Abstract
Introduction
Food insecurity is reported in approximately 28% of individuals with diabetes in the US, and is associated with poor glycemic and lipid control. The aim of this study was to understand the direct and indirect pathways through which food insecurity impacts glycemic control in individuals with diabetes.
Methods
615 adults with type 2 diabetes completed validated questionnaires after recruitment from two primary care clinics. Structural equation modeling (SEM) was used to investigate mechanisms through which food insecurity influences diabetes self-care behaviors and glycemic control, including investigation into possible direct and indirect effects of perceived stress and social support.
Results
The final model showed that higher food insecurity was directly significantly related to increased stress (r= 0.14, p<0.001) and increased HbA1c (r= 0.66, p=0.03). Higher stress was significantly related to poorer self-care (r= −0.54, p<0.001), and lower social support (r= −0.41, p<0.001). There was no significant direct association between food insecurity and self-care, or between perceived stress and glycemic control.
Conclusions
Food insecurity had both a direct effect on glycemic control, and an indirect effect on self-care through stress. The indirect pathway suggests efforts to address stress may influence the ability of individuals to perform diabetes self-care behaviors. The direct effect on glycemic control suggests pathways independent of self-care behaviors may also be necessary to improve diabetes outcomes. Results from this study suggest a multi-pronged approach is necessary to address food insecurity in individuals with diabetes.
Keywords: food insecurity, diabetes, stress, social support, self-care
Introduction
Diabetes affects over 9% of the US population, is the 7th leading cause of death, and is associated with significant morbidity, mortality, decreased quality of life, and increased health care utilization and cost. 1 Individuals diagnosed with diabetes have average medical expenditures that are approximately 2.3 times higher than what expenditures are for those not diagnosed with diabetes. 1 Additionally, the risk for death among people with diabetes is about twice that of people without diabetes of similar age.1 Despite significant research into lifestyle interventions focused on improving glycemic control and diabetes outcomes, national estimates suggest nearly half of those diagnosed with diabetes do not meet targets for glycemic control, and only 14% meet combined targets for glycemic control, blood pressure, and lipids.2
Significant evidence supports a relationship between food security, mental and physical health, and an individual’s ability to manage diabetes. 3 Food insecurity is defined by the USDA as an inability to or limitation in accessing nutritionally adequate foods, or dependence on emergency food supplies. 4 The overall age-standardized rate of food insecurity in the US population increased from 9.1% in 2005 to 18.3% in 2012, and is shown to be particularly prevalent for those with cardiometabolic diseases. 5 For example, food insecure adults are 2–3 times more likely to have diabetes than those who are food secure, after adjustment for income, employment, and lifestyle. 3,6
One hypothesized mechanism to explain the impact of food insecurity on those with diabetes is the change in dietary intake. 7–10 Overall, reliance on lower quality, energy-dense foods increases due to the higher cost of healthy alternatives. 3,11 However, it has also been hypothesized that food insecurity impacts an individual’s ability to make decisions regarding self-management of the disease. 8,12 For example, those with diabetes have noted diabetes specific diet behaviors such as counting carbohydrates and tracking servings were unrealistic, and meal planning became difficult with inconsistent income. 13 Self-care behaviors have been shown to be associated with food insecurity, particularly medication adherence, general diet, and blood sugar testing 14, but did not mediate the relationship with worse glycemic control 15. Food insecurity also has implications on a patient’s emotional and mental health, with food insecure patients with diabetes reporting lower overall health status, lower satisfaction with life, and higher prevalence of depression, diabetes distress, and self-perceived stress. 3, 10, 12, 16, 17 Low socioeconomic status has also been hypothesized to make developing and replenishing health promoting resources more difficult, decreasing the ability to manage stress. 18,19
Little work has been conducted investigating mediators of the relationship between food insecurity and glycemic control, and no study to our knowledge has investigated the direct and indirect influences of factors related to diet, psychological stress, and health promoting resources such as social support. Therefore, this study aimed to understand the direct and indirect pathways through which food insecurity impacts glycemic control in individuals with diabetes.
