Abstract
Purpose
Gene rearrangements involving NTRK1/2/3 can generate fusion oncoproteins containing the kinase domains of TRKA/B/C, respectively. These fusions are rare in non-small cell lung cancer (NSCLC), with frequency previously estimated to be <1%. Inhibition of TRK signaling has led to dramatic responses across tumor types with NTRK fusions. Despite the potential benefit of identifying these fusions, the clinicopathologic features of NTRK fusion-positive NSCLCs are not well characterized.
Methods
We compiled a database of NSCLC cases harboring NTRK fusions. We characterized the clinical, molecular, and histologic features of these cases with central review of histology.
Results
We identified 11 NSCLC cases harboring NTRK gene fusions verified by next-generation sequencing (NGS) and with available clinical and pathologic data, forming the study cohort. Fusions involved NTRK1 (7 cases) and NTRK3 (4 cases), with 5 and 2 distinct fusion partners, respectively. Cohort patients were 55% male, with a median age at diagnosis of 47.6 years (range 25.3–86.0) and a median pack year history of 0 (range 0–58). 73% of patients had metastatic disease at diagnosis. No concurrent alterations in KRAS, EGFR, ALK, ROS1, or other known oncogenic drivers were identified. Nine cases were adenocarcinoma, including 2 invasive mucinous adenocarcinomas and 1 adenocarcinoma with neuroendocrine features; one was squamous cell carcinoma; and one was neuroendocrine carcinoma. By collating data on 4872 consecutively screened NSCLC cases from unique patients, we estimate a frequency of NTRK fusions in NSCLC of 0.23% (95% CI 0.11–0.40).
Conclusion
NTRK fusions occur in NSCLCs across genders, ages, smoking histories, and histologies. Given the potent clinical activity of TRK inhibitors, we advocate that all NSCLCs be screened for NTRK fusions using a multiplexed NGS-based fusion assay.
INTRODUCTION
The neurotrophin kinase (NTRK) genes NTRK1, NTRK2 and NTRK3 encode the tropomyosin receptor tyrosine kinases TRKA, TRKB and TRKC, respectively, which function during normal neuronal development and maintenance. Gene rearrangements involving each of the NTRK genes have been described in a wide variety of adult and pediatric solid tumor malignancies, and are thought to drive tumor growth and survival via expression of a constitutively active fusion protein containing the TRK kinase domain. While the frequency of NTRK fusions is low in common cancer types, including non-small cell lung cancer (NSCLC), NTRK3 fusions are nearly ubiquitous among rare cancer types such as mammary analog secretory carcinoma and infantile fibrosarcoma.1,2 In NSCLC, NTRK fusions are estimated to occur at a frequency of approximately 0.1 to 1%.1,3,4 They are less common than other oncogenic gene rearrangements involving the anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), and RET proto-oncogene (RET), which occur at frequencies of approximately 4–6%, 1–2% and 1–2%, respectively.5–7
Much like ALK-, ROS1-, or RET-rearranged NSCLCs, NTRK-rearranged NSCLCs appear to be oncogene dependent. Targeted inhibition of TRK signaling in preclinical models results in cell death and tumor regressions.4 In early phase clinical trials, solid tumors harboring NTRK gene rearrangements have been highly sensitive to selective TRK tyrosine kinase inhibitors (TKIs), including larotrectinib, which is selective for TRKA/B/C, and entrectinib, which targets TRKA/B/C as well as ALK and ROS1. The objective response rate to larotrectinib across 55 adult and pediatric tumors with NTRK gene rearrangements was 75%.8 Responses were seen across tumor types and NTRK gene partners. Among 4 NSCLC cases, RECIST responses were seen in 3, and the fourth had an approximately 20% reduction in tumor size. One of four NTRK-rearranged tumors treated with entrectinib in an adult phase 1 trial was NSCLC, and this patient had a partial response, including a complete response in the central nervous system.9 Despite the potent activity of TRK inhibitors, the clinical and pathologic features of NTRK-rearranged NSCLCs are poorly defined. Here, we show that NTRK fusions occur across age and smoking status, and suggest that all patients with NSCLC should be screened for fusions using a multiplexed NGS assay.
