Abstract
Uniform amplification of low input DNA is important for applications across biology, including single-cell genomics, forensic science, and microbial and viral sequencing. However, the requisite biochemical amplification methods are prone to bias, skewing sequence proportions and obscuring signals relating to copy number. Digital droplet multiple displacement amplification enables uniform amplification, but requires expert knowledge of microfluidics to generate monodisperse emulsions. In addition, existing microfluidic methods are tedious and labor intensive for preparing many samples. Here, we introduce rapid emulsification multiple displacement amplification, a method to generate monodisperse droplets with a hand-held syringe and hierarchical droplet splitter. While conventional microfluidic devices require >10 minutes to emulsify a sample, our system takes tens of seconds and yields data of equivalent quality. We demonstrate the approach by using it to accurately measure copy number variation in single cancer cells.
Keywords: copy number variation, amplification bias, multiple displacement amplification, ddMDA, droplet microfluidics
Introduction
Sequencing is becoming an increasingly valuable tool in biology due to the universal importance of nucleic acids in living systems and the richness of the data it produces1–3. Often the system under investigation contains tiny quantities of DNA, for example in single-cell studies, and exponential amplification is required to obtain sufficient material for sequencing. However, exponential amplification reactions like polymerase chain reaction (PCR) or multiple displacement amplification (MDA) are prone to bias because molecules that begin amplifying sooner or have slightly higher doubling rates rapidly take over the system, so that they comprise an inordinate proportion of the final population. Biased regions are sequenced at depth at the expense of other regions, producing uneven coverage that conceals important biological features like copy number variation (CNV).
An effective way to address this challenge and enable accurate and quantitative sequencing of single cells is to compartmentalize the reaction in millions of equally-sized picoliter droplets, a process known as digital droplet MDA (ddMDA)4–7. In this approach, which derives from the concept of digital MDA8, a sample of starting templates is emulsified through a microfluidic device, such that each droplet contains a subset of the original template pool, typically one or a few molecules, with all the reagents necessary for MDA. The emulsion is then incubated, allowing the molecules to amplify. The compartmentalization eliminates competition between templates; molecules that happen to start amplification early or amplify faster quickly reach saturation but do not take over the system, and molecules that amplify at slower rates can catch up. This scheme yields extremely uniform amplification and quantitative sequence data, down to a single cell4–6.
A challenge with ddMDA is that it requires expert knowledge of microfluidics to emulsify the sample; moreover, even with this knowledge, >10 min are required to emulsify 50 μL, making it tedious and time consuming to prepare many samples. Parallelized emulsifiers may be employed to address this problem9–12, but the devices are complicated to fabricate and require expert optimization and operation. A simple alternative is to compartmentalize the sample into polydisperse droplets generated by vortexing or pipetting. However, because the number of amplicons of a given template is proportional to the volume of the droplet containing it, volume polydispersity translates into amplification bias. While this bias is substantially less than that of un-encapsulated MDA6, it nevertheless reduces the efficiency of the sequencing process, and the quality of the sequencing data. To enable broader access to ddMDA and its powerful features, a new method is needed to easily and rapidly generate monodisperse droplets from DNA samples.
In this paper, we describe rapid emulsification ddMDA (re-ddMDA), a method to generate monodisperse emulsions in a few seconds using a hand-operated microfluidic emulsifier. The sample to be emulsified is loaded into a syringe and injected by hand through the device, generating millions of monodisperse droplets in a few seconds. Because the droplets are monodisperse, amplification is uniform, yielding sequencing data comparable to painstakingly-generated pump-driven emulsions. To demonstrate the efficacy of our approach, we apply it to measure CNV in single cancer cells, and obtain results comparable to unamplified matched cancer genomes from millions of cells. Our method reduces the barrier to adopting ddMDA and enhances its scalability for preparing multiple samples, and should be valuable for implementation into high-throughput sequencing pipelines via interfacing with available liquid handling technologies such as pipetting robots.
