Abstract
Purpose
To investigate the pathophysiological effects of chronic kidney disease (CKD) on brain function in children with CKD by correlating cerebral blood flow (CBF) with clinical and behavioral indices.
Materials and Methods
In this prospective study, 73 pediatric patients with CKD (15.80 years ± 3.63; range, 9–25 years) and 57 controls (15.65 years ± 3.76; range, 9–25 years) were recruited. CBF measurements were acquired with a MR arterial-spin-labelling scheme. Neurocognitive measurements were performed using traditional and computerized neurocognitive batteries and clinical data were also collected. Group-level global and regional CBF differences between CKD and controls were assessed. Regression analyses were conducted to evaluate the associations between regional CBF, clinical variables and cognitive performance.
Results
Patients with CKD showed higher global CBF compared with controls that was attributable to reduced hematocrit (60.2±9.0 and 56.5±8.0 ml/100g/min, for CKD and control cohorts, respectively). White matter CBF and blood pressure (BP) were correlated (r=0.244, P=0.039), suggesting altered cerebrovascular autoregulation. Regional CBF differences between patients and controls included regions in the “default-mode’ network. CKD subjects with positive extrema in the precuneus showed a strong correlation with executive function (Rho=0.608, P=0.001).
Conclusion
Systemic effects of eGFR, Hct and BP on CBF and alterations in regional CBF may reflect impaired brain function underlying neurocognitive symptomatology in CKD. These findings further characterize the nature of alterations in brain physiology for children, adolescents, and young adults with CKD.
Introduction
Chronic kidney disease (CKD) is associated with alterations in blood pressure (BP), blood chemistry, and red blood cell production (1) that can potentially affect brain function. Previous studies in adults have shown that CKD is associated with subcortical ischemic lesions, atrophy, and deficits in cognitive performance (2, 3). However, the mechanisms and time course by which CKD affects brain function are unknown.
CKD-associated structural brain changes are characteristic of ischemic small vessel disease, which manifest as white matter hyperintensities on T2-weighted MRI and are most commonly seen in adults with chronic atherosclerotic vascular disease and hypertension. Moreover, even mild chronic kidney disease significantly increases the risk for transient ischemic attack and stroke (4). Given the high incidence of cardiovascular disease in CKD and the associations between CKD and neurological dysfunction (1, 5, 6), further characterization of cerebrovascular function in CKD is highly relevant, but studies of cerebrovascular function in adults with CKD are confounded by longstanding hypertension, itself a leading cerebrovascular risk factor. While CKD in adults is often a consequence of age-related disorders such as hypertension and diabetes, childhood CKD often occurs congenitally, yet still affects brain development and cognitive function (2, 7). Examining cerebrovascular function in a pediatric population with CKD provides the potential to dissociate CKD effects from those of associated chronic hypertensive vasculopathy.
Cerebral blood flow (CBF) provides a direct measure of cerebrovascular integrity by providing quantification of cerebral perfusion. Regional CBF also serves as a marker of regional neural activity owing to the tight coupling between regional CBF and neural activity and metabolism (8–10). However, existing data on regional CBF in CKD are limited, particularly for pediatric CKD. This limitation is partly due to the containdication for intravenous contrast in patients with kidney dysfunction, as the majority of MRI perfusion studies rely on dynamic contrast approaches.
Here we report the findings of ASL MRI data acquired in the NiCK cohort. The present report used ASL MRI in combination with individual Hct-corrected blood T1 to investigate CBF differences between pediatric patients and healthy control subjects. We hypothesized that CKD-associated systemic factors, such as anemia (18) and hypertension would cause global abnormalities in CBF, and that regional alterations in CBF would be associated with changes in neurocognitive performance in CKD patients.
Materials and methods
This prospective study was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia and informed consent was obtained from each participant. This study was Health Insurance Portability and Accountability Act compliant.
Participants
Between August 2011 and October 2014, seventy-three patients aged 8–25 years with CKD stage II to V and 57 age-matched control subjects were included (Table 1 and see supplementary materials)(17). The exclusion criteria were: (a) history of traumatic brain injury or other significant medical or neurological abnormality affecting motor or higher cortical functioning, (b) profound developmental disability or sensory-motor difficulties that would preclude valid use of diagnostic instruments or measurements based on eye tracking or MRI, (c) a DSM IV-TR Axis I disorder or other psychiatric symptoms that would interfere with the participant’s ability to participate in the study (e.g., active psychosis), and (d) known drug or alcohol use within 24 hours of any assessment. For controls, individuals matched in age were recruited. Participants in the typically developing control group had no history of CKD, nor any reported history of other neurological or psychiatric conditions.
Table 1.
Subject demographics and selected clinical measurements. Significant values are bolded.
| CKD (n=73) N, mean ±SD | Controls (n=57) N, mean ±SD | P | |
|---|---|---|---|
| Age (years) | 15.80±3.63 | 15.65±3.76 | 0.99a |
| Sex (M/F) | 48/25 | 29/28 | 0.06b |
| Race (White/Black/Asian) | 48/22/3 | 31/25/1 | 0.23c |
| Age of onset (years) | 6.33±6.44 | -- | |
| Length of disease (Months) | 112.15±73.82 | -- | |
| Hct (%) | 37.81±5.09 | 41.24±4.02 | <0.0001a |
| eGFR | |||
| CKiD | 45.71±24.22 | 98.83±20.04 | <0.0001a |
| MDRD (if >18yrs) | 48.60±23.01 | 109.58±13.22 | <0.0001a |
| Phosphate (mg/dl) | 4.65±1.00 | 4.42±0.62 | 0.09a |
| Calcium (mg/dl) | 9.42±0.48 | 9.46±0.33 | 0.57a |
| Calcium × phosphate | 43.56±8.22 | 41.91±6.75 | 0.22a |
| Total cholesterol (mm/dL) | 175.31±43.83 | 146.71±32.58 | <0.0001a |
| LDL cholesterol (mm/dL) | 97.37±33.83 | 78.78±25.15 | 0.004a |
| Triglycerides (mm/dL) | 131.41±71.30 | 80.46±59.72 | <0.0001a |
| Systolic BP (mm Hg) | 115.67±12.97 | 112.30±11.32 | 0.03a |
| Diastolic BP (mm Hg) | 65.97±10.32 | 62.60±8.74 | 0.02a |
CKD: chronic kidney disease, Hct: hematocrit, eGFR: estimated glomerular filtration rate, by CKiD equation, MDRD: modification of diet in renal disease if >18 years, LDL: low-density lipoprotein, BP: blood pressure,
a: Wilcoxon rank test,
b: Fisher’s exact test,
c: Chi-square test
Neurocognitive assessment
Assessments of the traditional neurocognitive battery (TNB) and a locally developed computerized neurocognitive battery (CNB) (19, 20) were performed. For TNB, a battery of age-specific standardized neurocognitive assessments was performed to assess targeted areas of neurocognition, including intellectual and executive functioning, attention, memory, and visual spatial processing (21). The CNB includes 14 tests assessing five neurobehavioral functions of executive control, episodic memory, complex cognition, social cognition and praxis speed. Each test provides measures of both accuracy and speed (22). All tests were administered by a trained examiner supervised by a licensed psychologist. The details of those measurements are described in the supplementary materials, along with laboratory and blood pressure measurements.
