Abstract
Background
Age-related macular degeneration (AMD) causes progressive and irreversible damage to the retina, resulting in loss of central vision. AMD is the third leading cause of irreversible visual impairment worldwide and the leading cause of blindness in industrialized countries. Since AMD is more common in older individuals, the number of affected individuals will increase significantly as the population ages. The implantable miniature telescope (IMT) is an ophthalmic device developed to improve vision in individuals who have lost vision due to AMD. Once implanted, the IMT is used to enlarge objects in the central visual field and focus them onto healthy areas of the retina not affected by AMD, allowing individuals to recognize objects that they otherwise could not see. It is unclear whether and how much the IMT can improve vision in individuals with end-stage AMD.
Objectives
To assess the effectiveness and safety of the IMT in improving visual acuity and quality of life in people with late or advanced AMD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2017, Issue 11); Ovid MEDLINE; Embase.com; PubMed; LILACS; AMED; Web of Science Conference Proceedings Citation Index-Science; OpenSIGLE; the metaRegister of Controlled Trials (mRCT) (last searched 27 June 2014); ClinicalTrials.gov; the ICTRP and the US Food and Drug Administration (FDA) Medical Devices database. The date of the search was 2 November 2017, with the exception of mRCT which is no longer in service.
Selection criteria
We planned to include randomized controlled trials (RCTs) and quasi-randomized trials that compared the IMT versus no IMT.
Data collection and analysis
Two review authors independently assessed all studies for inclusion, using standard methodological procedures expected by Cochrane.
Main results
Our search yielded 1042 unique records. We removed irrelevant studies after screening titles and abstracts, and evaluated five full-text reports from four studies; three were non-randomized studies. There was one ongoing RCT that compared the OriLens intraocular telescope with standard low vision training in eyes with end-stage AMD. Results for this study are expected in 2020.
Authors' conclusions
We found no RCT or quasi-RCT and can draw no conclusion about the effectiveness and safety of the IMT in improving visual acuity in individuals with late or advanced AMD. Since the IMT is typically implanted monocularly based upon which eye has better best-corrected distance visual acuity, randomization between eyes within an individual may not be acceptable. Studies are needed that compare outcomes between individuals randomized to the device versus individuals not implanted, at least during study follow-up, who serve as controls.
Plain language summary
Implantable miniature telescope for vision loss due to end-stage age-related macular degeneration
What is the aim of this review?
We conducted this Cochrane Review to determine if the implantable miniature telescope (IMT) can improve vision in individuals with end-stage age-related macular degeneration (AMD). End-stage AMD refers to advanced AMD that is no longer treatable by standard medication or surgery.
Key messages
It is uncertain whether and how much the IMT can improve vision in individuals with end-stage AMD.
What was studied in this review?
AMD causes damage to the central part of the retina and results in loss of vision. AMD is a leading cause of uncorrectable blindness worldwide. Loss of vision results in loss of independence and reduced quality of life (e.g. reduced ability to read or drive). Since AMD is more common in older individuals, the number of affected individuals will increase significantly as the population ages.
The IMT is a device that is implanted in only one eye of a person with poor vision. Typically, it is implanted in the eye with better vision. The IMT works with the cornea (in the front of the eye) to enlarge what is seen and to focus images onto healthy parts of the retina (in the back of the eye). By helping the eye to send images to the healthy parts of the retina, the IMT may improve both near and distance vision and thus quality of life.
What are the main results of the review?
Because we found no study that matched our selection criteria, we cannot draw any conclusion about the effectiveness and safety of the IMT in people with end-stage AMD. Studies are needed that compare results in individuals who receive the IMT to results in individuals who do not receive the IMT. We found one ongoing study that is expected to be completed in 2020.
How up-to-date is this review?
The review authors searched for studies that had been published up to 2 November 2017.
Background
Description of the condition
Age-related macular degeneration (AMD) is an age-associated disease that causes progressive and irreversible damage to the central part of the retina (macula) resulting in loss of central vision (National Eye Institute 2012). While the exact cause of AMD is unknown, risk factors include older age, family history, smoking, hypertension, and obesity (AAO 2014). AMD can be diagnosed with a comprehensive dilated eye exam, which may include an Amsler grid test, visual acuity test, fundus examination, and fluorescein angiography (Garcia-Layana 2017).
AMD has two forms and is diagnosed as either dry (non-neovascular) or wet (neovascular or exudative). Dry AMD is more prevalent than the wet form, and is responsible for approximately 90% of cases (Ferris 1984). Both forms can progress to advanced-stage (sometimes called late-stage) AMD and are associated with severe disability (Bennion 2012). In advanced dry AMD, central vision loss is caused by the breakdown of the light-sensitive cells in the macula (cones), which causes a blurry or blank spot to develop in the visual field (geographic atrophy). This spot can advance in size, further accelerating vision loss. In advanced wet AMD, central vision loss is caused by bleeding and leaking blood vessels that have grown under the macula (National Eye Institute 2012). The term end-stage AMD may also be used in cases of advanced AMD.
AMD is the third leading cause of irreversible visual impairment worldwide and the leading cause of blindness in industrialized countries (World Health Organization 2012). The prevalence of late AMD is estimated to be 1.4% (95% Credible Interval (CrI) 1.0% to 2.0%) at 70 years of age, 5.6% (95% CrI 3.9% to 7.7%) at 80 years of age, and 20% (95% CrI 14% to 27%) at 90 years of age (Rudnicka 2012). Since AMD prevalence rises with age, the number of cases worldwide is expected to increase as the population ages (Rein 2009). AMD also poses a significant societal economic burden. In 2006, direct medical costs for AMD were estimated to be USD 575 million. This figure is expected to rise to USD 845 million during the next 15 years (Rein 2006).
