Abstract
Rift Valley fever virus (RVFV) is a mosquito-borne pathogen endemic to sub-Saharan Africa and the Arabian Peninsula. There are no approved antiviral therapies or vaccines available to treat or prevent severe disease associated with RVFV infection in humans. The adenosine analogue, galidesivir (BCX4430), is a broad-spectrum antiviral drug candidate with in vitro antiviral potency (EC50 of less than 50 μM) in more than 20 different viruses across eight different virus families. Here we report on the activity of galidesivir in the hamster model of peracute RVFV infection. Intramuscular and intraperitoneal treatments effectively limited systemic RVFV (strain ZH501) infection as demonstrated by significantly improved survival outcomes and the absence of infectious virus in the spleen and the majority of the serum, brain, and liver samples collected from infected animals. Our findings support the further development of galidesivir as an antiviral therapy for use in treating severe RVFV infection, and possibly other related phleboviral diseases.
Keywords: BCX4430, Antiviral, Rift Valley Fever Virus, Phlebovirus, Bunyavirus, Viral Hemorrhagic Fever
1. Introduction
Rift Valley fever virus (RVFV; family Phenuiviridae, genus Phlebovirus) is a mosquito-borne pathogen that causes severe disease in humans and livestock and is endemic in sub-Saharan Africa and the Arabian Peninsula (Bird et al., 2009). Infection in humans occurs via mosquito bite or exposure to animal tissues during the processing or handling of infected animals, and typically results in a relatively mild febrile illness. However, a small percentage of cases result in severe and often fatal hemorrhagic fever that can be accompanied by retinitis, fulminant hepatitis, and encephalitis (Ikegami and Makino, 2011; McElroy and Nichol, 2012). Currently there are no approved vaccines or antivirals to prevent or treat RVFV infection (Kortekaas, 2014). The virus is also transmissible to humans via aerosolization, which underlines concerns regarding its potential use as a bioterror agent and its classification as a Category A priority pathogen (NIAID, 2016). In addition, the World Health Organization has listed RVF as a high priority disease of public health interest (WHO, 2018).
A number of arboviral human disease outbreaks in the Americas and Caribbean territories have occurred in recent years, presumably through introduction of foreign viruses by global travel and trade (Golnar et al., 2017). The potential also exists for RVFV to spread outside of established endemic areas due to the capacity of more than 40 species of mosquitoes, in 8 genera throughout the world, to serve as vectors (Turell et al., 2008). Introduction of the virus into naïve animal and human populations poses a significant risk to susceptible species of agricultural importance, as well as public health. Thus, there is an urgent need for the development of effective therapeutics and vaccines to treat and prevent RVFV infections.
Galidesivir is an adenosine analog that has a substitution of carbon for nitrogen at position 7 on the base and a substitution of nitrogen for oxygen at position 1 on the ribose ring (Warren et al., 2014). When the viral RNA polymerase substitutes the natural nucleotide with galidesivir triphosphate, the structural change alters its electrostatic interaction, resulting in premature termination of the elongating RNA strand. Galidesivir has demonstrated broad-spectrum antiviral activity against a wide range of viruses, including filoviruses, togaviruses, bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses, flaviviruses, one orthomyxovirus and one picornavirus (Warren et al., 2014), and is currently in clinical development as an antiviral therapy for Ebola virus disease (Taylor et al., 2016). In a pilot study, galidesivir was also shown to confer partial protection in a mouse model of RVFV infection (Warren et al., 2014). More recently, efficacy in Zika and tick-borne flavivirus infection models has been reported (Eyer et al., 2017; Julander et al., 2017). The first in-human Phase 1 study to evaluate the safety, tolerability and pharmacokinetics of intramuscular administration of galidesivir versus placebo in healthy subjects recently concluded with promising pharmacokinetics properties and good tolerability (Taylor et al., 2016).
To better define the antiviral activity of galidesivir as a potential therapy for Rift Valley fever (RVF) disease, we evaluated the pharmacokinetics of galidesivir in hamsters, and evaluated the efficacy of the compound by multiple routes in the RVFV hamster infection model. Our findings further support advancing the development of galidesivir as a broad-spectrum therapeutic with potential for application as a RVF treatment.
2. Materials and methods
2.1. Ethics statement
All animal procedures complied with USDA guidelines and were conducted at AAALAC-accredited facilities at Utah State University, and were approved by the Institutional Animal Care and Use Committee.
2.2. Animals
Female Syrian golden hamsters (81–90 g) were purchased from Charles River Laboratories (Wilmington, MA) and quarantined for at least 72 hours prior to virus challenge or drug administration.
