Abstract
The left ventral occipitotemporal cortex (vOT) is important in visual word recognition. Studies have shown that the left vOT is generally observed to be involved in spoken language processing in skilled readers, suggesting automatic access to corresponding orthographic information. However, little is known about where and how the left vOT is involved in the spoken language processing of young children with emerging reading ability. In order to answer this question, we examined the relation of reading ability in 5–6-year-old kindergarteners to the activation of vOT during an auditory phonological awareness task. Two experimental conditions: onset word pairs that shared the first phoneme and rhyme word pairs that shared the final biphone/triphone, were compared to allow a measurement of vOT’s activation to small (i.e., onsets) and large grain sizes (i.e., rhymes). We found that higher reading ability was associated with better accuracy of the onset, but not the rhyme, condition. In addition, higher reading ability was only associated with greater sensitivity in the posterior left vOT for the contrast of the onset versus rhyme condition. These results suggest that acquisition of reading results in greater specialization of the posterior vOT to smaller rather than larger grain sizes in young children.
Keywords: Left vOT, Grain size, Phonological awareness, Spoken language
1. Introduction
Reading starts from visual input, and involves translating print to sound. The left ventral occipitotemporal cortex (vOT) is an important brain region in reading that connects the visual word form of the stimuli to higher-level language brain areas. Even though the precise function of the left vOT is under debate (Dehaene and Cohen, 2011; Price and Devlin, 2011), it is a brain region that has consistently been found to be more sensitive to visual words compared to other stimuli such as faces and tools (Cohen et al., 2002; Gauthier et al., 2000). It has also been suggested that the sensitivity of the left vOT to visual word forms of the stimuli follows a posterior to anterior gradient, with the posterior part more sensitive to smaller grain sizes, such as letters. On the other hand, the anterior part, which has classically been referred to as the putative visual word form area (pVWFA), is in fact more sensitive to larger grain sizes, such as bigrams or trigrams (Dehaene et al., 2005).
The specialization of the left vOT for recognizing visual word forms is related to the acquisition of reading skill. An early influential theory of reading acquisition (Frith, 1985) argues that reading acquisition proceeds from the alphabetic to the orthographic stage. Early reading is marked by the acquisition of letter-sound relationships to form the alphabetic principle. Later reading is marked by the accumulation of knowledge of spelling patterns that recur across words to form larger grain orthographic representations. fMRI studies also suggest a developmental progression of vOT’s sensitivity to words from small grain sizes to large grain sizes. Brem et al. (2010) trained 6-year-old non-reading children to learn letter-speech sound correspondences by using the Graphogame, a phonics-intensive training program. After the training, the participants were asked to judge the modality of a stimulus in a multimodal task where both visually and auditorily presented words as well as false fonts and rotated speech sounds were presented as stimuli. They found that the posterior vOT (MNI, ± 46 −78 −12) showed greater activation to visually presented words compared to false fonts. In a study of older children, Brem et al. (2009) found that, similar to adults, 10-year-olds exhibited greater activation for visually presented words than symbol strings in the anterior left vOT in a repetition detection task, which required pressing a button after the immediate repetition of a stimulus. Taken together, these two studies suggest that young children utilize their posterior vOT to process visual words because they rely on letter-to-phoneme mapping, while older children and adults with more reading experience utilize their anterior vOT to process visual words because they rely on larger grain size information.
Unlike reading, which starts from visual input, spoken language processing begins with auditory input. Even though perceptual information comes through the auditory modality, a number of fMRI studies have consistently found activation in the left vOT during various spoken language tasks. Yoncheva et al. (2010) found selective attention to speech, relative to attention to melody, was associated with an activation increase in vOT (sphere ROI, MNI −35 −58 −15) for skilled adult readers. Dehaene et al. (2010) examined activation during an auditory lexical decision task in groups of adults with different reading abilities, and found that the illiterate group showed no activation in vOT, but all literate groups did (peak, MNI −44 −50 −14). Cone et al. (2008) tested typically achieving children aged 9- to 15- years old, and found vOT’s (peak, MNI −45 −51 −15) activation was greater for an auditory rhyme task than a pure tone task. They also found that an orthography-phonology conflicting (e.g. PINT-MINT) condition elicited greater activation in vOT (peak, MNI −54 −51 −15) than a non-conflicting (e.g. PRESS-LIST) condition, suggesting that orthographic representations are automatically activated in a spoken language task where all stimuli are only presented auditorily. Using the same auditory rhyme task, Desroches et al. (2010) found that typically achieving children showed significantly greater activation in vOT (peak, MNI −48 −51 −15) than those with reading difficulties. This is likely because reading acquisition drives the connection between oral and written language, resulting in the automatic activation of corresponding orthographic representations in vOT even during auditory tasks. The connections between phonological and corresponding orthographic representations may aid in the performance of a variety of spoken language tasks, including phoneme awareness ones (e.g. Castles et al., 2011). In sum, all of the above findings suggest that vOT can be activated in spoken language tasks that do not require access to corresponding orthographic information for correct performance, reflecting the automatic activation of corresponding orthographic representations, especially for those with higher reading abilities.
