Abstract
Purpose
Tumor genomic profiling for personalized oncology therapy is being widely applied in clinical practice even as it is being evaluated more formally in clinical trials. Given the complexities of genomic data and its application to clinical use, molecular tumor boards with diverse expertise can provide guidance to oncologists and patients seeking to implement personalized genetically targeted therapy in practice.
Methods
A multidisciplinary molecular tumor board reviewed tumor molecular profiling reports from consecutive referrals at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins over a 3-year period. The tumor board weighed evidence for actionability of genomic alterations identified by molecular profiling and provided recommendations including US Food and Drug Administration–approved drug therapy, clinical trials of matched targeted therapy, off-label use of such therapy, and additional tumor or germline genetic testing.
Results
One hundred fifty-five patients were reviewed. Actionable genomic alterations were identified in 132 patients (85%). Off-label therapies were recommended in 37 patients (24%). Eleven patients were treated off-label, and 13 patients were enrolled onto clinical trials of matched targeted therapies. Median progression-free survival of patients treated with matched therapies was 5 months (95% CI, 2.9 months to not reached), and the progression-free survival probability at 6 months was 43%(95% CI, 26% to 71%). Lack of locally available clinical trials was the major limitation on clinical actionability of tumor profiling reports.
Conclusion
The molecular tumor board recommended off-label targeted therapies for a quarter of all patients reviewed. Outcomes were heterogeneous, although 43% of patients receiving genomically matched therapy derived clinical benefit lasting at least 6 months. Until more data become available from precision oncology trials, molecular tumor boards can help guide appropriate use of tumor molecular testing to direct therapy.
INTRODUCTION
The advent of low-cost, next-generation DNA sequencing (NGS) technologies has led to an explosion in individual tumor molecular profiling with the goal of identifying personalized therapeutic matches for patients with cancer. Case reports and clinical trials attest to the clinical utility of targeting driver gene mutations in cancer types other than those in which the drug is approved for clinical use, such as BRAF V600 mutations in cancers other than melanoma.1 Ongoing efforts, such as the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH; ClinicalTrials.gov identifier: NCT02465060) and the ASCO Targeted Agent and Profiling Utilization Registry (TAPUR; ClinicalTrials.gov identifier: NCT02693535) trials, aim to identify genomic predictors of response for targeted therapies against diverse genetic variants that may be difficult to study clinically because of their rarity outside of specific disease types.2 However, the use of tumor sequencing in clinical practice has outpaced the implementation and completion of such trials. Surveys have indicated that many oncologists are unsure how to interpret tumor sequencing data and whether their patients will have access to targeted therapies on the basis of the reports (ie, how actionable the findings are in reality).3 In response to these concerns, we established a multidisciplinary Genetic Alterations in Tumors With Actionable Yields (GAITWAY) molecular tumor board at our institution (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD) to assist providers with interpretation and use of tumor molecular profiling data. Here, we present our approach to using tumor molecular profiling and our 3-year experience, with associated patient outcomes.
METHODS
The GAITWAY tumor board began meeting weekly in September 2013. Attendees included adult and pediatric medical oncologists representing diverse subspecialties, molecular pathologists with NGS expertise, genetic counselors, phase I clinical trial investigators, research coordinators, a patient advocate, and medical oncology fellows. Tumor testing was ordered by the referring oncologist without the board’s input. The referring oncologist provided the board with the molecular profiling report(s) and the patient’s oncologic and family history. A written summary of the board’s discussion and recommendations was provided to the referring oncologist.
The board reviewed Clinical Laboratory Improvement Amendments–approved NGS cancer gene panel tests from a variety of providers, including Foundation Medicine (Cambridge, MA; FoundationOne, n = 120), Personal Genome Diagnostics (Baltimore, MD; Cancer Select-203 or Cancer Select-88, n = 25), Memorial Sloan Kettering Cancer Center MSK-IMPACT (New York, NY; n = 5), Caris Life Sciences (Irving, TX; MI Profile, n = 3), and several others. Personal Genome Diagnostics and MSK-IMPACT included normal tissue control sequencing and filtered out most germline variants. A small number of reports included multiplatform testing, including NGS, fluorescence in situ hybridization, and protein immunohistochemistry. Only one test used circulating tumor DNA from plasma as the source material (Personal Genome Diagnostics, Lung Select).
Medical records for consecutive patients referred to the board from September 2013 through September 2016 were accessed under a protocol approved by the Johns Hopkins institutional review board. Patient characteristics were analyzed using descriptive statistics. Progression-free survival (PFS) was measured from the first date of treatment with a genomically matched therapy or next nonmatched therapy after tumor board evaluation until the date of disease progression or death, whichever came first. Progression was determined by imaging studies or clinician assessment. Responses were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Probability of PFS was estimated using the Kaplan-Meier method. Alive patients without progression were censored at the date of last radiographic assessment. Statistical analyses were performed using R software v.3.3.1.
Criteria for Actionability of Genomic Alterations
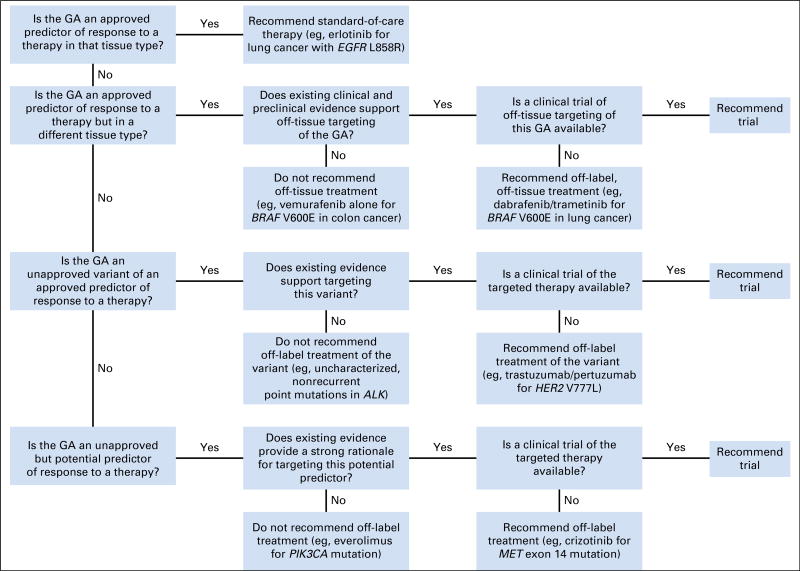
The board considered a genetic alteration actionable if one of the following conditions was met: it offered a target for a drug approved by the US Food and Drug Administration (FDA) for the patient’s tumor type; it offered a target for a drug approved by the FDA for another tumor type; it offered a target for a drug on a clinical trial; or it was a potential germline mutation for a hereditary cancer predisposition syndrome. The board’s approach to recommending genomically guided therapy is shown in Figure 1. The board had a relatively high threshold for considering a genetic variant as the basis for off-label use of a targeted therapy. Genetic alterations with drugs that specifically targeted the affected protein were of highest priority. In contrast, although many genes could potentially be linked to activation of the mammalian target of rapamycin (mTOR) path-way (eg, NF1, PIK3CA, FBXW7), the board generally did not recommend off-label use of mTOR inhibitors, given that the genetic link to mTOR was indirect or variants in these genes did not predict mTOR inhibitor benefit in clinical correlative studies.4,5 Similarly, CCND1 amplification and CDKN2A/B loss or mutation were not considered a high-level rationale for off-label use of the CDK4/6 inhibitor palbociclib because these alterations had not been shown to predict therapeutic benefit in breast cancer.6 Although multikinase inhibitors like pazopanib are often suggested as a match for FGFR1-3 amplifications,7 the board thought there was insufficient evidence to recommend off-label use and instead preferred clinical trials of fibroblast growth factor receptor (FGFR) inhibitors. The board was more liberal in recommending clinical trials for the alterations discussed earlier, even when the genetic alteration was not directly targeted by the investigational agent (eg, considering KRAS mutations as rationale for an MEK inhibitor trial). The rationale for some recommendations changed over time as new data and literature emerged relevant for a given target. The board’s approach to evaluating specific variants in genes is further described in the Appendix.
Fig 1.
Genetic Alterations in Tumors With Actionable Yields (GAITWAY) tumor board approach to therapeutic recommendations on the basis of tumor genomic analyses. GA, genomic alteration.
