Abstract
Anastasis (Greek for “rising to life”) is a recently discovered cell recovery phenomenon whereby dying cells can reverse late-stage cell death processes that are generally assumed to be intrinsically irreversible. Promoting anastasis could in principle rescue or preserve injured cells that are difficult to replace such as cardiomyocytes or neurons, thereby facilitating tissue recovery. Conversely, suppressing anastasis in cancer cells, undergoing apoptosis after anti-cancer therapies, may ensure cancer cell death and reduce the chances of recurrence. However, these studies have been hampered by the lack of tools for tracking the fate of cells that undergo anastasis in live animals. The challenge is to identify the cells that have reversed the cell death process despite their morphologically normal appearance after recovery. To overcome this difficulty, we have developed Drosophila and mammalian CaspaseTracker biosensor systems that can identify and permanently track the anastatic cells in vitro or in vivo. Here, we present in vivo protocols for the generation and use of the CaspaseTracker dual biosensor system to detect and track anastasis in Drosophila melanogaster after transient exposure to cell death stimuli. While conventional biosensors and protocols can label cells actively undergoing apoptotic cell death, the CaspaseTracker biosensor can permanently label cells that have recovered after caspase activation - a hallmark of late-stage apoptosis, and simultaneously identify active apoptotic processes. This biosensor can also track the recovery of the cells that attempted other forms of cell death that directly or indirectly involved caspase activity. Therefore, this protocol enables us to continuously track the fate of these cells and their progeny, facilitating future studies of the biological functions, molecular mechanisms, physiological and pathological consequences, and therapeutic implications of anastasis. We also discuss the appropriate controls to distinguish cells that undergo anastasis from those that display non-apoptotic caspase activity in vivo.
Keywords: Medicine, Issue 132, Anastasis, apoptosis, autophagy, biosensor, caspase, CaspaseTracker, necrosis, necroptosis, programmed cell death, reversal of apoptosis
Introduction
Programmed cell death, such as apoptosis, plays an essential role in embryonic development and normal homeostasis by eliminating unwanted, injured, or dangerous cells in multicellular organisms1,2,3. The loss of balance between cell death and survival can lead to fatal consequences such as cancer, heart failure, autoimmunity, and degeneration4,5,6,7,8. Activation of executioner caspases has traditionally been considered as the “point of no return” in apoptosis9,10,11, as it triggers rapid and massive cellular demolition12,13,14,15,16. Challenging this general dogma, we demonstrated that cultured dying primary cells and cancer cells can recover not only after caspase activation, but also following important cell death hallmarks including plasma membrane blebbing, cell shrinkage, mitochondrial fragmentation, release of mitochondrial cytochrome c into the cytosol, nuclear and chromatin condensation, DNA damage, nuclear fragmentation, cell surface exposure of phosphatidylserine (PS), and formation of apoptotic bodies17,18,19,20,21. We propose that anastasis is an intrinsic cell recovery phenomenon, as dying cells can recover after removal of cell death stimuli17,18,19,20,21. We coined the term “Anastasis” (Αναστάσης)18, which means “rising to life” in Greek, to describe this unexpected cell recovery phenomenon. Our observation of anastasis is further supported by recent independent studies that also reveal recovery of cells after phosphatidylserine externalization22,23,24, limited mitochondrial outer membrane permeabilization25, activation of mixed lineage kinase-like (MLKL), and cell shrinkage26.
Characterizing the mechanisms regulating anastasis will have paradigm-shifting physiological, pathological, and therapeutic implications. Anastasis could represent a previously unknown cytoprotective mechanism to rescue or preserve important postmitotic cells and tissues that are difficult to replace, and possibly account for heart failure reversal by ventricular unloading with left ventricular assist devices (LVADs)27,28, recovery of photoreceptor cells after transient exposure of excessive light29,30,31, or repair of neurons after brain injury32. If so promoting anastasis could enhance cell and tissue recovery. Conversely, anastasis could be an unexpected escape tactic used by cancer cells to survive cell-death-inducing therapy, causing cancer recurrence17,18. Therefore, suppressing anastasis in dying cancer cells during and after cancer treatment could be a novel therapeutic strategy to cure cancers by preventing their relapse.
During the process of anastasis, we have found that some recovered cells acquired permanent genetic alterations and underwent oncogenic transformation, likely due to DNA damage incurred during apoptosis18,20,21. Reversing the death process of DNA-damaged cells could be a mechanism of tumorigenesis, potentially underlying the observation that repeated tissue injury increases the risk of cancer in a variety of tissues, such as chronic thermal injury in the esophagus induced by the consumption of very hot beverages33,34,35, liver damage due to alcoholism36,37, tumor evolution after genotoxic cancer therapy38,39,40, and development of new cancers from normal tissues that arise during the intervals between cycles of anti-cancer therapy41,42,43,44. If true, targeting anastasis could prevent or arrest cancer development and progression. We have found that starvation-induced dying germ cells undergo anastasis in re-fed Drosophila19. If anastasis occurs in germ cells with DNA damage, it could be account for the observation that prolonged environmental stress promotes development of genetic diseases. For example, famines contribute to the development of transgenerational inheritable diseases such as diabetes and coronary heart diseases45. Therefore, understanding anastasis could lead to strategies for the prevention of developing inheritable diseases caused by this potential mechanism.
To harness the discovery of anastasis and direct it to develop innovative therapies, it is essential to study the cause and consequence of anastasis in live animals. However, it is technically challenging to identify and track anastatic cells in vivo, because the cells that recovered from cell death process appear morphologically indistinguishable from normal healthy cells, and there is no biomarker of anastasis identified yet17,18,21. To address these problems, we recently developed a new in vivo caspase biosensor designated “CaspaseTracker”19, to identify and track cells that survive apoptosis after caspase activation19,46, the hallmark of apoptosis10,14. Distinguishing it from the “real-time” caspase biosensors such as SCAT12,47, Apoliner48, CA-GFP49, ApoAlert18,50, C3AIs51 and iCasper52 that detect on-going caspase activity, the CaspaseTracker biosensor additionally features the ability to permanently label cells that express caspase activity even transiently. Therefore, the CaspaseTracker biosensor enables long term tracking of anastasis after reversal of caspase-mediated cell death process in vivo.
Protocol
1) Preparation of CaspaseTracker Biosensor Flies
-
Anesthetize flies with CO2, and use a paintbrush to transfer 7 to 10 caspase-sensitive Gal4 (DQVD)19 virgin females and 7 to 10 G-Trace53 Gal4 reporter young male flies (or vice versa) in the same vial with fly food and fresh yeast paste.
