulet.2012.10.006. [DOI] [PMC free article] [PubMed]
- Vanderwert RE, Simpson EA, Paukner A, Suomi SJ, Fox NA, Ferrari PF. Early social experience affects neural activity to affiliative facial gestures in newborn nonhuman primates. Dev Neurosci. 2015;37:243–252. doi: 10.1159/000381538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G, Philipp R, Lemon RN, Kraskov A. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr Biol. 2013;23:236–243. doi: 10.1016/j.cub.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C. An action perspective on motor development. Trends Cogn Sci. 2004;8:266–272. doi: 10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Woodward AL, Gerson SA. Mirroring and the development of action understanding. Philos Trans R Soc Lond B: Biol Sci. 2014;369:20130181. doi: 10.1098/rstb.2013.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KH, Cannon EN, Thorpe SG, Fox NA. Desynchronization in EEG during perception of means-end actions and relations with infants’ grasping skill. Br J Dev Psychol. 2016;34:24–37. doi: 10.1111/bjdp.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]