The above studies in older children and adults showed speech–related activation in the anterior left vOT, a brain region also sensitive to bigrams in written language. For younger pre-reading children, studies have also shown activation in the left vOT in auditory language tasks. Raschle et al. (2012) compared brain activation of pre-reading children aged 5–6 years with a familial history of developmental dyslexia (FHD +) versus children without a familial history (FHD−). Among these pre-reading children, only 2% were able to identify words. Children were asked to listen to two words and decide whether they started with the same initial sound. This onset task was compared to a control task that required the children to determine whether the two words were spoken by the same voice. In the comparison of the onset task to the control task, the FHD− children showed greater activation in the left vOT (peak, MNI −16 −86 −10) compared to the FHD+ children. This peak was in the lingual gyrus, which is more medial and posterior than the peaks in the studies of older children and adults. Powers et al. (2016) also used an onset task on 5-year-old children who could recognize less than 10 words. They found a positive correlation between home literacy environment and activation in the left vOT (peak MNI −36 −60 −20) in the onset compared to the control task. Hutton et al. (2015) found that, for even younger children aged 3–5 years, reading exposure, measured by a questionnaire assessing storybook reading, parental teaching and verbal interaction, was positively correlated with activation in the left vOT during a narrative story listening condition compared to a non-speech tone condition. This activation appeared to be located more posterior than the coordinates for older children and adults, but it is hard to determine as activation formed a large cluster that peaked in the angular gyrus. Finally, Dębska et al. (2016) found an effect of familial risk of dyslexia in the left vOT (peak, MNI −20 −68 −6) with decreased activation in FHD+ compared to FHD− in kindergarten children during an auditory rhyme task compared to a voice judgment task. In summary, these studies indicate that the posterior left vOT seems to be involved in spoken language processing in young children. Moreover, pre-reading children with better reading potential, such as an absence of familial history of developmental dyslexia or more reading exposure, showed greater activation in the posterior left vOT. Many early behavioral studies suggest more experience with learning to read changes the spelling-to-sound mapping from smaller grain size to larger grain size (e.g. Ziegler and Ferrand, 1998). Therefore, it is likely that the development of reading drives the connections between oral and written language, first at the phoneme-to-letter level and then at larger grain sizes such as the rhyme. In terms of the neural mechanism, recent fMRI evidence indicates the posterior vOT is engaged in spoken language more for young children, but the anterior vOT is engaged more for older children. However, little is known about whether this automatic activation of orthographic representations in the vOT during spoken language processing is related to reading skill and whether this vOT’s activation depends on grain sizes.
In order to answer these questions, the current study used the fMRI technique to examine whether reading skills in 5- to 6-year-old children at the onset of literacy were associated with automatic activation in the left vOT during an auditory phonological task that only requires a sound based judgment. Based on previous neuroimaging studies, we hypothesized that higher reading skills would be associated with greater activation in the left vOT. We also aimed to determine whether activation in the left vOT depended on grain sizes. We employed a small grain size onset condition that only contained the same first phoneme for the paired auditory words, and a large grain size rhyme condition that contained the same biphone or triphone for the paired auditory words. Previous studies have shown phoneme awareness is a better predictor of reading skill than the awareness of rhymes (Melby-Lervåg et al., 2012). Based on these behavioral studies, we hypothesized that the onset condition would be more difficult and more strongly correlated with reading skills, as compared to the rhyme condition. Based on the transition from alphabetic to orthographic reading (Frith, 1985), we expected that the young children in our study at their onset of literacy would be at the alphabetic stage due to little reading experience, and hypothesized that higher reading skills would be associated with greater sensitivity to the onset compared to the rhyme condition in the posterior left vOT that is presumably sensitive to smaller grain sizes, i.e. letters. Because the young children in our study are unlikely to be at the orthographic stage, we did not expect greater sensitivity for the rhyme compared to the onset condition in the anterior left vOT that is presumably sensitive to larger grain sizes, i.e. bigrams and trigrams.
2. Experimental procedures
2.1. Participants
Fifty-nine children (mean age = 5.9, range 5.5–6.5 years-old, 33 girls) were included in our study. Children were recruited from the Austin, Texas metropolitan area. Informed consent form was obtained from the parents. The Institutional Review Board approved all of the following procedures.
Participants were given developmental history questionnaires completed by their parents and a series of screening tests. The screening tests included the 5-handedness questions in which the children needed to pretend they write, draw, pick, open, and throw something, and the Diagnostic Evaluation of Language Variation (DELV) Part 1 Language Variation Status (Seymour et al., 2003). All the children met the following inclusionary criteria: (1) primarily right-handed, defined as performing at least 3 out of 5 items using their right hand; (2) Mainstream English speakers, defined as scoring above 8 out of 15 mainstream English items on DELV. (3) no diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), neurological disease, psychiatric disorders, learning disorder or specific language impairments as reported in the developmental history questionnaire completed by their parents; (4) normal hearing and normal or corrected-to-normal vision as reported in the developmental history questionnaire completed by their parents.