Materials and Methods
Device fabrication
The serial splitter device is fabricated using soft lithography. SU-8 3025 photoresist (MicroChem) is used to make a 45-μm-tall master mold structure on a 3-inch silicon wafer using standard photolithography techniques. PDMS prepolymer (Momentive, RTV 615) mixed with curing agent at 10:1 ratio is poured onto the master placed in a petri dish. After degassing under vacuum, the PDMS is cured at 65°C for 1 hour and cut out. Holes are punched at inlet and outlet ports using a 0.75 mm biopsy punch (Harris, Uni-Core 0.75). After cleaning with scotch tape, the PDMS channel structure is bonded to a glass substrate by treating with oxygen plasma for 60 s at 1 mbar in a plasma cleaner (Harrick Plasma, PDC-001). The channel surface is treated with Aquapel to make it hydrophobic. For easy access to device fabrication, the CAD design file (Fig. S1) and a list of microfluidics foundries (Table S1) are provided in Supplementary Information.
VCaP cell culture
VCaP cells (ATCC) are a prostate cancer cell line established from a vertebral metastatic lesion. They are maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 4 mM L-glutamine, 4500 mg/L glucose, 1 mM sodium pyruvate, 1500 mg/L sodium bicarbonate and 100 μg/ml streptomycin at 37°C in a 5% CO2 incubator.
FACS sorting of single cells
VCaP cells are released from culture with 0.25% trypsin, washed with PBS-0.2% BSA buffer and centrifuged at 1000 rpm for 5 minutes. The sample is re-suspended in ~300 μl PBS-0.2% BSA and analyzed by a FACS ARIA III (BD Biosciences) equipped with 407 nm, 488 nm, 561 nm and 633 nm lasers. One, ten, and fifty cell aliquots are sorted at a slow-speed under single-cell mode into 0.2 mL PCR tubes containing 5 μL of TE buffer placed in a 96 well plate holder.
Array comparative genomic hybridization (aCGH) protocol
DNA from 5 million VCaP cells is extracted with the QIAamp DNA Blood Mini kit (Qiagen). The final product is purified using the Qiagen PCR Purification Kit (Qiagen). DNA quality and quantity are checked by UV–Vis spectrophotometry. aCGH is carried out using a genome-wide oligonucleotide microarray platform (Human CGH 4×180K microarray kit, Agilent Technologies), following the manufacturer instructions. Human genomic DNA (G1471, Promega) is used as the control. Slides are scanned using an Agilent microarray scanner (model GC2505C), and images processed using Feature Extraction CytoGenomics software (Agilent Technologies).
re-ddMDA procedure
Before preparing reaction mixtures, all items that directly contact the reagents (syringes, tubings, PCR tubes, etc.) are UV-treated for at least 30 minutes. FACS-sorted single cells are collected into 5 μL of TE buffer in a 0.2 mL PCR tube (Accuflow, E&K Scientific). After adding 3 μL of D2 buffer (REPLI-g Single Cell, Qiagen), the tube is heated at 98°C in a thermocycler for 4 minutes to lyse the cells, heat-fragment, and denature gDNA. 3 μL of STOP buffer (REPLI-g kit) is added to the tube to neutralize. 40 μL of reaction mixture (29 μL Reaction Buffer, 9 μL water, 2 μL polymerase) is added to the tube on ice.
Pipette-push method.
55 μL of fluorinated oil (HFE, 3M Novec 7500) supplemented with 2% (w/w) 008-FluoroSurfactant (RAN Biotechnologies) is added to the MDA reaction mixture. The liquid is gently pipetted up and down 30 times using a 200 μL pipette (L-200×LS+, Ranin) set to 130 μL. 60-inch-long polyethylene tubing (SCI, PE/2, ID 0.38 mm, OD 1.09 mm) is attached to a 1-mL syringe (BD 1 mL Luer Lok ™ syringe and 27G 1/2 needle) with the plunger set to the 50 μL position. The pipetted MDA emulsion is withdrawn by moving the plunger to 200 μL position while keeping the end of the tubing at the bottom of the PCR tube so that the oil is loaded first and then the emulsion. Then the reagent-loaded tubing is inserted into the inlet of the splitter device. The syringe plunger is push down to the 0 μL position to initiate flow and the resulting split droplets are collected into a new PCR tube.