Description of the intervention
The implantable miniature telescope (IMT) is an ophthalmic device that works in conjunction with the cornea to improve near and distance vision in individuals who have lost bilateral central vision due to wet or dry end-stage AMD (FDA 2010). Once implanted, the telescope enlarges objects in the person's central visual field and focuses them onto healthy areas of the retina not affected by AMD, allowing individuals to recognize objects that they could not otherwise see (Hudson 2006). The telescope is implanted monocularly and eliminates peripheral vision in the eye in which it is implanted. As a result, the individual must rely solely on the non-implanted fellow eye for peripheral vision after surgery (Hudson 2006). Postoperatively, all recipients must undergo rehabilitation with a low vision specialist to learn how to adjust to the device. While the IMT will not restore vision to pre-AMD levels, it may improve vision enough to increase independence and decrease reliance on caregivers (Hau 2016).
The IMT is manufactured by VisionCare Ophthalmic Technologies (Saratoga, CA). The device received US Food and Drug Administration (FDA) approval in 2010 (FDA 2010). It is the only implantable telescope commercially available for treatment of end-stage AMD. The IMT is 4.4 mm long and 3.6 mm in diameter. It weighs 115 mg in air and 60 mg in aqueous humor. There are two IMT models, the WA (wide-angle) 2.2× and the WA 3.0× (FDA 2010).
How the intervention might work
The IMT is monocularly implanted by ophthalmic surgeons in an outpatient procedure under local anesthesia. The telescope is placed behind the pupil in the posterior chamber of the eye after the cataractous crystalline lens has been removed. The device is held in position by haptic loops. No intraocular lens is used in conjunction with the device (Lane 2004). Surgical preparation, surgery, and recovery take approximately two to three hours (VisionCare 2010).
The FDA has determined that the IMT is indicated in people with the following (FDA 2009; FDA 2014):
age 65 years or older with stable, moderate to profound central vision impairment caused by end-stage AMD;
retinal findings of geographic atrophy or disciform scar with foveal involvement;
evidence of cataract (unilateral), but no previous cataract surgery;
agreement to undergo training with an external telescope prior to surgery to determine whether adequate vision can be obtained (two to four sessions);
achieve at least a five-letter improvement on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart with an external telescope;
adequate peripheral vision in the eye not scheduled for surgery;
willingness to participate in a postoperative visual training program.
Why it is important to do this review
AMD causes loss of central vision, which is needed for daily activities such as recognizing faces, reading, and driving (Lane 2004). Individuals with AMD experience high levels of emotional distress and reductions in key aspects of quality of life (QoL) (SST 2005; Williams 1998). Financial costs associated with visual impairment are considerable, and include medical care, loss of income, and paid home help (Mitchell 2006). Currently, there are no effective treatments for advanced, dry AMD (National Eye Institute 2012). For wet AMD, options for preventing or slowing disease progression include laser photocoagulation, photodynamic therapy, and intravitreous anti-vascular endothelial growth factor (VEGF) injections. There is no surgical treatment that improves visual acuity (Hudson 2006). The heavy burden of the disease, the expected increase in the number of cases, the longer lifespan of affected individuals, and the current lack of effective alternatives underscore the need to examine new management options (Gehlbach 2016).
Objectives
To assess the effectiveness and safety of the IMT in improving visual acuity and quality of life in people with late or advanced AMD.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs) that compared participants randomized to receive the IMT with participants randomized to not receive the IMT (Gupta 2014). We also considered quasi-RCTs.
In the absence of the desired types of studies, we considered including randomized within-person study designs that compared eyes of individuals randomized to receive the IMT and fellow eyes that did not receive the IMT. For updates to this review, we will not consider within-person studies because in practice the dominant eye is usually chosen as the eye to receive the IMT.
Types of participants
We planned to include trials that recruited participants with late/advanced or end-stage bilateral wet or dry AMD. We defined late/advanced and end-stage AMD as retinal findings of geographic atrophy or disciform scar with foveal involvement.
Types of interventions
The intervention was an IMT of either model, 2.2× or 3.0×. We planned to compare each model separately against no IMT.
Types of outcome measures
Primary outcomes
Primary outcomes for the comparison of treatments
The proportion of participants who gained 2 or more lines of best-corrected distance visual acuity (BCDVA) (logMAR or equivalent) in the study eye 12 months after surgery (or study enrollment for control eyes). We selected a gain of 2 or more lines as an outcome measure because it is recognized as a clinically significant important difference when assessing change in visual acuity (Beck 2007).
Change in quality of life (QoL), measured as the difference in QoL scores from time of IMT implantation (or baseline) to 12 months following surgery (or study enrollment for control eyes). We planned to assess QoL as a continuous variable using the Activity of Daily Living Scale Questionnaire, the National Eye Institute Visual Functioning Questionnaire, or any other validated QoL instrument.
Secondary outcomes
Secondary outcomes for the comparison of treatments
The proportion of participants who gained 2 or more lines of BCDVA (logMAR or equivalent) in the study eye six and 18 months after surgery (or study enrollment for control eyes).
Change in QoL from time of IMT implantation (or baseline) to six and 18 months following surgery (or study enrollment).
Mean change in BCDVA (logMAR or equivalent) from time of IMT implantation (or baseline) to six, 12, and 18 months following surgery (or study enrollment).
The proportion of participants who lost 2 or more lines of BCDVA (logMAR or equivalent) in the study eye six, 12, and 18 months after surgery (or study enrollment for control eyes).
Adverse effects and surgical complications
Since the IMT is implanted monocularly, the following local adverse effects and surgical complications can occur only in the eye that received the intervention. We planned to provide a narrative summary when possible for the following events in such eyes:
The proportion of eyes with aborted surgery.
The proportion of eyes with device explantation.
The proportion of participants with BCDVA 20/200 or worse in the study eye six, 12, and 18 months after surgery.
The mean endothelial cell density (ECD) loss at 12 or more months after surgery. Due to the length of the IMT, it protrudes into the anterior chamber when implanted, and implantation can lead to loss of endothelial cells and diminished ECD. These cells are essential for maintaining the clarity of the cornea. We planned to report the mean ECD loss, measured as the change in ECD from baseline to 12 months or more following surgery among eyes that receive the implant, as a proxy measure to assess risk of corneal transplant.