Standardized testing was then administered to assess IQ and language. This included the Kaufman Brief Intelligence Test, Second Edition (KBIT-2, Kaufman and Kaufman, 2004) non-verbal scale subtest and the Clinical Evaluation of Language Fundamentals (CELF-5, Wiig et al., 2013) Core Language measure. All children scored greater than or equal to a standard score of 80 (9th percentile) on KBIT-2 and CELF-5 to be included in the study. The raw scores of the Woodcock-Johnson III Test of Achievement Letter-Word Identification subtest (Woodcock et al., 2001), which requires oral reading of presented letters and words, served as a measure of reading ability. Most of our children could recognize high frequency, single syllable words and they showed a wide range of reading skill, from recognition of letters to recognition of multi-syllable words. This variance allows us to capture the association between reading skill and activation in the vOT. Table 1 shows the mean, standard deviation, and the range of both the raw and standard scores for each standardized test.
Table 1.
Descriptive statistics of raw and standard scores for each standardized test.
| Raw Score | Standard Score | |||
|---|---|---|---|---|
|
|
|
|||
| Mean (SD) | Range | Mean (SD) | Range | |
| KBIT Non-verbal IQ | 19 (5) | 12–33 | 105 (14) | 80–141 |
| CELF-Core Language | 49 (7) | 36–64 | 114 (12) | 93–141 |
| WJ Letter Word Identification | 28 (11) | 9–57 | 124 (18) | 90–164 |
2.2. Auditory phonological task
Our task was an event related design. On each trial, children heard two sequentially presented auditory stimuli binaurally through earphones. There were four conditions of the pairs of stimuli: onset, rhyme, non-match, and noise (frequency modulated) as the auditory perceptual control (examples, see Table 2). The children were asked “do the two words have any of the same sound”. They were instructed to respond to all trials as quickly and accurately as possible with the right index finger for a yes response in the onset, rhyme as well as noise conditions, and with the right middle finger for a no response in the non-match condition. A blue circle remained on the screen during auditory stimuli presentation and it turned to yellow to remind the participants to respond. The duration of each word was between 500 and 700 milliseconds (ms) followed by a brief period of silence, with the second word beginning 1000 ms after the onset of the first. The length of the stimuli was counterbalanced across conditions. The blue circle turned to yellow 1000 ms after the onset of the second word, indicating the need to make a response. The duration of the response interval was 1800 ms. There were 24 trials for each of the four conditions, divided into two runs. The four conditions were pseudo-randomized so there were no more than 5 same responses in a row. To optimally deconvolve the hemodynamic response, inter-trial intervals were jittered by randomly adding 0, 450 or 900 ms to each trial, in equal proportions. For the second run, jitters of 0, 375 or 750 ms were similarly added to the trials. Each run lasted about 3 min.
Table 2.
Auditory stimuli conditions and examples.
| Condition | Response | Brief Explanation | Example |
|---|---|---|---|
| Onset | Yes | The two words start with the same sound | Coat – Cup |
| Rhyme | Yes | The two words rhyme | Wide – Ride |
| Non-match | No | The two words have no same sounds | Zip – Cone |
| Noise | Yes | Frequency modulated | Sh – Sh |
The three word conditions were designed according to the following standards. For the rhyme condition, the word pairs shared the same vowel and final phoneme/cluster (corresponding to 2–3 letters at the end of its written form). For the onset condition, the word pairs only shared the same initial phoneme (corresponding to one letter of its written form). The initial phoneme identity task appears to be at a reasonable difficulty level for 5- to 6-year old children, while the final phoneme identity task is too difficult (Carson et al., 2015). For the non-match condition, there were no shared phonemes (or letters of its written form). All the words were monosyllabic. Every paired word had no semantic association based on the University of South Florida Free Association Norms (Nelson et al., 1998). There were no significant differences (p > 0.1) between conditions in phonotactic frequency (Vitevitch and Luce, 2004), word frequency (Balota et al., 2007), part of speech (Balota et al., 2007), and the phonological and orthographic consistency (Bolger et al., 2008). Neither irregular forms nor inflected forms of words were used. The auditory perceptual control was made of frequency-modulated noise equated in length and amplitude with the word conditions.
Participants who scored within an acceptable accuracy range and had no response bias were included in our analysis. Acceptable accuracy was defined as the accuracy of the noise condition being greater than 60%. We used this above chance accuracy criterion to be more confident that the children were engaged in the task. The lack of a response bias was indicated by the accuracy difference between rhyme and non-match conditions being lower than 40%. Five participants were excluded from the fMRI analyses because of these criteria.
Prior to taking part in the fMRI scanning session, participants were required to complete the same task with different stimuli in the mock scanner and also before scanning in the practice room to make sure they understood the task and to acclimatize themselves to the scanner environment. All of the children were able to effectively press buttons using one hand.
