Abstract
Importance:
Oral anticancer medications are increasingly important but costly treatment options. By early 2017, 43 states and Washington, D.C. had passed laws to ensure privately-insured patients in fully-insured health plans pay no more for orally-administered than infused anticancer medications. Federal legislation is pending. Despite their rapid uptake, the effect of state oral chemotherapy parity laws has not been described.
Objective:
Estimate changes in oral anticancer medication use, out-of-pocket spending and total health plan spending associated with state adoption of oral chemotherapy parity laws.
Design:
Observational study using a difference-in-differences approach.
Setting:
Administrative health plan claims from 2008–2012 for three large nationwide insurers aggregated by the Health Care Cost Institute.
Participants:
Adults (18–64) living in one of sixteen states passing parity during the study period who received drug treatment for a cancer for which orally-administered treatment options were available (N=63,780).
Exposure:
Time before and after parity adoption, controlling for whether the patient was in a plan subject to parity (fully-insured) or not (self-funded, exempt via the Employee Retirement Income Security Act).
Outcomes:
Oral anticancer medication use, out-of-pocket spending, and total health care spending.
Results:
Oral anticancer medication use increased from 18 to 22% of anticancer medication treatments over time, regardless of parity status (p=0.34). In plans subject to parity, the proportion of fills for orally-administered therapy with no copayment increased from 15% to 53%, more than double that observed in plans not subject to parity (p<0.001). The proportion with out-of-pocket spending >$100/month increased from 8.4% to 11.1%, a larger increase than observed in plans not subject to parity (p=0.004). In plans subject to parity, estimated monthly out-of-pocket spending decreased by $19.44, $32.13 and $10.83 at the 25th, 50th and 75th percentiles of spending (all p<0.001), but increased at the 90th and 95th percentiles by $37.19 and $143.25 (both p<0.001). Parity did not increase six-month total spending overall or for enrollees using anticancer therapy.
Conclusions and Relevance:
While oral chemotherapy parity laws modestly improved financial protection for many patients without increasing total health care spending, these laws alone may be insufficient to ensure that patients are protected from high out-of-pocket costs.
BACKGROUND
Orally-administered anticancer medications are an increasingly important part of cancer treatment. By mid-2015 there were over fifty orally-administered anticancer medications approved by the U.S. Food and Drug Administration, with more anticipated in coming years, but they are expensive, with list prices often exceeding $100,000/year.1,2
Proponents of legislation aimed at limiting patient out-of-pocket expenditures suggest that anticancer medications obtained under a patient’s pharmacy benefit can require higher enrollee cost-sharing than infused therapy covered under the medical benefit,3 potentially impacting patient access to outpatient prescriptions.4–7 In response, since 2008 43 states and Washington D.C. have passed laws to ensure parity in cost-sharing for oral and intravenous anticancer therapies (i.e., “oral chemotherapy parity” laws).8 These laws are intended to ensure that cost-sharing for patients (e.g., copayments, coinsurance, benefit limits) is equivalent for anticancer drugs obtained with medical (intravenous) and pharmacy (oral) benefits. Despite their rapid uptake, the effect of the state chemotherapy parity laws on oral anticancer medication use and patient and health care spending is unknown.
METHODS
We used 2008–2012 national administrative health plan claims from the Health Care Cost Institute for privately-insured members of Aetna, Humana, and UnitedHealthcare to estimate the effect of oral chemotherapy parity laws on use of and out-of-pocket spending on orally-administered anticancer medications. We studied patients in states implementing parity between July 1, 2008-July 15, 2012 who were 18–64 years old, had prescription drug coverage, were treated with infused or orally-administered anticancer medication, and had diagnosis codes for a cancer for which orally-administered drugs were available (N=16 states; 72,500 patients). We excluded individuals without 3 months of continuous health plan enrollment before the observation month (for comorbidity measurement) (n=6,104) and those missing plan funding status (n=2,616). In total, 63,780 individuals with 375,387 person-months of anticancer medication use were included.
We identified infused anticancer therapy from outpatient and physician service claims and orally-administered anticancer medications from pharmacy claims. Following prior work, we included targeted orally-administered anticancer medications and capecitabine (which has an infused equivalent), excluding breast cancer endocrine therapies.9,10 We measured oral anticancer therapy use as a proportion of all anticancer therapy provided in each person-month.