NOTE: Cross of Caspase-sensitive (DQVD) Gal4 and G-Trace flies will produce CaspaseTracker progeny flies. Cross of Caspase-insensitive (DQVA)19 Gal4 and G-Trace flies will provide negative control flies (see discussion). Fresh yeast paste serves as protein source to enhance egg production, so that increases number of progeny. Select virgin females and young male flies according to their phenotypes54.
-
Incubate the flies at 18 degrees Celsius (°C) during the cross for 3 to 7 days, and then transfer the flies to a new vial to set up a new cross at 18 °C. Continue to incubate the original vial at 18 °C until progeny flies eclose.
NOTE: Transfer the parent flies to new vials to avoid overcrowding of progeny at the original vial. Parent flies can produce progeny with fresh food and yeast paste at the first 2 to 3 switches, and then the productivity will significantly decrease with time. Raising flies 18 °C can reduce non-specific signal of CaspaseTracker biosensor (see discussion).
-
Select progeny flies with correct phenotypes54 for following experiments.
NOTE: The transgenes of both caspase-sensitive Gal4 and G-Trace here are located at the second chromosome, balanced with CyO balancer. Select the non-curly wing progeny (without CyO), which has both transgenes of caspase-sensitive Gal4 and G-Trace.
2) Application of Transient Cell Death Induction to CaspaseTracker Biosensor Flies
-
Transfer 10 to 20 newly eclosed female flies to a new vial with fresh fly food and fresh yeast paste for 1 day at 18 °C to allow egg chamber production by oogenesis.
NOTE: Keeping the female with male flies might enhance egg chamber production.
-
To induce egg chambers to undergo apoptosis by cold shock, transfer the female flies to new empty vial, which is then placed at −7 °C for 1 h.
NOTE: Cold shock injures cells by inducing plasma membrane rupture55,56.
-
To induce egg chambers to undergo apoptosis by protein starvation, transfer the female flies to a new vial with 8% sucrose and 1% agar food at 18 °C for 3 days.
NOTE: Protein starvation (non-protein food) can trigger egg chambers to undergo apoptosis57,58,59 and autophagy60,61. Switch flies to a new vial with 8% sucrose and 1% agar food every day to keep optimal condition of the sucrose fly food.
-
Transfer the stressed flies back to a new vial with fresh fly food and fresh yeast paste for 3 days at 18 °C to allow them to recover. Dissect the starved and the starved-recovered flies to obtain egg chambers at ovaries as described62.
NOTE: To dissect Drosophila to obtain ovaries, anesthetize flies with CO2, and use 2 pairs of forceps to remove fly head, and use the forceps to pull the base of the abdomen to remove the ovaries of the flies.
3) Fixation and Staining of Dissected Egg Chambers for Imaging
-
Transfer the dissected egg chambers together with around 0.5 mL phosphate buffered saline (PBS) to 1 mL centrifuge tubes. Allow the eggs to settle down.
NOTE: Coat the plastic pipette tips with 1% bovine serum albumin (BSA) dissolved in water or PBS to prevent the egg chambers to stick on the plastic surface of the tips. Perform the following procedures in dark to avoid photobleaching of red fluorescent protein (RFP, also known as DsRed) and green fluorescent protein (GFP) in the egg chambers.
-
Remove the PBS by pipetting, and then apply 0.5 mL 4% paraformaldehyde in PBS to fix the egg chambers at room temperature in dark for 20 to 30 min.
NOTE: Apply gentle rotation in this and the following incubation steps.
-
Remove the paraformaldehyde by pipetting, and then washed the egg chamber with 0.5 mL PBST (PBS + 0.1% Triton X-100) for 3 times.
NOTE: Prolonged fixation could reduce the RFP and GFP signals.
-
Incubate the egg chambers with PBST for 1 to 2 h at room temperature or overnight at 4 °C with gentle rotation to permeabilize the egg chambers.
NOTE: PBST can also avoid egg chambers to stick to the non-BSA coated plastic surface.
-
Remove the PBST by pipetting, and then apply 0.5 mL of 10 μg/mL of blue nuclear Hoechst dye in PBST to egg chambers for 1 to 2 h at room temperature to stain for nucleus.
NOTE1: Avoid prolonged incubation with nuclear dye as this will increase non-specific signal.
NOTE2: Alternative approach to stain nucleus without Hoechst is to add 200 μL anti-bleaching mounting agent with DAPI (see materials) and incubate overnight63, before mounting the tissues on glass cover slip as described at protocol 3.8.
NOTE3: Perform the staining and the following procedures in dark to avoid photobleaching.
Remove the nuclear dye by pipetting, and then apply 0.5 mL PBST to wash the egg chambers in the 1 mL centrifuge tubes for 3 times, with 10 to 20 min incubation with gentle rotation between each washing step.
-
Remove all PBST with fine pipette, and then apply 200 μL anti-bleaching mounting agent (see materials) to incubate the egg chambers at room temperature for 3 h or overnight at 4 °C until the egg chambers sink to the bottom of the tube.
NOTE: Tissues that have fully absorbed the mounting agent sink to the bottom of the tube.
-
Mount the stained egg chambers by transferring them with 200 μL anti-bleaching mounting agent on a pre-cleaned glass slide for imaging by pipetting, cover the egg chambers with a 20 x 20 mm pre-cleaned glass cover slip, and seal the cover slip on glass slide by putting nail polish at the edge of the cover slip.
NOTE1: Pre-clean the glass slide and cover slip with water or 70% ethanol.
NOTE2: Apply petroleum jelly between the glass slide and the cover slip to avoid destroying the egg chambers by overcompression.
-
Image the egg chambers using fluorescence or confocal microscope, using a 20x, NA 0.8 Plan-Apochromat objective, with excitation light wavelength 405 nm for nuclear staining (detect emission ~461 nm), 561 nm for RFP (ongoing or recent caspase activity) signal (detect emission ~590 nm), and 488 nm for GFP (past caspase activity) signal (detect emission ~518nm).