2.3. Viruses
The molecular clone of the ZH501 strain of RVFV was obtained from Dr. Stuart Nichol (CDC, Atlanta, GA). The virus stock (1.1 × 108 plaque-forming units [PFU]/ml; 1 passage in BSRT7 cells, 3 passages in Vero E6 cells) used was from a clarified cell culture lysate preparation. It was diluted in sterile minimal essential medium (MEM; Hyclone, Logan, UT) and inoculated by subcutaneous (SC) injection of 0.1 ml containing 30 PFU (ventral, right side of the abdomen). The MP-12 vaccine strain of RVFV was obtained from Dr. Robert Tesh (World Reference Center for Emerging Viruses and Arboviruses, University of Texas Medical Branch, Galveston, TX) (Gowen et al., 2015). In vitro experiments with the MP-12 strain were conducted in biosafety level 2 (BSL-2) facilities and work with the pathogenic ZH501 strain was performed in BSL-3+ containment laboratories.
2.4. Test Compounds
Galidesivir was provided by BioCryst Pharmaceuticals, Inc. (Durham, NC). Ribavirin was from ICN Pharmaceuticals, Inc. (Costa Mesa, CA). Galidesivir was diluted in phosphate-buffered saline (PBS) and sterile filtered (0.45 μm) for administration by intramuscular (IM) injection. For intraperitoneal (IP) administration, galidesivir was diluted in sterile Lactated Ringer’s Solution (LRS) prior to filtration. Ribavirin was prepared in PBS or LRS.
2.5. Cell Culture Antiviral Assays
Vero 76 cells (ATCC, Manassas, VA) were maintained in MEM supplemented with 10 % fetal bovine serum (FBS). Varying concentrations of galidesivir (starting at 0.1 μg/ml with 6 serial dilutions up to 320 μg/ml) and ribavirin (starting at 0.32 μg/ml with 6 serial dilutions up to 1000 μg/ml) were added to test wells containing 70–80% confluent Vero 76 cells (in MEM containing 2% FBS and 50 μg/ml gentamycin) at the time of RVFV (MP-12 strain) infection at multiplicity of infection of approximately 0.001. For toxicity determinations done in parallel, the same galidesivir and ribavirin concentrations were added to uninfected Vero 76 cells. Plates were incubated at 37°C, 5% CO2 for 5 days, at which time culture supernatants were collected for endpoint titration of infectious virus and the plates processed to assess cell viability by neutral red (NR) vital dye uptake and virus yield reduction (VYR) as previously described (Gowen et al., 2007). The 50% effective concentration (EC50) based on the CPE reduction assay and the 90% effective concentration (EC90), the concentration of drug that reduced the virus yield by one log10, were determined by regression analysis. The 50% cell cytotoxic dose (CC50) was determined by neutral red dye uptake in uninfected, drug-treated cells. The selectivity index (SI) values were calculated using the formula: SI = CC50/EC50 or EC90.
2.6. Efficacy experiment 1: IM galidesivir against RVFV infection in hamsters
Hamsters were weighed the morning of infection and grouped so that the average weight per group across the entire experiment varied by less than 2 g. The experiment design is shown in Table 1. Animals in each group (n = 14 for treatment groups, n = 15 for placebo group) were treated twice daily (BID) by IM injection with different dosing regimens of galidesivir or the PBS vehicle placebo, with a day 0 loading dose beginning 30 min prior to challenge with 30 PFU of RVFV. Due to the higher volume requirement for the loading doses, injections were administered IP followed by the IM BID maintenance dosing. Ribavirin (100 mg/kg/day, IP) was included as a positive comparison control. Four animals from each infection group, and five from the placebo group, were designated for sacrifice on day 2 post-infection (p.i.), the optimal day for analysis of peak serum and tissue viral titers (Scharton et al., 2015). Serum was also analyzed for comprehensive blood chemistry parameters to measure liver and kidney function (Supplemental Table 2). The remaining animals were observed through day 21 for morbidity and mortality. In addition to the infection groups, non-infected animals (n = 5 per group) were treated in parallel as shown in Table 1 and observed for 21 days to assess drug tolerability.
Table 1.
Study design for the IM galidesivir efficacy experiment 1.