Suction-pull method.
A flow-focus device (Fig. S1) is connected to the splitter device with PE/2 tubing in tandem. Gel loading pipette tips are inserted into two inlet ports of the flow-focus device and serve as reservoirs. The MDA reaction mix and 110 μL of 2% (w/w) 008-FluoroSurfactant in HFE oil are added to the reservoirs. 7-inch-long PE/2 tubing is attached to a 1-mL syringe and the plunger is set to the 50 μL position. The tubing is inserted into the outlet of the splitter device and slowly pulled to the 200 μL position to initiate flow. As the emulsion fills the syringe, the plunger is pulled further to keep the suction pressure relatively constant. When all of the MDA mix is injected, 20 μL of surfactant oil is added to the aqueous reservoir to continue oil flow and flush all remaining droplets into the collection syringe.
The prepared emulsion (in a PCR tube for the pipette-push method and in a syringe for suction-pull) is incubated at 30°C for 16 hours. Then, the enzyme is deactivated by heating at 70°C for 20 minutes. The standard ddMDA samples in Fig. 4b and 4c are prepared as previously reported and the re-ddMDA sample in Fig. 4d is prepared using the suction-pull method. The estimated numbers of template molecules per droplet are 0.11 and 1.2 for ddMDA (6 pL) and re-ddMDA (65 pL) droplets, respectively, assuming ~10 kb fragments of triploid VCaP genome.
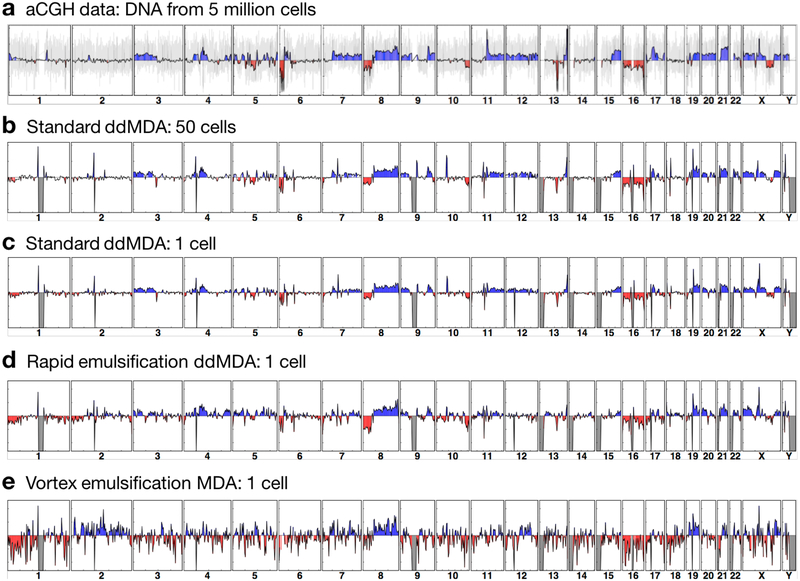
Fig. 4. Rapid-emulsification ddMDA produces CNV measurements comparable to microfluidic ddMDA and rivaling measurements based on unamplified DNA.