METHODS
Physicians across seven institutions contributed deidentified cases to an NTRK fusion NSCLC database. Clinical staging was performed by treating physicians using AJCC 7th edition. NTRK fusions were identified and validated as part of routine clinical testing at each institution.
Assays utilized identified fusions using variety of technologies as follows: RNA-based fusion targeted Anchored Multiplex PCR and Illumina sequencing10 (Massachusetts General Hospital (MGH) Solid Fusion Assay, Memorial Sloan Kettering (MSK) Solid Fusion Assay, ArcherDx FusionPlex performed at Caris Life Sciences); DNA hybridization capture with intron tiling and Illumina sequencing (Foundation One11, MSK IMPACT12,13); total nucleic acid extraction, PCR amplification, and Ion Torrent Sequencing (Paradigm PCDx14).
The Kaplan-Meier method was used to obtain estimates of overall survival from diagnosis of stage IV disease to death or last follow-up (censored). The Clopper-Pearson exact method for the binomial distribution was used to obtain confidence intervals for NTRK fusion frequencies.
Two central pathologists (M.S.T. and M.M.-K.) reviewed tumor histology. When utilized, immunohistochemistry was performed on a Leica BOND automated system (Leica Biosystems, Buffalo Grove, IL) with the standard chromogen 3,3′-diaminobenzidine tetrahydrochloride hydrate (DAB), using antigen retrieval solution ER1 (citrate buffer with surfactant, pH 6.0) or ER2 (EDTA buffer with surfactant, pH 9.0), with antibody incubated at room temperature: α-TTF1 (Leica RTU Cat # PA0364, ER2 for 30 min,); α-ΔNp63 (p40, Biocare Medical RTU Cat # API3066AA, Pacheco, California, USA, ER1 for 30 min); α-chromogranin (Leica RTU Cat # PA0430, ER2 for 20 min); α-synaptophysin (Leica RTU Cat # PA0299, ER2 for 20 min). Data collection and analysis were performed under IRB-approved protocols.
RESULTS
We reached out to physicians at 47 institutions in the United States who were actively participating in a TRK inhibitor clinical trial enrolling adult patients, and invited them to contribute living or deceased cases of patients with NSCLC harboring an NTRK gene rearrangement. Fourteen cases were initially contributed from seven institutions. Candidate fusions were initially identified using a combination of RNA- and DNA-based NGS assays, with validation by one or more of RNA-based NGS, FISH, and RT-PCR on a case-by-case basis. Among these cases, in-frame TRK fusions containing the kinase domain were verified in 11, forming the study cohort.