| No./ Group | Compound | Day 0 Loading Dose IP (mg/kg/day) | Dose, IM (mg/kg/day) | Treatment Regimen | Observations & Testing |
|---|---|---|---|---|---|
| Efficacy arm (30 PFU RVFV challenge) | |||||
| 10 | Galldeslvlr | 1000 | 250 | Loading dose 30 min preinfection, BID. Maintenance dose BID, 6 days | Observed for weight loss and mortality through day 21 |
| 10 | Galldeslvlr | 800 | 200 | ||
| 10 | Galldeslvlr | 600 | 150 | ||
| 10 | Galldeslvlr | 400 | 100 | ||
| 10 | Placebo | 0.1 ml PBS | |||
| 10 | Ribavirin | 100 | 30 min preinfection, BID, 8 days | ||
| 5 | Sham-infected, Normal controls for weight change | ||||
| 4 | Galldeslvlr | 1000 | 250 | Loading dose 30 min preinfection, BID. Maintenance dose BID, 1 day | Sacrificed for day 2 serum and tissue viral titers |
| 4 | Galldeslvlr | 800 | 200 | ||
| 4 | Galldeslvlr | 600 | 150 | ||
| 4 | Galldeslvlr | 400 | 100 | ||
| 5 | Placebo | 0.1 ml PBS | |||
| 4 | Ribavirin | 100 | 30 min preinfection, BID, 1 day | ||
| 2 | Sham-infected, Normal controls for viral titers | ||||
| Drug tolerability arm (uninfected) | |||||
| 5 | Galldeslvlr | 1000 | 250 | Loading dose 30 min preinfection, BID. Maintenance dose BID, 6 days | Observed for weight loss and mortality through day 21 |
| 5 | Galldeslvlr | 800 | 200 | ||
| 5 | Galldeslvlr | 600 | 150 | ||
| 5 | Galldeslvlr | 400 | 100 | ||
| 5 | Placebo | 0.1 ml PBS | |||
2.7. Pharmacokinetic (PK) analysis of IP galidesivir in uninfected Syrian golden hamsters
Hamsters were weighed 3 days prior to drug administration and grouped to achieve an even weight distribution across all experimental groups. The study was designed so that animals in each treatment group (n = 6) were treated once with the designated concentrations of galidesivir, or the vehicle placebo (n=3), by IP injection. As shown by the experiment design in Table 2, three animals from each dosage group were designated for whole blood collection by retro-orbital bleed at 15 min (right eye; 500 μl using lithium heparinized capillary tubes and lithium heparinized gel plasma tubes) and 2 h (left eye), with a terminal bleed (cardiac puncture) at 8 h post-treatment. Whole blood was collected from the second set of 3 animals in each treatment group at 30 min (right eye), 4 h (left eye), and 12 h post-treatment (cardiac puncture). The 3 animals treated with the LRS vehicle placebo were bled at the 15 min, 2 h, and 8 h time points. Plasma samples were processed by centrifugation and stored at −80°C prior to shipment to Alturas Analytics, Inc. (Moscow, ID) for bioanalysis. For PK analysis of the data, the individual data from all animals in a dosage group were pooled at each time point. A surrogate mean plasma profile was compiled from the 6 time points for each dose. The mean plasma concentrations were analyzed by noncompartmental analysis in Phoenix WinNonlin v 7.0 (Certara, USA) to determine the mean PK parameters for each dose group.
Table 2.
Study design for the IP galidesivir PK experiment.
| No./ Group | Compound | Dose (mg/kg) | Blood collection times |
|---|---|---|---|
| 3 | Galldeslvlr | 50 | 15 min (retro orbital) 2 h (retro orbital) 8 h (cardiac puncture) |
| 3 | Galldeslvlr | 100 | |
| 3 | Galldeslvlr | 150 | |
| 3 | Galldeslvlr | 200 | |
| 3 | Placebo | 0.1 ml LRS | |
| 3 | Galldeslvlr | 50 | 30 min (retro orbital) 4 h (retro orbital) 12 h (cardiac puncture) |
| 3 | Galldeslvlr | 100 | |
| 3 | Galldeslvlr | 150 | |
| 3 | Galldeslvlr | 200 | |
2.8. Efficacy experiment 2: IP galidesivir against RVFV infection in hamsters
Hamsters were weighed the morning of the infection and sorted to minimize weight variation across the experimental animal groups. The experiment design is shown in Table 3. Animals in each group (n = 14 for all treatment groups) were treated with different dosing regimens of galidesivir, or the vehicle placebo LRS, by IP injection with a day 0 loading dose for selected groups beginning 30 min prior to RVFV challenge. Ribavirin (100 mg/kg/day) was included as a positive control. Four animals from each infection group were designated for sacrifice on day 2 p.i. for analysis of serum and tissue viral titers. The remaining animals were observed for 21 days for morbidity and mortality.
Table 3.
Study design for the IP galidesivir efficacy experiment 2.
| No./ Group | Compound | Day 0 Loading Dose, BID (mg/kg/day) | Dose (mg/kg/day) |
Treatment Regimena | Observations & Testing |
|---|---|---|---|---|---|
| 10 | Galldeslvlr | 400 | 100 | BID, 6 days | Observed for weight loss and mortality through day 21 |
| 10 | Galldeslvlr | 240 | 60 | BID, 6 days | |
| 10 | Galldeslvlr | 120 | BID, 7 days | ||
| 10 | Galldeslvlr | 200 | 100 | QD, 6 days | |
| 10 | Placebo | 0.1 ml LRS | BID, 7 days | ||
| 10 | Ribavirin | 100 | BID, 7 days | ||
| 4 | Galldeslvlr | 400 | 100 | BID, 6 days | Sacrificed for day 2 viral titers |
| 4 | Galldeslvlr | 240 | 60 | BID, 6 days | |
| 4 | Galldeslvlr | 120 | BID, 7 days | ||
| 4 | Galldeslvlr | 200 | 100 | QD, 1 day | |
| 4 | Placebo | 0.1 ml LRS | BID, 2 days | ||
| 4 | Ribavirin | 100 | BID, 2 days | ||
| 3 | Normal controls for weight change and viral titers (sacrificed on day 21) | ||||
All treatments initiated 30 min pre-infection.