Additional outcomes of importance to participants
We planned to provide a narrative summary when possible for the following:
Proportion of participants with IMT surgery who had difficulty adjusting to loss of peripheral vision in the treated eye, as reported by study participants or masked observers.
Proportion of participants with IMT surgery who had postoperative pain.
Healing time following IMT surgery.
Proportion of participants who experienced falls.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 2 November 2017, with the exception of mRCT which is no longer in service.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 2 November 2017) (Appendix 1);
MEDLINE Ovid (1946 to 2 November 2017) (Appendix 2);
Embase.com (1980 to 2 November 2017) (Appendix 3);
PubMed (1948 to 2 November 2017) (Appendix 4);
LILACS (1982 to 2 November 2017) (Appendix 5);
AMED (1985 to November 2017) (Appendix 6);
Web of Science Conference Proceedings Citation Index-Science (CPCI-S) (1970 to November 2017) (Appendix 7);
OpenGrey (www.opengrey.eu; searched 2 November 2017) (Appendix 8);
metaRegister of Controlled Trials (mRCT) (last searched 27 June 2014) (Appendix 9);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov ; searched 2 November 2017) (Appendix 10);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)(www.who.int/ictrp/en/; searched 2 November 2017) (Appendix 11);
FDA Medical Devices database (www.fda.gov/MedicalDevices/ searched November 2017) (Appendix 12).
Searching other resources
We searched the reference lists of potentially relevant studies to identify any further additional trials. We did not handsearch journals or conference proceedings specifically for this review.
Data collection and analysis
Selection of studies
Two review authors independently evaluated all titles and abstracts obtained from the searches. We classified each record as definitely relevant, possibly relevant, or definitely not relevant. We obtained full-text reports of records classified as definitely relevant or possibly relevant by both review authors. Two review authors independently reviewed the full-text reports to determine final inclusion status. Papers that we excluded after full-text evaluation are described in the Characteristics of excluded studies table, with reasons for exclusion. We resolved discrepancies at all stages by discussion. In any case where the two review authors could not agree about inclusion or exclusion status, a third review author assisted in making the final decision. For studies written in languages not read by the review authors, we identified colleagues to help with assessing the eligibility and, when needed, to translate the report for further review.
Characteristics of studies.
Characteristics of excluded studies
| Brown 2011 | |
| Reason for exclusion | This study is a follow-up study of Hudson 2006 and is not an RCT. Within each individual, the determination of which eye received the intervention (IMT) was made by the surgeon and patient based on the patient's BCDVA. There was no randomization |
| Hudson 2006 | |
| Reason for exclusion | This study is not an RCT. Within each individual, the determination of which eye received the intervention (IMT) was made by the surgeon and patient based on BCDVA. There was no randomization |
| Lane 2004 | |
| Reason for exclusion | This study is not an RCT. Within each individual, the determination of which eye received the intervention (IMT) was made by the surgeon and patient based on the patient's BCDVA. There was no randomization |
Footnotes
BCDVA: best-corrected distance visual acuity
IMT: implantable miniature telescope
RCT: randomized controlled trial
We found no trials eligible for inclusion in our review. We will apply the methods described below to future updates of the review when eligible trials have been conducted and reported
Data extraction and management
Two review authors will independently extract data from all included studies using data abstraction forms developed by Cochrane Eyes and Vision. We will collect data pertinent to the study methods, participant characteristics, interventions, and outcomes for each included trial. We will compare results and resolve discrepancies by discussion between both review authors, referring back to the original article when necessary. When data are not available in the published report for primary or secondary outcomes of interest, we will contact the study authors and request relevant data in an effort to overcome any selective reporting biases. We will allow the authors four weeks to respond. When necessary, we will extract data from figures in the reports and contact the study authors to confirm or refute the accuracy of data so obtained, and will perform sensitivity analyses to determine the impact of using data extracted from figures. In cases where the review authors cannot agree, a third review author will help in decision-making. One review author will enter all data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author will verify these data.
Assessment of risk of bias in included studies
Two review authors will independently assess the potential risk of bias of each trial and will resolve any disagreement by discussion. We will use Chapter 8 of the Cochrane Handbook for Sysfemafic Reviews of Infervenfions to guide the assessment of the risk of bias of each trial included in the review (Higgins 2011). The two review authors will consider the following for each trial:
Sequence generation (selection bias).
Allocation concealment before randomization (selection bias).
Masking (blinding) of study personnel (performance bias).
Masking of outcome assessors (detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other potential biases.
We will assess each trial for each bias domain as 'low risk of bias', 'high risk of bias', or 'unclear risk of bias'. Since there may be variation in the risk of bias among different outcomes within a study, we will assess the risk of bias at the level of outcomes rather than for the entire study when appropriate (i.e. for masking of outcomes assessors and incomplete outcome data). We will consider any quasi-randomized study to be at high risk of selection bias due to inadequate sequence generation and unlikelihood of allocation concealment before assignment, as described in Chapter 8 of the Cochrane Handbook for Sysfemafic Reviews of Interventions (Higgins 2011). If we detect other types of potential bias, such as source of funding or conflict of interest, we will present these. We will discuss the potential impact on estimates of treatment effects from trials with high or unclear risks of bias.
Two review authors will independently assess bias for each study and for each relevant outcome. When there is disagreement about bias, the authors will discuss and reach consensus with help from a third review author. When there is an unclear or high risk of bias for a particular outcome within an individual study, we will conduct sensitivity analyses by removing that study from meta-analyses.
Measures of treatment effect
Primary outcomes
We will calculate the risk ratio (RR) and corresponding 95% confidence interval (CI) for gain of 2 or more lines of BCDVA in the study eye (dichotomous variable) from baseline to 12 months following surgery (or study enrollment). Ideally, BCDVA in participants randomized to receive the IMT in a predetermined study eye will be compared with BCDVA in the predetermined study eye of participants who did not receive the IMT implant in either eye. However, if these types of study designs are not available, we will consider within-person designs that compared BCDVA between eyes that received the implant and fellow eyes that did not received the implant in the same participant. We will calculate RRs following the criteria set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We will assess QoL by calculating the mean difference (MD) and corresponding 95% CI in the change from baseline to 12 months in participants randomized to receive the IMT and in participants who did not receive the implant, as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). If we include within-person studies, in which the eye with the IMT is compared with the fellow eye within the same participant, we will not be able to compare individual-level QoL outcomes between treatments (e.g. ability to read and drive); however, we will describe changes in QoL from baseline. We will calculate the standardized mean difference (SMD) and its 95% CI as the summary statistic for QoL when included studies used different QoL instruments and measurement scales.