Notably, three cases were excluded from the study cohort for the following reasons. The first case had an NTRK1 fusion detected by MSK-IMPACT, a DNA-based hybridization capture NGS assay12 but not by subsequent confirmatory testing with the MSK-Solid Fusion Assay, an RNA-based fusion-specific targeted NGS assay using anchored multiplex PCR.10 The candidate fusion contained P2RY8 exon 2 fused with NTRK1 exons 1–5. As NTRK1 exons 1–5 lack the kinase domain, this was thought to be a nonfunctional fusion. This case also had a concurrent KRAS G12C mutation, an established oncogenic driver. The second case had an NTRK2 intragenic deletion disrupting the exon 18 3′ splice site, which is predicted to disrupt the kinase domain and therefore be inactivating. The third case had an NTRK1 alteration detected by fluorescence in-situ hybridization (FISH) but not verified by NGS. This case also had a concurrent HER2 L755P mutation, which is predicted to be activating.15
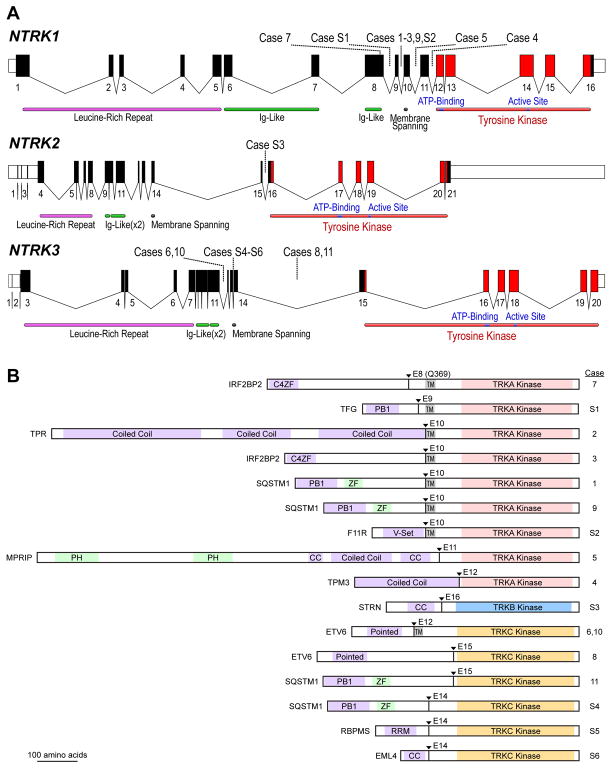
The clinical characteristics of the 11 cases in the study cohort are shown in Table 1. At the time of data analysis, 6 patients were living and 5 were deceased. The molecular characteristics of cases in the study cohort are shown in Table 2 and Figure 1 (cases 1–11). There were 7 cases of NTRK1 fusions, with 5 distinct fusion partners, and 4 cases of NTRK3 fusions with 2 distinct fusion partners. One case included in the cohort (case 4) had a candidate NTRK1 fusion detected by MSK-IMPACT with an equivocal partner, and the correct fusion partner was determined using the MSK-Solid Fusion Assay. All NTRK fusions couple the kinase domain of NTRK1 or NTRK3 (with or without the membrane-spanning helix) to an N-terminal gene fusion partner with domains known or predicted to mediate dimerization or oligomerization (Table 2 and Figure 1). Two of 9 cases tested had concurrent mutations in TP53. In all cases tested, potential oncogenic alterations in the following genes, when interrogated, were not detected: KRAS (0/10), EGFR (0/11), ALK (0/11), ROS1 (0/11), BRAF (0/11), PIK3CA (0/10), HER2 (0/8), MET (0/8).
Table 1.
Clinical characteristics of 11 patients with NTRK-rearranged NSCLC
| Age at diagnosis, median (range) | 47.6 (25.3–86.0) |
|
| |
| Gender, n (%) | |
| Male | 6 (55) |
| Female | 5 (45) |
|
| |
| Smoking history in pack years, n (%) | |
| 0–5 | 8 (73) |
| 5–20 | 0 |
| > 20 | 3 (27) |
|
| |
| Pack years, median (range) | 0 (0–58) |
|
| |
| Stage at diagnosis (AJCC 7th edition), n (%) | |
| I | 0 |
| II | 2 (18) |
| III | 1 (9) |
| IV | 8 (73) |
|
| |
| Histology (local assessment) | |
| Adenocarcinoma | 9 (82) |
| Squamous Cell Carcinoma | 1 (9) |
| Neuroendocrine Carcinoma | 1 (9) |
|
| |
| Sites of metastasis, n | |
| Lymph nodes | 8 |
| Bone | 6 |
| Pleura or malignant effusion | 5 |
| Lung | 5 |
| Liver | 4 |
| Brain | 4 |
| Adrenal | 2 |
| Skin/soft tissue | 1 |
| Pericardium | 1 |
| Trachea | 1 |
Table 2.