2.9. Tissue and serum virus titers
Virus titers were assayed using a previously described infectious cell culture assay (Gowen et al., 2007). Briefly, a specific volume of tissue homogenate or serum was serially diluted and added to triplicate wells of Vero cell (ATCC) monolayers in 96-well microtiter plates. The viral cytopathic effect (CPE) was determined 7 days after plating and the 50% endpoints were calculated as described (Reed and Muench, 1938). The lower limits of detection were 1.49 log10 CCID50/ml serum and 3.05–4.05 log10 CCID50/g tissue. The upper limits of detection were 8.24 and 9.8 log10 CCID50/ml serum or g of tissue, respectively. In samples presenting with virus outside the limits of detection, a value representative of the limit of detection was assigned for statistical analysis.
2.10. Statistical analysis
To determine group sizes for the primary outcome of survival in the efficacy studies, power analysis was performed using commonly accepted values for type I error (0.05) and power (80%). Survival was analyzed according to the method of Kaplan and Meier using the Mantel-Cox log-rank test. A one-way analysis of variance (ANOVA) with Dunnett’s method to correct for multiple comparisons was used to assess differences in virus titers. Differences in the number of survivors between compound-treated and placebo groups were analyzed by the Fisher’s exact (two-tailed) test. All statistical evaluations were done using Prism 7 (GraphPad Software, La Jolla, CA).
3. Results
3.1. In vitro antiviral activity of galidesivir.
The in vitro antiviral activity of galidesivir against RVFV (MP-12 strain) was evaluated in both primary CPE reduction (measured by NR uptake) and VYR assays. The cytotoxicity of galidesivir was minimal, with a CC50 of 280 μg/ml (105.6 μM). The EC50 determined by the CPE reduction assay was 54 μg/ml (20.4 μM), and the EC90 measured by VYR was 37 μg/ml (13.9 μM), resulting in SI values of 5.2 and 7.6, respectively.
The galidesivir EC50 and EC90 values previously reported for RVFV using an assay based on high-content image analysis were 41.6 μM and 98 μM (Warren et al., 2014). Before incorporation of galidesivir into the nascent viral RNA chain, the parent galidesivir compound must be phosphorylated by the host cell to galidesivir-triphosphate (galidesivir-TP); however, many cell lines, including Vero 76, are inefficient at anabolizing galidesivir to galidesivir-TP, resulting in EC50 values in the double digit micromolar range (Taylor et al., 2016). Therefore, the relatively high EC50 and EC90 values do not fully represent the compound’s potential in vivo antiviral activity.
3.2. Efficacy and tolerability of IM galidesivir in the hamster RVFV infection model.
Because of the peracute and severe nature of RVFV infection in hamsters (Scharton et al., 2015), we selected treatment regimens designed to rapidly achieve high concentrations of galidesivir. The IM route was selected based on previous animal studies (Warren et al., 2014). Given the highly lethal nature of the RVFV infection in hamsters, an IP loading dose on the day of infection was included according to Table 1. In addition to the groups challenged with RVFV, five groups of uninfected animals (n = 5/group) were treated in parallel to assess drug tolerability. Unexpectedly, the lowest dose of galidesivir (400/100 mg/kg/day) was the most effective treatment paralleling the efficacy observed with the positive control drug, ribavirin (Figure 1A, B and Supplemental Table 1). Animals treated with higher doses of the drug had lower survival rates, which was a consequence of reduced drug tolerability with only the 400/100 mg/kg/day dosage being well-tolerated (Figure 1C, D). All of the animals treated with the vehicle placebo succumbed to the disease by day 4 p.i.
Figure 1. Efficacy of galidesivir against RVFV infection and tolerability in Syrian golden hamsters (experiment 1).
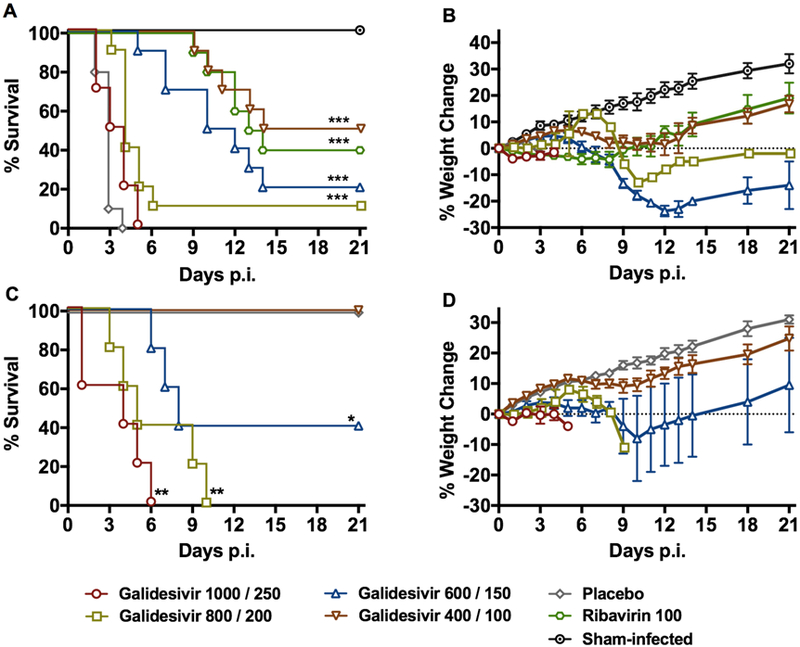
A) survival outcome and B) percent weight change of animals challenged SC with RVFV (n=10/group) that were treated BID with the indicated loading (IP) and maintenance (IM) doses of galidesivir (mg/kg/day) or placebo for 6 days according to Table 1. Ribavirin was administered IP, BID, for 8 days. C) survival outcome and D) percent weight change of un-infected animals (n=5/group) treated as described in Table 1 to assess the tolerability of galidesivir treatments. The weight data are represented as the group mean and standard error of the mean of the percent change in weight of surviving animals relative to their starting weights on day 0. *P < 0.05, **P < 0.01, ***P < 0.001 compared to animals receiving placebo.