Secondary outcomes
Gain of 2 or more lines of BCDVA.
Change in QoL.
Mean change in BCDVA.
Loss of 2 or more lines of BCDVA.
We will calculate the RR and corresponding 95% CI for gain of 2 or more lines of BCDVA from baseline to six months and 18 months following surgery (or study enrollment). We will assess QoL by calculating the MD or SMD and corresponding 95% CI of the change from baseline to six months and 18 months following surgery (or study enrollment).
We will also calculate the MD and 95% CI for the change in BCDVA (continuous variable, expressed as logMAR) from baseline (time of surgery) to six, 12, and 18 months of follow-up. When BCDVA is not expressed as logMAR, we will calculate the logMAR equivalent from visual acuity data reported. For continuous variables, we will calculate the MD as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We will also calculate the RR and corresponding 95% CI for loss of 2 or more lines of BCDVA from baseline to six, 12, and 18 months following surgery (or study enrollment).
We will perform statistical analyses using Cochrane's Review Manager 5 (RevMan 5) software (Review Manager 2014).
Adverse effects and surgical complications
Local adverse effects and surgical complications of the IMT can occur only in the eye receiving the intervention. For adverse effects and surgical complications (see Secondary outcomes), we will provide a narrative summary that reports the proportion of eyes experiencing these events.
Additional outcomes of importance to participants
For additional outcomes of importance to participants, we will provide a narrative summary that reports the proportion of participants experiencing these outcomes (see Secondary outcomes).
Unit of analysis issues
If RCTs are available that assess differences between participants who receive the implant and participants who do not receive the implant, the unit of analysis will be the individual. Ideally, such trials should report the criteria used to determine which eye was to receive the IMT implant, as well as which eye was to serve as the study eye in the control group, that is, the eye with the best or worse visual acuity at baseline, or determined randomly when both eyes had equal baseline visual acuity. However, if only randomized within-person designs are available, the unit of analysis for the primary intervention (BCDVA) will be the eye. We will document whether studies using within-person designs accounted for intra-person correlations.
Dealing with missing data
Whenever possible, we will contact study authors for missing data that were not available in published reports. We will give the authors four weeks to reply to our requests, after which time we will use the information reported. We will not attempt any imputation for missing data. When visual acuity outcomes have not been reported, we will examine the reasons given for missing data, the amount of missing data, and the comparability of missing data among treatment groups. We will conduct analyses with only the available data, and then qualitatively assess the potential impact of the missing data. Whenever sufficient data are not available for quantitative analysis (e.g. missing measures of variability, number of participants at risk), we will not include a trial in a meta-analysis but will describe results in a narrative form.
Assessment of heterogeneity
We will assess clinical and methodologic heterogeneity among studies qualitatively by examining differences in participant characteristics, differences in method of follow-up and length of follow-up periods, and differences in how outcomes were measured. We will assess statistical heterogeneity among trial results using a Chi2 test, as well as by consideration of the direction of effect of individual studies and visual inspection of overlap in the 95% CIs in forest plots generated in Review Manager 5. We will use the I2 statistic to assess statistical inconsistency across studies (Deeks 2011). We will consider an I2 value greater than 50% to be indicative of substantial statistical heterogeneity.
Assessment of reporting biases
We will present a funnel plot for each outcome when 10 or more studies are included in a meta-analysis. For each trial, we will plot effect estimates on the horizontal axis and the standard error on the vertical axis. We will judge funnel plot asymmetry by visual inspection. We will try to judge whether any asymmetry is due to publication bias or due to the tendency of smaller studies to produce different effect sizes for various reasons, as outlined in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). We will present a full description and interpretation of each funnel plot with the cautionary note that such interpretation will be subjective and probably speculative.
Data synthesis
When there is great variation among studies with respect to baseline characteristics, how the intervention was provided, assessment of outcomes, or follow-up periods, we will not conduct a meta-analysis, but will provide a qualitative summary. Whenever there is no evidence of clinical, methodologic, or statistical heterogeneity (I2 = 50% or less), we will use a fixed-effect model when fewer than three studies are included in a meta-analysis and a random-effects model when three or more studies are included, as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). In order to analyze data collected using different QoL instruments, we will calculate the SMD and its 95% CI as the summary statistic for QoL.
Subgroup analysis and investigation of heterogeneity
When we find substantial heterogeneity, we will investigate the effects of different implant models and type of macular lesion at baseline. If we can identify no apparent reasons and we cannot explain the cause of heterogeneity, we will not conduct meta-analyses.
Sensitivity analysis
We have described previously sensitivity analyses for using data extracted from figures and for handling missing participant outcome data. When applicable, we will conduct additional sensitivity analyses to determine the impact of changes in inclusion criteria such as:
exclusion of within-person studies that did not appropriately account for intra-person correlation;
exclusion of studies judged at high risk of bias for attrition bias;
exclusion of unpublished data; and
exclusion of industry-funded studies.
If the only data available are unpublished or industry-funded, we will not perform sensitivity analyses.
Summary of findings
We will present a 'Summary of findings' table of the main outcomes of this review when sufficient data are available. The main outcomes, assessed at 12 months follow-up, will include the proportion of participants who gained 2 or more lines of BCDVA, the mean change from baseline in quality of life scores, the mean change from baseline in BCDVA, the proportion of participants who lost 2 or more lines of BCDVA, the proportion of eyes with aborted surgery, the proportion of eyes with device explantation, and the proportion of participants with BCDVA 20/200 or worse in the study eye.