RESULTS
From September 2013 through September 2016, 157 cases representing 155 patients were reviewed by the GAITWAY tumor board (two patients had subsequent tumor sequencing and were reviewed twice). Patient demographic characteristics and distribution of tumor types are listed in Table 1. The most frequent tumor types were breast cancer (n = 56), lung cancer (n = 21), and squamous cell carcinoma of the head and neck (n = 11). The breast cancer cases included 20 patients with triple-negative disease previously reviewed as part of Individualized Molecular Analyses Guide Efforts (IMAGE), an institutional precision oncology feasibility trial.8 The mean number of lines of prior systemic therapy for metastatic disease was two lines (range, zero to 11 lines). Follow-up information was available for 129 patients (83%). The main reason for lack of follow-up was that the patient was seen only once for a second opinion.
Table 1.
Patient Demographic Characteristics and Distribution of Tumor Types
| Characteristic | Patients, No. (%) |
|---|---|
| Total | 155 |
| Sex | |
| Female | 101 (65) |
| Male | 54 (35) |
| Median age, years (range) | 56 (17–89) |
| Race | |
| White | 118 (76) |
| African American/African | 24 (15) |
| Asian | 6 (4) |
| Other | 5 (3) |
| Unknown | 3 (2) |
| No. of prior lines of therapy for metastatic disease, mean (range) | 2.0 (0–11) |
| Tumor type | |
| Breast triple negative | 32 |
| Breast ER positive/HER2 negative | 21 |
| Breast HER2 positive (ER positive or negative) | 3 |
| Lung adenocarcinoma | 14 |
| Lung squamous carcinoma | 6 |
| Lung adenosquamous | 1 |
| Head and neck squamous carcinoma | 11 |
| Neuroendocrine carcinoma/small-cell carcinoma/atypical carcinoid | 8 |
| Salivary gland/duct | 7 |
| Glioblastoma/anaplastic astrocytoma | 6 |
| Unknown primary site | 5 |
| Adenoid cystic carcinoma | 5 |
| Hepatobiliary/ampullary/duodenal | 5 |
| Pancreas adenocarcinoma | 4 |
| Cholangiocarcinoma | 4 |
| Sarcoma | 4 |
| Endometrial | 3 |
| Prostate | 2 |
| Stomach | 2 |
| Colon | 2 |
| Ovary | 1 |
| Other* | 11 |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Sinonasal undifferentiated carcinoma, anaplastic ependymoma, appendiceal mucinous carcinoma, testicular choriocarcinoma, esthesioneuroblastoma, inflammatory myofibroblastic tumor, ameloblastoma, medullary kidney cancer, lacrimal gland carcinoma, skin adnexal carcinoma, and acinar pancreas cancer.
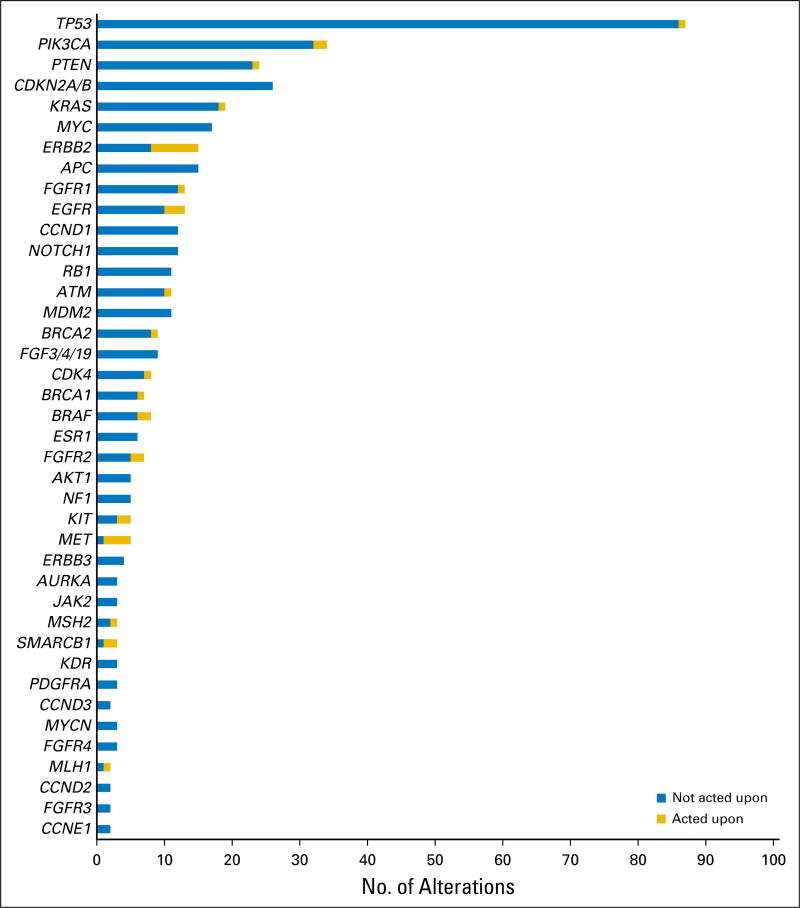
The average number of genetic alterations per tumor was 4.8 (range, zero to 16 alterations), excluding variants of uncertain significance or equivocal amplifications. The average number of genetic alterations considered actionable by the board was 1.9 (range, zero to six alterations). Genes recurrently altered across samples were similar to those reported from other series,9–11 although the relative frequencies were influenced by the case mix seen by our tumor board (Fig 2). In terms of targetable pathways, the phosphatidylinositol-3 kinase (PI3K) pathway was most frequently altered (63 alterations), followed by the G1 cell cycle checkpoint (61 alterations) and FGFR pathways (30 alterations).
Fig 2.
Frequency of genomic alterations. Actionable genomic alterations and selected nonactionable alterations are shown. For actionable genes, the number of patients in whom an action was taken is shown. Actions taken included therapy assignment, germline testing, or microsatellite instability evaluation.
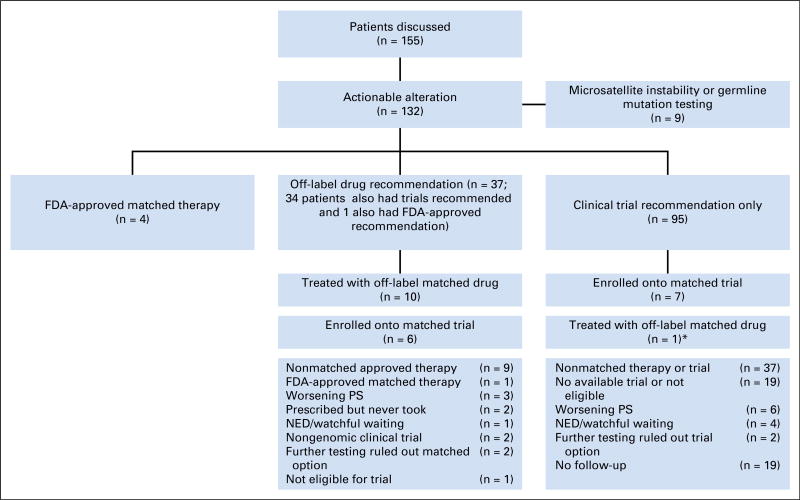
The board identified a potentially actionable genomic alteration in 132 patients (85%; Fig 3). Four patients (3%) received therapies approved by the FDA for their tumor type. Thirty-seven patients (24%) had a recommendation for off-label use of an FDA-approved therapy (Table 2 and Appendix Table A1). Twenty-four patients (15%) received a nonstandard, genetically matched therapy off-label (n = 11) or on a clinical trial (n = 13). Reasons patients did not receive recommended off-label therapy are detailed in Figure 3. In no case was the treating oncologist unable to prescribe or obtain a recommended off-label drug as a result of denial by insurance.
Fig 3.
CONSORT diagram of patients discussed at the molecular tumor board. Note, all numbers do not add up because some patients are counted in more than one category (eg, had an actionable alteration for a clinical trial and also were recommended off-label use or had an actionable alteration for therapy and also for germline analysis). (*) One patient with KRAS mutation for whom the board recommended a clinical trial only but who was prescribed off-label trametinib by his primary oncologist. FDA, US Food and Drug Administration; NED, no evidence of disease; PS, performance status.
Table 2.