We summarized per-fill out-of-pocket spending on oral anticancer medications, including copayment, coinsurance and deductibles, adjusting to reflect spending on a median monthly dose. We also summarized six-month total health care spending beginning with the patient’s first observed anticancer therapy.11
Statistical Analysis
We used a propensity score weighted difference-in-differences approach to estimate the net impact of parity among individuals in fully-insured plans, controlling for changes over time among individuals in self-funded plans (not subject to parity) in those states.12
For our three models with binary outcomes – the probability of using oral anticancer medications; paying $0/month; and paying >$100/month – we used generalized estimating equations with log links and binomial distributions to account for repeated observations.13 Because we observed non-linear changes in out-of-pocket spending as a result of parity, we used quantile regression to estimate changes at the 25th, 50th, 75th, 90th, 95th percentiles of monthly out-of-pocket spending.14,15 Finally, for six-month total health care spending, we used generalized estimating equations with log links and gamma distributions and retransformed model estimates to 2012 USD. For further model descriptions and sensitivity analyses see the online appendix.
RESULTS
Of 63,780 individuals using anticancer medications in states that passed parity during the study period, 51.4% were in fully-insured plans and 48.6% were in self-funded plans. After propensity score weighting, patients were well balanced on measured characteristics (eTable 1).
Oral anticancer medication use as a proportion of all anticancer medication use increased from approximately 18% to 22%, with no significant differences attributed to parity (adjusted difference-in-differences Risk Ratio [aDDRR]:1.04,95%CI:0.96−1.13; p=0.34).
In both fully-insured and self-funded health plans, monthly out-of-pocket spending was $50 or less for most fills for orally-administered and infused anticancer medications both before and after parity (eFigure 1). After parity, the probability of paying nothing for orally-administered anticancer medications more than doubled in fully-insured plans as compared with self-funded plans (aDDRR:2.42,95%CI:2.03−2.89). However, there was an increase in the proportion of fills with out-of-pocket spending of >$100 in fully-insured plans relative to self-funded plans over that same period (aDDRR:1.36,95%CI:1.11−1.68). In contrast, among infused treatments, there was no difference in the probability of paying nothing (aDDRR:0.99,95%CI:0.89−1.10) or the probability of paying >$100 (aDDRR:1.05, 95%CI:0.79−1.39).
When considering the distribution of spending, parity was associated with modest but statistically significant decreases in estimated monthly out-of-pocket spending on orally-administered anticancer medications at the 25th, 50th and 75th percentiles of the out-of-pocket spending by $19.44, $32.13 and $10.83, respectively (all p<0.001). However, spending increased at the 90th and 95th percentiles by $37.19 (both p<0.001) and $143.25 (Figure 1). In analyses excluding deductibles, results were consistent but out-of-pocket spending increases at the 90th and 95th percentiles were somewhat lower ($24.32 and $49.43, respectively) and not statistically significant for the 95th percentile (not shown).
Figure 1. Association of Parity Laws with Monthly Out-of-Pocket Spending for Orally-Administered Anticancer Medications.
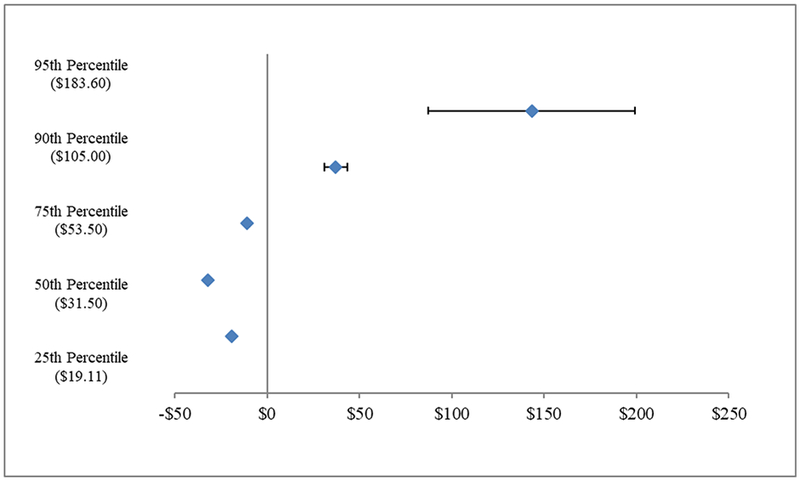
Source: Authors analysis of Health Care Cost Institute Claims, 2008–2012. N=85,107 observations
Quantile regression analyses in propensity-weighted cohorts to predict changes in the distribution of patient out-of-pocket spending on a single fill of orally-administered anticancer therapy. Per fill medication costs were adjusted to reflect a standardized dose of therapy and inflation adjusted to 2012 dollars using the medical component of the consumer price index. Models were estimated using PROC QUANTREG in SAS 9.4. Values in parentheses represent baseline per-fill spending for each percentile.