Using the GRADE approach (GRADEpro 2014), we will assess the certainty of evidence for each outcome. Two review authors will independently grade each outcome as providing very low, low, moderate, or high certainty of evidence. We will resolve discrepancies by discussion. We will use the following five criteria on which to base our judgements:
Risk of bias in individual trials
Indirectness
Heterogeneity
Imprecision of estimate (wide confidence intervals)
Publication bias.
Results
Description of studies
Results of the search
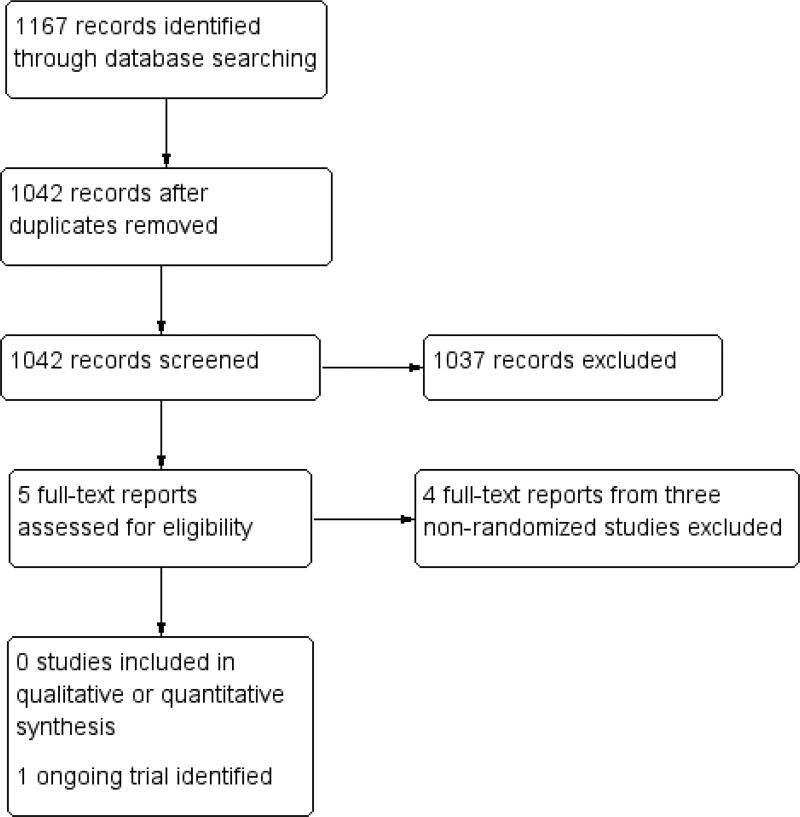
The electronic searches yielded 1167 references (Figure 1). We removed 125 duplicate records and eliminated 1038 irrelevant studies. We reviewed the full-text reports for the remaining five records (four studies). We excluded four reports from three studies and found one ongoing RCT (MIRROR 2017).
Figure 1.
Study flow diagram.
Included studies
No completed study met our inclusion criteria. We identified one ongoing RCT that compared the OriLens intraocular telescope with standard low vision training in eyes with end-stage AMD (MIRROR 2017). Results for this study are expected in 2020.
Excluded studies
We excluded three non-randomized studies (Brown 2011; Hudson 2006; Lane 2004). Reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
We found no study that met the inclusion criteria.
Effects of interventions
We found no study that met the inclusion criteria.
Discussion
Summary of main results
We found no study that met the inclusion criteria.
The purpose of the IMT is to improve distance and near vision in people who have lost central vision due to end-stage AMD. The IMT is implanted monocularly and is intended to improve central vision. Once implanted, peripheral vision is lost in the eye that receives the device, and individuals must rely on the fellow eye for peripheral vision. In clinical practice, to maximize central and peripheral vision, BCDVA and patient preference are used to determine which eye receives the prosthesis. Randomization between eyes is therefore unlikely, and unbiased estimates cannot be calculated of the effectiveness of the IMT for improving visual acuity between implanted and fellow eyes. Furthermore, within-person studies cannot investigate differences between IMT and control regarding quality-of-life measures and adverse effects, such as the inability to cope with the disparity in retinal images between the two eyes. Studies are needed that evaluate differences in outcomes between individuals who are randomized to device or control.
Potential biases in the review process
We followed procedures expected by Cochrane. We did not include non-randomized studies in our review, due to the high risk of bias of these studies for interpreting intervention effectiveness.
Agreements and disagreements with other studies or reviews
We are not aware of any other review of the effectiveness of the IMT in improving visual acuity and safety in individuals with late or advanced AMD and there are no randomized trials. As no randomized or quasi-randomized trials have been conducted, non-randomized studies may provide the best available evidence for the IMT in people with AMD. Non-randomized studies that we found include Hudson 2006 and Lane 2004, which are prospective, open-label, multicenter studies that provided up to 12 months of follow-up data on BCDVA in people who were implanted with the device. Investigators from both studies suggested that further research is warranted. Based on information on quality of life from Hudson 2006, a modeling study suggested that improvements in BCDVA and quality of life due to the IMT could be cost-effective (Brown 2011).
Authors' conclusions
Implications for practice
Based on the results of this review, no evidence from RCTs was available to assess the effectiveness of the IMT in improving visual acuity and safety in people with late or advanced AMD.
Implications for research
Since the IMT is implanted monocularly and randomization between eyes within an individual may not be acceptable, studies are needed that evaluate differences in outcomes between individuals who are randomized to device or control (or delayed device implantation). Such studies would randomize participants with late/advanced or end-stage bilateral wet or dry AMD with central vision loss to IMT or to control (or no delayed device), and would then compare differences in visual acuity (BCVA) and quality of life between groups 12 to 24 months after device implantation. While no RCTs are currently available for the IMT, an RCT for a different type of implantable telescope (OriLens) is currently underway (MIRROR 2017). This multicenter study is evaluating differences in visual acuity and quality of life between individuals with end-stage AMD randomized to OriLens or standard care. Results are expected in 2020.