Representative Results
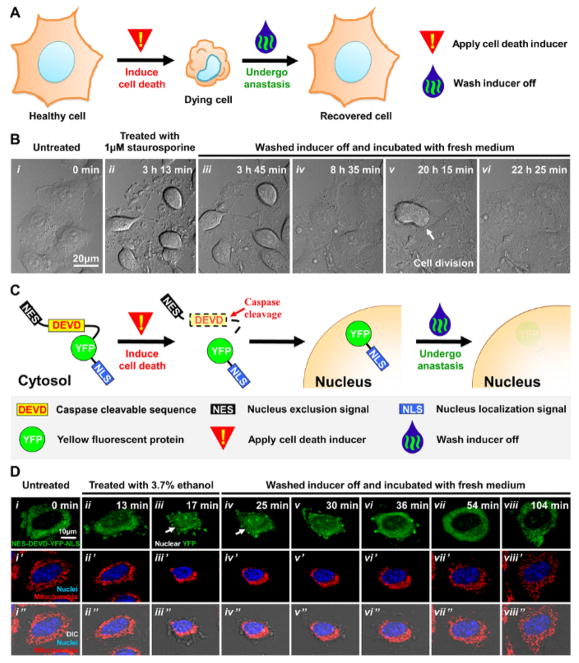
While time-lapse live cell microscopy is a reliable method to tract anastasis in cultured cells20, it is challenging to identify which cells have undergone anastasis in animals, because the recovered cells appear morphologically indistinguishable from normal healthy cells that have not attempted cell death. For example, human cervical cancer HeLa cells display morphological hallmarks of apoptosis1,2,14, such as cell shrinkage, nuclear condensation, and plasma membrane blebbing in response to the cell death stimulus of 1μM staurosporine17 (Figure 1A, Figure 1B i–ii). After removing the cell death stimulus and incubation in fresh medium, the dying cells reverse the cell death process by anastasis17,18, as indicated by morphological recovery (Figure 1B iii–iv), followed by proliferation (Figure 1B v–vi). Our previous studies have also used “real-time” caspase biosensors, such as ApoAlert (NES-DEVD-YFP-NLS) to demonstrate reversal of apoptosis after caspase activation18,20. This biosensor localizes to the cytosol in healthy cells (Figure 1C, 1D i). Upon caspase activation triggered by the cell death stimulus 3.7% ethanol, this YFP-based biosensor is cleaved by caspases and translocated to nucleus, where it accumulates and the resulting nuclear fluorescence of its YFP tag identifies cells with on-going caspase activity (Figure 1C, 1D ii–iii). The dying cells also display morphological hallmarks of apoptosis during ethanol-induction1,14,18,20, such as fragmentation of tubular mitochondria, nuclear condensation, cell shrinkage, and plasma membrane blebbing (Figure 1D ii–iii). Interestingly, after removal of the cell death stimulus, the same cells can recover, and regain normal morphology (Figure 1D iv–viii). Notably, the nuclear fluorescence of the ApoAlert (cleaved biosensor) is gone within 1 hour in the recovered cells (Figure 1D iv–viii), possibly due to similar processes that eliminate damaged components in anastatic cells18, such as cleaved caspase-3, PARP, and ICAD generated during apoptosis. Therefore, a new strategy is required for tracking anastasis over a longer time frame, especially in vivo.
Figure 1. Recovery of HeLa cells after cell death induction.
(A) Schematic diagram of the approach to induce cell death and subsequently allow dying cells to recover after removal of cell death inducer.
(B) Time-lapse live cell DIC microscopy of healthy HeLa cells (i), the same group of cells after treating with 1μM staurosporine (ii), and then washed and further incubated with fresh culture medium to remove the staurosporine (iii–vi). White arrow indicates a dividing cell.
(C) Schematic diagram of caspase biosensor fusion protein NES-DEVD-YFP-NLS, and the subcellular localization of YFP during cell death induction and after anastasis.
(D) Time-lapse live cell confocal microscopy of one HeLa cell expressing the caspase biosensor fusion protein NES-DEVD-YFP-NLS before (i), during exposure to 3.7% ethanol (ii – iii), and after removing ethanol from the culture medium (iv – viii). Shown here are images of the caspase biosensor only (green fluorescence, top row); merged images of the Hoechst-stained nucleus (blue fluorescence) and mitochondria (red fluorescence) (middle row), and images in the middle row further merged with DIC images (bottom row). White arrows in top row indicate nuclear localized YFP. Please click here to view a larger version of this figure.
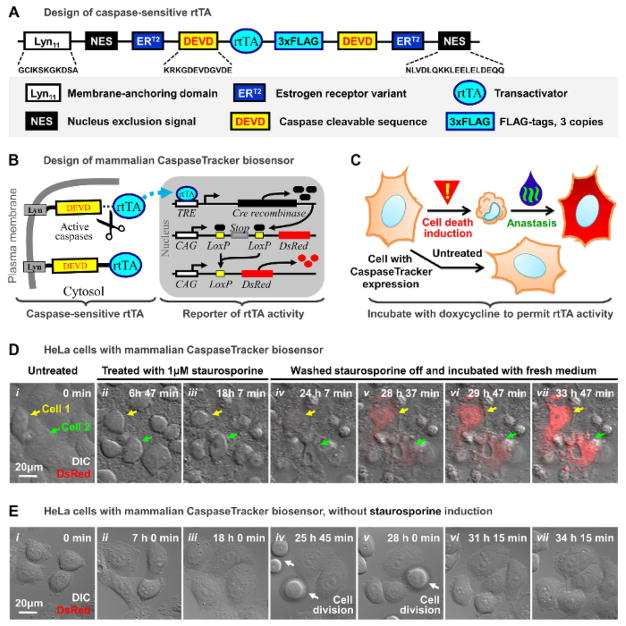
To track the fate of anastatic cells, we developed the mammalian CaspaseTracker biosensor system, which permanently labels the cells after being cleaved by executioner caspase activity. This biosensor is composed of a caspase-sensitive rtTA (reverse tetracycline-controlled transactivator), and a Cre-LoxP-based reporter for rtTA activity (Figure 2A–B). In healthy cells with no caspase activity, the transactivator rtTA65 is tethered to the plasma membrane anchor (Lyn11)66,67, nucleus exclusion signal (NES) of Map Kinase Kinase (MAPKK)68, and estrogen receptor variant (ERT2)69 through caspase-cleavable (DEVD)70 linkers derived from PARP (Figure 2A, 2B). As tethered rtTA cannot translocate from the cytosol to the nucleus, it cannot activate the rtTA reporter. However, upon activation in response to a cell death stimulus, caspases cleave the DEVD linkers, freeing the rtTA to translocate to the nucleus (Figure 2B). Once in the nucleus, the rtTA binds to the tet response element (TRE) and triggers transient expression of Cre recombinase, which leads to an irreversible recombination event that removes the stop codon cassette between the CAG promoter and the coding sequences for red fluorescent protein (DsRed). This results in permanent expression of DsRed (Figure 2B), which serves as the permanent fluorescent marker of those cells that can remain alive after they have experienced caspase activity, as well as their progeny (Figure 2C).