Molecular characteristics of NTRK rearrangements in NSCLC tumors included in the study cohort.
| Case # | NTRK | Fusion Partner | Position of fusion | Pack years | Histology | Concurrent genetic alteration(s) detected** | Molecular Assays |
|---|---|---|---|---|---|---|---|
| 1 | NTRK1 | SQSTM1 | SQSTM1 exon 6 – NTRK1 exon 10 | 30 | AC | None | MGH Solid Fusion, FISH3 |
| 2 | NTRK1 | TPR | TPR exon 21 – NTRK1 exon 10 | 0 | AC-NE | MDM4 amp | FoundationOne, MGH Snapshot, MGH Solid Fusion, FISH |
| 3 | NTRK1 | IRF2BP2 | IRF2BP2 exon 1 – NTRK1 exon 10 | 0 | AC | None | MSK-IMPACT, FoundationOne |
| 4 | NTRK1 | TPM3 | TPM3 Exon 8 – NTRK1 Exon 12 | 2 | AC | None | MSK-IMPACT, MSK-Solid Fusion |
| 5 | NTRK1 | MPRIP | MPRIP exon 21 – NTRK1 exon 11† | 0 | AC | ATM L745fs*8 | Hybridization capture DNA NGS, FISH, RT-PCR, RNAseq4 |
| 6 | NTRK3 | ETV6 | ETV6 exon 4 – NTRK3 exon 12 | 0 | AC | None | Caris ArcherDx FusionPlex, RT-PCR |
| 7 | NTRK1 | IRF2BP2 | IRF2BP2 exon 1 – NTRK1 exon 8 | 30 | AC | SMARCB1 Q368* | FoundationOne |
| 8 | NTRK3 | ETV6 | ETV6 exon 5 – NTRK3 exon 15 | 58 | SCC | TP53 E258K, CREBBP P248fs*3, MLL3 L325fs*30. Amplifications: CCND2, RICTOR, FGF6, FGF23. | FoundationOne |
| 9 | NTRK1 | SQSTM1 | SQSTM1 exon 5 – NTRK1 exon 10 | 0 | AC | None | Paradigm PCDx |
| 10 | NTRK3 | ETV6 | ETV6 exon 4 – NTRK3 exon 12 | 0 | AC | CTNNB1 (D32N), CDKN2A/B loss | FoundationOne |
| 11 | NTRK3 | SQSTM1 | SQSTM1 exon 6 – NTRK3 exon 15 | 1 | NE | ARID1A R892GfsTer27 | MGH Solid Fusion, MGH Snapshot |
AC, adenocarcinoma; SCC, squamous cell carcinoma; NE, neuroendocrine carcinoma; AC-NE, adenocarcinoma with neuroendocrine features.
Variants of unknown significance not shown.
This fusion position is referred to as exon 14 in reference 9, but is exon 11 in current nomenclature (NTRK1 RefSeq Variant 1 NM_001012331.1).
Figure 1.
A: Schematic of the human NTRK loci. Exon numbers are shown below their respective boxes for RefSeq NTRK1 transcript variant 1 (NM_001012331.1), NTRK2 transcript variant a (NM_006180.4), and NTRK3 transcript variant 1 (NM_001012338.2). Fusion breakpoints are shown as dotted lines for the indicated cases; Case 7 has an exonic breakpoint; all others are intronic. Note that exons are drawn at a larger scale than introns, and introns are not drawn to the same scale for each gene (NTRK1 locus is ~21 kB, NTRK2 is ~358 kB, and NTRK3 is ~384 kB). B: Schematic of predicted fusion protein products (see also Tables 2 and 3). Triangles and Ex notation indicate the fusion breakpoints and subsequent TRK exon. Purple shaded domains are those predicted or shown to induce dimerization in the fusion partner (C4ZF: C4 zinc finger; PB1: Phox and Bem1p interaction domain; CC: coiled coil; Pointed: sterile alpha motif (SAM) / helix loop helix (HLH) oligomerization domain); RRM: RNA recognition motif. Green shaded domains are other annotated sequence features (ZF: zinc finger; PH: pleckstrin homology). Gray shaded TM: transmembrane domain. Proteins are drawn to scale (MPRIP-NTRK1 fusion = 1332 AA).