Data acquisition
MRI Scanning
MRI data were acquired on a 3-T whole-body Siemens Verio scanner (Erlangen, Germany) with an 8-channel receive-only head coil and body coil transmission. High-resolution structural MRI (MPRAGE sequence, 0.9×0.8×0.8 mm3, TR/TE=2000/3.3 ms) and T2-FLAIR data were collected for each participant. Resting CBF measurements were acquired with a pseudocontinuous ASL labeling scheme (23) implemented with 2D gradient-echo echo-planar imaging (EPI) sequence. The labeling duration was 1.5 sec with post-labeling delay of 1.2 sec. Multi-slice perfusion maps were acquired with the following parameters: repetition time (TR)/ echo time (TE) = 4000/12 ms, flip angle=900, bandwidth = 3005 Hz/pixel, slice thickness = 5 mm with 25% distance factor, matrix size = 64×64, FOV = 220×220 mm2, slice number = 20, and GRAPPA factor = 2 in phase-encoding direction. The total ASL MRI acquisition time was approximately 5 minutes for 40 label/control pairs.
Data processing and analysis
ASL CBF and hematocrit effects
MRI data were processed using Statistical Parametric Mapping (SPM8) and customized MATLAB scripts (The Mathworks Inc., Natick, MA). CBF maps were calculated using a modified perfusion data processing toolbox, ASLtbx (24). Global CBF values of whole brain (WB), gray matter (GM), and white matter (WM) areas were obtained from non-normalized segmented images (Figure S2). To optimally address the confound of Hct changes in ASL CBF quantification in CKD, we used the method of hematocrit-based T1 estimation (16) for the CBF quantification.
Statistical analysis
Sample size justification
The NiCK study had a targeted recruitment of 90 normal controls and 90 persons diagnosed with CKD (eGFR <60 ml/min/1.73m2). If we assume that in the 90 CKD patients the prevalence of MRI abnormalities is 12.5% and it is 1% in normal controls, we will have 80% power to detect such a difference with 95% confidence. This estimate was based on a review article citing a 12–50% prevalence of either white matter lesions or atrophy in children with kidney disease (25). The NiCK study ultimately recruited 92 patients and 70 controls. Data from study subjects with successful ASL MRI acquisitions were included in the present report.
Statistical mapping of group comparison in CBF maps
Group-level voxel-wise differences in normalized CBF maps between CKD patients and controls were assessed using a cluster-wise permutation-based method thresholded at P<0.05, with Hct, age, and sex as covariates of no interest. Subject-specific voxel-wise analysis was also performed to delineate regions with abnormally increased or decreased CBF (extreme voxels) in each individual participant using the distribution-corrected Z-score (DisCo-Z) method (26) (see Supplementary materials).
General linear model (GLM, type III sum of squares) was utilized to evaluate differences of global CBF values between CKD and control subjects, comparing group, sex, group*sex and adjusting for age and hematocrit. To further explore if group differences in global CBF were driven by Hct changes in CKD, we performed a stepwise regression analysis to examine the relationship between CBF outcome and factors of CKD (group), Hct, age and sex. The regression analysis was utilized to predict each of the CBF outcome (GM, WM, and WB) using group, age, sex, Hct and the interaction terms of group*gender, group*age and group*Hct as predictors. Because CKD has been associated with WM disease and WM was reported to be particular susceptible to ischemic injury with impaired CBF autoregulation due to its blood supply (27, 28), we further investigated the effect of altered BP on CBF changes, focusing on the white matter. We examined the correlation between BP and WM CBF by controlling Hct, age and sex.
To examine how regional CBF related to cognitive performance between the two groups, we performed correlation analyses on memory, executive function, language reasoning and spatial processing. Wilcoxon rank sum tests were used to evaluate the group differences in neurocognitive performance for each test between CKD and control subjects. Extreme value maps calculated from the subject-specific voxel-wise analysis were used as masks to extract CBF for each subject. The method of Generalized Estimating Equations was utilized to test the difference of neurocognitive performance between CKD subgroups with and without the presence of positive extrema in CBF. Spearman rank correlation analysis was then used to evaluate the association of extracted CBF and neurocognitive performance for both CKD and control subjects. Qualitative inspection of common locations of subject-specific increases in CBF for CKD patients showed high inter-subject overlap of clusters in the precuneus, which is similar to the group-level voxel-wise comparison (Figures 1 and 2). Therefore, an exploratory analysis was conducted to investigate the relationship between the CBF changes in the precuneus and neurocognitive impairment.
Figure 1.