Total health care spending for 6-month treatment episodes averaged $87,328 for patients in fully-insured plans and $84,103 for patients in self-funded plans (Figure 2) with no differences by parity status.
Figure 2. Association of Parity Laws with Six Month Total Health Care Spending.
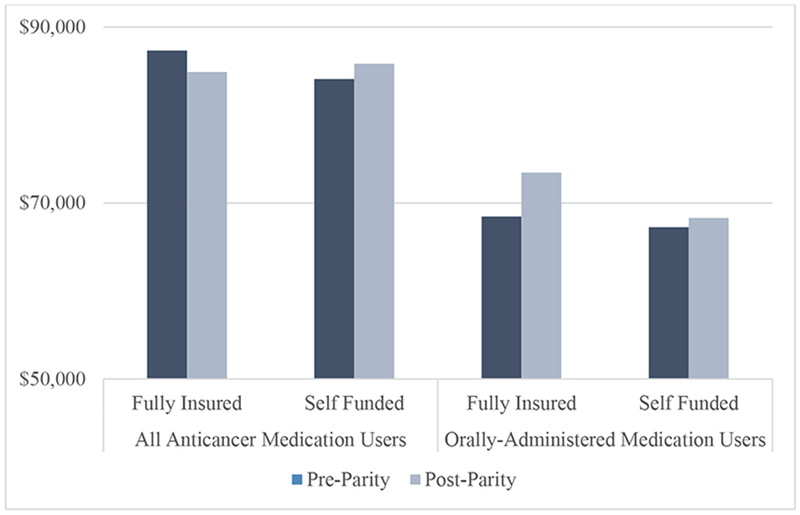
Source: Authors analysis of Health Care Cost Institute Claims, 2008–2012. N = 63,780 (all users) N = 11,141 (oral users only)
Propensity score weighted generalized estimating equations with log links and gamma distributions were used to estimate six-month spending on health care services. Models were estimated using PROC GENMOD in SAS 9.4. There were no significant difference in total health care spending as a result of parity for all anticancer medication users (adjusted difference-in-differences [aDD] Risk Ratio:0.96, 95%CI:0.90−1.02; p=0.09) or orally-administered anticancer medication users (aDD Risk Ratio: 1.06, 95%CI:0.93−1.20; p=0.40).
DISCUSSION
Although oral chemotherapy parity laws have been widely adopted by states, these laws have not consistently reduced out-of-pocket spending for orally-administered anticancer medications. Specifically, parity laws reduced monthly out-of-pocket spending on fills at the lower end of the out-of-pocket spending distribution, but increased spending for those at the top of the spending distribution. This is evidenced by the more than doubling of medication fills with no cost-sharing and the simultaneous increase in fills with at least $100 in cost-sharing post-parity.
Our findings illuminate several important issues for privately insured patients. First plans typically required relatively modest cost-sharing before and after parity (<$50 monthly). However, approximately 5% of fills had out-of-pocket spending of ≥$500 in fully-insured plans after parity, suggesting that parity requirements alone may not address high out-of-pocket spending for some patients. Second, as expected, estimated out-of-pocket spending did not change for patients in self-funded plans, which are exempt from state mandates. Federal legislation would be required to extend parity to individuals in self-funded plans. Finally, opposition to state efforts have centered on concerns that improved coverage for orally-administered chemotherapy would increase health care spending overall, but we found no evidence of this for six-month health care spending.
Our study has some important limitations. First, we were limited to studying sixteen states that passed parity during from 2008–2012 so our findings may not represent all states with parity or more recent time periods. However, the laws passed more recently have been nearly identical to laws in the sixteen states studied. Second, we could not observe use of manufacturer coupons, and patients with very high cost-sharing who do not fill their prescriptions are unobserved. Third, we studied patients in three health plans, so results may not generalize to other insurers. However, Aetna, UnitedHealthcare and Humana are among the largest private insurers in the US. Finally, the orally-administered anticancer medications included were all branded products and out-of-pocket spending requirements may differ for generic or biosimilar medications.