The effect of galidesivir treatments on the inhibition of viral replication in hamsters sacrificed on day 2 p.i. is shown in Figure 2. RVFV was undetectable in the serum, spleen, or brain of any animal, and only a single animal in the galidesivir 800/200 mg/kg/day group had a detectable viral load in the liver. As expected, samples from animals treated with ribavirin were also devoid of virus. The dramatic reductions in viral titers were highly significant compared to hamsters that were treated with the PBS placebo (P < 0.001). Serum collected on day 2 p.i. was also analyzed for comprehensive biochemistry parameters (Supplemental Table 2). With the exception of decreases in total protein and albumin in the galidesivir-treated animals, all other blood chemistry values did not differ significantly compared to the sham-infected, normal controls.
Figure 2. Analysis of day 2 serum and tissue viral titers in RVFV-infected hamsters treated with galidesivir (efficacy experiment 1).
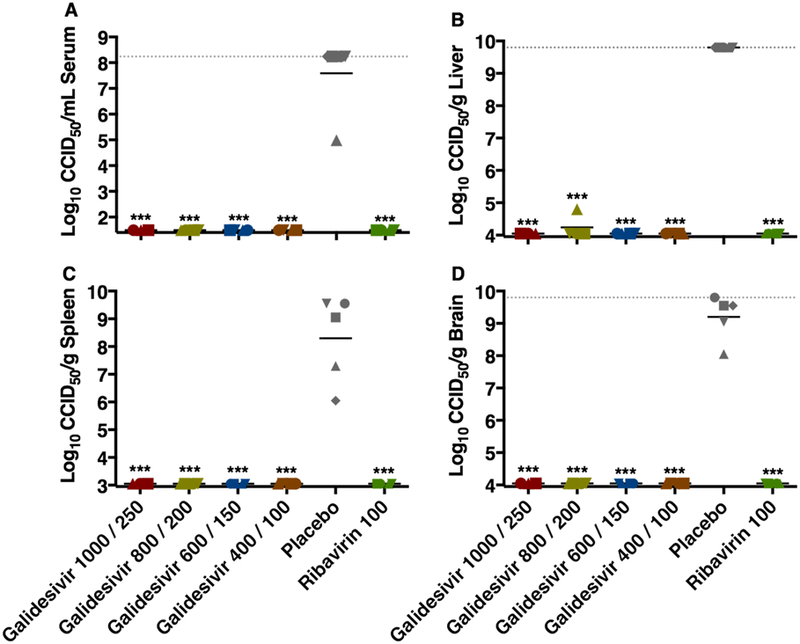
Hamsters were treated as described in Table 1 and Figure 1. Four (5 for placebo) animals in each group were designated for sacrifice on day 2 p.i. for analysis of A) serum, B) liver, C) spleen, and D) brain virus titers. Unique symbols in each treatment group represent values for the same animal across all parameters. The x-axis represents the lower limit of detection while the grey-hashed lines indicate the assay upper limits of detection. One animal receiving the highest dose of galidesivir succumbed prior to sacrifice, and thus is not included in the analysis. ***P < 0.001 compared to animals receiving placebo.
3.3. PK analysis indicates high concentrations of galidesivir are achieved in plasma.
In addition to the IM route, we investigated the IP administration route, another treatment model that approximates intravenous delivery route. A recent study in hamsters deemed 200 mg/kg/day galidesivir administered IP BID for 7 days to be the maximum tolerated dose (Julander et al., 2014). Based on this data, we analyzed the PK of escalating doses (50, 100, 150, 200 mg/kg) of galidesivir, solubilized in LRS, delivered by IP injection. Whole blood samples were collected at multiple time points within a 12-h window following administration as shown in Table 2.
Following a single dose administration, exposure of galidesivir increased greater than proportional to the increase in dose (Figure 3). There was a 7.6-fold increase in peak plasma concentration (Cmax) with a 4-fold increase in dose, while area under curve (AUC) increased 6.3-fold with a 4-fold increase in dose. Consistent with previous data obtained in rats (Warren et al., 2014), there appeared to be a second Cmax at 8 h and 12 h. Based on previous rat data, the results are likely the outcome of a rapid initial uptake of galidesivir into cells and conversion to the active triphosphate form of the drug, followed by slower catabolism back to the parent compound, which is then excreted. The biphasic kinetics of galidesivir with a rapid uptake into cells, conversion to the active moiety, and much slower excretion is reflected in the very large volume of distribution (Vd) of the drug, which is exemplified in studies conducted in albino rats and in Wistar-Han female rats (BioCryst, unpublished data), where the Vd was >45 L/kg and >34 L/kg, respectively.