Methods
Sample population
Data was collected between 2013–2014 as part of a cross-sectional study of adults with type 2 diabetes. 615 adults with type 2 diabetes, recruited from primary care clinics in the Southeast United States, completed a series of validated questionnaires intended to measure the impact of social determinants of health on individuals with diabetes. Eligibility to participate included age 18 or older, diagnosis of type 2 diabetes noted in their medical record, and the ability to communicate in English. If after interaction with the research coordinators, patients were determined to be cognitively impaired, they were considered ineligible. Participants were recruited through two methods: letters of invitation sent to their home describing the study and inviting them to participate, and direct invitation by a research coordinator in the clinic waiting room. Prior to consent, all participants were provided a detailed explanation of the study, and procedures were approved by the local institutional review board before initiation of enrollment. Following completion of the self-administered survey, hemoglobin A1c (HbA1c) levels were abstracted from the medical record.
Measures
Food Insecurity
Food insecurity was measured using the US Household Food Security Survey Module: Six-Item Short Form scale developed by the National Center for Health Statistics. 4,20 This sub-set of the original 18 question survey reduces respondent burden and is used by several national health surveys, as well as the US Department of Agriculture Economic Research Service as a more parsimonious way to measure food insecurity. 20 The 6-item scale was shown to identify food-insecure households with good specificity and sensitivity relative to the 18-item scale, particularly for studies focused on adult populations. 20 Questions ask about whether participants did not have enough money to buy more food in the past 12-months, if they could not afford to eat balanced meals, ever had to cut the size of their meals because there was not enough money, or if they ever ate less than they felt they should because there was not enough food. Scoring increases for increased frequency of these experiences. 20 Raw scores of 0–1 indicate high or marginal food security, raw scores of 2–4 indicate low food security, and raw scores of 5–6 indicate very low food security. 20
Perceived Stress
The perceived stress scale (PSS) is a brief 4-item scale to assess the degree to which the respondent finds situations stressful. 21 As this study was focused on psychosocial influences on food insecurity, the perception of stress, rather than a laboratory measure of stress, for example, cortisol level, was chosen. Participants are asked about the frequency of feelings over the last month related to feeling unable to control important things, lack of confidence in ability to handle problems, and difficulties piling up so high they were not able to overcome them. 21 Responses range from “0” (never) to “4” (very often) with higher scores indicating more perceived stress. 21 The Cronbach’s alpha value for the scale is reported as 0.69, and scores are highly correlated with stress, depression and anxiety. 22
Social Support
Three items representing positive social interaction from the Medical Outcomes Study (MOS) Social Support Survey was used to measure social support. 23 The three-item positive social interaction portion of the MOS survey measures frequency with which the respondent has someone to have a good time with, someone to get together for relaxation, and someone to do something enjoyable with. 23 The Cronbach’s alpha for the social interaction subscale was 0.94, and it was shown to have good reliability (0.77), had high correlation with other sub-scales (0.65 to 0.88), and had one-year test-retest reliability exceeding the 0.50 standard (0.72). 23
Diabetes Self-care
Behavioral skills were assessed with the 12-item Summary of Diabetes Self-Care Activities (SDSCA) scale, a brief validated self-report questionnaire of diabetes self-management actions. 24 Questions assess frequency over the past 7 days respondents followed a healthy diet, ate fruits and limited fat in their diet, exercised, completed blood glucose testing, and checked their feet. The average inter-item correlations within scales are high, and correlations with other measures of diet and exercise support the validity of the SDSCA subscales. 24 Medication adherence was measured with the 8-item self-report Morisky Medication Adherence Scale (MMAS), with each item measuring a specific medication-taking behavior. 25 The scale has high reliability (α = 0.83), with higher scores indicating lower adherence. 25
Glycemic control
HbA1c provides an average glycemic control over the past 3 months, and is the clinical standard of care to determine diabetes control. The most recent HbA1c measure within the previous 6 months was abstracted from the medical record.
Demographics
Previously validated questions from the National Health Interview Survey 26 were asked to collect age, gender, race/ethnicity, marital status, employment status, education level, household income, health insurance, and duration of diabetes. In addition, the single item self-rated health question used in the National Health Interview Survey, was asked to collect health status.