To estimate the overall frequency of NTRK fusions in NSCLC, we reviewed consecutively tested clinical NSCLC cases from MGH and MSKCC, where NGS screening of a total of 4872 unique patients identified 11 total NTRK fusions (0.23%; Table 3). The frequencies of NTRK1, NTRK2, and NTRK3 fusions were 0.12%, 0.02%, and 0.08%, respectively. Five of these cases had available clinical and pathologic data for inclusion in our study cohort, and we report the molecular details of the additional 6 cases, S1–S6, in Supplemental Table 1. We diagram the fusion positions of all 17 of these cases (study cohort cases 1–11 plus cases S1–S6) in Figure 1.
Table 3.
Frequency of NTRK fusions among consecutively tested NSCLC clinical cases from unique patients. MGH, Massachusetts General Hospital; MSKCC, Memorial Sloan-Kettering Cancer Center. This group includes cases 1–4, 11, and S1–S6.
| MGH | MSKCC | Total | Frequency, % (95% CI) | |
|---|---|---|---|---|
| NSCLC screened | 1804 | 3068 | 4872 | |
| NTRK1 fusion | 2 | 4 | 6 | 0.12 (0.05–0.27) |
| NTRK2 fusion | 0 | 1 | 1 | 0.02 (0.005–0.11) |
| NTRK3 fusion | 2 | 2 | 4 | 0.08 (0.02–0.21) |
| All NTRK fusions | 4 | 7 | 11 | 0.23 (0.11–0.40) |
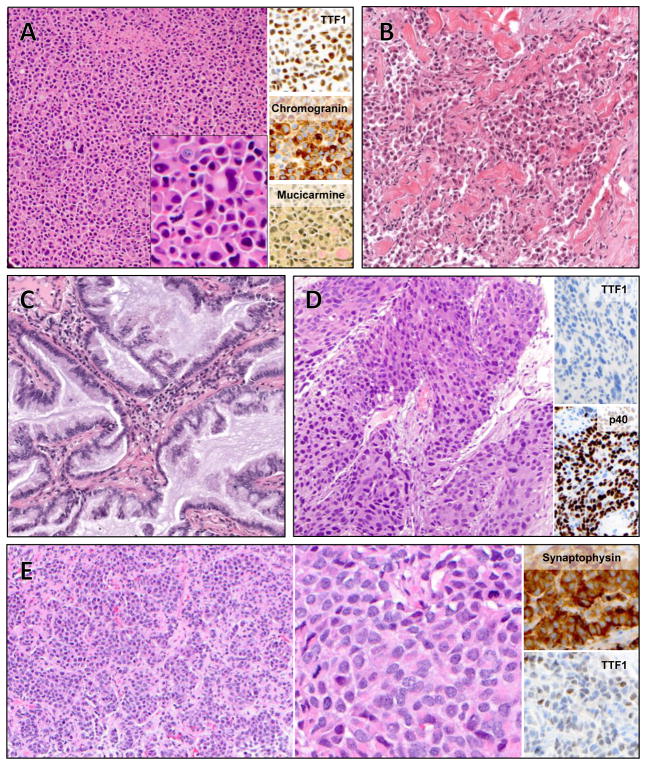
We next examined the histologic features of the 11 cases forming the study cohort. Nine were adenocarcinoma, one was squamous cell carcinoma, and one was neuroendocrine carcinoma. Among the cases of adenocarcinoma, we observed a range of histologic subtypes, including adenocarcinoma with neuroendocrine features (case 2, figure 2A), poorly differentiated adenocarcinoma with solid pattern and signet ring cells (Figure 2B, case 1), and invasive mucinous adenocarcinoma (cases 4 and 6, Figure 2C). Squamous cell histology was observed in case 8, and was confirmed with adequate sampling and by immunohistochemical expression of p40 and absence of TTF1 (case 8, Figure 2D). Case 11 was a morphologically well-differentiated neuroendocrine tumor (equivalent to atypical carcinoid) with an increased mitotic index of 12 per 10 high power fields and a brain metastasis; this tumor was classified as large cell neuroendocrine carcinoma in accordance with the current WHO criteria.16
Figure 2.