Characteristics of ongoing studies
| MIRROR 2017 | |
|
| |
| Study name | Efficacy of the Telescopic Mirror Implant for Age-related Macular Degeneration: The MIRROR Trial |
|
| |
| Methods | Parallel-group, randomized clinical trial |
|
| |
| Participants | End-stage age-related macular degeneration |
|
| |
| Interventions | OriLens device vs. optimized low vision training with the opportunity to try external telescopes |
|
| |
| Outcomes | Primary outcome: best-corrected distance visual acuity, measured using number of letters improvement on ETDRS chart at 12 months |
| Secondary outcomes: best-corrected distance visual acuity at one, three and six months; best-corrected near visual acuity, reading speed and contrast sensitivity, measured by MNRead chart and MARS chart at 12 months; vision-specific quality of life, measured using the IVI at six and 12 months; health-related quality of life status, measured using the EQ-5D-5L questionnaire at six and 12 months; health service use and associated costs, measured by a Health Service Use Questionnaire at six and 12 months | |
|
| |
| Starting date | 2015 |
|
| |
| Contact information | Dr Catherine Adams |
| The Royal Hospitals | |
| Belfast, UK | |
|
| |
| Notes | Intention to publish date: 28 February 2020 |
Acknowledgments
We would like to thank the Cochrane Eyes and Vision US Project for their assistance in preparing the review and developing the search strategies. We thank Barbara Hawkins and other peer reviewers for comments on the review.
Sources of support
Internal sources
Kaiser Permanente, USA
External sources
Methodologic support provided by the Cochrane Eyes and Vision US Project, funded by Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
-
◦Richard Wormald, Co-ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
◦This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
◦
Appendices
1 CENTRAL search strategy
#1 MeSH descriptor: [Retinal Degeneration] explode all trees
#2 MeSH descriptor: [Macular Degeneration] explode all trees
#3 MeSH descriptor: [Retinal Neovascularization] explode all trees
#4 MeSH descriptor: [Choroidal Neovascularization] explode all trees
#5 MeSH descriptor: [Macula Lutea] explode all trees
#6 ((macul* or retina* or choroid*) near/4 degener*)
#7 ((macul* or retina* or choroid*) near/4 neovasc*)
#8 maculopath*
#9 (macul* near/2 lutea*)
#10 (macul* near/3 dystroph*)
#11 (macul* near/2 syndrome)
#12 ((macul* or geographic) near/2 atroph*)
#13 ((macul* or retina*) near/2 edema*)
#14 (AMD or ARMD or CNV)
#15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 MeSH descriptor: [Prostheses and Implants] explode all trees
#17 MeSH descriptor: [Prosthesis Implantation] explode all trees
#18 MeSH descriptor: [Miniaturization] explode all trees
#19 MeSH descriptor: [Telescopes] explode all trees
#20 IMT*
#21 Prosthe*
#22 Telescop*
#23 microtelescop*
#24 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #15 and #24
2 MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt.
2. Controlled Clinical Trial.pt.
3. (randomized or randomised).ab,ti.
4. placebo.ab,ti.
5. drug therapy.fs.
6. randomly.ab,ti.
7. trial.ab,ti.
8. groups.ab,ti.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. exp animals/ not humans.sh.
11. 9 not 10
12. exp Macular Degeneration/
13. exp Retinal Degeneration/
14. exp Retinal Neovascularization/
15. exp Choroidal Neovascularization/
16. exp Macula Lutea/
17. ((macul* or retina* or choroid*) adj4 degener*).tw.
18. ((macul* or retina* or choroid*) adj4 neovasc*).tw.
19. Maculopath*.tw.
20. (macul* adj2 lutea*).tw.
21. (macul* adj3 dystroph*).tw.
22. (macul* adj2 syndrome).tw.
23. ((macul* or geographic) adj2 atroph*).tw.
24. ((macul* or retina*) adj2 edema*).tw.
25. (AMD or ARMD or CNV).tw.
26. or/13–24
27. exp "Prostheses and Implants"/
28. exp Prosthesis Implantation/
29. exp Miniaturization/
30. exp Telescopes/
31. IMT*.tw.
32. Prosthe*.tw.
33. Telescop*.tw.
34. microtelescop*.tw.
35. or/27–34
36. 11 and 26 and 35
3 Embase.com search strategy
#1 'randomized controlled trial'/exp
#2 'randomization'/exp
#3 'double blind procedure'/exp
#4 'single blind procedure'/exp
#5 random*:ab,ti
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 'animal'/exp OR 'animal experiment'/exp
#8 'human'/exp
#9 #7 AND #8
#10 #7 NOT #9
#11 #6 NOT #10
#12 'clinical trial'/exp
#13 (clin* NEAR/3 trial*):ab,ti
#14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti
#15 'placebo'/exp
#16 placebo*:ab,ti
#17 random*:ab,ti
#18 'experimental design'/exp
#19 'crossover procedure'/exp
#20 'control group'/exp
#21 'latin square design'/exp
#22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
#23 #22 NOT #10
#24 #23 NOT #11
#25 'comparative study'/exp
#26 'evaluation'/exp
#27 'prospective study'/exp
#28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti
#29 #25 OR #26 OR #27 OR #28
#30 #29 NOT #10
#31 #30 NOT (#11 OR #23)
#32 #11 OR #24 OR #31
#33 'retina maculopathy'/exp
#34 'retina degeneration'/exp
#35 'retina macula degeneration'/exp
#36 'retina neovascularization'/exp
#37 'subretinal neovascularization'/exp