Patients Who Received Matched Targeted Therapies or for Whom the Board Recommended Off-Label Use of Targeted Therapies
| Cancer Type | Actionable Alteration | Board Recommendation | Treatment and Outcome |
|---|---|---|---|
| Inflammatory myofibroblastic tumor | ALK G1202R, RANBP2-ALK fusion | Cabozantinib off label or clinical trial | Cabozantinib; SD for 5 months, hospice |
| Breast, triple negative* | BAP1 E498fs*38, loss; EGFR R108K | PARP inhibitor trial or lapatinib/capecitabine off label | Olaparib/carboplatin trial: SD for 4 months |
| Ameloblastoma | BRAF V600E | Dabrafenib/trametinib off label | Dabrafenib/trametinib; PR for 8 months, then PD after medication supply ran out; now back on therapy |
| Glioblastoma | BRAF G469R | Trametinib off label | Trametinib prescribed but never started as a result of intervening medical complications and worsening PS |
| Stomach | BRAF D594N | Trametinib or sorafenib off label | PD, PS decline, hospice |
| Lung adenocarcinoma | BRAF G469S | Trametinib or sorafenib off label | Nivolumab with SD, then died |
| Lung adenocarcinoma | BRAF V600E | Dabrafenib/trametinib off label | Immunotherapy, then chemotherapy with PR |
| Pancreas acinar cell carcinoma† | SND1-BRAF fusion | Trametinib or selumetinib off label | NED, not requiring therapy |
| Supraglottic larynx squamous carcinoma | BRCA1 Q905* | PARP inhibitor trial or olaparib off label (qualified recommendation given uncertainty of BRCA1 germline status) | Chemotherapy, PD, hospice within 1 month |
| Poorly differentiated INI-1–deficient carcinoma | BRCA1 C61G, SMARCB1 loss | PARP inhibitor trial or olaparib off label (qualified recommendation given uncertainty of BRCA1 germline status) or EZH2 inhibitor trial or single-patient IND for alisertib | Tazemetostat (EZH2 inhibitor) trial targeting SMARCB1 loss; response assessment pending |
| Breast, metaplastic, ER positive, PgR negative, HER2 negative | BRCA1 Q94* | PARP inhibitor trial or olaparib off label | Olaparib; PR at 2 months, then PD after 5 months |
| Glioblastoma | BRCA2 F1546fs*22 | PARP inhibitor trial or olaparib off label or platinum | Pursued alternative clinical trial, radiation, bevacizumab |
| Pancreas adenocarcinoma | BRCA2 S599fs*1, splice site 794-37_794-del37 | PARP inhibitor trial or olaparib off label or platinum chemotherapy | Not eligible for PARP inhibitor trial; no further follow-up available |
| Lung squamous carcinoma | CDK4 amplification, CDKN2A/B loss | CDK4/6 inhibitor trial only | Palbociclib trial; PD after 12 weeks |
| Lung adenocarcinoma | EGFR G719S, S768I | Afatinib (FDA indication) | Afatinib for 8 months; mixed response (PR of mass originally sequenced, PD contralateral mass) |
| Lung adenocarcinoma | EGFR H773_V774insH | No recommendation at time of review; was receiving definitive chemoradiation | AUY922 (HSP90 inhibitor) trial upon recurrence; SD for 9 months, ongoing |
| Lung adenocarcinoma | EGFR D770_N771insY | AUY922 (HSP90 inhibitor) trial | AUY922 trial; PD, off study after 1 month |
| Malignant peripheral nerve sheath tumor | EGFR duplication exons 18–26 | Afatinib off label | Had already started temozolomide/pazopanib; died within 2 months |
| Biliary tract adenocarcinoma | EGFR amplification 19-fold (KRAS wild type) | Cetuximab or panitumumab off label | Had neoadjuvant chemotherapy, resection, and more chemotherapy |
| Breast, triple negative* | ERBB2 D769H, V777L | Trastuzumab/lapatinib; trastuzumab/chemotherapy; lapatinib/capecitabine | Trastuzumab/capecitabine (after 6 weeks of trastuzumab alone); SD for 9 months |
| Breast, HER2 positive, ER positive | ERBB2 amplification (equivocal) | Trastuzumab with endocrine therapy (FDA indication) | Trastuzumab with endocrine therapy; was and remains NED after radiation for brain metastatic recurrence |
| Breast, HER2 positive | ERBB2 amplification; MTOR E2104K | Trastuzumab/pertuzumab/docetaxel (FDA indication) (qualified recommendation for everolimus off label on the basis of MTOR variant) | Trastuzumab/pertuzumab for 3 months, PD, added docetaxel: near CR of nodes (now not measurable) |
| Lung adenocarcinoma | ERBB2 L755S | Various HER2-targeted therapies: trastuzumab ± chemotherapy, afatinib, T-DM1 | Trastuzumab/pertuzumab on trial; PR at 12 months, continues on therapy |
| Skin adnexal carcinoma | ERBB2 FISH ratio 2.1 | Various HER2-targeted therapies | Trastuzumab/pertuzumab trial; CR after 2 cycles |
| Lung adenocarcinoma | ERBB2 exon 20 insertion E770_A771insAYVM (equivalent to A775_G776insYVMA) | Afatinib off label (but approved for lung cancer) | Was benefitting from chemotherapy, then died |
| Lung adenocarcinoma (known EGFR mutated) | ERBB2 amplification, EGFR E746_A750del | Trastuzumab/pertuzumab/taxane or trastuzumab/pertuzumab or trastuzumab/lapatinib or T-DM1 or trastuzumab + EGFR kinase inhibitor off label | PS declined; died within several weeks of tumor board |
| Salivary duct | ERBB2 S310F | Trastuzumab + pertuzumab or afatinib or trastuzumab + chemotherapy; off label or on trial | Trastuzumab/pertuzumab on trial; response assessment pending |
| Lacrimal gland | ERBB2 amplification | Trastuzumab ± pertuzumab ± taxane off label | Awaiting assessment of current chemotherapy |
| Squamous cell carcinoma hypopharynx | ERBB2 amplification | Trastuzumab ± pertuzumab ± taxane off label or on trial | HER2 1+ by IHC; received chemotherapy |
| High-grade neuroendocrine carcinoma | ERBB3 G284R | Afatinib or trastuzumab + lapatinib; off label | Immunotherapy trial for 2 cycles; died |
| Rhabdomyosarcoma | FGFR1 N577K | Pazopanib (FDA indication) | Pazopanib; on therapy 4 months, no restaging; died of PD |
| Breast, triple negative | FGFR2 amplification | FGFR inhibitor trial | Lucitanib (FGFR inhibitor) trial; PD after 1 cycle |
| Cholangiocarcinoma | FGFR2-WAC fusion | FGFR inhibitor trial | BGJ398 (FGFR inhibitor) trial; SD for 6 months |
| Duodenal adenocarcinoma‡ | JAK2 V617F | Ruxolitinib (qualified recommendation pending verification that mutation was present in tumor) | Mutation determined to be from contaminating blood cells (patient had polycythemia vera) |
| Breast, ER positive | KIT V560G | Imatinib off label | Imatinib; worsening respiratory failure, discontinued therapy after 7 days, comfort care |
| Appendiceal mucinous carcinoma | KRAS G12V, GNAS amplification | MEK inhibitor on trial only | Trametinib off label for 4 months with PD |
| Breast, triple negative* | MAP2K1 amplification | MEK inhibitor trial or off label | Trametinib off label; early clinical response (skin nodules), then PD after several weeks |
| Lung adenocarcinoma | MET exon 14 splice; MET amplification (equivocal) | Crizotinib off label | Crizotinib off label; SD for 21 months, ongoing |
| Lung adenocarcinoma | MET amplification | Crizotinib off label | Prescribed crizotinib, but never started as a result of medical and social circumstances |
| Anaplastic astrocytoma | MET amplification 8.9-fold, MET R731Q, KIT amplification 5-fold | Crizotinib or cabozantinib off label | Cabozantinib off label; clinical and radiographic response after 3 weeks, treatment stopped during episode of zoster, followed by rapid PD (on treatment 1 month) |
| Lung adenocarcinoma (known EGFR exon 21 mutation) | MET exon 14 splice (EGFR mutation not found in biopsy specimen) | Add crizotinib to erlotinib | Erlotinib/crizotinib; SD for 6 months, ongoing |
| Breast, ER positive | PIK3CA H1047R, R88Q | Continue on BKM120 (PI3K inhibitor)/fulvestrant trial | BKM120/fulvestrant trial; SD for 6 months |
| Breast, ER positive, PgR positive | PIK3CA E545K | PI3K inhibitor trial | BYL719 (PI3K inhibitor)/protein-bound paclitaxel trial; on cycle 3 with tumor marker decline and SD |
| Colon | PTCH1 P681L | Vismodegib on trial or off label | PS worsened, hospice |
| Lung adenosquamous carcinoma | RET KIF5B-RET fusion | Cabozantinib off label or on trial | Cabozantinib clinical trial; PR after 2 months, then off study for debility; continued cabozantinib off label for 1 month, then leptomeningeal PD |
| Lung adenocarcinoma | RICTOR amplification, STK11 G276fs*11 | Everolimus on trial or off label | Received chemotherapy then radiation |
| Medullary kidney cancer | SMARCB1 Y47* | Alisertib single-patient IND | Received chemotherapy then nivolumab and experienced progression; died before alisertib could be given (IND obtained) |
| Cholangiocarcinoma | STK11 spl598-2A>C | Everolimus or temsirolimus; on trial or off label | Temsirolimus added to capecitabine; PD after 1 month |
| Mucoepidermoid carcinoma | TSC2 V1711M | Everolimus (qualified recommendation; mutation not well characterized) on trial or off label | Received chemotherapy then pembrolizumab |
NOTE. A more detailed version of this table including all genomic alterations in the tumors is available as Appendix Table A1.