Statistical Analysis
Structural equation modeling (SEM) was used to investigate mechanisms through which food insecurity influences diabetes self-care behaviors and glycemic control. SEM combines regression, path analysis and factor analysis, allowing estimation and modeling of closely related predictors while taking measurement error into account. 27–29 Cross-sectional designs can be analyzed using SEM, however, do not provide evidence of causation. 27 Therefore, the results are interpreted within the context of data collection. By incorporating multiple independent and dependent variables in the same model, SEM allows simultaneous testing of direct and indirect effects. 30 After descriptive statistics were completed to ensure multivariate normal and linearly related variables, confirmatory factor analysis (CFA) was used to test latent factors for food insecurity, perceived stress, social support, and self-care. Alpha statistics and loading were used to test the goodness of fit for each factor using principal component factor analysis. 31
First, descriptive statistics were run to provide demographic information (Table 1) and details on the scales used in the analysis (Table 2). Second, pairwise correlations were completed between measures included in the initial SEM model (Table 3). Finally, SEM was used to investigate the hypothesized model based on a priori specifications. The hypothesized model can be seen in Figure 1, with the final model shown in Figure 2. Direct, indirect, and total effects were assessed in the final model (Table 4). Analyses were completed using Stata v14 with standardized estimates, and the ‘mlmv’ option, which retains variables rather than using listwise deletion for missing data. Standardized estimates can be interpreted as the change in standard deviation of the outcome resulting from 1 standard deviation of the predictor, and allows comparison between estimates. The fit of individual paths was determined by a significance of p < 0.05. The model fit was investigated using multiple fit statistics, as recommended by best practices for SEM. 32 As chi2 is sensitive to sample size, we used the root square mean error of approximation (RSMEA), comparative fit index (CFI), and Tucker fit index (TFI). (Hooper) RSMEA values lower than 0.05, CFI values above 0.9, and TFI values above 0.9 indicate good fit. 33 A sample size of 615 provided the recommended 20:1 (subject to variable) ratio needed to maintain 80% power while estimating stable parameters, and minimizing the possibility of oversaturating the model. 28,32
Table 1.
Sample demographics for adults with diabetes included in study (n=615)
| mean± standard deviation or % | |
|---|---|
|
| |
| Age (years) | 61.3±10.9 |
| Diabetes Duration (years) | 12.3±9.1 |
| Education (years of school) | 13.4±2.8 |
| Employment (hours per week) | 12.5±19 |
| Race | |
| White | 33.0 |
| Black | 64.9 |
| Other | 2.1 |
| Gender | |
| Women | 38.4 |
| Men | 61.6 |
| Marital Status | |
| Never married | 11.2 |
| Married | 49.7 |
| Separated/Divorced | 28.2 |
| Widowed | 10.9 |
| Income | |
| <$10,000 | 20.2 |
| $10,000–$14,999 | 11.3 |
| $15,000–$19,999 | 10.1 |
| $20,000–$24,999 | 10.4 |
| $25,000–$34,999 | 14.7 |
| $35,000–$49,999 | 13.8 |
| $50,000–$74,999 | 10.1 |
| $75,000 or more | 9.4 |
| Insurance | |
| None | 9.3 |
| Private | 20.2 |
| Medicare | 24.7 |
| Medicaid | 10.2 |
| VA | 23.9 |
| Other | 11.7 |
| Health Status | |
| Excellent | 1.3 |
| Very Good | 12.0 |
| Good | 38.2 |
| Fair | 38.7 |
| Poor | 9.8 |
Table 2.
Descriptive Statistics for Variables of Interest included in final model
| Factors | Mean Values ± Standard Deviation |
|---|---|
|
| |
| Food Insecurity (FI) | |
| FI-1 | 0.4±0.5 |
| FI-2 | 0.3±0.5 |
| FI-3 | 0.2±0.4 |
| FI-4 | 0.1±0.3 |
| FI-5 | 0.2±0.4 |
| FI-6 | 0.1±0.3 |
| Perceived Stress (PSS) | |
| PSS-1 | 1.2±1.1 |
| PSS-2 | 1.4±1.3 |
| PSS-3 | 1.5±1.1 |
| PSS-4 | 1.3±1.2 |
| Social Support (MOS) | |
| MOS-16 | 4.0±1.2 |
| MOS-17 | 3.9±1.3 |
| MOS-18 | 3.9±1.3 |
| Glycemic Control (HbA1c) | 7.9±1.8 |
| Self-Care | |
| Medication Adherence | 5.9±2.0 |
| General Diet | 4.7±2.0 |
| Special Diet | 4.0±1.6 |
| Exercise | 2.6±2.2 |
| Blood Sugar Testing | 4.6±2.5 |
| Foot Care | 4.3±2.5 |
Note: numbers indicate item in scale (i.e. FI – 1 is the first item in the food insecurity scale)
Table 3.