Figure 2. Mammalian CaspaseTracker biosensor system.
(A) Schematic diagram of the caspase-sensitive rtTA.
(B) Schematic diagram of the mammalian CaspaseTracker rtTA biosensor system.
(C) Flowchart of using the CaspaseTracker rtTA biosensor system to detect anastasis. Red/yellow triangle (apply cell death inducer) and green/ blue (wash inducer off) symbols are as in Figure 1A.
(D) Time-lapse live cell confocal microscopy of a cluster of HeLa cells expressing the mammalian CaspaseTracker rtTA biosensor before (i), and during exposure to 1μM staurosporine (ii – iii), and after removing staurosporine from the culture medium (iv – vii). Merged images of DIC and DsRed signals. Arrows indicate the cell 1 (yellow) and cell 2 (green).
(E) Time-lapse live cell confocal microscopy of the untreated biosensor-expressing HeLa cells. Merged images of DIC and DsRed signals. White arrows indicate dividing cells.
Doxycycline (1ug/mL) was present in the medium throughout the experiments shown in panels (D) and (E). Please click here to view a larger version of this figure.
To test the mammalian CaspaseTracker biosensor, we introduced it into HeLa cells by transient transfection, treated the cells with a cell death stimulus (1μM staurosporine), and monitored recovery of the cells by time-lapse live cell confocal microscopy as we have described20. Doxycycline (1μg/mL final concentration), which is essential to permit rtTA activity65,71, was added to the cell culture medium to turn on the biosensor throughout the experiment. In response to the cell death stimulus, the treated cells display hallmarks of apoptosis, including cell shrinkage and plasma membrane blebbing as expected (Figure 2D i–iii). After removing the cell death stimulus, rinsing the cell layer once, and adding fresh culture medium, anastasis can be observed in the dying cells, as indicated by their return to a normal morphology (Figure 2D iv–vii). Diagnostically, only recovered cells express the DsRed fluorescent marker during and after anastasis (Figure 2D iv–vii), distinguishing them from both non-recovered cells (Figure 2D iv–vii), and control cells not exposed to the cell death stimulus (Figure 2E). This demonstrates the application and utility of the mammalian CaspaseTracker as a novel new tool for identifying and permanently labeling anastatic cells recovering from caspase-activation, and provides a way to track and study their longer-term fate.
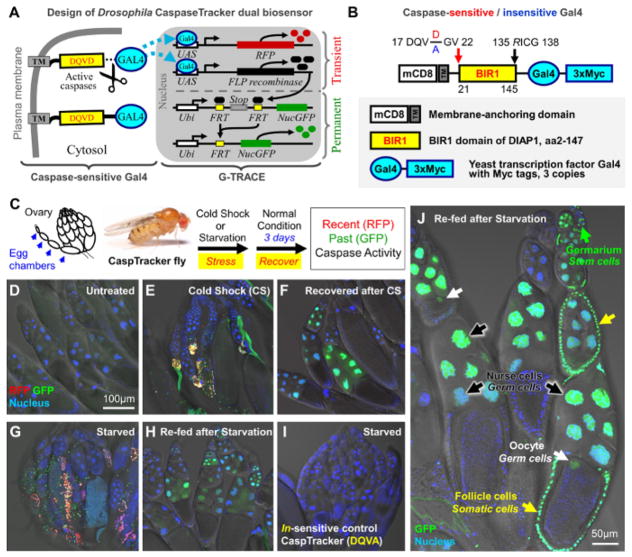
To detect and track anastasis in live animals, CaspaseTracker biosensor transgenic animals are required. Therefore, we first used Drosophila melanogaster as a model system19, because as it is an important genetically tractable organism for study of animal development and human diseases72,73,74,75. As modified from the mammalian CaspaseTracker biosensor, the Drosophila dual biosensor can identify and distinguish “recent/on-going” from “past” caspase activity. This dual biosensor is composed of a caspase-sensitive Gal419, and the Gal4 reporter G-Trace53 (Figure 3A). In cells with no caspase activity, the yeast transcription factor Gal4 is tethered to a plasma membrane anchor (mCD8) domain through a caspase-cleavable linker (DQVD) derived from DIAP1 (Figure 3B), having a 21NN/GV22 mutation to prevent degradation of Gal4 by the N-end rule upon caspase cleavage of the linker afterAsp2076, and a D135R mutation to abolish the drICE caspase inhibitory function in the BIR1 domain77. As tethered Gal4 cannot translocate to nucleus without cleavage by caspase to release it, the Gal4 reporter G-Trace remains inactive in the cells having no caspase activity19. Upon caspase activation, however, activated caspases cleave the DQVD linker, freeing Gal4 to translocate into the nucleus to activate the G-Trace reporter (Figure 3A)19. Gal4 binds to specific upstream activating sequences (UAS) to trigger transient transcription and expression of RFP, which then serves as a fluorescent reporter of recent or current caspase activity until the Gal4 (caspase) activity stops and then RFP protein is degraded. Gal4 also triggers expression of FLP recombinase from the transgene, leading to a recombination event that removes the stop codon cassette between a ubiquitin (Ubi) promoter and a coding sequence for nucleus-targeted GFP (nucGFP). This results in permanent expression of nucGFP, which serves as the permanent marker of those cells that have experienced caspase activity and remain alive.
Figure 3. Drosophila CaspaseTracker dual biosensor system(Adopted with permission from Tang et al., Scientific Reports 2015, 9: 901519).
(A) Schematic diagram of the Drosophila CaspaseTracker Gal4 biosensor system.
(B) Schematic diagram of the caspase-sensitive (DQVD) and caspase-insensitive control (DQVA) Gal4.
(C) Schematic of Drosophila ovary, and flowchart for cell death-induction in 1-day-old flies, followed by 3-days recovery at normal condition. Drosophila image provided by Darren Obbard.
(D) Representative confocal image of egg chambers from the ovary of a female biosensor fly fed with normal fly food for 6 days (untreated).
(E) Representative confocal image of egg chambers from the ovary of a cold shocked female biosensor fly placed at −7 °C for 1 h and then switched to normal culture condition for 1 day (Cold Shock, CS).
(F) Like panel E except the cold shocked flies were switched to normal culture condition for 3 days (Recovered after CS).