#38 'retina macula lutea'/exp
#39 ((macul* OR retina* OR choroid*) NEAR/4 degener*):ab,ti
#40 ((macul* OR retina* OR choroid*) NEAR/4 neovasc*):ab,ti
#41 maculopath*:ab,ti
#42 (macul* NEAR/2 lutea*):ab,ti
#43 (macul* NEAR/3 dystroph*):ab,ti
#44 (macul* NEAR/2 syndrome):ab,ti
#45 ((macul* OR geographic) NEAR/2 atroph*):ab,ti
#46 ((macul* OR retina*) NEAR/2 edema*):ab,ti
#47 amd:ab,ti OR armd:ab,ti OR cnv:ab,ti
#48 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47
#49 'prostheses and orthoses'/exp
#50 'implantation'/exp
#51 'telescope'/exp
#52 imt*:ab,ti
#53 prosthe*:ab,ti
#54 telescop*:ab,ti
#55 microtelescop*:ab,ti
#56 #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55
#57 #32 AND #48 AND #56
4 PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh])
#2 ((macul*[tiab] OR retina*[tiab] OR choroid*[tiab]) AND degener*[tiab]) NOT Medline[sb]
#3 ((macul*[tiab] OR retina*[tiab] OR choroid*[tiab]) AND neovasc*[tiab]) NOT Medline[sb]
#4 Maculopath*[tiab] NOT Medline[sb]
#5 (macul*[tiab] AND lutea*[tiab]) NOT Medline[sb]
#6 (macul*[tiab] AND dystroph*[tiab]) NOT Medline[sb]
#7 (macul*[tiab] AND syndrome[tiab]) NOT Medline[sb]
#8 ((macul*[tiab] OR geographic[tiab]) AND atroph*[tiab]) NOT Medline[sb]
#9 ((macul*[tiab] OR retina*[tiab]) AND edema*[tiab]) NOT Medline[sb]
#10 (AMD[tiab] OR ARMD[tiab] OR CNV[tiab]) NOT Medline[sb]
#11 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 IMT*[tiab] NOT Medline[sb]
#13 Prosthe*[tiab] NOT Medline[sb]
#14 Telescop*[tiab] NOT Medline[sb]
#15 microtelescop*[tiab] NOT Medline[sb]
#16 #12 OR #13 OR #14 OR #15
#17 #1 AND #11 AND #16
5 LILACS search strategy
((Macul$ OR Macul$ OR Retina$ OR Retiniana OR Choroid$ OR Coroide) AND (Degenera$ OR Neovasculariza$) OR MH:C11.768.585$ OR MH:C11.768.585.439$ OR MH: C11.768.725$ OR MH:C23.550.589.500.725$ OR MH:C11.941.160.244$ OR MH:C23.550.589.500.145$ OR MH:A09.371.729.522$ OR maculopath$ OR AMD OR ARMD OR CNV) AND (Prosthe$ OR Protes$ OR MH:E07.695$ OR MH:VS2.006.002.010$ OR MH:E04.650$ OR Miniaturization OR Miniaturizacion OR Miniaturizagao OR MH:J01.897.520$ OR Telescop$ OR Telescopios OR MH:E07.632.875$ OR IMT$ OR microtelescop$)
6 AMED Ovid search strategy
1. exp eye disease/
2. ((macul* or retina* or choroid*) adj4 degener*).tw.
3. ((macul* or retina* or choroid*) adj4 neovasc*).tw.
4. Maculopath*.tw.
5. (macul* adj2 lutea*).tw.
6. (macul* adj3 dystroph*).tw.
7. (macul* adj2 syndrome).tw.
8. ((macul* or geographic) adj2 atroph*).tw.
9. ((macul* or retina*) adj2 edema*).tw.
10. (AMD or ARMD or CNV).tw.
11. or/1–10
12. exp prosthesis/
13. Implants artificial/
14. IMT*.tw.
15. Prosthe*.tw.
16. Telescop*.tw.
17. microtelescop*.tw.
18. or/12–17
19. 11 and 18
7 CPCI-S search strategy
# 1 Topic=(((macul* OR retina* OR choroid*) NEAR/4 degener*))
# 2 Topic=(((macul* OR retina* OR choroid*) NEAR/4 neovasc*))
# 3 Topic=(maculopath*)
# 4 Topic=((macul* NEAR/2 lutea*))
# 5 Topic=((macul* NEAR/3 dystroph*))
# 6 Topic=((macul* NEAR/2 syndrome))
# 7 Topic=(((macul* OR geographic) NEAR/2 atroph*))
# 8 Topic=(((macul* OR retina*) NEAR/2 edema*))
# 9 Topic=(amd OR armd OR cnv)
# 10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
# 11 Topic=(imt*)
# 12 Topic=(prosthe*)
# 13 Topic=(telescop*)
# 14 Topic=(microtelescop*)
# 15 #14 OR #13 OR #12 OR #11
# 16 #15 AND #10
8 OpenGrey search strategy
(macul* OR retina* OR choroid*) AND (degenerat* OR neovascula* OR lutea* OR dystroph* OR syndrome OR atroph* OR edema*) AND (telescop* OR implant* OR IMT* OR prosthe* OR microtelescop*)
9 metaRegister of Controlled Trials search strategy
(macular OR retina OR choroidal) AND (telescope OR prosthetic OR IMT)
10 ClinicalTrials.gov search strategy
(Condition) macular degeneration OR retinal degeneration OR retinal neovascularization OR choroidal neovascularization OR macula lutea OR Maculopathy OR macular dystrophy OR macular syndrome OR macula edema OR retinal edema OR AMD OR ARMD OR CNV
(Intervention) telescope OR telescopes OR microtelescope OR prostheses OR prosthesis OR prosthetic OR IMT
11 ICTRP search strategy
(Condition) macular degeneration OR retinal degeneration OR retinal neovascularization OR choroidal neovascularization OR macula lutea OR Maculopathy OR macular dystrophy OR macular syndrome OR macula edema OR retinal edema OR AMD OR ARMD OR CNV
(Intervention) telescope OR telescopes OR microtelescope OR prostheses OR prosthesis OR prosthetic OR IMT
12 FDA search strategy
combinations of the following key words: "implantable miniature telescope, "IMT," retina AND implant," "Vision Care Ophthalmic Technologies," "macular degeneration," and "AMD"
Footnotes
Contributions of authors
Amisha Gupta and Jessica Lam wrote the review and screened the search results against the eligibility criteria.
Peter Custis, Stephen Munz, Donald Fong, and Marguerite Koster provided substantial and critical comments to the review. All authors approved the final version of the review.
Declarations of interest
Amisha Gupta: None known.
Jessica Lam; None known.
Peter Custis: None known.
Stephen Munz: None known.
Donald Fong: None known.