Figure 3. PK analysis of plasma samples from uninfected hamsters dosed IP with escalating doses of galidesivir.
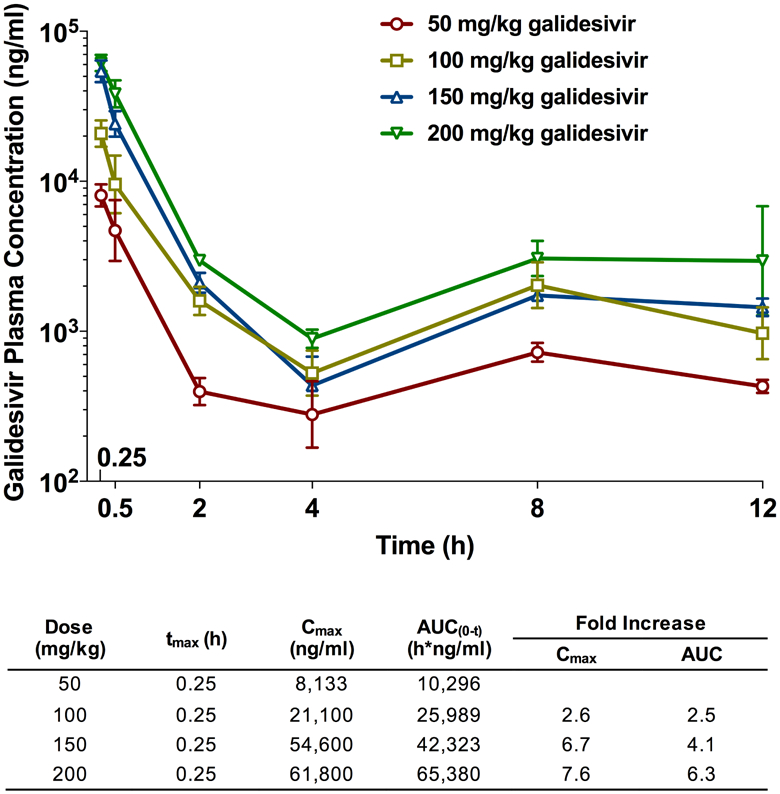
Animals in each treatment group (n=6) were treated with 50, 100, 150 or 200 mg/kg of galidesivir or the vehicle placebo (n=3), by IP injection. Whole blood was collected at the designated times (Table 2) and arithmetic mean and standard deviation plasma concentrations and mean composite PK parameters (Table inset) are shown.
3.4. Treatment with IP galidesivir significantly reduces viral replication and improves survival outcome in hamsters challenged with RVFV.
Based on the IP galidesivir PK analysis data, we selected various dosage regimens, with or without a day 0 loading dose, as a second evaluation in the RVFV hamster infection model according to Table 3. All of the groups treated with galidesivir had significantly improved survival outcomes compared to the placebo-treated animals, and as the administered dose was lowered, a dose-dependent effect was observed with a lower percentage of animals surviving the infection in the 240/60 mg/kg/day treatment group compared to the 400/100 mg/kg/day dose group (Figure 4A and Supplemental Table 2). Galidesivir dosed at 400/100 mg/kg/day provided the greatest protection with 70% of the animals surviving the uniformly lethal RVFV challenge. Notably, the ribavirin treatment resulted in a lengthy delay in the time of death, the most dramatic weight loss, and only 30% survival (Figure 4 and Supplemental Table 2). All but one of the animals treated with the LRS vehicle placebo succumbed to the disease by day 3 p.i., with an outlier expiring on day 8.
Figure 4. Effect of galidesivir on A) survival outcome and B) percent weight change in hamsters challenged SC with RVFV (efficacy experiment 2).
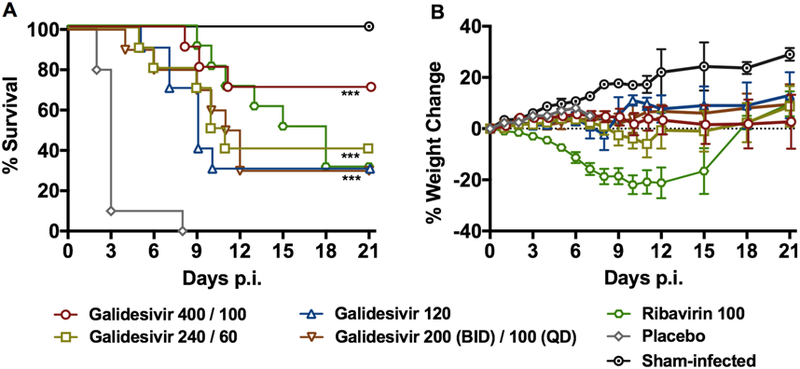
Animals in each group (n=10) were treated with the indicated loading and maintenance doses of galidesivir (mg/kg/day), ribavirin or placebo administered by IP injection for 7 days according to Table 3. The weight data are represented as the group mean and standard error of the mean of the percent change in weight of surviving animals relative to their starting weights on day 0. ***P < 0.001 compared to animals receiving placebo.