2.3. Data acquisition
Participants lay in the scanner with a response button box placed in their right hand. Visual stimuli were projected onto a screen, viewed via a mirror attached to the inside of the head coil, to keep participants focused on the task so that they would respond in time. Participants wore earphones to hear the auditory stimuli and two ear pads were used to attenuate the scanner noise. The two phonological task runs were counterbalanced across participants.
Images were acquired using 3.0T Skyra Siemens scanner with a 64-channel headcoil. The blood oxygen level dependent (BOLD) signal was measured using a susceptibility weighted single-shot echo planar imaging (EPI) method. Functional images were acquired with multiband EPI (TE = 30 ms, flip angle = 80, matrix size = 128 × 128, FOV = 256 mm, slice thickness = 2 mm without gap, number of slices = 56, TR = 1250 ms, Multi-band accel.factor = 4, voxel size = 2 × 2 × 2). A high resolution, T1 weighted 3D image was acquired. The following scan parameters were used: TR = 1900 ms, TE = 2.34 ms, matrix size = 256 × 256, field of view = 256 mm, slice thickness = 1 mm, number of slices = 192.
2.4. Data analysis
fMRI data was analyzed using Statistical Parametric Mapping (SPM8 http://www.fil.ion.ucl.ac.uk/spm). Images were realigned and ArtRepair (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to correct for participant movement, which identified and used the interpolated values from the 2 adjacent non-outlier scans to replace outlier volumes, defined as those with head movement exceeding 4 mm in any direction or deviations of more than 1.5% from the mean global signal. No more than 10% of the volumes from each run and no more than 6 consecutive volumes for any individual run were interpolated in this way. Interpolated volumes were then partially deweighted when first-level models were calculated on the repaired images (Mazaika et al., 2009). Functional images were normalized to a segmented pediatric template (5–9 years old) from the Imaging Research Center at Cincinnati Children's Hospital Medical Center (CCHMC2_y, www.irc.chmcc.org) using both linear and non-linear transformation. The templates are based on brain imaging data from 67 healthy young children. The functional data was smoothed using a 4 mm isotropic Gaussian kernel.
Statistical analyses at the first level were calculated using an event-related design with the four conditions as conditions of interest. A high pass filter with a cutoff of 128 s and the default artificial mask threshold of 0.6 compared to the SPM default of 0.8 were applied. All experimental trials were included in the analysis. Word and noise pairs were treated as individual events for analysis and modeled using a canonical hemodynamic response function (HRF). Contrast maps were generated for onset versus rhyme condition for each individual, and then we used regression analysis at the group level for one-sample t-test model to examine the correlation between reading ability and the onset versus rhyme contrast. We combined the anatomical left fusiform gyrus (FG) and left inferior temporal gyrus (ITG) from aal template to form the left vOT mask. According to the model proposed by Dehaene et al. (2005), the letter sensitive left vOT is around y = −68, whereas the bigram sensitive left vOT is around y = −54 in MNI coordinates. We divided the left vOT mask into two parts around these two y-coordinates ± 7 mm to make the posterior and anterior left vOT adjacent to each other. The anterior and posterior masks were used to define the vOT sections according to Dehaene’s hierarchical model. We then tested our hypothesis by analyzing data within these two masks.
Cluster significance was determined by using AFNI’s 3dClustSim (December 2015) based on 10,000 interactions and spatial autocorrelation function (ACF) of mixed Gaussian and mono-exponential form. Parameters to the ACF were the average of all 59 individual subject’s values using AFNI's 3dFWHMx (Cox et al., 2016). We included voxels significant at p < .005 uncorrected level and family-wise error corrected at p < .05 for significant clusters. The voxel size was 2 × 2 × 2 mm3.
3. Results
3.1. Behavior results
Table 3 presents the mean accuracy and reaction time of the onset and rhyme conditions from the in-scanner auditory phonological task and its relationship with behavioral measures of the reading skill. Paired t-tests showed that the accuracy for the onset condition was significantly lower than that of the rhyme condition [t(58) = −7.416, p < .001], and the reaction time for the onset condition was significantly longer than that of the rhyme condition [t(58) = −3.056, p < .005], suggesting the onset condition was harder than the rhyme condition. Pearson correlation analysis showed that children’s reading score was correlated only with the accuracy of the onset condition, showing that the higher reading ability was associated with better performance in the onset condition.
Table 3.