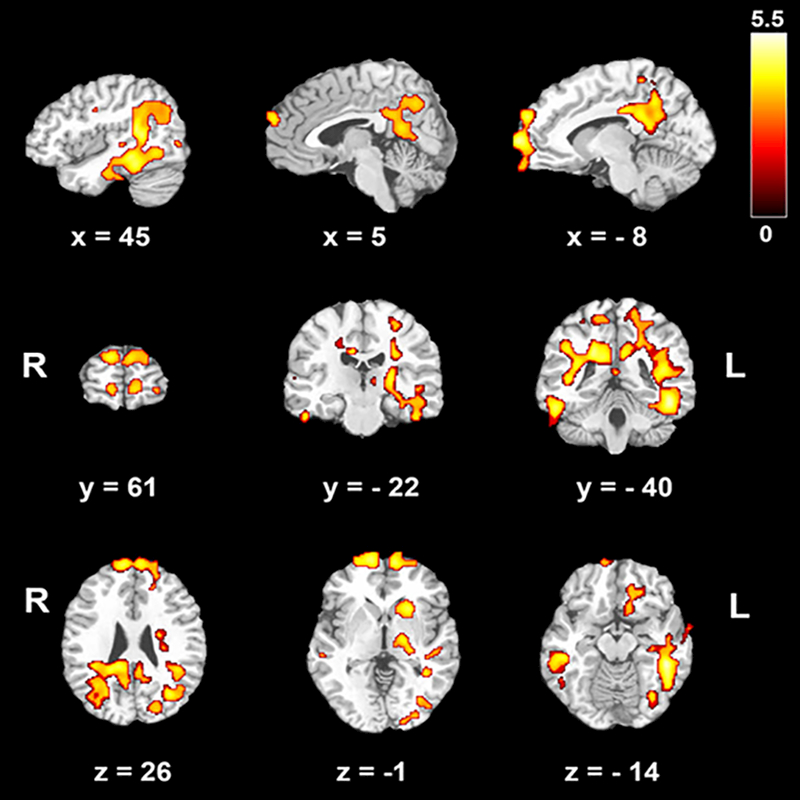
Voxel-wise group comparison in cerebral blood flow (CBF) after removing the effects of hematocrit, age and sex. The contrast chronic kidney disease (CKD) > Control is shown. There were no regions where controls showed greater CBF than CKD patients. The color bar indicates t scores. R: right, L: left. x, y, z: coordinates in Monteal Neurological Institute (MNI) space.
Figure 2.
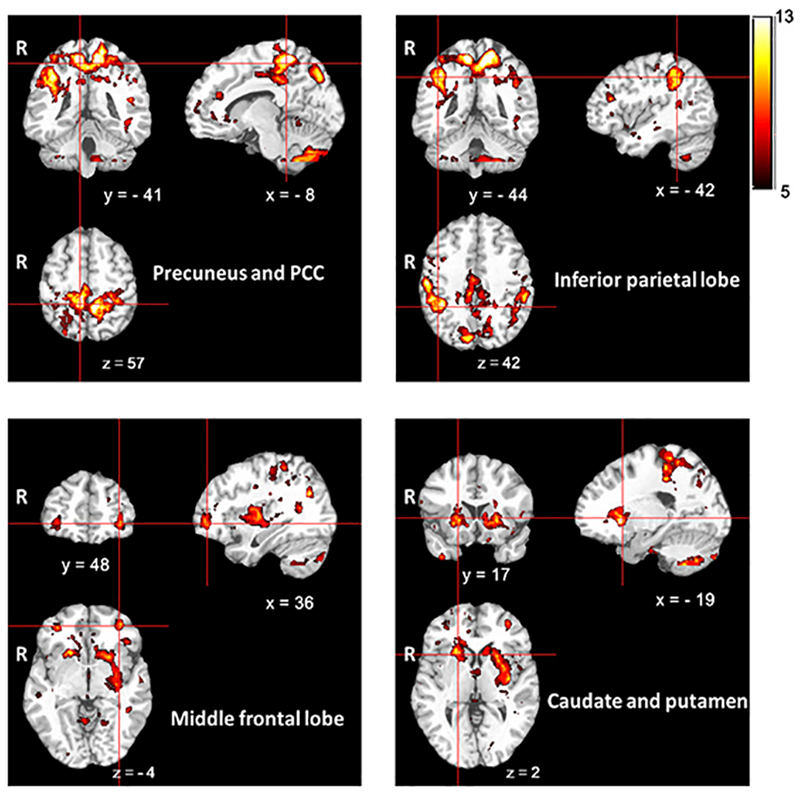
Overlapped clusters from all individual CKD subjects with positive extrema in CBF in the subject-specific voxel-wise analysis. The color bar indicates the total number of subjects who had subject-specific clusters with increased CBF. R: right. x, y, z: coordinates in Monteal Neurological Institute (MNI) space.
Results
Demographic and clinical characteristics
Table 1 shows the demographic and clinical characteristics of the subjects recruited for the study. As expected, CKD patients showed lower Hct and eGFR compared with controls (P<0.0001). Patients with CKD showed significantly elevated lipid levels (P<0.005). A significant difference was also noted between these two groups in systolic (P=0.03) and diastolic (P=0.02) BP.
Global CBF group comparison and clinical correlates
Patients showed a trend with higher mean GM and WB CBF values compared with controls (Table 2). However, GLM type III analysis showed that group differences in CBF were not significant after controlling for Hct (Table 2). Both CKD and control subjects showed significant correlations of CBF with age and Hct. Significant correlations of eGFR with Hct (Rho=−0.456, P<0.0005) and CBF (P < 0.0005, Table 3) were observed in control subjects but, after controlling for Hct, significant correlations between CBF and eGFR became much weaker in GM (Rho=0.342, P=0.01) and WB (Rho=0.291, P=0.03). For CKD patients, there was no significant correlation between eGFR and HCT (Rho=0.221, P=0.06), and no significant correlation between eGFR and CBF (P > 0.05, Table 3). WM and WB CBF were negatively correlated with the length of disease in CKD patients. CKD patients also showed a significant correlation of CBF with phosphate in GM, WM and WB areas, but not after controlling for Hct.
Table 2.
Cerebral blood flow (CBF, ml/100g/min) differences between chronic kidney disease (CKD) patients and control subjects in group comparison for, gray matter (GM), white matter (WM), and whole brain (WB) using general linear model (GLM) type III sum of squares (SS). Mean (SD) and estimated marginal mean values are presented.
| CBF | CKD (n=73) | Control (n=57) | (Pr>F)a |
|---|---|---|---|
| GM | 71.0 (11.1), 69.9 | 66.5 (9.9), 67.2 | 0.2 |
| WM | 31.8(4.6), 31.6 | 30.7(4.7), 30.9 | 0.4 |
| WB | 60.2(9.0), 59.4 | 56.5(7.9), 57.0 | 0.1 |
a:GLM type III SS, comparing group, sex, group*sex adjusting for age and hematocrit.