Pairwise Correlations for Glycemic Control, Self-Care Behaviors, Food Insecurity, Perceived Stress, and Social Support Variables included in Model
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. General diet | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 2. Specific diet | 0.36* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 3. Exercise | 0.29* | 0.15* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 4. BST | 0.21* | 0.19* | 0.11* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5. Foot care | 0.22* | 0.22* | 0.12* | 0.28* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 6. Med Adh | 0.28* | 0.26* | 0.13* | 0.17* | 0.23* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 7. FI -1 | −0.17* | −0.14* | −0.11* | −0.09* | −0.03 | −0.19* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 8. FI -2 | −0.19* | −0.13* | −0.08* | −0.08 | 0.03 | −0.20* | 0.71* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 9. FI -3 | −0.15* | −0.10* | −0.09* | −0.10* | −0.03 | −0.20* | 0.52* | 0.51* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 10. FI - 4 | −0.12* | −0.10* | −0.09* | −0.09* | −0.01 | −0.15* | 0.46* | 0.42* | 0.76* | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 11. FI - 5 | −0.17* | −0.12* | −0.07 | −0.09* | −0.04 | −0.24* | 0.05* | 0.55* | 0.76* | 0.67* | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 12. FI - 6 | −0.16* | −0.08* | −0.03 | −0.4* | −0.07 | −0.21* | 0.42* | 0.41* | 0.63* | 0.59* | 0.73* | -- | -- | -- | -- | -- | -- | -- | -- |
| 13. PSS - 1 | −0.18* | −0.23* | −0.18* | −0.13* | −0.11* | −0.31* | 0.36* | 0.32* | 0.30* | 0.27* | 0.37* | 0.27* | -- | -- | -- | -- | -- | -- | -- |
| 14. PSS - 2 | −0.07 | −0.07 | −0.03 | −0.02 | 0.03 | −0.13* | 0.18* | 0.19* | 0.15* | 0.12* | 0.15* | 0.16* | 0.11* | -- | -- | -- | -- | -- | -- |
| 15. PSS - 3 | −0.18* | −0.11* | −0.06 | −0.03 | −0.04 | −0.19* | 0.25* | 0.24* | 0.16* | 0.15* | 0.19* | 0.20* | 0.27* | 0.56* | -- | -- | -- | -- | -- |
| 16. PSS - 4 | −0.19* | −0.21* | −0.11* | −0.13* | −0.10* | −0.29* | 0.37* | 0.34* | 0.28* | 0.26* | 0.31* | 0.26* | 0.64* | 0.11* | 0.26* | -- | -- | -- | -- |
| 17. MOS - 1 | 0.19* | 0.15* | 0.13* | 0.07 | 0.09* | 0.17* | −0.30* | −0.28* | −0.19* | −0.25* | −0.27* | −0.21* | −0.37* | −0.14* | −0.29* | −0.36* | -- | -- | -- |
| 18. MOS - 2 | 0.22* | 0.18* | 0.14* | 0.08* | 0.12* | 0.16* | −0.30* | −0.28* | −0.21* | −0.25* | −0.26* | −0.21* | −0.42* | −0.14* | −0.28* | −0.37* | 0.91* | -- | -- |
| 19. MOS - 3 | 0.22* | 0.17* | 0.15* | 0.09* | 0.12* | 0.18* | −0.29* | −0.25* | −0.20* | −0.25* | −0.25* | −0.20* | −0.41* | −0.14* | −0.30* | −0.38* | 0.92* | 0.94* | -- |
| 20. HbA 1c | −0.12* | −0.07 | −0.10* | 0.10* | 0.03 | −0.20* | 0.10* | 0.11* | 0.12* | 0.10* | 0.17* | 0.12* | 0.14* | 0.04 | 0.001 | 0.15* | −0.08* | −0.07 | −0.05 |
p<0.05.