(G) Representative confocal image of egg chambers from the ovary of a starved female biosensor fly fed with 8% sucrose in 1% agar without protein for 3 days (Starved).
(H) Like panel G except the treated flies were switched to normal fly food for 3 days after protein starvation treatment (Re-fed).
(I) Like panel G except showing the starvation of caspase insensitive CaspaseTracker (DQVA) female biosensor flies, which served as negative control.
(J) Representative confocal image of egg chambers from a starved and re-fed female Drosophila. Arrows indicate nuclear GFP expressing in the nurse cells (black), oocytes (white) and follicle cells (yellow) of egg chambers, and in the germarium (green). Please click here to view a larger version of this figure.
To test the Drosophila CaspaseTracker biosensor for detecting apoptosis and anastasis in vivo, CaspaseTracker female flies were subjected to physiological stress (Figure 3C), such as cold shock19, which can efficiently trigger cell death, including apoptosis as indicated by caspase activation, nuclear condensation and cell shrinkage19,78, and also necrosis due to chilling that causes loss of membrane integrity and leakage of cytoplasmic contents55,56. As expected, CaspaseTracker was not activated in the egg chambers of control flies where stress-related cell death was not induced (Figure 3D). In contrast, the egg chambers of stressed flies one day after cold shock showed not only characteristic apoptotic cell shrinkage and nuclear condensation, but also RFP and GFP expression, indicating the presence of recent or on-going (RFP+) and past (GFP+) caspase activity (Figure 3E). However, at 3 days after returning the stressed flies to normal non-stressing condition, GFP, but no RFP, expression was detected in the recovered egg chambers (Figure 3F). This indicates that egg chambers that had expressed caspase activity following stress were able to overcome the cell death process and survive.
To further test reversibility of cell death process in egg chambers19, CaspaseTracker female flies were stressed by protein starvation after being fed 8% sucrose in 1% agar for 3 days. Previous studies demonstrated that protein starvation can trigger caspase-mediated apoptosis and autophagy in tissues with somatic and germ cells, including egg chambers57,60,61. As expected, CaspaseTracker was activated in egg chambers after 3 days of protein starvation (Figure 3G). The dying egg chambers exhibited both apoptotic morphologies and expression of the RFP and GFP biosensor markers, indicating recent or on-going (RFP+) and past (GFP+) caspase activity (Figure 3G). To demonstrate that CaspaseTracker can track recovered cells that previously experienced caspase activation after a death stimulus, the starved flies were then transferred to normal protein-containing fly food. As expected, the recovered egg chambers of these re-fed flies lack the RFP transient caspase reporter, indicating no recent or ongoing caspase activity (Figure 3H). However, the egg chambers in these flies after relieving stress by returning them to normal food displayed the GFP caspase reporter (Figure 3H), indicating that the cells in these egg chambers had reversed the initiated cell death process at a point after caspase activation. Verification that the CaspaseTracker biosensor activity is triggered by caspase was obtained by replacing the cleavage sequence DQVD with the sequence of DQVA, which caspase is unable to cleave and which abolish biosensor activity (Figure 3B, 3I).
After the CaspaseTracker Drosophila recovered from protein starvation, we found that multiple types of cell in the egg chambers, such as somatic (follicle) cells and germline cells (nurse cells and oocytes), displayed only GFP, but not RFP (Figure 3J)19, indicating that these cells can undergo anastasis after caspase activation. Interestingly, the GFP also labeled cells in the germarium (Figure 3J)19, which contains stem cells, along with its associated egg chambers in the same ovariole chain. Therefore, these GFP-positive egg chambers may have been derived from stem cells that have recovered from cell death process after caspase activation. Importantly, the starved and re-fed female flies lay fertile eggs that can produce GFP-expressing progeny flies19, suggesting that potentially the cells reversed cell death process after caspase activation can regain apparently normal function. Future studies are needed to determine if progeny flies that survive as a consequence of anastasis exhibit permanent sequelae.
Discussion
The CaspaseTracker dual biosensor system is a novel and unique tool that allows detection of recent or ongoing caspase activity, and tracking of cells that have reversed cell death process and survive after experiencing caspase activity in vivo. While caspase activity has been traditionally assumed as a hallmark of apoptosis, growing studies reveal that non-apoptotic caspase activity plays potential roles in diverse normal cell functions, such as regulation of neuronal activity79,80, learning and memory81,82,83,84, suppression of necroptotic cell death85,86, spermatid individualization87,88, microRNA processing89, cell proliferation90, and cell fate patterning91. In addition to apoptosis and anastasis, the CaspaseTracker biosensor system can therefore detect non-apoptotic caspase activity, which is present in the brain and optic lobes, cardia, gut, Malpighian tubules, trachea, muscles, and other tissues of Drosophila19,46,64. This biosensor signal could also represent the current or past anastatic activity during embryo development or normal homeostasis in these tissues. To study anastasis after transient cell death-inducing environmental stress in live animals, it is critical to choose tissues with cells that exhibit no caspase biosensor activity under normal physiological conditions, but that can be induced to undergo caspase activation by transient cell death induction. Egg chambers are ideal for this purpose, because they typically have no caspase activity from germarium to stage 10 during oogenesis58,59,92.
Exposing female Drosophila to transient environmental stresses, such as protein starvation and cold shock, can efficiently trigger caspase activation and cell death in egg chambers19,57,58,78. Critical steps within the protocol include avoiding prolonged cell death induction to flies. The optimized conditions of protein starvation (8% sucrose in 1 % agar for 3-days) and cold shock (1 h at −7 °C) to female flies can trigger caspase-activated cell death process in egg chambers, and allow them to recover after the stressed flies are returned to normal condition19. Prolonged treatment with a cell death stimulus can trigger more egg chambers to undergo the cell death process, but the recovery rate is also reduced, presumably because the dying egg chambers experience massive damage beyond repair.
An additional critical step in this protocol is to reduce the CaspaseTracker background signal in egg chambers by crossing, raising and maintaining the CaspaseTracker flies at a lower temperature, such as 18 °C. While the majority of egg chambers from optimally reared flies do not display caspase activity in the germarium through stage 10 during oogenesis58, about 1% of egg chambers could exhibit caspase biosensor activity without cell death induction19. This may reflect the normal attrition rate due to innate errors or may be triggered unintentionally during oogenesis by standard laboratory conditions. As Gal4 displays less activity in flies at low temperature93, raising flies at 18 °C, rather than at room temperature, can reduce the endogenous signal that activates the CaspaseTracker system. Alternatively, switching flies to a higher temperature, such as 29 °C, can increase the sensitivity of CaspaseTracker system, due to increase in the Gal4 activity93, and potentially other endogenous temperature-dependent enzymatic activities.