Table 3.
Univariate Spearman correlation coefficients for cerebral blood flow and clinical and demographic variables. Significant values are bolded.
| Characteristic | CKD (Rho, p-value) | Control (Rho, p-value) |
|---|---|---|
| Age (years) |
GM (−0.50, <0.001)* WM(−0.30, 0.01) WB(−0.53, <0.001) * |
GM (−0.40, 0.002) WM (−0.38, 0.003) WB (−0.51, <0.001)* |
| Race (White/Black/Asian) | GM (−0.16, 0.180) WM (−0.12, 0.331) WB (−0.14, 0.251) |
GM (0.17, 0.201) WM (0.21, 0.124) WB (0.21, 0.112) |
| Sex (male/female) | GM (0.15, 0.207) WM (0.07, 0.580) WB (0.13, 0.278) |
GM (−0.25, 0.057) WM (−0.09,0.487) WB (−0.28,0.037) |
| Age of onset (years) | GM (−0.01,0.95) WM (0.16, 0.19) WB (−0.02,0.86) |
|
| Length of disease (months) | GM (−0.23, 0.054) WM (−0.27, 0.021) WB (−0.24, 0.040) |
|
| Phosphate (mg/dl) |
GM (0.32, 0.007) WM (0.28, 0.021) WB (0.37, 0.001) |
GM (0.15, 0.257) WM (0.15, 0.266) WB(0.23, 0.084) |
| Calcium (mg/dl) | GM (−0.16, 0.187) WM (−0.10, 0.383) WB (−0.15, 0.202) |
GM (0.13, 0.334) WM (0.03, 0.812) WB (0.15, 0.259) |
| Total cholesterol (mm/dL) | GM (0.09, 0.466) WM (0.05, 0.650) WB (0.08, 0.477) |
GM (−0.06, 0.683) WM (0.03, 0.817) WB (−0.03, 0.844) |
| LDL cholesterol (mm/dL) | GM (0.08, 0.517) WM (0.07, 0.578) WB (0.08, 0.504) |
GM (−0.14, 0.297) WM (−0.03, 0.803) WB (−0.17, 0.218) |
| Triglycerides (mm/dL) | GM (0.05, 0.656) WM (0.04, 0.734) WB (0.03, 0.777) |
GM (−0.23, 0.091) WM (−0.14, 0.307) WB (−0.26, 0.060) |
| Hct (%) |
GM (−0.32, 0.005) WM (−0.21, 0.072) WB(−0.32, 0.006) |
GM (−0.44, <0.001)* WM (−0.38, 0.003) WB (−0.51, <0.001)* |
| eGFR (ml/min/1.73m2) | GM (−0.18, 0.127) WM (−0.07, 0.572) WB (−0.18, 0.120) |
GM (0.48,<0.001)* WM (0.23,0.086) WB (0.52,<0.001)* |
Note.--CKD: chronic kidney disease, Hct: hematocrit, eGFR: estimated glomerular filtration rate, LDL: low-density lipoprotein, GM: gray matter, WM: white matter, WB: whole brain.
P<0.001, statistical significant after Bonferroni correction.
We found that age, Hct and interaction of CKD by sex explained most of the variability in CBF for both GM and WB (GM CBF: regression coefficient= −1.09, −0.52 and 3.89, and P=5×10−6, 0.003 and 0.02 for age, Hct and CKD × sex, respectively; WB CBF: regression coefficient= −1.00, −0.44 and 2.82, and P=10−7, 0.001 and 0.03 for age, Hct and CKD × sex, respectively) (Table S1). Age explained most of the variability in WM CBF (regression coefficient= −0.43 and P=6.2×10−5). The effect of sex on CBF within each group was further explored using ANCOVA, controlling for age. Table S2 presents results comparing participants’ sex within each study group. In controls, females showed higher WB CBF values than males. However, the effect of sex on global CBF was not significant after controlling for Hct (ANCOVA, F=1.021, 0.962 and 1.384, P=0.317, 0.331 and 0.245 for CBF in GM, WM and WB areas, respectively). In the control group, males had a significant higher Hct than females (Table S2). In CKD patients, no significant sex differences in either CBF or Hct were observed (Table S2).
The CKD group showed a marginally positive correlation between BP and CBF in white matter (r=0.244, P=0.039, controlling for Hct, age and sex; Figure S3), whereas no significant correlation between BP and WM CBF was observed in control subjects (r=−0.11, P=0.411).
Regional CBF differences between CKD and control groups
In a group-level analysis, after removing the effects of Hct, age, and sex, we found residual clusters in bilateral prefrontal cortices, middle and inferior temporal cortices, posterior cingulate cortices (PCC), precuneus, left hippocampus, striatum and thalamus. CKD patients showed higher CBF than controls in these regions (Figure 1). There were no regions where controls showed significantly higher CBF than CKD patients.
Using the subject-specific “DisCo-Z” method, we detected regions of positive extrema in subsets of 26/73 and 7/57 subjects for CKD and control groups, respectively, and negative extrema in 11/73 and 12/57 subjects for CKD and control groups, respectively (Table S3). Patients with CKD showed a significantly higher incidence (P=0.002, Table S4) and larger total volume (P=0.0016, Table S5) of positive extreme clusters with increased CBF as compared with control group. There was a non-significant trend (P=0.071) for a greater mean CBF within positive extreme clusters in CKD relative to control subjects (Table S5). There was no difference in the incidence, total volume, or mean CBF values of negative extreme clusters between patients and controls. Qualitative inspection of common locations of subject-specific increases in CBF for patients with CKD showed high inter-subject overlap of clusters in bilateral precuneus, PCC, middle cingulate cortex, inferior parietal lobe, middle frontal lobe, caudate and left putamen (Figure 2).