Abbreviations: HbA1c=Hemoglobin A1c, Med Adh=medication adherence, BSG=blood sugar testing, FI=food insecurity, PSS=perceived stress scale, MOS=social support scale, numbers indicate item in scale (i.e. PSS – 1 is the first item in the PSS scale)
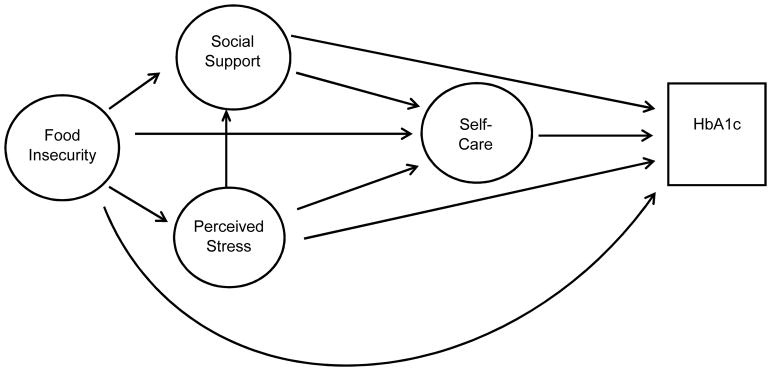
Figure 1.
Hypothesized Model of Influence of Food Insecurity, Perceived Stress, and Social Support on Self-Care and Glycemic Control
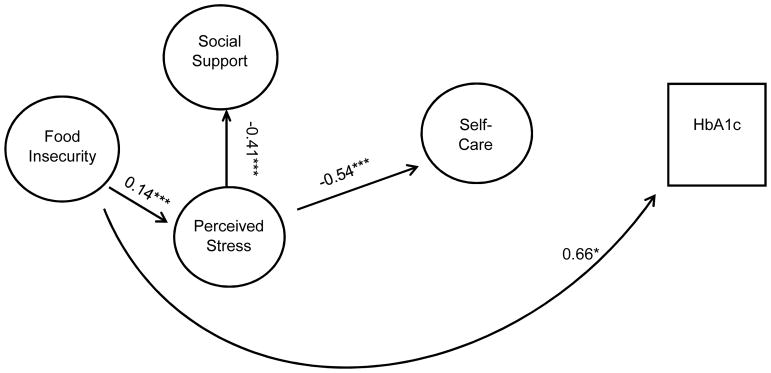
Figure 2.
Final Model of Influence of Food Insecurity, Perceived Stress, and Social Support on Self-Care and Glycemic Control
Note: Coefficients are standardized path coefficients.
Overall Model Fit: Chi2 (158)=301.97, p<0.001; R2=0.98, RMSEA=0.038, CFI=0.977, TFI=0.972) *p<0.05, **p<0.01, ***p<0.001
Table 4.
Standardized Direct, Indirect, and Total Effects for Relationship between Food Insecurity, Perceived Stress, Self-Care Behaviors and Glycemic Control in SEM model
| Direct Effects | Indirect Effects | Total Effects | |
|---|---|---|---|
| Self-Care Behaviors | |||
| → Food Insecurity | −0.36 | - | −0.36 |
| → Perceived Stress | −0.54*** | −0.05 | −0.59*** |
| → Social Support | 0.11 | 0.25*** | 0.36*** |
| Perceived Stress | |||
| → Social Support | −0.41*** | - | −0.41*** |
| Food Insecurity | |||
| → Perceived Stress | 0.14*** | - | 0.14*** |
| → Social Support | −0.02 | −0.06*** | −0.07*** |
| Glycemic Control | |||
| → Self-Care | −0.13 | - | −0.13 |
| → Food Insecurity | 0.66* | 0.05 | 0.71* |
| → Perceived Stress | 0.21 | 0.17* | 0.38** |
| → Social Support | 0.08 | −0.18*** | −0.10 |
p<0.05,
p<0.01,
p<0.001
Note: Structural Equation Modeling (SEM) was used to investigate direct and indirect effect. Significant direct effects indicate direct association between variables. For example, increased food insecurity is associated with poorer glycemic control (i.e., higher glycosylated HbA1c). Significant indirect effects indicate pathways through which variables influence outcomes. For example, increased food insecurity is associated with social support through perceived stress.