Histology of select cases. A: Case 2, adenocarcinoma with solid growth pattern, diffuse neuroendocrine differentiation, and signet ring cells. Inset, high magnification. B: Case 1, poorly differentiated adenocarcinoma, with solid and single-cell growth patterns. C: Case 4, mucinous adenocarcinoma. Case 6 had similar histology (not shown). D: Case 8, squamous cell carcinoma. E: Case 11, neuroendocrine carcinoma with well-differentiated morphology and increased mitotic activity (left) and high power (middle).
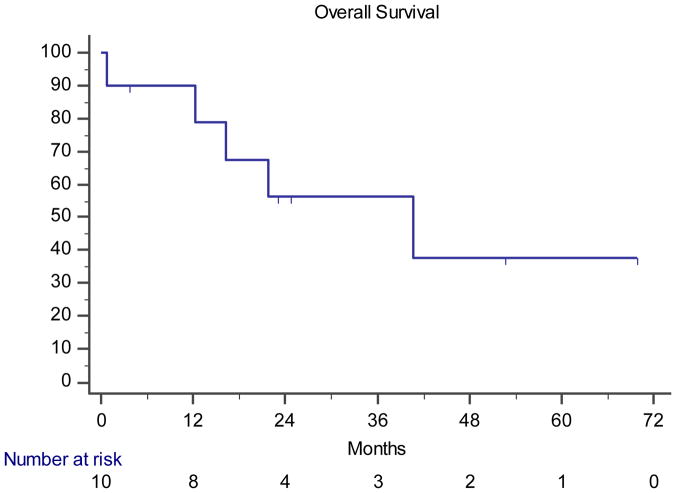
Although analysis of our cohort is limited by size and the fact that this is a retrospective review across multiple institutions, we sought to describe clinical outcomes in these patients. Across the cohort of 11 patients, 8 patients (73%) received at least one TRK tyrosine kinase inhibitor at some point in their treatment course, and 10 received a platinum doublet (91%). One patient (9%) received no treatment. The median overall survival of the 10 patients with metastatic disease was 40.8 months (95% CI 0.79-NR) with a median follow up of 52.8 months (Figure 3).
Figure 3.
Overall survival of the 10 patients with metastatic disease, measured from the date of stage IV diagnosis to date of death or last known follow up. Censored cases are marked with vertical marks.
Three patients had early stage disease at the time of diagnosis. Case #4 had stage IIB (T3N0) disease at diagnosis, was treated with surgery followed by adjuvant cisplatin and pemetrexed, and remained recurrence-free at the most recent follow up 30.0 months after initial diagnosis. Case #6 had stage IIA (T1bN1) disease at diagnosis, was treated with surgery followed by cisplatin and pemetrexed, and developed metastatic disease 24.5 months after initial diagnosis. Case #10 had stage IIIB (T4N2M0) disease at diagnosis, was treated with chemotherapy and radiation, and developed metastatic disease 10 months after initial diagnosis. The remaining 8 patients had metastatic disease at the time of diagnosis.
DISCUSSION
TRK tyrosine kinase inhibitors have shown tremendous promise in NTRK fusion-positive solid tumors across cancer types8,9, following the paradigm now well-established for EGFR-mutant and ALK or ROS1 fusion-positive NSCLCs. Although NTRK fusions are rare in NSCLC, uncertainty remains about which patients should undergo testing for these alterations. Here, we describe the clinicopathologic features of a cohort of 11 cases of NSCLCs harboring NTRK gene rearrangements resulting in the fusion of the TRK tyrosine kinase domain with a dimerization-inducing partner. These are predicted to be activating or previously reported to be activating.4,17 This cohort includes both men and women across a range of ages, histologies, and smoking histories. Although this is a small cohort, the only defining pattern of clinical characteristics that emerges is the lack of an alternate canonical driver mutation in all cases, similar to other kinase fusion-positive NSCLCs.18 Importantly, NTRK rearrangements were identified in patients with and without a history of smoking; while the majority of patients (8 of 11, 73%) had a minimal to never smoking history, 3 of the 11 cases had a history of 30 pack-years or higher. Similarly, ALK-, ROS1-, and RET-driven NSCLCs are enriched in never-smokers, though can be seen in current and former smokers as well.5,19,20 Nine of 11 cases were adenocarcinoma, and tended to be mucinous or poorly differentiated, including one case of a TPR-NTRK1 fusion with neuroendocrine differentiation. However, other histologies were also observed; we identified one squamous cell carcinoma with an ETV6-NTRK3 fusion and one neuroendocrine carcinoma with an SQSTM1-NTRK3 fusion.