It is important to distinguish the CaspaseTracker-positive cells that undergo cell death process or anastasis from cells that exhibit non-apoptotic caspase activity. Apoptotic cells express RFP (transient marker for on-going or recent caspase activity), and often GFP (permanent maker for past caspase activity) in treatment of apoptosis induction, as these dying cells have ongoing caspase activity that cleave-activated Gal4, which then activates the transient (Gal4 activity-dependent RFP) and permanent (Gal4 triggered FLPase-FRT mediated GFP) reporters of the G-Trace system. Apoptosis of these cells can be confirmed by morphological and biochemical hallmarks such us nuclear condensation detected by staining with nuclear dyes14,19,58, and caspase cleavage detected by immunostaining19,94. Cells that have already undergone anastasis display permanent GFP expression due to the FLPase-mediated recombination event of G-Trace system. These cells express no RFP as they have no on-going caspase activity, nor other hallmarks of apoptosis19. These cells also display normal nuclear morphology. Cells do have on-going non-apoptotic caspase activity often display both RFP and GFP expression, and also have a normal nuclear morphology19.
It can be difficult to distinguish cells that experienced anastasis from those cells that had past non-apoptotic caspase activity, because both display only GFP and have no hallmark of cell death. Therefore, careful control experiments must be included19. For examples, to study anastasis in egg chambers, it is essential to examine GFP expression in both stressed-recovered flies and non-stressed flies (negative control). Recovered flies should show more GFP-expressing cells than unstressed flies, if anastasis has occurred after caspase activation. It is also important to distinguish CaspaseTracker fluorescent signals from nonspecific auto-fluorescence, as observed in cuticle, pigments, and fat bodies19. We generated negative control biosensor flies by mutating the caspase cleavage site of the biosensor (DQVD) to a non-cleavable sequence (DQVA) and thereby rendering the control biosensor non-responsive to caspase activity19. The signal detected in the caspase sensitive (DQVD) but not in the negative control (DQVA) biosensor flies is the signal of interest, triggered by caspase activity, rather than autofluorescence.
Our current Drosophila dual CaspaseTracker biosensor can identify cells with “recent” caspase activity by expression of RFP, and cells with “past” caspase activity by expression of GFP19. We note that RFP is not a “real-time” caspase activity indicator, because it takes a few hours of reaction time for Gal4 to drive the expression of RFP in response to caspase activity. To add a “real time” function, the Drosophila CaspaseTracker biosensor can be combined with the recently developed iCasper biosensor52, a “real-time” and “dark to bright” in vivo biosensor that only shows its far-red signal when it is cleaved by caspases.
Apart from apoptosis, CaspaseTracker could potentially also be activated during alternative types of cell death as cells can switch cell death pathways during their course that directly or indirectly involves caspase activation, such as autophagy after starvation95,96, necrosis by the cold shock-induced plasma membrane rupture19,55,56,78, and staurosporine-induced necroptosis97,98. Our present and previous studies demonstrated that the CaspaseTracker biosensors can label cells recovered from these cell death inductions in vitro or in vivo17,18,19,20,21. As these cell death inductions alone could simultaneously activate multiple cell death pathways in the same cells, the recovery of these dying cells suggests that anastasis is a general cell recovery phenomenon that can reverse different cell death processes including apoptosis, autophagy, necrosis and necroptosis, individually or simultaneously.
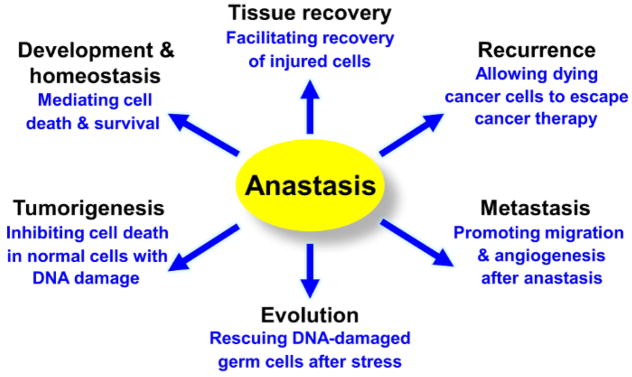
The in vivo CaspaseTracker biosensor will facilitate pursuit of the yet unknown functions, mechanisms, and therapeutic implications of anastasis (Figure 4)21. To reveal the molecular signature of anastasis, we performed time-course whole-genome gene expression microarray studies to analyze mouse primary liver cells during reversal of ethanol-induced apoptosis, and interestingly, found striking changes in transcription of genes involved in multiple pathways including pro-survival, anti-oxidation, DNA damage response, histone modification, angiogenesis, cell migration, and transformation18,21. This finding is supported by our validation study of the recovery of human liver cancer HepG2 cells from apoptosis18,21, and also a recent independent RNA-sequencing study of HeLa cells in recovery of apoptosis99. Interestingly, up-regulation of some pro-survival factors found in anastatic cells are also observed in heart failure reversal, tumor progression, and cancer recurrence100,101,102, suggesting the potential participation of anastasis. To study the physiological, pathological and therapeutic potentials of anastasis, it is important to identify the anastatic cells and track their fate in small animals, as this will enable mechanistic and therapeutic studies. Our Drosophila and mammalian CaspaseTracker biosensors will be useful tools for testing the potential contributions of anastasis in normal development, homeostasis, tissue recovery, tumor evolution, cancer recurrence, and metastasis. Finding from these studies will increase our understanding of the natural role of anastasis, and offer potential to identify revolutionary new therapeutic approaches for intractable diseases by mediating cell death and survival through controlling anastasis.
Figure 4. Physiological, pathological and therapeutic implications of anastasis.
(Adopted with permission from Tang et al., F1000Res 2017, 6: 4321). Please click here to view a larger version of this figure.
Acknowledgments
We thank Darren Obbard for Drosophila image in Figure 3C and in video manuscript; J. Marie Hardwick, Wade Gibson, and Heather M. Lamb for valuable discussion of this manuscript. This work was supported by a Sir Edward Youde Memorial Fellowship (H.L.T.), Dr. Walter Szeto Memorial Scholarship (H.L.T.), Fulbright grant 007-2009 (H.L.T.), Life Science Research Foundation fellowship (H.L.T.), and NCI K22 grant CA204458 (H.L.T.). Ho Lam Tang was a Shurl and Kay Curci Foundation Fellow of the Life Sciences Research Foundation (2014–2017).