Results
Sample demographics are reported in Table 1. The mean age of participants was 61.3 years (standard deviation ±10.9), and mean duration of diabetes was 12.3 years (standard deviation ±9.1). The sample consisted of 38.4% women and 64.9% non-Hispanic blacks. 52% of the sample reported an income below $25,000. 13.3% reported excellent or very good health, 38.2% and 38.7% reported good and fair health, respectively, and 9.8% reported poor health.
Table 2 presents descriptive statistics for the variables of interest including food insecurity, perceived stress, social support, glycemic control, and self-care behaviors. Table 3 presents pairwise correlations between the measures included in these variables.
Figure 1 shows the hypothesized relationships tested, and Figure 2 shows the final model. Table 4 presents the standardized direct, indirect, and total effects for the relationship between food insecurity, self-care behaviors, and glycemic control from the final model (chi2 (158) =301.97, p<0.001, R2=0.98, RMSEA=0.038, CFI=0.977, TFI=0.972). The final model showed that higher food insecurity was directly significantly related to increased stress (r= 0.14, p<0.001) and increased HbA1c (r= 0.66, p=0.03). Higher perceived stress was significantly related to poorer self-care (r= −0.54, p<0.001), and lower social support (r= −0.41, p<0.001). There was no significant direct association between food insecurity and self-care, or between perceived stress and glycemic control.
Discussion
Using a sample of primary care patients with diabetes, we found that food insecurity has a direct relationship to glycemic control outside the influence of self-care, and that stress serves as a pathway through which food insecurity influences self-care behaviors. The indirect relationship on self-care suggests efforts to address stress may help improve the ability of food insecure individuals with diabetes to perform self-care behaviors. The direct effect on glycemic control suggests pathways independent of self-care behaviors may also be necessary to improve diabetes outcomes. Therefore, results from this study suggest a multi-pronged approach is necessary to address food insecurity in individuals with diabetes, including both support in obtaining healthy food and support in coping with stress that can impact self-management behaviors.
The findings of this study provide important information regarding mechanisms to explain the relationship between food insecurity and health outcomes for individuals with diabetes. A better understanding of the factors that lead to poor outcomes can inform more targeted support interventions to address individuals with food insecurity. 34 Current intervention efforts have focused on decreasing the cost of fresh vegetables and improving the diet quality of food insecure patients. 9 While programs like the Supplemental Nutrition Assistance Program (SNAP) and emergency food banks can be effective at increasing food availability, some individuals may not be eligible and the food choices available may not be ideal for those with diabetes. 5,10 Pathways for interventions aimed at improving health outcomes may exist beyond improving the nutrition of food available to individuals, but currently the majority of interventions in food insecure populations have focused on this pathway. 34 This study suggests that a focus on nutrition alone will not address the needs of individuals with diabetes, and recommends additional pathways be targeted with future interventions.