Mean accuracy and reaction time on the task and its correlation with reading
| Onset ACC (%) |
Rhyme ACC (%) |
Onset RT (ms) |
Rhyme RT (ms) |
|
|---|---|---|---|---|
| Mean (SD) | 59 (20) | 78 (16) | 1739 (328) | 1649 (255) |
| Correlation with reading | .413* | .068 | .113 | .170 |
3.2. Brain activation
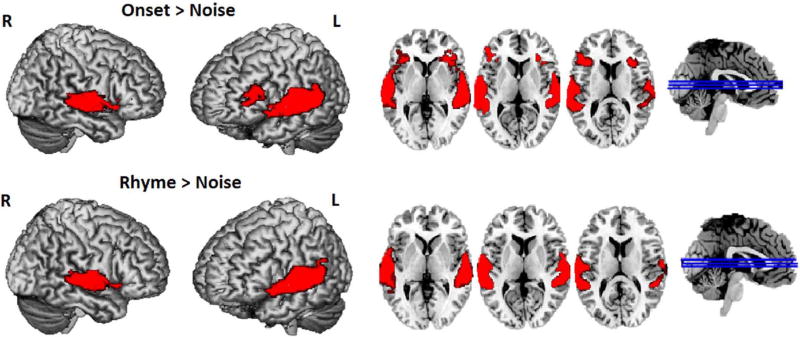
For the whole brain analysis, comparisons of onset > noise conditions and rhyme > noise conditions were conducted. The threshold for a significant cluster (pthr = 0.005, alpha = 0.05) within the whole brain mask (133,192 voxels) was 307 voxels. As shown in Fig. 1 and Table 4, the comparison of onset > noise revealed activation in the bilateral superior temporal gyrus that extended to left inferior frontal gyrus (BA 44/45) and bilateral insula (BA 13), whereas the comparison of rhyme > noise showed activation in the bilateral superior temporal gyrus only. No significant clusters were found when directly contrasting the onset versus rhyme conditions both at the whole brain level and within the vOT mask.
Fig. 1.
Brain activation in the onset > noise (upper panel) and rhyme > noise (lower panel) conditions, threshold at p < 0.005 k > 307.
Table 4.
Brain activation of onset > noise and rhyme > noise conditions
| Region | L/R | Brodmann Area | MNI Coordinates | Voxels (2 × 2 × 2 mm3) | Z |
|---|---|---|---|---|---|
| Onset > Noise | |||||
| Superior Temporal Gyrus | L | 22 | −64 −10 8 | 3586 | 13.64 |
| Superior Temporal Gyrus | R | 22 | 60–28 4 | 2182 | 11.21 |
| Rhyme > Noise | |||||
| Superior Temporal Gyrus | L | 22 | −62 −10 8 | 2494 | 13.33 |
| Superior Temporal Gyrus | R | 22 | 62 −30 4 | 1510 | 11.13 |
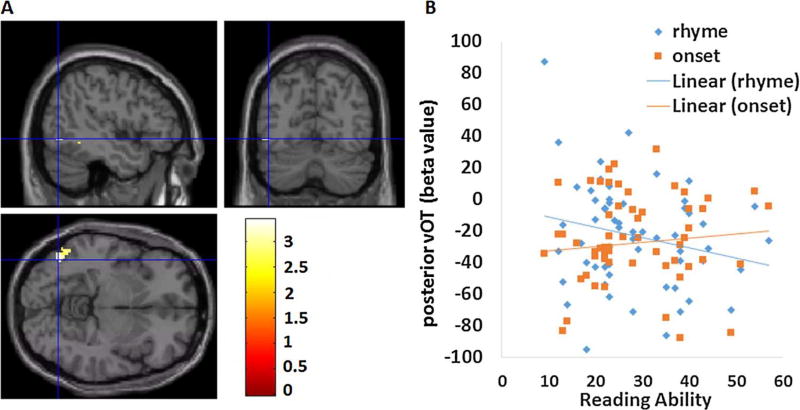
In order to examine activation as a function of reading ability, we conducted regression analyses using reading scores as a predictor for the onset > rhyme contrast and the rhyme > onset contrast within our hypothesized left vOT masks. The regression analysis for the onset > rhyme contrast showed, as expected, a cluster within the posterior left vOT mask (see Table 5, Fig. 2A). The threshold for a significant cluster (pthr = 0.005, alpha = 0.05) within the posterior left vOT mask (484 voxels) was 12 voxels. As shown in the scatterplot of Fig. 2B, increasing reading ability was associated with greater activation in the onset condition, whereas it was associated with less activation in the rhyme condition. In order to rule out the effect of difficulty on the activation of vOT, we repeated this analysis regressing out accuracy or RTs on the onset and rhyme conditions, and still found significant interaction effects at a similar site in the posterior vOT (coordinate −48 −66 −4, cluster size 18 voxels after regressing out accuracies, coordinate −38 −66 −6, cluster size 23 voxels after regressing out RTs). Follow-ups for each condition showed there was not a significant effect of the rhyme or onset condition. Therefore, the significant interaction with reading ability in the posterior left vOT is not only due to the increasing activation of the posterior vOT in the onset condition, but also due to the decreasing activation of the posterior vOT in the rhyme condition. These two trends suggest that the posterior vOT is becoming more responsive to small grain size while less responsive to large grain size as reading ability increases. Further analyses showed no significant effects in the anterior left vOT for rhyme > onset contrast. In addition, no significant clusters were found in the regression analysis for whole brain mask (outside the left vOT) in all the above regression analyses.
Table 5.