Abbreviations: CR, complete response; ER, estrogen receptor; FDA, US Food and Drug Administration; FGFR, fibroblast growth factor receptor; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; IND, investigational new drug application; NED, no evidence of disease; PARP, poly (ADP-ribose) polymerase; PD, progressive disease; PgR, progesterone receptor; PR, partial response; PS, performance status; SD, stable disease; T-DM1, trastuzumab emtansine.
Previously reported.8
This patient’s BRAF fusion has been previously reported.12
Details of this patient’s case are discussed in another report.13
A clinical trial was recommended as an option for 129 patients (83%; including 34 patients who also had a recommendation for off-label therapy), but only 13 patients were enrolled onto are commended trial (Fig 3). In a number of patients, the trial recommendation was qualified, because there was no clinical evidence that the alterations were predictive of benefit. The most frequent alterations that led to a qualified recommendation were KRAS or MDM2 mutations and alterations activating the G1 cell cycle checkpoint (CDKN2A/B, CCND1, and CDK4). Stronger recommendations were made for trials targeting the FGFR or PI3K pathways, but only four patients were treated on such trials, largely because of lack of availability. Twenty-five patients who investigated clinical trial options were not eligible, could not access a trial for reasons of geographical proximity, or had worsening performance status (Fig 3).
Outcomes of Patients Receiving Matched Targeted Therapy
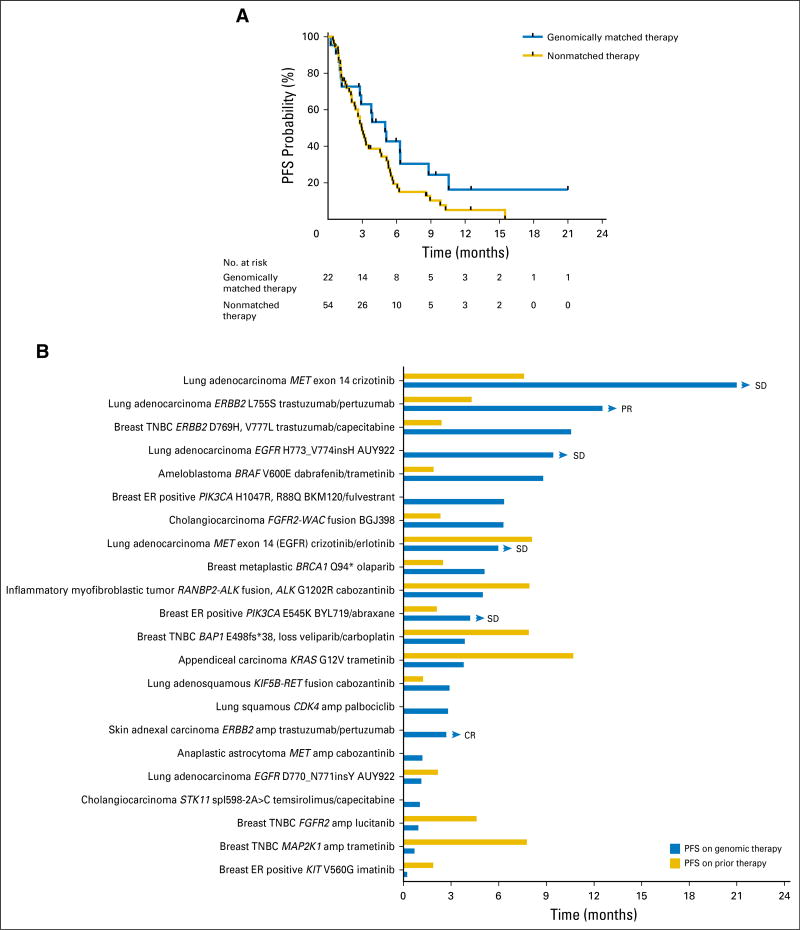
Follow-up information was available for all 24 patients treated with a matched therapy on a clinical trial or off-label. Two patients had recently initiated therapy, and response assessment was not yet available. With a median follow-up of 7.0 months, the median PFS of patients treated with genomically matched therapy was 5 months (95% CI, 2.9 months to not reached), and the 6-month PFS probability was 43% (95% CI, 26% to 71%; Fig 4A). The median PFS for 54 patients with available follow-up information whose next therapy was nonmatched was 2.97 months (95% CI, 2.4 to 5.13 months), and 6-month PFS was 20% (95% CI, 11% to 35%). Demographic characteristics of the two cohorts are listed in Appendix Table A2. Fifty percent of patients receiving genomically matched therapy had a PFS more than double their PFS on prior therapy (Fig 4B). Two patients with ERBB2 missense mutations, one with triple-negative breast cancer (reported previously8) and one with lung adenocarcinoma, have had prolonged disease control on human epidermal growth factor receptor 2 (HER2) antibody–based therapy. The patient with lung cancer had progression after four cycles of platinum doublet chemotherapy and has achieved a partial response after 12 months on a clinical trial of trastuzumab and pertuzumab. This is notable because there have been few reports of ERBB2-mutant tumors benefiting from HER2 antibody–based therapy in the absence of chemotherapy or small-molecule HER2 kinase inhibitors.14,15 Two patients with lung adenocarcinoma with MET exon 14 skipping splice site mutations have had prolonged stable disease (21 months and 6 months, ongoing) on crizotinib, in line with recent reports.16,17 A patient with BRAF V600E–mutated ameloblastoma experienced a partial response lasting 8 months to dabrafenib and trametinib, again consistent with a previous case report.18
Fig 4.
(A) Progression-free survival (PFS) of patients treated with genomically matched therapy (n = 22) or nonmatched therapy (n = 54). All patients with follow-up who were treated with genomically matched therapy are included in the analysis, including one patient who was treated with off-label trametinib for a KRAS mutation when the board had recommended a clinical trial. (B) PFS and PFS on immediate prior therapy for individual patients treated with genomically matched therapies. Eight patients either had no prior systemic therapy for metastatic disease or insufficient documentation of prior therapy. Arrowheads indicate patients continuing on treatment. amp, amplification; CR, complete response; ER, estrogen receptor; PR, partial response; SD, stable disease; TNBC, triple-negative breast cancer.
Evidence for Tumor Evolution and Heterogeneity
Five patients had tumor sequencing performed more than once (in three patients, the multiple reports were reviewed at a single tumor board session). Sequencing of metastatic sites of disease progression on targeted therapy revealed new genetic alterations consistent with pre-existing tumor heterogeneity or acquired drug resistance. A patient with an inflammatory myofibroblastic tumor with an RANBP2-ALK rearrangement at diagnosis was initially treated with crizotinib with an excellent response, followed by ceritinib. Repeat sequencing at the time of progression on ceritinib showed the same RANBP2-ALK fusion as well as an acquired ALK G1202R mutation, which confers resistance to both crizotinib and ceritinib.19 The board reviewed preclinical data on the G1202R mutation and identified activity of cabozantinib as well as newer investigational ALK inhibitors against G1202R or the analogous mutation in ROS1.20,21 The patient was treated with off-label cabozantinib, with stable disease lasting 5 months.