Prior investigation into pathways explaining the relationship between food insecurity and health outcomes in patients with diabetes is limited. Our findings of stress as a pathway are supported by the results of Seligman et al., which found that difficulty following a diet and emotional distress met formal criteria as a mediator of the relationship. 12 Emotional distress mediated 34% of the relationship between food insecurity and glycemic control; whereas difficulty following a diet only mediated 20%. 12 Maintaining a healthy diet while dealing with food insecurity may require considerable more time, planning, and knowledge than patients have with the resources currently provided. 12 This can include the information and skills necessary to support good self-care, as well as the social support necessary for long-term success. After completion of a diabetes self-management intervention, individuals who were food insecure showed a significant improvement in glycemic control, whereas those who were food secure did not. 8 They also showed significant improvement in diabetes self-efficacy and increased fruit intake, suggesting despite challenges faced obtaining food, the support of a self-management intervention was particularly important for food insecure patients. 8 Social support was shown to serve as a buffer against increased prevalence of depressive symptoms in individuals with food insecurity 35, a finding that supports our results of a relationship between social support and perceived stress. Individuals with diabetes reporting food insecurity noted that lack of funds limited socializing and made it difficult to eat and drink differently from friends. 13 And while community programs, such as community kitchens and gardens, food distribution centers, and meal programs, allow interaction with others, they were not always able to attend. 13
Limitations
While this study used sophisticated methodology to investigate pathways for the relationship between food insecurity and glycemic control, there are some limitations. First, the analysis was based on cross-sectional data, and therefore findings cannot speak to causality. While SEM is appropriate for non-experimental designs, its interpretation is linked to the method of data collection.27 Secondly, the sample population was collected from primary care clinics in the Southeastern United States and may not be representative of all areas within the United States. Though there is no indication the impact of food insecurity on individuals with diabetes differs by region, additional studies should be conducted throughout the US. In addition, this sample was 65% Non-Hispanic Black, 62% male, and had a mean age of 61. Generally, racial/ethnic minorities, women, and younger individuals report higher food insecurity. 6,9 Therefore, additional studies should test if the strength of these relationships is maintained across populations with different representation of demographic factors. Finally, while SEM is a powerful technique for investigating mechanisms, it validates models as hypothesized prior to the analysis and additional variables not included in the model will be important to explore in the future.
Conclusion
In conclusion, we found that food insecurity influences health outcomes in patients with diabetes both through a direct influence on glycemic control, and an indirect influence on self-care through perceived stress. Results suggest future interventions to address food insecurity in patients with diabetes should consider factors that impede their ability to perform self-care behaviors, including eating a healthy diet.
Acknowledgments
Financial Support: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant K24DK093699, Principal Investigator: Leonard Egede, MD) and the study sponsor had no role in study design, collection, analysis, interpretation, or writing of report.
Conflict of Interest: The authors declare no conflict of interest.
Authorship: LEE obtained funding for the study. RJW and LEE acquired, analyzed and interpreted the data. RJW, JSW, and LEE designed the study, drafting the article, and critically revised the manuscript for intellectual content. All authors approved the final manuscript.
Ethical Standards Disclosure: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Review Board of the Medical University of South Carolina, Charleston, SC, USA. Written informed consent was obtained from all subjects/patients.
References
- 1.Centers for Disease Control and Prevention (CDC) National Diabetes Statistics Report, 2017. Atlanta, GA: U.S. Department of Health and Human Services; 2017. [Google Scholar]
- 2.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement in goals in US diabetes care, 1999–2010. N Engl J Med. 2013;368:287–288. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 3.Gucciardi E, Vahabi M, Norris N, Del Monte JP, Farnum C. The Intersection between Food Insecurity and Diabetes: A Review. Curr Nutr Rep. 2014;3(4):324–332. doi: 10.1007/s13668-014-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel Gary, Nord Mark, Price Cristofer, Hamilton William, Cook John. Guide to Measuring Household Food Security, Revised 2000. U.S. Department of Agriculture, Food and Nutrition Service; Alexandria VA: [Google Scholar]