Regression analyses for onset > rhyme contrast.
| Region | L/R | Brodmann Area | coordinates (x y z) |
voxels | Z |
|---|---|---|---|---|---|
| Occipito-Temporal Cortex | L | 37 | −46 −68 −6 | 28 | 3.26 |
Fig. 2.
Regression analyses for onset > rhyme condition. (A) Significant effect of onset > rhyme in the regression analysis at posterior left vOT, threshold at p < 0.005, k > 12 voxels (corrected). (B) Scatter plot illustrates the correlation for each condition.
4. Discussion
Reading connects oral and written language. Previous behavioral studies have suggested that the development of reading skill drives the connection between phonology and its orthographic representations first at phoneme level and then at larger grain size level (e.g. Ziegler and Ferrand, 1998). Therefore, the goal of the present fMRI study was to determine whether reading skill was associated with automatic activation of orthographic representations in the left vOT during spoken language processing in 5–6-year-old children. More importantly, we aimed to determine whether this association was regionally specific depending on the grain size of the phonological awareness judgment. In terms of behavior, we found that the smaller grain size onset judgments were more difficult and more strongly related to reading skill than the larger grain size rhyme judgments. In terms of neural activation, we found higher reading ability was associated with greater sensitivity in the posterior left vOT that is proposed to be associated with processing small orthographic grain sizes, such as letters. Moreover, this correlation was specific for the onset compared to rhyme contrast, suggesting that better reading skill in young readers is associated with greater engagement of the posterior vOT for smaller grain sizes and less engagement for larger grain sizes, which is supportive of greater specialization of this region with increasing reading skill in beginning readers. In contrast, there was no correlation with reading skill in the anterior left vOT for rhyme compared to onset conditions. The anterior left vOT is proposed to be sensitive to larger orthographic grain sizes, such as bigrams and trigrams, so this lack of a correlation suggests that the coupling between phonology and orthography at a larger grain size has not been robustly established for young readers.
The main finding of our study was that higher reading skill in 5–6-year-old children was associated with greater sensitivity for the onset > rhyme contrast in the posterior left vOT during an auditory phonological task. Small grain size was measured by an onset judgment while large grain size was measured by a rhyme judgment to word pairs. This finding suggests that the posterior left vOT shows preference for small compared to large grain size for beginning readers with higher reading skill and is consistent with the hypothesis that left vOT is sensitive to orthographic grain size along posterior to anterior gradient (Dehaene et al., 2005). Our results are also consistent with many previous fMRI studies on older readers that showed the left vOT was activated in auditory language tasks and that the level of activation in this region was associated with reading ability (Yoncheva et al., 2010; Dehaene et al., 2010; Cone et al., 2008; Desroches et al., 2010). However, unlike these studies, we didn’t find significant vOT activation in the whole brain level analysis across the entire sample. We only found activation modulated by reading skill. This difference is likely because the vOT activation of older readers tends to be more robust than the younger children in our sample who are at the onset of literacy. Because higher reading skill drives greater automatic coupling between orthographic and phonological representations, overall the left vOT is likely well developed in older readers but underdeveloped in beginning readers.
Our results are broadly consistent with previous fMRI studies in young children that have shown associations of reading skill with automatic activation of the vOT during spoken language processing. Hutton et al. (2015) study found that more extensive reading exposure was associated with greater activation in posterior left vOT in a story listening task. Compared to our results, the vOT in Hutton et al. (2015) study was even more posterior, which may reflect more basic visual processing. The narrative story listening task in the Hutton et al. (2015) study is likely to elicit a visually imagined scene of the stories rather than the visual word forms. Therefore, the results of the study by Hutton et al. (2015) do not likely reflect activation of the corresponding orthographic representation. Our results are also broadly consistent with two previous fMRI studies of 5–6-year-old children that found FHD− children showed greater activation in the vOT than FHD+ children during an onset judgment task (Raschle et al., 2012) and that there was a positive correlation between left vOT and home literacy environment (Powers et al., 2016). However, Raschle’s study found that risk status was associated with activation in a region about 2 cm posterior to our region. This could be because only 2% of the children in the Raschle et al. (2012) study could recognize words, whereas most of our participants could read words. It could be that the children in Raschle et al. (2012) study were still at logographic stage where they treat words as visual symbols and attach them to their corresponding phonology and semantics, so they were relying on non-linguistic visual representations in occipital cortex. In contrast, the left vOT in Powers’ study was slightly anterior to our results perhaps because they used onsets sharing biphones (e.g., bed & belt), which are of a larger grain size than the onset task of our study that only shared one phone (e.g. nut & nail). Finally, Dębska et al. (2016) found FHD− kindergarteners showed greater activation in posterior left vOT as compared to FHD+ children, suggesting an effect of at-risk reading status on the posterior vOT activation during spoken language processing. Like the participants in our study, the Polish kindergarteners in the Dębska et al. (2016) study were also beginning readers with some letter knowledge, word reading and pseudo-word reading ability. However, our study showed posterior vOT sensitivity to onset judgments, whereas the Dębska et al. (2016) study showed sensitivity to an auditory rhyme task, a larger grain size phonological task. This difference may be because Polish is an orthographically transparent language with regular letter-sound mappings, so these Polish-speaking kindergarteners are more likely to use automatic letter-sound mapping even for larger grain sizes due to the greater consistency of the Polish language. In contrast, English is an opaque language with inconsistent letter-sound mappings, and therefore young English-speaking children are not as likely to rely on letter-sound mapping for larger grain sizes because they are less useful. Even though the rhyming word pairs could be processed at large grain size in Polish, the children in the Dębska et al. (2016) study were likely to only have well developed phoneme-to-letter mappings and rely on this phoneme-to-letter mapping to process larger grain sizes. Therefore, the skill level of the readers and the nature of the orthography both lead to greater involvement of letter sensitive posterior vOT. In summary, our study is broadly consistent with previous studies showing that the automatic activation of the vOT during spoken language processing is related to reading skills in young children. We specifically showed that the posterior vOT implicated in small orthographic grain size letter processing is more selectively involved in onset judgments in 5–6-year-old children who are better readers.