Marguerite Koster: None known.
Differences between protocol and review
Many methodological aspects of our protocol (Gupta 2014) were not implemented because we found no eligible trials.
References to studies
- Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS, Lane SS. Comparative effectiveness and cost-effectiveness of the implantable miniature telescope. Ophthalmology. 2011;118(9):1834–43. doi: 10.1016/j.ophtha.2011.02.012. [CRSREF: 8394542] [DOI] [PubMed] [Google Scholar]
- *.Hudson HL, Lane SS, Heier JS, Stulting RD, Singerman L, Lichter PR, et al. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology. 2006;113(11):1987–2001. doi: 10.1016/j.ophtha.2006.07.010. [CRSREF: 8394544] [DOI] [PubMed] [Google Scholar]
- Hudson HL, Stulting RD, Heier JS, Lane SS, Chang DF, Singerman LJ, et al. Implantable telescope for end-stage age-related macular degeneration: long-term visual acuity and safety outcomes. American Journal of Ophthalmology. 2008;146(5):664–73. doi: 10.1016/j.ajo.2008.07.003. [CRSREF: 8394545] [DOI] [PubMed] [Google Scholar]
- Lane SS, Kuppermann BD, Fine IH, Hamill MB, Gordon JF, Chuck RS, et al. A prospective multicenter clinical trial to evaluate the safety and effectiveness of the implantable miniature telescope. American Journal of Ophthalmology. 2004;137(6):993–1001. doi: 10.1016/j.ajo.2004.01.030. [CRSREF: 8394547] [DOI] [PubMed] [Google Scholar]
- [last accessed 14 May 2018];How well does the OriLens (Hubble-type) implant work in improving vision in age-related macular degeneration? Available at https://doi.org/10.1186/ISRCTN47403123. [CRSREF: 8394549]
- The Foundation of the American Academy of Ophthalmology. [accessed 3 April 2018];Top 5 Risk Factors for AMD. www.aao.org/eye-health/news/top-5-risk-factors-amd.
- Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114(10):1804–9. doi: 10.1016/j.ophtha.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Bennion AE, Shaw RL, Gibson JM. What do we know about the experience of age related macular degeneration? A systematic review and meta-synthesis of qualitative research. Social Science and Medicine. 2012;75(6):976–85. doi: 10.1016/j.socscimed.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG, Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [updated March 2011]. Chapter 9: Analysing data and undertaking meta-analyses. Available from handbook.cochrane.org. [Google Scholar]
- FDA. FDA Ophthalmic Devices Advisory Panel information package for March 27, 2009. [accessed 3 April 2018];Review of P050034. VisionCare Ophthalmic Technologies implantable miniature telescope. Volume I of III. www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4423b1-04-Sponsor%20Executive%20Summary%20Ophthalmic%20Device.pdf.
- FDA. Summary of safety and effectiveness data, July 2010. PMA P050034. [accessed 3 April 2018];Implantable miniature telescope. www.accessdata.fda.gov/cdrh_docs/pdf5/P050034b.pdf.
- FDA. PMA Supplement. [accessed 3 April 2018];Implantable Miniature Telescope Models Wide Angle 2.2X and Wide Angle 2.7X. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma_template.cfm?id=p050034.
- Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Archives of Ophthalmology. 1984;102(11):1640–2. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- García-Layana A, Cabrera-López F, García-Arumí J, Arias-Barquet L, Ruiz-Moreno JM. Early and intermediate age-related macular degeneration: update and clinical review. Clinical Interventions in Aging. 2017;12:1579–87. doi: 10.2147/CIA.S142685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database of Systematic Reviews. 2016;(8) doi: 10.1002/14651858.CD006927.pub5. Art. No.: CD006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADEpro GDT [Computer program]. Version. Hamilton (ON): GRADE Working Group, McMaster University; [accessed prior to 10 January 2018]. 2014. [Google Scholar]
- Hau VS, London N, Dalton M. The treatment paradigm for the implantable miniature telescope. Ophthalmology and Therapy. 2016;5(1):21–30. doi: 10.1007/s40123-016-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Sterne JA, Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. Chapter 8: Assessing risk of bias in included studies. Available from handbook.cochrane.org. [Google Scholar]
- Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health and Quality of Life Outcomes. 2006;4:97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Eye Institute. [accessed 3 April 2018];Facts about age-related macular degeneration. nei.nih.gov/health/maculardegen/armd_facts.
- Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. The economic burden of major adult visual disorders in the United States. Archives of Ophthalmology. 2006;124(12):1754–60. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Archives of Ophthalmology. 2009;127(4):533–40. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- Review Manager 5 (RevMan 5) [Computer program]. Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119(3):571–80. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Submacular Surgery Trials Research Group. Health- and vision-related quality of life among patients with ocular histoplasmosis or idiopathic choroidal neovascularization at enrollment in a randomized trial of submacular surgery: Submacular Surgery Trials Report No. 5. Archives of Ophthalmology. 2005;123(1):78–88. doi: 10.1001/archopht.123.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Egger M, Moher D, Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. Chapter 10: Addressing reporting biases. Available from handbook.cochrane.org. [Google Scholar]
- VisionCare Ophthalmic Technologies. VisionCare's Implantable Miniature Telescope. An Intraocular Telescope for Treating Severe to Profound Vision Impairment due to Bilateral End-Stage Age-Related Macular Degeneration. Patient Information Booklet. VisionCare Ophthalmic Technologies. 2010 [Google Scholar]
- Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Archives of Ophthalmology. 1998;116(4):514–20. doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Prevention of blindness and visual impairment. Priority eye diseases. [accessed 3 April 2018];Age-related macular degeneration. www.who.int/blindness/causes/priority/en/index7.html.
- Gupta A, Lam J, Custis P, Munz S, Fong D, Koster M. Implantable miniature telescope (IMT) for vision loss due to end-stage age-related macular degeneration. Cochrane Database of Systematic Reviews. 2014;(6) doi: 10.1002/14651858.CD011140. Art. No.: CD011140. [DOI] [PMC free article] [PubMed] [Google Scholar]