Footnotes
Disclosures
The authors have nothing to disclose.
Video Link
The video component of this article can be found at https://www.jove.com/video/54107/
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–54. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Narula J, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–9. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 6.Nagata S. Apoptosis and autoimmune diseases. Ann N Y Acad Sci. 2010;1209:10–6. doi: 10.1111/j.1749-6632.2010.05749.x. [DOI] [PubMed] [Google Scholar]
- 7.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–9. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 10.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 11.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemoto K, Nagai T, Miyawaki A, Miura M. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J Cell Biol. 2003;160:235–43. doi: 10.1083/jcb.200207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luthi AU, Martin SJ. The CASBAH: a searchable database of caspase substrates. Cell Death Differ. 2007;14:641–50. doi: 10.1038/sj.cdd.4402103. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 15.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julien O, Wells JA. Caspases and their substrates. Cell Death Differ. 2017;24:1380–1389. doi: 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang HL, Yuen KL, Tang HM, Fung MC. Reversibility of apoptosis in cancer cells. Br J Cancer. 2009;100:118–22. doi: 10.1038/sj.bjc.6604802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang HL, et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol Biol Cell. 2012;23:2240–52. doi: 10.1091/mbc.E11-11-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang HL, Tang HM, Fung MC, Hardwick JM. In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity. Sci Rep. 2015;5:9015. doi: 10.1038/srep09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang HL, Tang HM, Hardwick JM, Fung MC. Strategies for tracking anastasis, a cell survival phenomenon that reverses apoptosis. J Vis Exp. 2015 doi: 10.3791/51964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang HM, Talbot CC, Jr, Fung MC, Tang HL. Molecular signature of anastasis for reversal of apoptosis. F1000Res. 2017;6:43. doi: 10.12688/f1000research.10568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammill AK, Uhr JW, Scheuermann RH. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp Cell Res. 1999;251:16–21. doi: 10.1006/excr.1999.4581. [DOI] [PubMed] [Google Scholar]
- 23.Geske FJ, Lieberman R, Strange R, Gerschenson LE. Early stages of p53-induced apoptosis are reversible. Cell Death Differ. 2001;8:182–91. doi: 10.1038/sj.cdd.4400786. [DOI] [PubMed] [Google Scholar]
- 24.Kenis H, et al. Annexin A5 uptake in ischemic myocardium: demonstration of reversible phosphatidylserine externalization and feasibility of radionuclide imaging. J Nucl Med. 2010;51:259–67. doi: 10.2967/jnumed.109.068429. [DOI] [PubMed] [Google Scholar]
- 25.Ichim G, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell. 2015;57:860–72. doi: 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong YN, et al. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell. 2017;169:286–300. e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narula J, Haider N, Arbustini E, Chandrashekhar Y. Mechanisms of disease: apoptosis in heart failure--seeing hope in death. Nat Clin Pract Cardiovasc Med. 2006;3:681–8. doi: 10.1038/ncpcardio0710. [DOI] [PubMed] [Google Scholar]
- 28.Drakos SG, et al. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126:230–41. doi: 10.1161/CIRCULATIONAHA.111.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKechnie NM, Foulds WS. Recovery of the rabbit retina after light damage (preliminary observations) Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;212:271–83. doi: 10.1007/BF00410521. [DOI] [PubMed] [Google Scholar]
- 30.Milligan SC, Alb JG, Jr, Elagina RB, Bankaitis VA, Hyde DR. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J Cell Biol. 1997;139:351–63. doi: 10.1083/jcb.139.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2002;43:3511–21. [PubMed] [Google Scholar]
- 32.Blennow K, et al. Traumatic brain injuries. Nat Rev Dis Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- 33.Castellsague X, et al. Influence of mate drinking, hot beverages and diet on esophageal cancer risk in South America. Int J Cancer. 2000;88:658–64. doi: 10.1002/1097-0215(20001115)88:4<658::aid-ijc22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Islami F, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loomis D, et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016;17:877–8. doi: 10.1016/S1470-2045(16)30239-X. [DOI] [PubMed] [Google Scholar]
- 36.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 37.McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol. 2005;35:195–203. doi: 10.1016/j.alcohol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Davis AJ, Tannock JF. Repopulation of tumour cells between cycles of chemotherapy: a neglected factor. Lancet Oncol. 2000;1:86–93. doi: 10.1016/s1470-2045(00)00019-x. [DOI] [PubMed] [Google Scholar]
- 39.Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J. 2010;35:202–15. doi: 10.1183/09031936.00105009. [DOI] [PubMed] [Google Scholar]
- 40.Wagle N, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–96. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RE, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 42.Travis LB, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 43.Chaturvedi AK, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst. 2007;99:1634–43. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 44.Moteabbed M, Yock TI, Paganetti H. The risk of radiation-induced second cancers in the high to medium dose region: a comparison between passive and scanned proton therapy, IMRT and VMAT for pediatric patients with brain tumors. Phys Med Biol. 2014;59:2883–99. doi: 10.1088/0031-9155/59/12/2883. [DOI] [PubMed] [Google Scholar]
- 45.Painter RC, et al. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–9. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 46.Ding AX, et al. CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. Elife. 2016;5 doi: 10.7554/eLife.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takemoto K, et al. Local initiation of caspase activation in Drosophila salivary gland programmed cell death in vivo. Proc Natl Acad Sci U S A. 2007;104:13367–72. doi: 10.1073/pnas.0702733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bardet PL, et al. A fluorescent reporter of caspase activity for live imaging. Proc Natl Acad Sci U S A. 2008;105:13901–5. doi: 10.1073/pnas.0806983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholls SB, Chu J, Abbruzzese G, Tremblay KD, Hardy JA. Mechanism of a genetically encoded dark-to-bright reporter for caspase activity. J Biol Chem. 2011;286:24977–86. doi: 10.1074/jbc.M111.221648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golbs A, Nimmervoll B, Sun JJ, Sava IE, Luhmann HJ. Control of programmed cell death by distinct electrical activity patterns. Cereb Cortex. 2011;21:1192–202. doi: 10.1093/cercor/bhq200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, et al. Visualization of caspase-3-like activity in cells using a genetically encoded fluorescent biosensor activated by protein cleavage. Nat Commun. 2013;4:2157. doi: 10.1038/ncomms3157. [DOI] [PubMed] [Google Scholar]