Although we demonstrated a robust effect in the posterior left vOT, we did not show that reading skill was associated with activation in the anterior left vOT for the comparison of rhyme versus onset conditions. One possible reason is that children in our study have not yet established the automatic connection between large grain size phonology and orthography. Reading acquisition appears to drive the connection between orthography and phonology initially at the letter-phoneme level and then at larger grain sizes. According to the Frith (1985) model, the orthographic stage is only reached after much reading practice that builds the knowledge of spelling patterns that recur across words. Most children in our sample are likely in the alphabetic stage of reading, solidifying the phoneme-to-letter correspondences. Even though children are proficient at rhyming at quite an early age, even before reading, their phonological representations at this larger grain size are weakly interconnected with orthography.
Apart from the findings in our left vOT region of interest, our analyses at the whole brain level revealed that bilateral superior temporal gyri were activated in both the onset and rhyme judgments compared to noise. Bilateral superior temporal gyri are engaged in the analysis of physical features of speech and activation in this region has consistently been found for various auditory phonological processing tasks (Liebenthal et al., 2005). In addition, we found that left inferior frontal gyrus and bilateral insula were involved in onset but not rhyme judgments, which may reflect additional effort needed in the onset judgment. These two areas have been shown to be associated with verbal working memory (Kurth et al., 2010; Liakakis et al., 2011 for review), and activation in the inferior frontal gyrus has often been shown in tasks involving effortful selection, retrieval or manipulation of phonological representations (Fiez et al., 1999). Even though these regions were active in the onset and not rhyme condition, when directly contrasting onset versus rhyme, there were no significant effects. However, as discussed above, we did find an effect in the posterior vOT in onset > rhyme contrast as a function of reading ability, suggesting the sensitivity of the posterior vOT to small grain size is reading ability dependent in young children.
In terms of behavior, we found that onset was more difficult than rhyme judgment as shown by lower accuracy and longer reaction time. This is consistent with an extensive body of research showing that children first master phonological awareness at larger grain sizes, and then acquire smaller grain size awareness (Ziegler and Goswami, 2005). In addition, our study revealed that better reading was only associated with higher accuracy for the onset but not rhyme. This is consistent with previous studies in young children using similar explicit phonological tasks that showed phoneme awareness is a better predictor of current and future reading ability than the awareness of rhyme (Melby-Lervåg et al., 2012). Phonological awareness and reading appear to have bi-directional influences (Wagner et al., 1994). Learning to read influences the development of phonology, especially phoneme awareness (Anthony and Francis, 2005). Phonemes produced in speech are acoustically inseparable because of co-articulation, so higher reading ability in young children might be helpful in discovering phonemes in spoken words. This may explain why higher reading skill was associated with better onset judgments in the current study.
In summary, we investigated where and how reading acquisition influences neural activation during spoken language processing, specifically, during a phonological awareness task. Our results show that for young 5–6-year-old children, better reading skill was correlated with greater sensitivity in posterior left vOT for small phonological grain sizes. Because reading acquisition appears to drive the connection between phonological and orthographical representations, our results indirectly suggest that better reading skill leads to greater sensitivity in the posterior vOT to small orthographic grain sizes, such as letters. In contrast, reading skill had no influence on the sensitivity in anterior left vOT for larger orthographic grain sizes, such as rhymes. Because our study only included young children, their anterior vOT might have not yet developed sensitivity to bigrams or trigrams. For future studies, it is important to examine whether reading skill tunes the sensitivity to large grain sizes in older children during spoken language processing in anterior left vOT. This would suggest a developmental progression of first sensitivity to smaller grain size in posterior vOT and later sensitivity to larger grain sizes in anterior vOT.
Supplementary Material
Acknowledgments
This research was supported by R01 DC013274 to James R. Booth.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.01.011.