A patient with EGFR exon 21–mutated lung adenocarcinoma with an excellent response to erlotinib developed a new progressing lesion, which was biopsied and sequenced. Sequencing showed a MET exon 14 skipping splice site mutation that had not been present when the tumor was initially sequenced, but no EGFR mutation. The board noted that distinct tumor clones with EGFR and MET mutations could account for the mixed response to erlotinib, and the patient was treated with erlotinib plus crizotinib on the basis of tolerability of the combination in a phase I trial.22 His disease has been stable for 6 months, and treatment is ongoing.
A second patient who had lung cancer with an EGFR exon 19 deletion mutation had tumor sequencing after experiencing progression on many lines of targeted therapy including erlotinib, afatinib and cetuximab, and osimertinib. Sequencing showed the EGFR exon 19 deletion as well as amplification of ERBB2, which has been described as an acquired resistance mechanism in EGFR-mutated lung cancer treated with EGFR inhibitors.23 The board recommended HER2-targeted therapy; unfortunately, the patient’s clinical condition deteriorated, and she died without receiving another line of therapy.
Germline Evaluation Prompted by Tumor Sequencing
The board recommended further clinical or genetic evaluation for a possible germline cancer-predisposing variant for 46 patients. Many of the patients with breast cancer had already had germline testing. The board examined mutant allele frequencies, if available, to determine the likelihood of the alterations being germline. However, because tumor sequencing platforms have not been validated specifically for germline testing, the board recommended genetic counseling to consider dedicated germline analysis. Three patients had additional tumor testing for microsatellite instability, and all were microsatellite stable. Six patients had germline testing for BRCA1/2, PTEN, TP53, and other genes; all were germline nonmutated.
DISCUSSION
This report adds to the growing literature on precision oncology guided by a molecular tumor board. Our series is one of the largest to date to include patient outcomes. Although this was not a prospective clinical trial and treating oncologists were not bound by the board’s recommendations, the molecular tumor board attempted to apply a consistent standard of principles when assessing each patient. Our criteria for evaluating individual mutations and levels of supporting evidence for actionability are similar to those applied in the NCI-MATCH trial.2,24 The board preferred direct rather than indirect drug-gene matches and rarely advocated multikinase inhibitors off-label when the target had not been extensively validated as predictive. We found a high frequency of actionable genomic alterations, similar to previous reports9,11,25,26; however, lack of available clinical trials and a higher threshold for recommending off-label use of targeted therapies reduced the rate of therapeutic use in our patients.
A limitation of our study is that the board reviewed only those patients referred to it. Thirty-eight percent of our patients had genomic variants that could have made them eligible for one of the NCI-MATCH treatment arms, which is higher than the estimated match rate of 23% with the current NCI-MATCH arms open.27 This likely reflects the case mix of patients seen at our cancer center and referred to our tumor board, which was perhaps enriched for variants the referring physician considered actionable.
Lack of locally available trials was a leading reason for lack of application of matched, targeted therapies, as noted by others.8–10 Immunotherapy also emerged as a compelling therapeutic alternative for many patients. Almost all of our patients were reviewed before the NCI-MATCH trial opened at our institution. NCI-MATCH and TAPUR offer access to genomically targeted therapies for a variety of targets that would be difficult for any single cancer center to have in its clinical trial portfolio. Such trials could partially solve the leading barrier to actionability noted here.
Despite these limitations, 15% of our patients were treated with matched targeted therapies on a clinical trial or with off-label drugs. Although the PFS of patients treated with genomically matched therapies compared favorably with that of patients receiving nonmatched therapies, our study was not designed to test the superiority of a genomically guided approach for all patients. The small sample size, retrospective nature of the analysis, and heterogeneous patient population subject to referral bias all limit the utility of such a comparison. The non–genomically treated patients had fewer actionable alterations than the genomically treated cohort and had a different distribution of tumor types. Nonetheless, half of the patients receiving genomically matched therapy had a PFS more than double their prior PFS, and several had prolonged duration of benefit. The patients who benefited most from matched therapy had alterations in genes such as ERBB2, BRAF, EGFR, and MET, for which there is currently patient-level response data from clinical trials, case series, and case reports. As with prospective trials of precision oncology,28 outcomes of series such as ours will be impacted by the criteria used to match targeted therapies to genetic variants found in patients’ tumors. We cannot rule out that our conservative approach toward actionability prevented us from observing greater clinical benefit, but we believe that more liberal off-label use of targeted agents is unlikely to produce better outcomes than we observed. Reasons for the limited benefit of single-agent targeted therapies have been reviewed elsewhere.29
Even when off-label drug use was recommended for a highly actionable mutation, some patients experienced rapid disease progression that precluded them from starting or continuing such therapies. The optimal timing of tumor sequencing has yet to be determined, but the experience of these patients suggest that the review of genomic actionability should take place earlier in the disease course. However, we documented several patients in whom repeat tumor sequencing demonstrated new genomic findings consistent with either pre-existing heterogeneity or clonal evolution. Improvements in the use of NGS panels on cell-free circulating tumor DNA from plasma may allow sampling of tumor heterogeneity and enable serial monitoring of acquired resistance and clonal dynamics in the face of targeted therapy.8,30,31
The board also reviewed patients for whom it disagreed with a prior interpretation and use of tumor profiling data. In one instance, a patient had discontinued an approved chemotherapy drug to which she had been responding (docetaxel) because a tumor profiling biomarker (TUBB3 immunohistochemistry) predicted lack of benefit. A patient with HER2-positive breast cancer was treated ineffectively for several months with enzalutamide on the basis of the tumor staining positive for androgen receptor. The board instead recommended treating the patient with pertuzumab, an FDA-approved therapy, to which she responded. The circumstances of these patients show the value a molecular tumor board can add and highlight that standard-of-care therapies should still be considered as viable and often preferred options, because approved therapies for a given cancer type have high levels of evidence to support their use.
Our standard for recommending off-label use may be more stringent than that used at some other centers or by individual practicing oncologists. Whereas 73% of our patients had a variant for which off-label drug use was suggested by the commercial sequencing provider and 55% had a variant that would have enabled use of an FDA-approved drug on the TAPUR trial, our board recommended off-label use in only 24% of patients. In the absence of more data or practice guidelines, patients and providers have some discretion about where to set the threshold. Large data-sharing efforts and publication of series such as this one will be essential to expand public knowledge about the clinical actionability of genetic variants and outcomes associated with precision oncology as currently practiced.32 We support ongoing trials like NCI-MATCH and TAPUR in the belief that, as much as possible, precision oncology should be carried out in the context of clinical trials so we can replace educated guesswork with evidence.
Acknowledgments
Support
Supported by National Cancer Institute Grant No. P30 CA006973 to the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center.
APPENDIX
Methods
For uncommon variants in well-established cancer driver genes (eg, non-V600 BRAF mutations), the board considered factors including recurrence of that specific mutation in databases such as Catalogue of Somatic Mutations in Cancer (Zhan F, et al: Blood 108:2020–2028, 2006), sequencing data accessed at cBioPortal (Cerami E, et al: Cancer Discov 2:401–404, 2012), and in published studies; location in a known protein domain; evidence that the variant could be a germline single nucleotide polymorphism; and preclinical functional characterization—to show whether the variant was biologically activating and whether it demonstrated sensitivity or resistance to known drugs. The board generally did not recommend targeting amplifications reported as equivocal or mutations that were clearly subclonal relative to other mutations in the tumor. In some cases, reported variants of uncertain significance were evaluated for potential treatment implications.
Table A1.