CBF correlations with neurocognitive performance
CKD patients performed worse than controls in TNB measures of verbal memory, working memory and executive function. CKD participants scored worse in CNB measures of language reasoning, nonverbal reasoning and spatial processing (Table 4, Wilcoxon rank sum test, P<0.05). In general, CKD subjects with positive extrema in CBF measurements showed poorer neurocognitive function than those without presence of positive extrema (Generalized Estimating Equations, B=0.883, Exp(B)=2.417, P=0.003). CKD subjects with positive extrema performed significantly worse scores in TNB measures of verbal memory (Table 4, Wilcoxon rank sum test, P=0.003). CBF extracted from the positive extreme clusters in CKD patients showed a strong correlation with executive function deficits (Rho=0.670, P=4.76☓10−4, controlling for age, sex and Hct, Table 5). Additional adjustments for the additional confounders listed in Table 1 did not change this association (Rho=0.696, P=1.61×10−4, controlling for age, sex, Hct, eGFR, cholesterol levels, triglycerides and BPs). An exploratory correlation analysis was restricted to the precuneus as this region exhibited reliable differences for both the group-level and subject-specific methods. A significant correlation between executive function and CBF in the precuneus for patients with CKD was also observed (Figure 3, Rho=0.608, P=0.001).
Table 4.
Neurocognitive performance (age-normalized z scores) for patients with chronic kidney disease (CKD), controls, the subgroups of CKD with presence and absence of positive extrema cerebral blood flow (CBF). In general, CKD subjects showed poorer neurocognitive functions than controls. CKD subjects with presence of positive extrema in CBF measurements showed poorer neurocognitive functions than those without presence.
| TNB verbal memory | TNB verbal working memory | TNB executive function | CNB language reasoning | CNB non-verbal reasoning | CNB spatial processing | ||
|---|---|---|---|---|---|---|---|
| CKD | mean±SD | 0.15±1.60 | 0.05±1.29 | −0.67±1.18 | −0.61±1.14 | −0.38±0.93 | −0.46±1.06 |
| Control | mean±SD | 0.90±1.90 | 0.68±1.57 | −0.11±1.11 | −0.19±1.01 | 0.002±1.02 | 0.10±1.12 |
| Wilcoxon rank sum test (CKD vs. Control) | P-value | 0.03 | 0.02 | 0.005 | 0.02 | 0.04 | 0.006 |
| CKD subgroup with positive extrema CBF | mean±SD | −0.61±1.12 | −0.21±1.36 | −0.68±1.10 | −0.48±1.02 | −0.60±0.86 | −0.67±1.06 |
| CKD subgroup without positive extrema CBF | mean±SD | 0.57±1.65 | 0.20±1.23 | −0.66±1.23 | −0.68±1.21 | −0.26±0.95 | −0.35±1.06 |
| Wilcoxon rank sum test (with vs. without positive extrema CBF in CKD) | P-value | 0.003 | 0.188 | 0.689 | 0.568 | 0.191 | 0.222 |
Note.--CNB: computerized neurocognitive battery, TNB: traditional neurocognitive battery. Both CNB and TNB are age adjusted z-scores.
Table 5.
Partial correlation analyses for cerebral blood flow (CBF) extracted from DiscoZ positive extrema and neurocognitive performance for subjects with positive extrema in CBF measurements (controlling for age, sex and hematocrit). Significant values are bolded.
| TNB verbal memory |
TNB verbal working memory |
TNB executive function |
CNB Language Reasoning |
CNB nonverbal reasoning |
CNB spatial processing |
||
|---|---|---|---|---|---|---|---|
| Control Group (n=7) | Correlation Coefficient |
−0.87 |
−0.46 |
0.32 |
0.13 |
−0.32 |
0.34 |
| P-value |
0.13 |
0.54 |
0.69 |
0.87 |
0.68 |
0.66 |
|
| CKD Group (n=26) | Correlation Coefficient |
0.37 |
0.33 |
0.67 |
−0.04 |
0.16 |
0.38 |
| P-value | 0.08 | 0.13 | p<0.001 | 0.84 | 0.48 | 0.08 |
CKD: chronic kidney disease, CNB: computerized neurocognitive battery, TNB: traditional neurocognitive battery.
Figure 3.
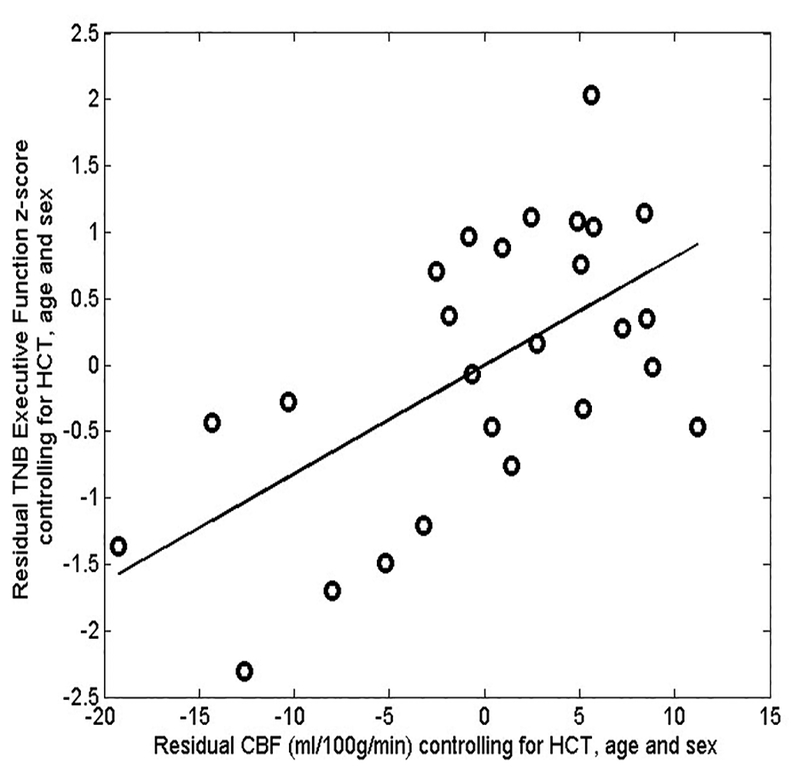
Scatter plot of partial residual values of precuneus cerebral blood flow (CBF) and executive function in CKD patients with presence of positive extrema CBF, indicating a significant correlation between precuneus CBF and executive function after controlling for hematocrit (HCT), age and sex (Rho=0.608, P=0.001). TNB: traditional neurocognitive battery.