References
- Anthony JL, Francis DJ. Development of phonological awareness. Curr. Dir. Psychol. Sci. 2005;14(5):255–259. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The english lexicon project. Behav. Res. Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Hornickel J, Cone NE, Burman DD, Booth JR. Neural correlates of orthographic and phonological consistency effects in children. Hum. Brain Mapp. 2008;29(12):1416–1429. doi: 10.1002/hbm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: distinct developmental ERP and fMRI effects. Hum. Brain Mapp. 2009;30(6):1833–1844. doi: 10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Kujala JV, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proc. Natl. Acad. Sci. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson K, Boustead T, Gillon G. Content validity to support the use of a computer-based phonological awareness screening and monitoring assessment (Com-PASMA) in the classroom. Int. J. Speech-Lang. Pathol. 2015;17(5):500–510. doi: 10.3109/17549507.2015.1016107. [DOI] [PubMed] [Google Scholar]
- Castles A, Wilson K, Coltheart M. Early orthographic influences on phonemic awareness tasks: evidence from a preschool training study. J. Exp. Child Psychol. 2011;108(1):203–210. doi: 10.1016/j.jecp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cone NE, Burman DD, Bitan T, Bolger DJ, Booth JR. Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. Neuroimage. 2008;41(2):623–635. doi: 10.1016/j.neuroimage.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: false positive rates redux. BioRxiv. 2016:065862. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dębska A, Łuniewska M, Chyl K, Banaszkiewicz A, Żelechowska A, Wypych M, Marchewka A, Pugh KR, Jednoróg K. Neural basis of phonological awareness in beginning readers with familial risk of dyslexia—results from shallow orthography. Neuroimage. 2016;132:406–416. doi: 10.1016/j.neuroimage.2016.02.063. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cognit. Sci. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cognit. Sci. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene-Lambertz G, Kolinsky R, Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Desroches AS, Cone NE, Bolger DJ, Bitan T, Burman DD, Booth JR. Children with reading difficulties show differences in brain regions associated with orthographic processing during spoken language processing. Brain Res. 2010;1356:73–84. doi: 10.1016/j.brainres.2010.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24(1):205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Frith U. Beneath the surface of developmental dyslexia. Surf. Dyslexia. 1985;32:301–330. [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform face area is part of a network that processes faces at the individual level. J. Cognit. Neurosci. 2000;12(3):495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Hutton JS, Horowitz-Kraus T, Mendelsohn AL, DeWitt T, Holland SK C-MIND Authorship Consortium. Home reading environment and brain activation in preschool children listening to stories. Pediatrics. 2015;136(3):466–478. doi: 10.1542/peds.2015-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, second edition. Pearson, Bloomington, MN: 2004. [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behav. Brain Res. 2011;225(1):341–347. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Binder JR, Spitzer SM, Possing ET, Medler DA. Neural substrates of phonemic perception. Cereb. Cortex. 2005;15(10):1621–1631. doi: 10.1093/cercor/bhi040. [DOI] [PubMed] [Google Scholar]
- Mazaika PK, Hoeft F, Glover GH, Reiss AL. Methods and software for fMRI analysis of clinical subjects. Neuroimage. 2009;47:S58. [Google Scholar]
- Melby-Lervåg M, Lyster SAH, Hulme C. Phonological skills and their role in learning to read: a meta-analytic review. Psychol. Bull. 2012;138(2):322–352. doi: 10.1037/a0026744. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida Word Association, Rhyme, and Word Fragment Norms. 1998 doi: 10.3758/bf03195588. http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Powers SJ, Wang Y, Beach SD, Sideridis GD, Gaab N. Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Ann. Dyslexia. 2016;66(3):337–360. doi: 10.1007/s11881-016-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends Cognit. Sci. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc. Natl. Acad. Sci. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour HN, Roeper T, De Villiers JG. DELV: Diagnostic Evaluation of Language Variation: Screening Test. PsychCorp 2003 [Google Scholar]
- Vitevitch MS, Luce PA. A web-based interface to calculate phonotactic probability for words and nonwords in English. Behav. Res. Methods Instrum. Comput. 2004;36:481–487. doi: 10.3758/bf03195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Development of reading-related phonological processing abilities: new evidence of bidirectional causality from a latent variable longitudinal study. Dev. Psychol. 1994;30(1):73. [Google Scholar]
- Wiig EH, Semel E, Secord WA. Clinical Evaluation Language Fundamentals (CELF-5) 5. Pearson, San Antonio: 2013. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Riverside Publishing. Itasca, IL: 2001. Woodcock-Johnson III. [Google Scholar]
- Yoncheva YN, Zevin JD, Maurer U, McCandliss BD. Auditory selective attention to speech modulates activity in the visual word form area. Cereb. Cortex. 2010;20(3):622–632. doi: 10.1093/cercor/bhp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Ferrand L. Orthography shapes the perception of speech: the consistency effect in auditory word recognition. Psychonomic Bull. Rev. 1998;5(4):683–689. [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol. Bull. 2005;131(1):3. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.