- 52.To TL, et al. Rationally designed fluorogenic protease reporter visualizes spatiotemporal dynamics of apoptosis in vivo. Proc Natl Acad Sci U S A. 2015;112:3338–43. doi: 10.1073/pnas.1502857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans CJ, et al. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6:603–5. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chyb S, Gompel N. Atlas of Drosophila morphology : wild-type and classical mutants. Academic Press; London ; Waltham, MA: 2013. p. xviii.p. 224. [Google Scholar]
- 55.Drobnis EZ, et al. Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J Exp Zool. 1993;265:432–7. doi: 10.1002/jez.1402650413. [DOI] [PubMed] [Google Scholar]
- 56.Quinn PJ. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology. 1985;22:128–46. doi: 10.1016/0011-2240(85)90167-1. [DOI] [PubMed] [Google Scholar]
- 57.Drummond–Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–78. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 58.Pritchett TL, Tanner EA, McCall K. Cracking open cell death in the Drosophila ovary. Apoptosis. 2009;14:969–79. doi: 10.1007/s10495-009-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenkins VK, Timmons AK, McCall K. Diversity of cell death pathways: insight from the fly ovary. Trends Cell Biol. 2013;23:567–74. doi: 10.1016/j.tcb.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McPhee CK, Baehrecke EH. Autophagy in Drosophila melanogaster. Biochim Biophys Acta. 2009;1793:1452–60. doi: 10.1016/j.bbamcr.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barth JM, Szabad J, Hafen E, Kohler K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 2011;18:915–24. doi: 10.1038/cdd.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong LC, Schedl P. Dissection of Drosophila ovaries. J Vis Exp. 2006:52. doi: 10.3791/52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarkissian T, Timmons A, Arya R, Abdelwahid E, White K. Detecting apoptosis in Drosophila tissues and cells. Methods. 2014;68:89–96. doi: 10.1016/j.ymeth.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang HL, Tang HM, Fung MC, Hardwick JM. In Vivo Biosensor Tracks Non-apoptotic Caspase Activity in Drosophila. J Vis Exp. 2016 doi: 10.3791/53992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gossen M, et al. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–9. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 66.Yamanashi Y, et al. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987;7:237–43. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods. 2005;2:415–8. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–8. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- 69.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–7. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 70.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–7. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 71.Mansuy IM, et al. Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron. 1998;21:257–65. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 72.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez C. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat Rev Cancer. 2013;13:172–83. doi: 10.1038/nrc3461. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto S, et al. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. 2014;159:200–14. doi: 10.1016/j.cell.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wangler MF, Hu Y, Shulman JM. Drosophila and genome-wide association studies: a review and resource for the functional dissection of human complex traits. Dis Model Mech. 2017;10:77–88. doi: 10.1242/dmm.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ditzel M, et al. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat Cell Biol. 2003;5:467–73. doi: 10.1038/ncb984. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Wang J, Shi Y. Structural mechanisms of DIAP1 auto-inhibition and DIAP1-mediated inhibition of drICE. Nat Commun. 2011;2:408. doi: 10.1038/ncomms1418. [DOI] [PubMed] [Google Scholar]
- 78.Yi SX, Moore CW, Lee RE., Jr Rapid cold-hardening protects Drosophila melanogaster from cold-induced apoptosis. Apoptosis. 2007;12:1183–93. doi: 10.1007/s10495-006-0048-2. [DOI] [PubMed] [Google Scholar]
- 79.Jonas EA, et al. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci U S A. 2004;101:13590–5. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–71. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 82.Maor-Nof M, Yaron A. Neurite pruning and neuronal cell death: spatial regulation of shared destruction programs. Curr Opin Neurobiol. 2013;23:990–6. doi: 10.1016/j.conb.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Yu F, Schuldiner O. Axon and dendrite pruning in Drosophila. Curr Opin Neurobiol. 2014;27:192–8. doi: 10.1016/j.conb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neukomm LJ, Freeman MR. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 2014;24:515–23. doi: 10.1016/j.tcb.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–97. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 88.Kaplan Y, Gibbs-Bar L, Kalifa Y, Feinstein-Rotkopf Y, Arama E. Gradients of a ubiquitin E3 ligase inhibitor and a caspase inhibitor determine differentiation or death in spermatids. Dev Cell. 2010;19:160–73. doi: 10.1016/j.devcel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Weaver BP, et al. CED-3 caspase acts with miRNAs to regulate non-apoptotic gene expression dynamics for robust development in C. elegans. Elife. 2014;3 doi: 10.7554/eLife.04265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fogarty CE, Bergmann A. Killers creating new life: caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017;24:1390–1400. doi: 10.1038/cdd.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weaver BP, Weaver YM, Mitani S, Han M. Coupled Caspase and N-End Rule Ligase Activities Allow Recognition and Degradation of Pluripotency Factor LIN-28 during Non-Apoptotic Development. Dev Cell. 2017;41:665–673. e6. doi: 10.1016/j.devcel.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baum JS, Arama E, Steller H, McCall K. The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ. 2007;14:1508–17. doi: 10.1038/sj.cdd.4402155. [DOI] [PubMed] [Google Scholar]
- 93.Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 94.Fan Y, Bergmann A. The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 2010;17:534–9. doi: 10.1038/cdd.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–18. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 96.Tsapras P, Nezis IP. Caspase involvement in autophagy. Cell Death Differ. 2017;24:1369–1379. doi: 10.1038/cdd.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunai ZA, et al. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS One. 2012;7:e41945. doi: 10.1371/journal.pone.0041945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simenc J, Lipnik-Stangelj M. Staurosporine induces different cell death forms in cultured rat astrocytes. Radiol Oncol. 2012;46:312–20. doi: 10.2478/v10019-012-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun G, et al. A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J Cell Biol. 2017 doi: 10.1083/jcb.201706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milting H, et al. Altered levels of mRNA of apoptosis-mediating genes after mid-term mechanical ventricular support in dilative cardiomyopathy--first results of the Halle Assist Induced Recovery Study (HAIR) Thorac Cardiovasc Surg. 1999;47:48–50. doi: 10.1055/s-2007-1013108. [DOI] [PubMed] [Google Scholar]
- 101.Haider N, et al. Concurrent upregulation of endogenous proapoptotic and antiapoptotic factors in failing human hearts. Nat Clin Pract Cardiovasc Med. 2009;6:250–61. doi: 10.1038/ncpcardio1452. [DOI] [PubMed] [Google Scholar]
- 102.Strik H, et al. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg Psychiatry. 1999;67:763–8. doi: 10.1136/jnnp.67.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]