Patients Who Received Matched Targeted Therapies or for Whom the Board Recommended Off-Label Use of Targeted Therapies
| Cancer Type | Alterations | Board Recommendation | Treatment and Outcome |
|---|---|---|---|
| Inflammatory myofibroblastic tumor | ALK G1202R, RANBP2-ALK fusion PALB2 splice site 2748+1G>T SMAD4 loss | Cabozantinib off label or clinical trial | Cabozantinib; SD for 5 months, hospice |
| Breast, triple negative | BAP1 E498fs*38, loss EGFR R108K TP53 A276G PALB2 L1045F | PARP inhibitor trial or lapatinib/capecitabine off label | Olaparib/carboplatin trial: SD for 4 months |
| Ameloblastoma | BRAF V600E NF1 N2676D SMO L412F | Dabrafenib/trametinib off label | Dabrafenib/trametinib; PR for 8 months, then PD after medication supply ran out; now back on therapy |
| Glioblastoma | BRAF G469R NF1 S436* | Trametinib off label | Trametinib prescribed but never started as a result of intervening medical complications and worsening PS |
| Stomach | BRAF D594N SMO P352L | Trametinib or sorafenib off label | PD, PS decline, hospice |
| Lung adenocarcinoma | BRAF G469S TP53 Y220C LRB1B Q3419* SF3B1 V701F | Trametinib or sorafenib off label | Nivolumab with SD, then died |
| Lung adenocarcinoma | BRAF V600E PDGFRB V125Wfs*18 TP53 R175H | Dabrafenib/trametinib off label | Immunotherapy, then chemotherapy with PR |
| Pancreas acinar cell carcinoma | SND1-BRAF fusion MCL1 amplification SF3B1 K700E subclonal | Trametinib or selumetinib off label | NED, not requiring therapy |
| Supraglottic larynx squamous carcinoma | BRCA1 Q905* DNMT3A A910V CDKN2A p16 H83Y/p14ARF A97V TP53 P142fs*7 APC W685* CREBBP R1446L EPHB1 R865W RB1 V520fs*3 | PARP inhibitor trial or olaparib off label (qualified recommendation given uncertainty of BRCA1 germline status) | Chemotherapy, PD, hospice within 1 month |
| Poorly differentiated INI-1–deficient carcinoma | BRCA1 C61G SMARCB1 loss CHEK2 truncation intron 2 | PARP inhibitor trial or olaparib off label (qualified recommendation given uncertainty of BRCA1 germline status) or EZH2 inhibitor trial or single-patient IND for alisertib | Tazemetostat (EZH2 inhibitor) trial targeting SMARCB1 loss; response assessment pending |
| Breast, metaplastic, ER positive, PgR negative, HER2 negative | BRCA1 Q94* TP53 C238Y | PARP inhibitor trial or olaparib off label | Olaparib; PR at 2 months, then PD after 5 months |
| Glioblastoma | BRCA2 F1546fs*22 NF1 loss PIK3CA R88Q CDKN2A/B loss EPHB1 V322I SETD2 rearrangement exon 3 | PARP inhibitor trial or olaparib off label or platinum | Pursued alternative clinical trial, radiation, bevacizumab |
| Pancreas adenocarcinoma | BRCA2 S599fs*1, splice site 794-37_794-del37 KRAS G12V CDKN2A deletion p16INK4a exons 1–2 and p14ARF exon 2 APC G2070fs*1, P2018fs*26 SMAD4 P198fs*4 | PARP inhibitor trial or olaparib off label or platinum chemotherapy | Not eligible for PARP inhibitor trial; no further follow-up available |
| Lung squamous carcinoma | CDK4 amplification CDKN2A/B loss KDR amplification KIT amplification PDGFRA amplification GLI1 amplification MDM2 amplification PRKCI amplification (equivocal) TP53 K164E APC S1362F FRS2 amplification | CDK4/6 inhibitor trial only | Palbociclib trial; PD after 12 weeks |
| Lung adenocarcinoma | EGFR G719S, S768I | Afatinib (FDA indication) | Afatinib for 8 months; mixed response (PR of mass originally sequenced, PD contralateral mass) |
| Lung adenocarcinoma | EGFR H773_V774insH APC Q1067* | No recommendation at time of review; was receiving definitive chemoradiation | AUY922 (HSP90 inhibitor) trialupon recurrence; SD for 9 months, ongoing |
| Lung adenocarcinoma | EGFR D770_N771insY EGFR amplification equivocal CDK4 amplification MYC amplification GLI1 amplification NFKBIA amplification NKX2-1 amplification TP53 V197E | AUY922 (HSP90 inhibitor) trial | AUY922 trial; PD, off study after 1 month |
| Malignant peripheral nerve sheath tumor | EGFR duplication exons 18–26 ATRX K2174fs*7 CDKN2A/B loss CIC truncation exon 5 RPTOR amplification | Afatinib off label | Had already started temozolomide/pazopanib; died within 2 months |
| Biliary tract adenocarcinoma | EGFR amplification 19-fold TP53 R213Q BMPR1A R417C GNA11 R38_E39 dup HIST1H2BD ex1 K13fs XPO1 T118S SLC12A7-TERT rearrangement | Cetuximab or panitumumab off label | Had neoadjuvant chemotherapy, resection, and more chemotherapy |
| Breast, triple negative* | ERBB2 D769H, V777L PIK3CA M1043V GNAS amplification (equivocal) AURKA amplification (equivocal) TP53 R273H ARFRP1 amplification (equivocal) EMSY amplification | Trastuzumab/lapatinib; trastuzumab/chemotherapy; lapatinib/capecitabine | Trastuzumab/capecitabine (after 6 weeks of trastuzumab alone); SD for 9 months |
| Breast, HER2 positive, ER positive | ERBB2 amplification (equivocal) IGF1R R595H TP53 R248Q MAP2K4 G111fs*22 | Trastuzumab with endocrine therapy (FDA indication) | Trastuzumab with endocrine therapy; was and remains NED after radiation for brain metastatic recurrence |
| Breast, HER2 positive | ERBB2 amplification MTOR E2104K EGFR R108K (subclonal) CRKL amplification FGFR1 amplification GNAS amplification SRC amplification TOP1 amplification AURKA amplification TP53 R110L mutation ZNF217 amplification ZNF703 amplification | Trastuzumab/pertuzumab/docetaxel (FDA indication) (qualified recommendation for everolimus off label on the basis of MTOR variant) | Trastuzumab/pertuzumab for 3 months, PD, added docetaxel: near CR of nodes (now not measurable) |
| Lung adenocarcinoma | ERBB2 L755S ATM N2267I, 4901+1G>T FGFR1 448+3G>A FGFR2 D334Y NOTCH1 G1015D | Various HER2-targeted therapies: trastuzumab ± chemotherapy, afatinib, T-DM1 | Trastuzumab/pertuzumab on trial; PR at 12 months, continues on therapy |
| Skin adnexal carcinoma | ERBB2 FISH ratio 2.1 EGFR amplification (equivocal) PIK3CA E545K AURKA amplification ARFRP1 amplification (equivocal) FAT1 R806H GNAS amplification (equivocal) NFKBIA amplification (equivocal) TP53 S241F ZNF217 A671T, amplification | Various HER2-targeted therapies | Trastuzumab/pertuzumab trial; CR after 2 cycles |
| Lung adenocarcinoma | ERBB2 exon 20 insertion E770_A771insAYVM (equivalent to A775_G776insYVMA) | Afatinib off label (but approved for lung cancer) | Was benefitting from chemotherapy, then died |
| Lung adenocarcinoma (known EGFR mutated) | ERBB2 amplification EGFR E746_A750del FANCD2 2716-4A>T splice site TP53 V173L | Trastuzumab/pertuzumab/taxane or trastuzumab/pertuzumab or trastuzumab/lapatinib or T-DM1 or trastuzumab + EGFR kinase inhibitor off label | PS declined; died within several weeks of tumor board |
| Salivary duct | ERBB2 S310F FANCA R1400C FANCB M1? FANCM Y1918C MAP2K4 D263Cfs*4 TSC2 N187H Microsatellite stable | Trastuzumab + pertuzumab or afatinib or trastuzumab + chemotherapy; off label or on trial | Trastuzumab/pertuzumab on trial; response assessment pending |
| Lacrimal gland | ERBB2 amplification IDH2 amplification 3.5-fold indeterminate APC K1593Sfs*57 (3%) K1878Rfs*4 (3%) AR Q58L (5%) CDKN2A W15X (2%) GNAQ T96S (3%) POLE 1924-4C>T (12%) SUFU P24Rfs*72 (3%) TET2 K655Nfs*45 (3%) TP53 L330Dfs*5 (32%) MSI high | Trastuzumab ± pertuzumab ± taxane off label | Awaiting assessment of current chemotherapy |