The effect of galidesivir treatments on reducing viral titers in hamster cohorts sacrificed on day 2 p.i. is shown in Figure 5. Across all tissues and serum, galidesivir treatment largely resulted in undetectable levels of virus with mean titers that were reduced by 4 to >7 orders of magnitude. The dramatic reductions in viral loads in the serum and tested tissues were highly significant compared to the placebo group (P < 0.001). Notably, in contrast to most of the galidesivir-treated hamsters, all animals that received ribavirin were found to have 4–5 log10 of infectious RVFV in their liver and brain tissues (Figure 5B, D).
Figure 5. Analysis of day 2 serum and tissue viral titers in RVFV-infected hamsters treated with galidesivir (efficacy experiment 2).
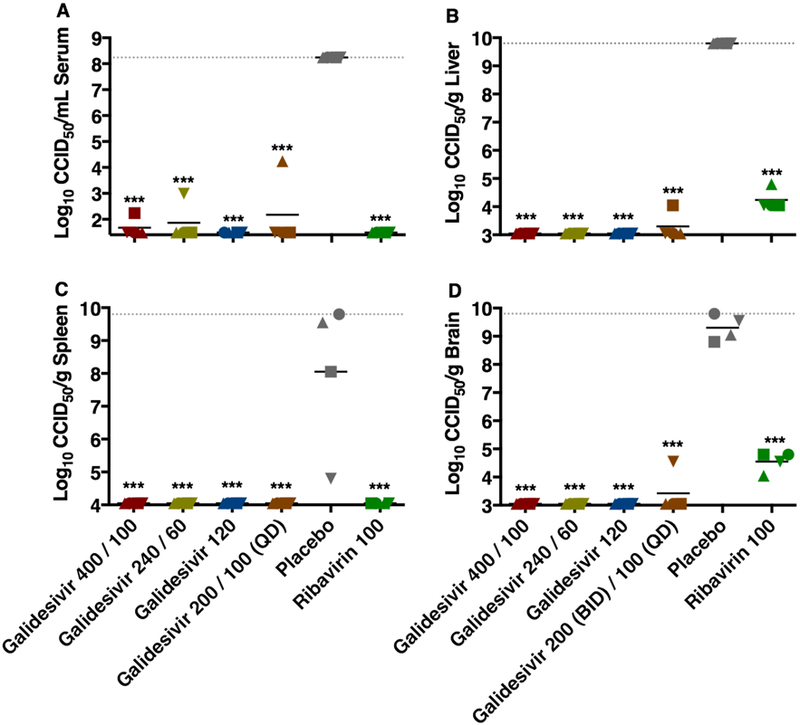
Hamsters were treated as described in Table 3 and Figure 4. Four animals per group were designated for sacrifice on day 2 p.i. for analysis of A) serum, B) liver, C) spleen, and D) brain virus titers. Unique symbols in each treatment group represent values for the same animal across all parameters. The x-axis represents the lower limit of detection and the grey-hashed lines indicate the assay upper limits of detection. ***P < 0.001 compared to animals receiving placebo.
In addition to the animals analyzed on day 2 p.i., surviving animals on day 21 were analyzed for end-of-study RVFV titers in serum, liver, spleen, and brain. No virus was found in any of the tested samples (data not shown), indicating that all of the survivors had cleared the viral infection to below the limits of detection by the infectious titer assay used.
4. Discussion
Galidesivir is an adenosine analog designed to block viral RNA synthesis by inhibiting the RNA-dependent RNA polymerase (RdRP) activity via non-obligate RNA chain termination (Warren et al., 2014). To achieve this, the galidesivir parent compound first must be converted to galidesivir-triphosphate and then incorporated into nascent viral RNA by the RdRP, causing premature termination of transcription of the viral RNA via the 3’-hydroxyl group that permits further nucleotide addition. This direct action against the viral RdRP allows galidesivir to exert broad-spectrum antiviral activity against a wide range of viruses (Taylor et al., 2016). Galidesivir has been shown to ameliorate hemorrhagic disease manifestations in Marburg virus-infected cynomolgus macaques (Warren et al., 2014), and could potentially be of value for other indications involving life-threatening viral diseases including RVF.