Ascertainment bias due to selective testing has historically limited an accurate assessment of frequency of NTRK fusions in NSCLC. Here we combine the clinical experience from multiplexed targeted NGS screening of 4872 unique consecutive NSCLC cases at both MGH and MSKCC to estimate an NTRK fusion frequency of 0.23% in NSCLC. These assays are generally utilized at the time of tissue diagnosis in both institutions, and therefore it is likely that this population largely represents a previously unscreened group, in which patients were not already selected to be negative for other known driver mutations in lung cancer. We note that cases selected for molecular testing may be enriched for patients with metastatic disease, as there is no established role for targeted therapies in early stage lung cancer to date. Although NTRK fusions are rare in lung cancer, we estimate that with approximately 234,000 new NSCLC cases annually in the United States, over 500 of these patients may be candidates for highly effective TRK inhibitor therapy. Significantly more patients NTRK fusion NSCLC cases may exist when considering the global incidence of lung cancer.
The natural history of NTRK fusion NSCLCs, compared to NSCLCs in general, is not well-established. While we observe a median overall survival of 40.8 months among the 10 patients with metastatic disease, we acknowledge that this is a small retrospective cohort among whom 8 received at least one TRK tyrosine kinase inhibitor. The observation that one of two stage II patients and one stage III patient developed relapsed metastatic disease is consistent with the natural history of NSCLCs in general, though there may be selection bias against screening early-stage cases that did not develop metastatic disease.
As there appears to be no uniform defining clinical or pathologic feature of NTRK fusion-positive NSCLCs, we recommend screening all NSCLCs for NTRK gene rearrangements. In our experience, RNA-based fusion assays such as the MGH or MSK Solid Fusion Assays or related ArcherDX FusionPlex have a number of advantages over DNA-based methods, including high sensitivity, confident identification of breakpoints and in-frame fusions, and deeper coverage.10 Importantly, three cases of predicted non-functional NTRK alterations were also identified in this study, emphasizing the added value of NGS-based sequencing and attention to the breakpoints. Although immunohistochemical assays for the detection of TRK expression are in development21, allocation of an unstained slide for TRK immunohistochemistry may be impractical given the need to test for a wide range of molecular alterations on often limited tissue samples. Similarly, given the apparent lack of concurrent canonical driver mutations in these cases, it may be reasonable to consider initial DNA-based NGS for mutational profiling, with reflex multiplexed fusion-targeted RNA-based NGS in cases lacking such a driver. However, sequential testing for possible gene alterations can delay the ultimate molecular diagnosis, may be problematic for small samples, and relies on mutual exclusivity of a kinase fusion and oncogenic driver mutation. Therefore, we favor concurrent NGS-based mutational analysis with multiplexed NGS-based targeted RNA sequencing for identification of gene fusions in NSCLC rather than sequential mutation testing or immunohistochemistry, which consumes more time and tissue. Ultimately, we anticipate that more widespread and comprehensive NTRK fusion testing in NSCLC cases will lead to expanded treatment options for NTRK fusion positive patients.
Supplementary Material
Acknowledgments
Sources of support:
This work was supported by the President and Fellows of Harvard College (CMeRIT to AFF), the National Institute of Health and National Cancer Institutes (Chabner K12CA087723 to AFF), and the NIH/NCI University of Colorado Lung Cancer SPORE (P50 CA058187) to RCD.