Our findings suggest that, while state oral chemotherapy parity laws modestly improved financial protection for many patients without increasing total health care spending, these laws alone may be insufficient to ensure that patients are protected from high out-of-pocket costs.
Supplementary Material
KEY POINTS
Question:
How have state oral chemotherapy parity laws changed use of and spending on orally-administered anticancer drugs?
Findings:
In this observational study we found that parity laws appeared to reduce monthly out-of-pocket spending on fills at the lower end of the out-of-pocket spending distribution, but appeared to increase spending for those with the highest out-of-pocket spending. Parity had no impact on six month total spending.
Meaning:
Although oral chemotherapy parity laws have been widely adopted by states, these laws have not consistently reduced out-of-pocket spending for orally-administered anticancer medications.
TWEET:
Oncology parity laws reduce out-of-pocket price for some, but not all, cancer patients.
ACKNOWLEDGMENT
Dr. Dusetzina had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the design, analysis, drafting and critical revision of this manuscript and all authors have approved the final version. This project was supported by a Research Scholar Grant, RSGI-14-030-01-CPHPS from the American Cancer Society (Dr. Dusetzina). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Dusetzina serves on the National Academy of Sciences, Engineering, and Medicine Committee “Ensuring Patient Access to Affordable Drug Therapies.”
The authors acknowledge the assistance of the Health Care Cost Institute (HCCI) and its data contributors, Aetna, Humana, and UnitedHealthcare, in providing the claims data analyzed in this study.
Dr. Keating is also supported by K24CA181510 from the National Cancer Institute.
Drs. Dusetzina, Huskamp, Basch, Keating and Mr. Winn have no conflicts to disclose.
REFERENCES
- 1.Bach PB. Cancer drug costs for a month of treatment at initial Food and Drug Administration approval. Cost of Cancer Drugs 2014; http://www.mskcc.org/research/health-policy-outcomes/cost-drugs. Accessed 3/31/2014, 2014. [Google Scholar]
- 2.Dusetzina SB. Drug Pricing Trends for Orally Administered Anticancer Medications Reimbursed by Commercial Health Plans, 2000–2014. JAMA oncology. 2016;2(7):960–961. [DOI] [PubMed] [Google Scholar]
- 3.Andrews M Some states mandate better coverage of oral cancer drugs. 2012; http://www.kaiserhealthnews.org/features/insuring-your-health/2012/cancer-drugs-by-pill-instead-of-iv-michelle-andrews-051512.aspx. Accessed 9/18/2013, 2013.
- 4.Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. Journal of oncology practice. 2011;7(3 Suppl):46s–51s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncology. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 6.Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncology. 2012;30(14):1608–1614. [DOI] [PubMed] [Google Scholar]
- 7.Winn AN, Keating NL, Dusetzina SB. Factors Associated With Tyrosine Kinase Inhibitor Initiation and Adherence Among Medicare Beneficiaries With Chronic Myeloid Leukemia. J Clin Oncology. 2016;34(36):4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Joffe S, Kesselheim AS. Chemotherapy parity laws: a remedy for high drug costs? JAMA internal medicine. 2014;174(11):1721–1722. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute Targeted Cancer Therapies. 2016; http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Accessed 03/30/2016, 2016.
- 10.Shih YC, Smieliauskas F, Geynisman DM, Kelly RJ, Smith TJ. Trends in the Cost and Use of Targeted Cancer Therapies for the Privately Insured Nonelderly: 2001 to 2011. J Clin Oncology. 2015;33(19):2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Medicaire and Medicaid Innovation Oncology Care Model. Centers for Medicare & Medicaid Services 2016, 2016. [Google Scholar]
- 12.Wooldridge JM. What’s New in Econometrics? Difference-in-Differences Estimation. http://www.nber.org/WNE/slides7-31-07/slides_10_diffindiffs.pdf 2007.
- 13.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 14.Heckman JJ, Smith J, Clements N. Making the most out of programme evaluations and social experiments: Accounting for heterogeneity in programme impacts. Rev Econ Stud. 1997;64(4):487–535. [Google Scholar]
- 15.Heckman JJ. The scientific model of causality. Sociol Methodol. 2005;35:1–97. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