Here, we have expanded the preclinical characterization of in vivo antiviral activity of galidesivir through evaluation in a lethal hamster model of RVFV infection for which no antiviral has proven to be 100% protective. Hamsters are exquisitely sensitive to the ZH501 strain of RVFV as reflected by the rapid, uniform lethality (within 2 – 3 days of challenge) with as little as 10 PFU of virus, high titer viremia, and substantial viral loads in most tissues examined (Scharton et al., 2015). Our findings show that galidesivir is able to significantly delay disease progression in all animals and protect up to 70% from mortality due to the rapidly progressing, highly lethal peracute nature of the RVFV-induced disease in hamsters. This high level of efficacy is comparable to that observed with another RdRP inhibitor, favipiravir, which was able to protect 70–80% of hamsters from lethal RVFV challenge when treatment was initiated 1 h p.i. (Scharton et al., 2014). Our results are highly encouraging and support the continued development of galidesivir through further studies in well-characterized nonhuman primate models (Hartman et al., 2013; Smith et al., 2012).
Supplementary Material
Highlights.
Intramuscular and intraperitoneal administration of galidesivir reduced severity of RVFV infection in hamsters.
Galidesivir significantly improved survival outcome and prevented RVFV replication in the serum and many tissues.
Our findings support the continued development of galidesivir through further studies in advanced models of RVF disease.
Acknowledgments
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (HHSN272201000039I and HHSN272201100019I). We are grateful for the technical support provided by Angela Clyde, Michael Bertolio, and Brittney Downs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interest
AM, RT, and WS are employed by BioCryst Pharmaceuticals, Inc., the manufacturer of galidesivir. All other authors declare that no competing interests exist.
References
- Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ, 2009. Rift Valley fever virus. J Am Vet Med Assoc 234, 883–893. [DOI] [PubMed] [Google Scholar]
- Eyer L, Zouharová D, Širmarová J, Fojtíková M, Štefánik M, Haviernik J, Nencka R, de Clercq E, Růžek D, 2017. Antiviral activity of the adenosine analogue BCX4430 against West Nile virus and tick-borne flaviviruses. Antiviral research 142, 63–67. [DOI] [PubMed] [Google Scholar]
- Golnar AJ, Kading RC, Hamer GL, 2017. Quantifying the potential pathways and locations of Rift Valley fever virus entry into the United States. Transboundary and Emerging Diseases 65, 85–95. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Westover JB, Sefing EJ, Bailey KW, Nishiyama S, Wandersee L, Scharton D, Jung KH, Ikegami T, 2015. MP-12 virus containing the clone 13 deletion in the NSs gene prevents lethal disease when administered after Rift Valley fever virus infection in hamsters. Frontiers in Microbiology 6, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW, 2007. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother 51, 3168–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Powell DS, Bethel LM, Caroline AL, Schmid RJ, Oury T, Reed DS, 2013. Aerosolized rift valley fever virus causes fatal encephalitis in african green monkeys and common marmosets. Journal of virology 88, 2235–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Makino S, 2011. The Pathogenesis of Rift Valley Fever. Viruses 3, 493–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Bantia S, Taubenheim BR, Minning DM, Kotian P, Morrey JD, Smee DF, Sheridan WP, Babu YS, 2014. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a Hamster model. Antimicrobial Agents and Chemotherapy 58, 6607–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Siddharthan V, Evans J, Taylor R, Tolbert K, Apuli C, Stewart J, Collins P, Gebre M, Neilson S, Van Wettere A, Lee YM, Sheridan WP, Morrey JD, Babu YS, 2017. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral research 137, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas J, 2014. One Health approach to Rift Valley fever vaccine development. Antiviral research 106, 24–32. [DOI] [PubMed] [Google Scholar]
- McElroy AK, Nichol ST, 2012. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 422, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAID, 2016. NIAID Emerging Infectious Diseases/Pathogens. National Insitute of Allergy and Insfectious Diseases, https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogens.
- Reed LJ, Muench H, 1938. A simple method of estimating fifty percent endpoints. American Journal of Hygiene 27, 493–497. [Google Scholar]
- Scharton D, Bailey KW, Vest Z, Westover JB, Kumaki Y, Van Wettere A, Furuta Y, Gowen BB, 2014. Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment. Antiviral research 104, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton D, Van Wettere AJ, Bailey KW, Vest Z, Westover JB, Siddharthan V, Gowen BB, 2015. Rift Valley fever virus infection in golden Syrian hamsters. PloS one 10, e0116722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Bird BH, Lewis B, Johnston SC, McCarthy S, Keeney A, Botto M, Donnelly G, Shamblin J, Albariño CG, Nichol ST, Hensley LE, 2012. Development of a novel nonhuman primate model for Rift Valley fever. Journal of virology 86, 2109–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Kotian P, Warren T, Panchal R, Bavari S, Julander J, Dobo S, Rose A, El-Kattan Y, Taubenheim B, Babu Y, Sheridan WP, 2016. BCX4430 - A broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. Journal of Infection and Public Health 9, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Mores CN, Terracina L, Wallette DLJ, Hribar LJ, Pecor JE, Blow JA, 2008. Potential for North American mosquitoes to transmit Rift Valley fever virus. Journal of the American Mosquito Control Association 24, 502–507. [DOI] [PubMed] [Google Scholar]
- Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S, 2014. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508, 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2018. 2018 annual review of the Blueprint list of priority diseases. World Health Organization, http://www.who.int/blueprint/priority-diseases/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