| Squamous cell carcinoma hypopharynx | ERBB2 amplification PTCH1 F109fs*27 CCND1 amplification CDKN2A/B loss IGF1R amplification MYCL1 amplification TP53 E180* FGF19/3/4 amplification NOTCH1 E1595* RAD51 truncation exon 10 | Trastuzumab ± pertuzumab ± taxane off label or on trial; vismodegib on trial or off label (qualified on the basis of uncertainty about status of other PTCH1 allele) | HER2 1+ by IHC; received chemotherapy |
| High-grade neuroendocrine carcinoma | ERBB3 G284R ATM R3008C CDKN2A/B loss APC H929fs*26, T1556fs*3 FAM123BQ335* MAP3K1 splice site 2369+1G>G | Afatinib or trastuzumab + lapatinib; off label | Immunotherapy trial for 2 cycles; died of PD |
| Rhabdomyosarcoma | FGFR1 N577K APC R1858Q NF1 H2123N PIK3CA G118D, T1025A PTPN11 A5775 | Pazopanib (FDA indication) | Pazopanib; on therapy 4 months, no restaging; died of PD |
| Breast, triple negative | FGFR2 amplification PTEN D24G TP53 R175H LRP1B duplication exons 68–72 | FGFR inhibitor trial | Lucitanib (FGFR inhibitor) trial; PD after 1 cycle |
| Cholangiocarcinoma | FGFR2-WAC fusion PTEN loss CDKN2A/B loss | FGFR inhibitor trial | BGJ398 (FGFR inhibitor) trial; SD for 6 months |
| Duodenal adenocarcinoma | JAK2 V617F CCND3 amplification CDK4 amplification ERBB3 amplification VEGFA amplification GLI1 amplification MDM2 amplification SMAD4 E538* | Ruxolitinib (qualified recommendation pending verification that mutation was present in tumor) | Mutation determined to be from contaminating blood cells (patient had polycythemia vera) |
| Breast, ER positive | KIT V560G AKT1 amplification EGFR amplification PTEN loss exon 1 RICTOR amplification TP53 splice site 920-2A>G ESR1 D538G | Imatinib off label | Imatinib; worsening respiratory failure, discontinued therapy after 7 days, comfort care |
| Appendiceal mucinous carcinoma | KRAS G12V GNAS amplification ATR L610* AURKA amplification CCND2 amplification TP53 R175H | MEK inhibitor on trial only | Trametinib off label for 4 months with PD |
| Breast, triple negative* | MAP2K1 amplification CCNE1 amplification MCL1 amplification MYC amplification TP53 N247K, R175H-subclonal, T253N CREBBP TRAP1-CREBBP fusion SETD2 T2354A | MEK inhibitor trial or off label | Trametinib off label; early clinical response (skin nodules), then PD after several weeks |
| Lung adenocarcinoma | MET exon 14 splice MET amplification (equivocal) CDKN2A/B loss MDM2 amplification | Crizotinib off label | Crizotinib off label; SD for 21 months, ongoing |
| Lung adenocarcinoma | MET amplification JAK2 K607N ATR K829* TP53 G266V ARID1A R391* TOP2A R736L | Crizotinib off label | Prescribed crizotinib, but never started as a result of medical and social circumstances |
| Anaplastic astrocytoma | MET amplification 8.9-fold MET R731Q KIT amplification 5-fold IDH1 R132H NF1 F2714Vfs*16 TP53 R273C | Crizotinib or cabozantinib off label | Cabozantinib off label; clinical and radiographic response after 3 weeks, treatment stopped during episode of zoster, followed by rapid PD (on treatment 1 month) |
| Lung adenocarcinoma (known EGFR exon 21 mutation) | MET exon 14 splice (EGFR mutation not found in biopsy specimen) AKT1 E40K RB1 T774Pfs*36 | Add crizotinib to erlotinib | Erlotinib/crizotinib; SD for 6 months, ongoing |
| Breast, ER positive | PIK3CA H1047R, R88Q FGFR1 amplification TP53 E285K CDH1 D746fs*24 RB1 splice ZNF703 amp | Continue on BKM120 (PI3K inhibitor)/fulvestrant trial | BKM120/fulvestrant trial; SD for 6 months |
| Breast, ER positive, PR positive | PIK3CA E545K CCND1 amplification FGF19 amplification FGF3 amplification FGF4 amplification PRSS8 amplification TET2 Q943*, subclonal* | PI3K inhibitor trial | BYL719 (PI3K inhibitor)/protein-bound paclitaxel trial; on cycle 3 with tumor marker decline and SD |
| Colon | PTCH1 P681L ATM R189K TP53 R248W APC E1295*, W1049* FGF10 N159K | Vismodegib on trial or off label | PS worsened; hospice |
| Lung adenosquamous carcinoma | RET KIF5B-RET fusion ERBB3 amplification CDKN2A/B loss MDM2 amplification CDK4 amplification | Cabozantinib off label or on trial | Cabozantinib clinical trial; PR after 2 months, then off study for debility; continued cabozantinib off label for 1 month, then leptomeningeal PD |
| Medullary kidney cancer | SMARCB1 Y47* | Alisertib single-patient IND | Received chemotherapy then nivolumab and experienced progression; died before alisertib could be given (IND obtained) |
| Lung adenocarcinoma | STK11 G276fs*11 RICTOR amplification FGFR4 amplification KRAS amplification CCNE1 amplification TP53 G154V, M237_C238del FGF10 amplification | Everolimus on trial or off label | Received chemotherapy then radiation |
| Cholangiocarcinoma | STK11 spl598-2A>C KRAS amp BRCA2 K3326* ATM S1905* CDKN2A/B loss | Everolimus or temsirolimus on trial or off label | Temsirolimus added to capecitabine; PD after 1 month |
| Mucoepidermoid carcinoma | TSC2 V1711M | Everolimus (qualified recommendation; mutation not well characterized) on trial or off label | Received chemotherapy then pembrolizumab |
NOTE. Alterations serving as the basis for board recommendation of off label use or as basis of matched therapy actually received are shown in bold. Other alterations in the tumor are also shown.
Abbreviations: CR, complete response; ER, estrogen receptor; FDA, US Food and Drug Administration; FGFR, fibroblast growth factor receptor; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; IND, investigational new drug application; MSI, microsatellite instability; NED, no evidence of disease; PARP, poly (ADP-ribose) polymerase; PD, progressive disease; PgR, progesterone receptor; PR, partial response; PS, performance status; SD, stable disease; T-DM1, trastuzumab emtansine.
Table A2.
Demographic Characteristics of the Genomically Matched and Non–Genomically Matched Cohorts of Patients Analyzed for Progression-Free Survival in Figure 4
| Characteristic | No. of Patients (%) | P | |
|---|---|---|---|
| Genomic (n = 22) | Nongenomic (n = 54) | ||
| Age, years, median (range) | 58.5 (17–73) | 56 (25–81) | 1.00 |
| No. of prior lines of therapy for metastatic disease, mean (range) | 2.2 (0–8) | 1.8 (0–10) | .43 |
| Sex | |||
| Male | 7 (32) | 34 (37) | .79 |
| Female | 15 (68) | 20 (63) | |
| No. of alterations, mean (range) | 5.5 (2–10) | 4.4 (1–10) | .08 |
| No. of actionable alterations, mean (range) | 2.5 (0–5) | 1.7 (0–6) | .04 |
| Tumor type | |||
| Breast triple negative | 4 (18) | 13 (24) | .76 |
| Breast ER positive/HER2 negative | 4 (18) | 8 (15) | .74 |
| Lung adenocarcinoma | 5 (23) | 4 (7) | .11 |
| Lung other | 2 (9) | 3 (6) | .62 |
| Cholangiocarcinoma | 2 (9) | 2 (4) | .57 |
| SCC head and neck | 0 (0) | 6 (11) | .17 |
| Salivary gland | 0 (0) | 4 (7) | .32 |
| Brain tumor | 1 (5) | 3 (6) | 1.00 |
| Endometrial | 0 (0) | 3 (6) | .55 |
NOTE. Continuous data were compared by t test, and categorical data were compared by Fisher’s exact test using GraphPad version 5.0 software.
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; SCC, squamous cell carcinoma.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Personalized Medicine in the Oncology Clinic: Implementation and Outcomes of the Johns Hopkins Molecular Tumor Board
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted.
I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or po.ascopubs.org/site/ifc.