Discussion
We characterized regional CBF alterations in pediatric and young adult patients with CKD using ASL perfusion MRI. To minimize the potential confound of Hct changes on CBF quantification, we used a Hct-corrected blood T1 to derive CBF from ASL MRI data. We found markedly higher CBF in patients with CKD as compared to control subjects and confirmed known effects of age and Hct on global CBF (29, 30). Our results also confirmed an association between kidney function as manifested in eGFR and CBF in control subjects, while the association between these two physiological parameters was disrupted in CKD patients. Similarly, sex effects on global CBF were seen in controls, but not CKD patients. CKD patients showed a subtle association between BP and WM CBF.
Hct is a well-known modulator of CBF, and CKD is associated with anemia due to a decrease in erythropoietin production (31). Hct-related effects on CBF explained most of the observed group differences in CBF. Chronic anemia could potentially cause endothelial damage due to increased flow as well as deficits in tissue oxygen delivery, and is a potential therapeutic target. In adult patients with CKD, anemia has been associated with deterioration in cardiac function, decreased mental acuity, and increased mortality (32, 33), while an improvement in GFR was demonstrated after correcting anemia with erythropoietin (32, 34–36). The significant correlations between Hct and CBF in our cohorts underscore the importance of accounting for Hct both in ASL MRI quantification and in the interpretation of CBF changes more generally. After applying the Hct-corrected blood T1 in CBF calculations, residual correlations between ASL CBF and Hct likely reflect true hyperperfusion rather than purely artifactual hyperperfusion due to T1 underestimation. The absence of sex differences in CBF in patients with CKD might reflect delayed sexual differentiation that is known to occur in this condition (37), but may also be partly or even wholly attributable to the absence of sex difference in Hct in this group.
We observed a correlation between eGFR and CBF in our control cohort, consistent with a previous report (38), and implying that kidney function is associated with the regulation of blood flow in the brain. However, the association between eGFR and CBF was largely explained by Hct differences, further underscoring the importance of Hct as a determinant of CBF and suggesting that this association is largely mediated through renal effects on hematopoiesis. The lack of association between eGFR and CBF in CKD patients may have resulted from the heterogeneity of CKD in the group, which included patients with variable duration of CKD and some who had undergone kidney transplantation. Additionally, our subjects were on a variety of treatments for the comorbidities of CKD, i.e. anemia, hypertension, and acidosis, which could have further obscured associations between eGFR and brain function. A recently published study has shown that reduced eGFR was associated with higher CBF for patients with CKD. However, Hct was not assessed and corrected in their ASL CBF measurements (27).
Cerebrovascular disease is highly prevalent in adult patients with CKD, primarily in subcortical WM (1, 5). Our patients with CKD were found to have elevated blood pressure, dyslipidemia and anemia. We observed a weak but significantly positive correlation between systolic BP and WM CBF in CKD patients, suggesting the possibility of an alteration in cerebral microvascular autoregulation that may ultimately contribute to the development of WM ischemic injury in CKD, though minimal WM ischemic injury was seen in the cohort (see supplementary material). Cerebral autoregulation is thought to protect the brain from ischemia caused by acute arterial pressure fluctuations (39–41) and may become impaired during ischemia or other chronic insults such as hypertension (4, 5, 39). Such early changes in WM physiology may also interfere with developmental trajectories of myelination and synaptic pruning thought to be responsible for decreasing CBF and metabolism that occurs in late childhood and adolescence during healthy human development (29, 42, 43).
The neuropsychological impairment in CKD has been reported to closely resemble a cerebral microangiopathy wherein chronic disturbances of regional CBF are known to occur (44). In addition to the global CBF differences between CKD and control, which were largely driven by group differences in Hct, we also observed residual increases in CBF involving in the prefrontal cortex, PCC, precuneus, basal ganglia, and parts of the limbic system. This observed distribution of regional CBF changes includes regions of the “default mode” network (DMN) (45), which is consistent with a prior report of reduced DMN functional connectivity in adult patients with CKD (46).
To better classify how spatially heterogeneous brain injury varies across patients with CKD, we used an individualized approach to characterize the spatial distribution of CBF changes in each patient with CKD (47). The subset of patients with CKD who showed positive extreme clusters of CBF demonstrated a larger incidence and total volume of positive extreme clusters along with higher CBF extracted from those clusters as compared with control subjects. CKD patients with positive extreme clusters showed significantly worse neurocognitive performance as compared with patients without positive extreme clusters. Altered brain perfusion in CKD was correlated with reduced executive function suggesting that CBF differences between CKD and controls reflects a neural correlate of observed neurocognitive deficits and highlighting the potential utility of CBF as an objective and physiological-based biomarker of brain dysfunction in this disorder.
We also identified a significant association between precuneus CBF changes and executive function. Precuneus represents the functional core of the DMN and has been implicated in the integration of both internally and externally driven information (48). Structural changes in this brain region have been associated with the executive dysfunction for patients with dementia (49). Note however that in this study, significant regional differences between groups were only observed for regions showing increased CBF in CKD versus controls. This may reflect either compensatory hyperactivity of these regions in the presence of normal coupling between regional CBF and regional neural activity, or potentially a pathological dysregulation of the normal coupling between regional CBF and regional neural activity in highly metabolic brain regions such as precuneus. Our findings are consistent with a previous report in adult CKD using ASL(50), showing that abnormal CBF change in CKD is associated with a poor performance in cognitive tests although those findings may not have fully accounted for effects of anemia on ASL CBF.
Our study had limitations. First, as described in the statistical analysis, in the absence of any previous report concerning CBF changes in pediatric CKD, this study was powered for structural MRI changes. The lack of associations after Hct correction in the correlation analyses may reflect a power issue with the limited statistical justification for sample size in ASL CBF measurements. Second, this study included a relatively heterogeneous population of patients with CKD, with a wide range of kidney function and post-transplant and dialysis patients (21, 22). Third, although we controlled for the effect of age in our CBF group analysis, we could not exclude possible residual confounding effects of age in our results. Future work with much larger number of participants in each of these subgroups will be needed for additional analyses accounting for those potentially confounding factors. Fourth, all results presented here are cross-sectional. Ongoing longitudinal data will allow developmental changes in regional brain function to be assessed in this population. Finally, ASL MRI data were acquired using a 2D EPI readout scheme without the background suppression, in which the instabilities in the background signal can potentially affect the accuracy and precision of the ASL measurements (51–53). Although we obtained generally excellent ASL image quality, the use of 3D readout sequences combined with background suppression will increase sensitivity of future ASL MRI studies (12, 51–56).