We thank Roxana Azimi for administrative support, and the patients and families who contributed to this study.
Footnotes
Previous presentations of this work:
This study has been previously presented, in part, at the ASCO annual meeting 2017, Farago et al., Abstract # 11580.
Relevant conflicts of interest:
AFF has received consultant fees from AbbVie, PharmaMar, Loxo Oncology; research funding (to institution) from Loxo Oncology, Ignyta, AstraZeneca, AbbVie, Merck, Bristol Myers-Squibb, Novartis.
RCD has received consultant/advisory board fees from Ariad, Takeda, AstraZeneca, Spectrum, and Ignyta; licensing fees from Abbott Molecular and Ignyta; stock ownership in Rain Therapeutics.
VWZ has received honoraria from AstraZeneca, Roche/Genentech, Takeda, and Biocept, and consultant fees from TP Therapeutics.
DLA has received consultant fees from Bristol-Myers Squibb and AbbVie
LPL has equity interest and royalties from exclusive license of AMP technology to ArcherDx and has received consultant fees from ArcherDx
AJI has received consulting fees for Debiopharm Group, Constellation Pharmaceuticals, Chugai Pharmaceutical, and Roche, research Funding from Blueprint Medicines, and has equity interest and royalties from exclusive license of AMP technology to ArcherDx. SHIO has received consultant/advisory board fees from ARIAD/Takeda, Pfizer, Genentech/Roche, Astra Zeneca, Novartis, Ignyta, Foundation Medicine Inc, member of the speaker bureau of Genentech/Roche, AstraZeneca, ARIAD/Takeda, and Merck; a member of the scientific advisory board of TP Therapeutics Inc and stock ownership in TP Therapeutics Inc.
ATS has received consultant fees from Pfizer, Novartis, Ariad/Takeda, Genentech/Roche, Ignyta, Loxo Oncology, Blueprint medicines, KSQ therapeutics, Natera.
MMK has received consultant fees from Merrimack Pharmaceuticals and H3 Biomedicine.
AD has received consultant/advisory board fees from Ignyta, Loxo Oncology, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Takeda/Ariad.
MST, SK, AIS, TAB, EBH, MEA, RB, NKH, JKL have no relevant disclosures.
References
- 1.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farago AF, Azzoli CG. Beyond ALK and ROS1: RET, NTRK, EGFR and BRAF gene rearrangements in non-small cell lung cancer. Transl Lung Cancer Res. 2017;6:550–559. doi: 10.21037/tlcr.2017.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farago AF, Le LP, Zheng Z, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol. 2015;10:1670–4. doi: 10.1097/01.JTO.0000473485.38553.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19:1469–72. doi: 10.1038/nm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–9. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 7.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–70. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drilon A, Siena S, Ou SI, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7:400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–84. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 11.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss GJ, Hoff BR, Whitehead RP, et al. Evaluation and comparison of two commercially available targeted next-generation sequencing platforms to assist oncology decision making. Onco Targets Ther. 2015;8:959–67. doi: 10.2147/OTT.S81995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra R, Hanker AB, Garrett JT. Genomic alterations of ERBB receptors in cancer: clinical implications. Oncotarget. 2017;8:114371–114392. doi: 10.18632/oncotarget.22825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res. 2016;22:3618–29. doi: 10.1158/1078-0432.CCR-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardini E, Bosotti R, Borgia AL, et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol Oncol. 2014;8:1495–507. doi: 10.1016/j.molonc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19:4273–81. doi: 10.1158/1078-0432.CCR-13-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautschi O, Milia J, Filleron T, et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. Journal of Clinical Oncology. 2017 doi: 10.1200/JCO.2016.70.9352. JCO.2016.70.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–71. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman DM, Laetsch TW, Kummar S, et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. Journal of Clinical Oncology. 2017;35:LBA2501–LBA2501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.