W. Brian Dalton
No relationship to disclose
Patrick M. Forde
Research Funding: Bristol-Myers Squibb, AstraZeneca, Novartis, Kyowa
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, Novartis, Celgene
Hyunseok Kang
Consulting or Advisory Role: AstraZeneca
Research Funding: Boehringer Ingelheim, Merck Sharp & Dohme, Plexxikon, AstraZeneca, VentiRx Pharmaceuticals
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Eli Lilly
Roisin M. Connolly
Research Funding: Genentech (Inst), Merck (Inst), Merrimack (Inst), Puma Biotechnology (Inst), Novartis (Inst), Clovis (Inst)
Vered Stearns
Research Funding: Abbvie, Celgene, Merck, Medimmune, Novartis, Pfizer, Puma
Christopher D. Gocke
No relationship to disclose
James R. Eshleman
No relationship to disclose
Jennifer Axilbund
Employment: Invitae Corporation
Stock and Other Ownership Interests: Invitae Corporation
Dana Petry
No relationship to disclose
Cindy Geoghegan
Consultant or Advisory Role: Celgene, Pfizer
Antonio C. Wolff
Research Funding: Myriad (Inst)
David M. Loeb
No relationship to disclose
Christine A. Pratilas
Consultant or Advisory Role: Genentech
Christian F. Meyer
Consulting or Advisory Role: Janssen, Eli Lilly, Eisai (Inst)
Travel, Accommodations, Expenses: Plexxicon, Eli Lilly
Eric S. Christenson
No relationship to disclose
Shannon A. Slater
No relationship to disclose
Jennifer Ensminger
No relationship to disclose
Heather A. Parsons
No relationship to disclose
Ben H. Park
Leadership: Horizon Discovery, Foundation Medicine, Genomic Health, Loxo Oncology
Stock and Other Ownership Interests: Loxo Oncology
Consulting or Advisory Role: Horizon Discovery, Genomic Health, Foundation Medicine, Loxo Oncology
Research Funding: Foundation Medicine, Genomic Health
Josh Lauring
Speakers’ Bureau: Galderma(I), Abbvie(I)
Patents, Royalties, Other Intellectual Property: Royalty payments from sales of products under a licensing agreement between Horizon Discovery and Johns Hopkins University
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: W. Brian Dalton, Patrick M. Forde, Christopher D. Gocke, Jennifer Axilbund, David M. Loeb, Heather A. Parsons, Ben H. Park, Josh Lauring
Financial support: Ben H. Park, Josh Lauring
Administrative support: Ben H. Park, Josh Lauring
Provision of study material or patients: Patrick M. Forde, Hyunseok Kang, Roisin M. Connolly, Vered Stearns, Antonio C. Wolff, David M. Loeb, Christine A. Pratilas, Christian F. Meyer, Heather A. Parsons, Ben H. Park
Collection and assembly of data: W. Brian Dalton, Patrick M. Forde, Hyunseok Kang, Roisin M. Connolly, Vered Stearns, Christopher D. Gocke, Jennifer Axilbund, Cindy Geoghegan, Antonio C. Wolff, Shannon A. Slater, Jennifer Ensminger, Heather A. Parsons, Ben H. Park, Josh Lauring
Data analysis and interpretation: W. Brian Dalton, Patrick M. Forde, Hyunseok Kang, Christopher D. Gocke, James R. Eshleman, Jennifer Axilbund, Dana Petry, Antonio C. Wolff, David M. Loeb, Christine A. Pratilas, Christian F. Meyer, Eric S. Christenson, Heather A. Parsons, Ben H. Park, Josh Lauring
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
Contributor Information
W. Brian Dalton, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.
Patrick M. Forde, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Hyunseok Kang, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.
Roisin M. Connolly, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Vered Stearns, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.
Christopher D. Gocke, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
James R. Eshleman, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Jennifer Axilbund, Invitae Corporation, San Francisco, CA.
Dana Petry, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.
Cindy Geoghegan, Patient and Partners, Madison, CT.
Antonio C. Wolff, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
David M. Loeb, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Christine A. Pratilas, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Christian F. Meyer, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Eric S. Christenson, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Shannon A. Slater, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Jennifer Ensminger, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.
Heather A. Parsons, Susan F. Smith Center for Women’s Cancers, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA
Ben H. Park, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Josh Lauring, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD.
References
- 1.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conley BA, Doroshow JH. Molecular analysis for therapy choice: NCI MATCH. Semin Oncol. 2014;41:297–299. doi: 10.1053/j.seminoncol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Kurzrock R, Colevas AD, Olszanski A, et al. NCCN Oncology Research Program’s Investigator Steering Committee and NCCN Best Practices Committee molecular profiling surveys. J Natl Compr Canc Netw. 2015;13:1337–1346. doi: 10.6004/jnccn.2015.0163. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi GN, Chen D, Piccart M, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from BOLERO-2. J Clin Oncol. 2016;34:419–426. doi: 10.1200/JCO.2014.60.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jardim DL, Wheler JJ, Hess K, et al. FBXW7 mutations in patients with advanced cancers: Clinical and molecular characteristics and outcomes with mTOR inhibitors. PLoS One. 2014;9:e89388. doi: 10.1371/journal.pone.0089388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 7.Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. doi: 10.1371/journal.pgen.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons HA, Beaver JA, Cimino-Mathews A, et al. Individualized Molecular Analyses Guide Efforts (IMAGE): A prospective study of molecular profiling of tissue and blood in metastatic triple negative breast cancer. Clin Cancer Res. 2017;23:379–386. doi: 10.1158/1078-0432.CCR-16-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirshfield KM, Tolkunov D, Zhong H, et al. Clinical actionability of comprehensive genomic profiling for management of rare or refractory cancers. Oncologist. doi: 10.1634/theoncologist.2016-0049. [epub ahead of print on August 26, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meric-Bernstam F, Brusco L, Shaw K, et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol. 2015;33:2753–2762. doi: 10.1200/JCO.2014.60.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwaederle M, Parker BA, Schwab RB, et al. Molecular tumor board: The University of California-San Diego Moores Cancer Center experience. Oncologist. 2014;19:631–636. doi: 10.1634/theoncologist.2013-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JS, Wang K, Chmielecki J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer. 2016;138:881–890. doi: 10.1002/ijc.29825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Axilbund J, Dalton WB, et al. A polycythemia vera JAK2 mutation masquerading as a duodenal cancer mutation. J Natl Compr Canc Netw. 2016;14:1495–1498. doi: 10.6004/jnccn.2016.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann Oncol. 2016;27:281–286. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 15.Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 16.Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 17.Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5:842–849. doi: 10.1158/2159-8290.CD-14-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaye FJ, Ivey AM, Drane WE, et al. Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J Natl Cancer Inst. 2014;107:378. doi: 10.1093/jnci/dju378. [DOI] [PubMed] [Google Scholar]
- 19.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davare MA, Vellore NA, Wagner JP, et al. Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proc Natl Acad Sci USA. 2015;112:E5381–E5390. doi: 10.1073/pnas.1515281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015;21:166–174. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou SI, Govindan R, Eaton KD, et al. Phase I results from a study of crizotinib in combination with erlotinib in patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2017;12:145–151. doi: 10.1016/j.jtho.2016.09.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: A potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meric-Bernstam F, Johnson A, Holla V, et al. A decision support framework for genomically informed investigational cancer therapy. J Natl Cancer Inst. 2015;107:djv098. doi: 10.1093/jnci/djv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker BA, Schwaederlé M, Scur MD, et al. Breast cancer experience of the molecular tumor board at the University of California, San Diego Moores Cancer Center. J Oncol Pract. 2015;11:442–449. doi: 10.1200/JOP.2015.004127. [DOI] [PubMed] [Google Scholar]
- 26.Tsimberidou AM, Wen S, Hong DS, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: Validation and landmark analyses. Clin Cancer Res. 2014;20:4827–4836. doi: 10.1158/1078-0432.CCR-14-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conley BA, Gray R, Chen A, et al. NCI-Molecular Analysis for Therapy Choice (NCI-MATCH) clinical trial: Interim analysis. Cancer Res. 2016;76(suppl 14) doi: 10.1158/1538-7445.AM2016-CT101. abstr CT101. [DOI] [Google Scholar]
- 28.Le Tourneau C, Delord JP, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 29.Tannock IF, Hickman JA. Limits to personalized cancer medicine. N Engl J Med. 2016;375:1289–1294. doi: 10.1056/NEJMsb1607705. [DOI] [PubMed] [Google Scholar]
- 30.Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815. doi: 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwaederle M, Husain H, Fanta PT, et al. Use of liquid biopsies in clinical oncology: Pilot experience in 168 patients. Clin Cancer Res. 2016;22:5497–5505. doi: 10.1158/1078-0432.CCR-16-0318. [DOI] [PubMed] [Google Scholar]
- 32.Siu LL, Lawler M, Haussler D, et al. Facilitating a culture of responsible and effective sharing of cancer genome data. Nat Med. 2016;22:464–471. doi: 10.1038/nm.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]