In conclusion, our results confirmed systemic effects of eGFR, Hct and BP on CBF and alterations in regional CBF that may reflect impaired brain function underlying neurocognitive symptomatology in CKD.
Supplementary Material
Implications for Patient Care.
Quantification and interpretation of perfusion imaging data acquired using arterial spin labeled magnetic resonance imaging in chronic kidney disease patients and other populations with anemia must be carried out with careful attention to hematocrit effects.
Correlations between regional cerebral blood flow and alterations in cognitive performance suggest the potential value of cerebral blood flow as an objective and physiological-based biomarker of cognitive decline in pediatric chronic kidney disease.
Summary Statement.
Our results confirmed systemic effects of estimated glomerular filtration rate, hematocrit and blood pressure on cerebral blood flow and alterations in regional cerebral blood flow may reflect impaired brain function underlying neurocognitive symptomatology in pediatric patients with chronic kidney disease.
Acknowledgements
We would like to thank the patients and families who have participated in the study.
Funding
The sources of support:
This project is funded, in part, under a Commonwealth Universal Research Enhancement grant with the Pennsylvania Department of Health, # SAP 4100054843. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Study data were collected and managed using REDCap electronic data capture tools hosted at The Children’s Hospital of Philadelphia. REDCap (Research Electronic Data Capture) (Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377–81.) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
The Clinical and Translational Research Center at the Children’s Hospital of Philadelphia is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants UL1RR024134 and UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was also supported in part by the National Institutes of Health (grants MH080729 and EB015893).
Hua-Shan Liu was supported by Taipei Medical University (grants TMU103-AE1-B29), Taipei Medical University Hospital (grants 105TMU-TMUH-05 and 106TMU-TMUH-21) and Ministry of Science and Technology, R.O.C (grants MOST105–2218-E-038–003-MY2 and MOST 106–2221-E-038 −004 -MY2).
Footnotes
Disclosure
All of the authors declared no competing interests.
Contributor Information
Hua-Shan Liu, Email: HeatherLiu2016@gmail.com, School of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan; Department of Neurology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA; International Ph.D. Program in Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan; Research Center of Translational Imaging, College of Medicine, Taipei Medical University, Taipei, Taiwan..
Erum A. Hartung, Email: hartunge@email.chop.edu, Division of Nephrology, Department of Pediatrics, Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA..
Abbas F. Jawad, Email: jawad@email.chop.edu, Department of Pediatrics, Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA..
Jeffrey B. Ware, Email: Jeffrey.Ware2@uphs.upenn.edu, Department of Radiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Nina Laney, Email: laneyn@email.chop.edu, Division of Nephrology, Children’s Hospital of Philadelphia, Philadelphia, PA..
Allison M. Port, Email: alliport@mail.med.upenn.edu, Brain Behavior Laboratory, Department of Psychiatry, University of Pennsylvania, Philadelphia, PA..
Ruben C. Gur, Email: gur@upenn.edu, Brain Behavior Laboratory, Department of Psychiatry, University of Pennsylvania, Philadelphia, PA..
Stephen R. Hooper, Email: stephen_hooper@med.unc.edu, Department of Allied Health Sciences, University of North Carolina School of Medicine, Chapel Hill, NC..
Jerilynn Radcliffe, Email: radcliffe@email.chop.edu, Division of Developmental and Behavioral Pediatrics, Department of Pediatrics, Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA..
Susan L. Furth, Email: furths@email.chop.edu, Division of Nephrology, Departments of Pediatrics and Epidemiology, Perelman School of Medicine at the University of Pennsylvania; Division of Nephrology, Children’s Hospital of Philadelphia, Philadelphia, PA..
John A. Detre, Email: detre@mail.med.upenn.edu, Departments of Neurology and Radiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA..
References
- 1.Moodalbail DG, Reiser KA, Detre JA, et al. Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol. 2013;8(8):1429–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper SR, Gerson AC, Butler RW, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidel UK, Gronewold J, Volsek M, et al. The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney Int. 2014;85(3):693–702. [DOI] [PubMed] [Google Scholar]
- 4.Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67(2):224–8. [DOI] [PubMed] [Google Scholar]
- 5.Yahalom G, Schwartz R, Schwammenthal Y, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. 2009;40(4):1296–303. [DOI] [PubMed] [Google Scholar]
- 6.Drew DA, Weiner DE. Cognitive impairment in chronic kidney disease: keep vascular disease in mind. Kidney Int. 2014;85(3):505–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartung EA, Matheson M, Lande MB, et al. Neurocognition in children with autosomal recessive polycystic kidney disease in the CKiD cohort study. Pediatr Nephrol. 2014;29(10):1957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reivich M Blood flow metabolism couple in brain. Res Publ Assoc Res Nerv Ment Dis. 1974;53:125–40. [PubMed] [Google Scholar]
- 9.Sokoloff L Relationships among local functional activity, energy metabolism, and blood flow in the central nervous system. Fed Proc. 1981;40(8):2311–6. [PubMed] [Google Scholar]
- 10.Gur RC, Ragland JD, Reivich M, Greenberg JH, Alavi A, Gur RE. Regional differences in the coupling between resting cerebral blood flow and metabolism may indicate action preparedness as a default state. Cereb Cortex. 2009;19(2):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. [DOI] [PubMed] [Google Scholar]
- 12.Günther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med. 2005;54(2):491–8. [DOI] [PubMed] [Google Scholar]
- 13.Tamura MK, Pajewski NM, Bryan RN, et al. Chronic kidney disease, cerebral blood flow, and white matter volume in hypertensive adults. Neurology. 2016;86(13):1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang XL, Wen JQ, Zhang LJ, et al. Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: an arterial-spin labeling MR imaging. Metab Brain Dis. 2016;31(4):929–36. [DOI] [PubMed] [Google Scholar]
- 15.Gevers S, Nederveen AJ, Fijnvandraat K, et al. Arterial spin labeling measurement of cerebral perfusion in children with sickle cell disease. J Magn Reson Imaging. 2012;35(4):779–87. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52(3):679–82. [DOI] [PubMed] [Google Scholar]