- 5.Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005–2012. PLoS One. 2017 Jun 7;12(6):e0179172. doi: 10.1371/journal.pone.0179172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seligman HK, Bindman AB, Vittinghoff E, et al. Food insecurity is associated with diabetes mellitus: results from the NHANES 1999–2002. J Gen Intern Med. 2007 Jul;22(7):1018–23. doi: 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. New Eng J Med. 2010;363(1):6–9. doi: 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- 8.Lyles CR, Wolf MS, Schillinger D, et al. Food insecurity in relation to changes in hemoglobin A1c, self-efficacy, and fruit/vegetable intake during a diabetes educational intervention. Diabetes Care. 2013;36(6):1448–53. doi: 10.2337/dc12-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014 Apr;127(4):303–310.e3. doi: 10.1016/j.amjmed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Essien UR, Shahid NN, Berkowitz SA. Food Insecurity and Diabetes in Developed Societies. Curr Diab Rep. 2016 Sep;16(9):79. doi: 10.1007/s11892-016-0774-y. [DOI] [PubMed] [Google Scholar]
- 11.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010 Feb;140(2):304–10. doi: 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan J, DeMelo M, Gingras J, Gucciardi E. Challenges of Diabetes Self-Management in Adults Affected by Food Insecurity in a Large Urban Centre of Ontario, Canada. Int J Endocrinol. 2015:903468. doi: 10.1155/2015/903468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smalls BL, Gregory CM, Zoller JS, Egede LE. Assessing the relationship between neighborhood factors and diabetes related health outcomes and self-care behaviors. BMC Health Serv Res. 2015 Oct 1;15:445. doi: 10.1186/s12913-015-1086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heerman WJ, Wallston KA, Osborn CY, Bian A, Schlundt DG, Barto SD, Rothman RL. Food insecurity is associated with diabetes self-care behaviours and glycaemic control. Diabet Med. 2016 Jun;33(6):844–50. doi: 10.1111/dme.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverman J, Krieger J, Kiefer M, et al. The Relationship Between Food Insecurity and Depression, Diabetes Distress and Medication Adherence. J Gen Intern Med. 2015 Oct;30(10):1476–80. doi: 10.1007/s11606-015-3351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery J, Lu J, Ratliff S, Mezuk B. Food Insecurity and Depression Among Adults With Diabetes: Results From the National Health and Nutrition Examination Survey (NHANES) Diabetes Educ. 2017 Jun;43(3):260–271. doi: 10.1177/0145721717699890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 19.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22:337–56. [PubMed] [Google Scholar]
- 20.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the household food security scale. Am J Public Health. 1999;89:1231–1234. doi: 10.2105/ajph.89.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Williamson G. Perceived Stress in a Probability Sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 22.Andreou E, Alexopoulos EC, Lionis C, Varvogli L, Gnardellis C, Chrousos GP, Darviri C. Percieved stress scale: reliability and validity study in Greece. Int J Environ Res Public Health. 2011;8:3287–3298. doi: 10.3390/ijerph8083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherbourne CD, Stewart AL. The MOS Social Support Survey. Social Science and Medicine. 1991;32:705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 24.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care Jul. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 25.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. Survey Questionnaire, National Health Interview Survey, 2002. National Center for Health Statistics; Hyattsville, Maryland: 2004. [Google Scholar]
- 27.Kline RB. Principles and Practice of Structural Equation Modeling. 4. New York: Guilford Press; 2016. [Google Scholar]
- 28.Schumacker RE, Lomax RG. A Beginner’s Guide to Structural Equation Modeling. 3. New York: Taylor and Francis Group; 2010. [Google Scholar]
- 29.Hoyle R. Confirmatory factor analysis. In: Tinsley HEA, Brown SD, editors. Handbook of Applied Multivariate Statistics and Mathematical Modeling. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 30.Sanchez BN, Budtz-Jorgensen E, Ryan LM, Hu H. Structural equation models: a review with applications to environmental epidemiology. J Am Stat Assoc. 2005;100:1443–1455. [Google Scholar]
- 31.Byrne B. Factor analytic models: viewing the structure of an assessment instrument from three perspectives. J Personality Assess. 2005;85:17–32. doi: 10.1207/s15327752jpa8501_02. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber JB. Core reporting practices in structural equation modeling. Res Soc Admin Pharm. 2008;4:83–97. doi: 10.1016/j.sapharm.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Hooper D, Caughlan J, Mullen MR. Structural equation modeling: guidelines for determining model fit. Electron J Business Res Methods. 2008;6:53–60. [Google Scholar]
- 34.Barnard LS, Wexler DJ, DeWalt D, Berkowitz SA. Material need support interventions for diabetes prevention and control: a systematic review. Curr Diab Rep. 2015 Feb;15(2):574. doi: 10.1007/s11892-014-0574-1. [DOI] [PubMed] [Google Scholar]
- 35.Kollannoor-Samuel G, Vega-López S, Chhabra J, Segura-Pérez S, Damio G, Pérez-Escamilla R. Food insecurity and low self-efficacy are associated with health care access barriers among Puerto-Ricans with type 2 diabetes. J Immigr Minor Health. 2012 Aug;14(4):552–62. doi: 10.1007/s10903-011-9551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]