- 17.Hartung EA, Laney N, Kim JY, et al. Design and methods of the NiCK study: neurocognitive assessment and magnetic resonance imaging analysis of children and young adults with chronic kidney disease. BMC Nephrol. 2015;16(66):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottesman RF, Sojkova J, Beason-Held LL, et al. Patterns of Regional Cerebral Blood Flow Associated With Low Hemoglobin in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2012;67(9):963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gur R, Richard J, Hughett P, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gur RC, Richard J, Calkins ME, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruebner RL, Laney N, Kim JY, et al. Neurocognitive Dysfunction in Children, Adolescents, and Young Adults With CKD. Am J Kidney Dis. 2016;67(4):567–75. [DOI] [PubMed] [Google Scholar]
- 22.Hartung EA, Kim JY, Laney N, et al. Evaluation of Neurocognition in Youth with CKD Using a Novel Computerized Neurocognitive Battery. Clin J Am Soc Nephrol. 2016;11(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu WC, Fernández-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58(5):1020–7. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gipson DS, Duquette PJ, Icard PF, Hooper SR. The central nervous system in childhood chronic kidney disease. Pediatr Nephrol. 2007;22(10):1703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer AR, Bedrick EJ, Ling JM, Toulouse T, Dodd A. Methods for identifying subject-specific abnormalities in neuroimaging data. Hum Brain Mapp. 2014;35(11):5457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura MK, Pajewski NM, Bryan RN, et al. Chronic kidney disease, cerebral blood flow, and white matter volume in hypertensive adults. Neurology. 2016;86(13):1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28(3):652–9. [DOI] [PubMed] [Google Scholar]
- 29.Biagi L, Abbruzzese A, Bianchi MC, Alsop DC, Del Guerra A, Tosetti M. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging. 2007;25(4):696–702. [DOI] [PubMed] [Google Scholar]
- 30.Vorstrup S, Lass P, Waldemar G, et al. Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab. 1992;12(5):745–9. [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara Y, Sasaki M, Hirakata H, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61(2):564–9. [DOI] [PubMed] [Google Scholar]
- 32.Cană-Ruiu D, Moţa E, Istrate N, Văduva C, Trican E. Renal anemia - risk factor for chronic kidney disease. Curr Health Sci J. 2013;39(4):214–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Fishbane S. Anemia and cardiovascular risk in the patient with kidney disease. Heart Fail Clin. 2008;4(4):401–10. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg D Outcomes of anaemia management in renal insufficiency and cardiac disease. Nephrol Dial Transplant. 2003;18(Suppl 2):ii7–12. [PubMed] [Google Scholar]
- 35.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38(4):955–62. [DOI] [PubMed] [Google Scholar]
- 36.Hsu CY, Bates DW, Kuperman GJ, Curhan GC. Relationship between hematocrit and renal function in men and women. Kidney Int. 2001;59(2):725–31. [DOI] [PubMed] [Google Scholar]
- 37.Salas P, Pinto V, Rodriguez J, Zambrano MJ, Mericq V. Growth retardation in children with kidney disease. Int J Endocrinol. 2013;2013:970946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedaghat S, Vernooij MW, Loehrer E, et al. Kidney Function and Cerebral Blood Flow: The Rotterdam Study. J Am Soc Nephrol. 2016;27(3):715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010;41(11):2697–704. [DOI] [PubMed] [Google Scholar]
- 40.Ruland S, Aiyagari V. Cerebral autoregulation and blood pressure lowering. Hypertension. 2007;49(5):977–8. [DOI] [PubMed] [Google Scholar]
- 41.Ono M, Joshi B, Brady K, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109(3):391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satterthwaitea TD, Shinohara RT, Wolf DH, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014;102(49):17804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taki Y, Hashizume H, Sassa Y, et al. Correlation between gray matter density-adjusted brain perfusion and age using brain MR images of 202 healthy children. Hum Brain Mapp. 2011;32(11):1973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermann DM, Kribben A, Bruck H. Cognitive impairment in chronic kidney disease: clinical findings, risk factors and consequences for patient care. J Neural Transm. 2014;121(6):627–32. [DOI] [PubMed] [Google Scholar]
- 45.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni L, Wen J, Zhang LJ, et al. Aberrant default-mode functional connectivity in patients with end-stage renal disease: a resting-state functional MR imaging study. Radiology. 2014;271(2):543–52. [DOI] [PubMed] [Google Scholar]
- 47.Meier TB, Bergamino M, Bellgowan PS, et al. Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum Brain Mapp. 2016;37(2):833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34(3):932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasconcelos Lde G, Jackowski AP, Oliveira MO, et al. The thickness of posterior cortical areas is related to executive dysfunction in Alzheimer’s disease. Clinics (Sao Paulo). 2014;69(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang XL, Wen JQ, Zhang LJ, et al. Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: an arterial-spin labeling MR imaging. Metab Brain Dis. 2016;31(4):929–36. [DOI] [PubMed] [Google Scholar]
- 51.Ye FQ, Frank JA, Weinberger DR, A.C. M. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST). Magn Reson Med. 2000;44(1):92–100. [DOI] [PubMed] [Google Scholar]
- 52.Duyn JH, Tan CX, van Gelderen P, Yongbi MN. High-sensitivity single-shot perfusion-weighted fMRI. Magn Reson Med. 2001;46(1):88–94. [DOI] [PubMed] [Google Scholar]
- 53.Maleki N, Dai W, Alsop DC. Optimization of background suppression for arterial spin labeling perfusion imaging. MAGMA. 2012;25(2):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen JF, Hernandez-Garcia L. Functional perfusion imaging using pseudocontinuous arterial spin labeling with low-flip-angle segmented 3D spiral readouts. Magn Reson Med. 2013;69(2):382–90. [DOI] [PubMed] [Google Scholar]
- 55.Vidorreta M, Wang Z, Rodríguez I, Pastor MA, Detre JA, Fernández-Seara MA. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage. 2013;66(C):662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernández-Seara MA, Wang Z, Wang J, et al. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magn Reson Med. 2005;54(5):1241–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