(a) An aCGH microarray is used to measure CNV in gDNA extracted from 5 million VCaP cells (2.5 Mb averaging window); the y-axis is log scale, indicating the signal difference between VCaP gDNA and the control gDNA from a normal cell line, and the grey background shows the raw signals from individual probes before averaging. (b) Similar maps can be generated from low-coverage sequencing data for 50 cells subjected to ddMDA with microfluidic flow focusing emulsions. (c) The low bias of ddMDA even allows accurate CNV measurements from a single cancer cell. (d) Suction-generated emulsions are also uniform and, thus, produce data of equivalent quality. (e) Emulsions prepared by vortexing produce data with a large degree of noise due to amplification bias. (c-e) show average data from two independent measurements of single cells.
Library preparation & NGS
Droplets are coalesced by adding 100 μL perfluorooctanol (Sigma, 370533) and centrifuging at 1000g for 1 minute. The aqueous phase is transferred to a spin column for purification (Zymo Research, DNA Clean & Concentrator). The purified DNA is quantified with fluorescence (Thermo Fisher Scientific, Qubit dsDNA HS Assay Kit). 1 ng DNA is tagmented following the manufacturer’s protocol (Illumina, Nextera XT DNA Library Prep Kit) and purified with beads to select for ~300-bp fragments (AMPure XP, Beckman Coulter). The library is characterized with a Bioanalyzer (Agilent, High Sensitivity DNA Analysis Kit) and quantified with qPCR (New England Biolabs, NEBNext Library Quant Kit for Illumina). 15 pM library concentration is used for NGS runs on MiSeq sequencer (Illumina).
Bioinformatics
The Fastq files are down-sampled using R (ShortRead package) to adjust total read counts for each sample to the same value (~2.8 million reads) and aligned to the human reference genome (UCSC hg19) using BWA Aligner (Illumina BaseSpace Labs, version 1.1.4). The coverage maps (averaging window size of 2.5 Mb) are calculated from BAM files and visualized using R (GenomicAlignments and ggplot2 packages). The global mean coverage values for samples in Fig. 4b, 4c and 4d are 0.098×, 0.089×, and 0.099×, respectively. The Pearson correlation coefficients are calculated using cor() function of R’s stats package.
Results & Discussion
MDA is based on an enzymatic reaction catalyzed by φ29 DNA polymerase13,14. This highly processive polymerase has strand displacement activity, enabling isothermal amplification of input DNA with random hexamer primers. φ29 produces long amplicons (~10 kb) with low error rates, making MDA the method of choice for many low-input sequencing applications15,16. However, like most exponential reactions, MDA is prone to bias, skewing sequence proportions due to stochastic binding of the enzyme to the templates, and preferential amplification of early-bound sequences17. To reduce bias, the amplification can be constrained by performing the reaction in microfluidic chambers18,19 or droplets20 that nevertheless yield sufficient DNA for sequencing. Alternatively, the sample can be divided and amplified in millions of monodisperse droplets, a method known as ddMDA, that produces superbly-uniform sequencing data4–7.
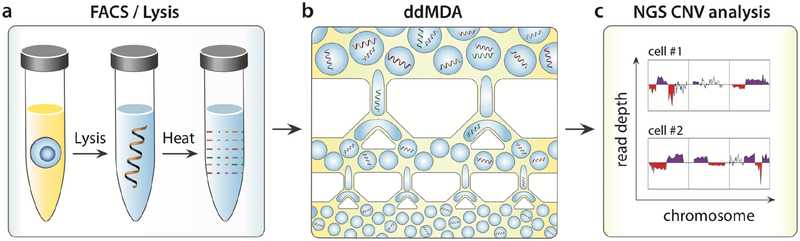
To apply the ddMDA method to single cells, the first step is to isolate the cells in wells via fluorescence-activated cell sorting (FACS). Then, the cells are lysed and their genomes are fragmented with high alkalinity and temperature (98°C) for 4 minutes, cleaving genomic DNA (gDNA), into ~10-kilobase fragments (Fig. 1a)21,22. The alkaline buffer is neutralized and the MDA reagents are added, and the sample is emulsified into millions of monodisperse droplets. With re-ddMDA, emulsification is accomplished by first generating a rough pipetted emulsion comprising large droplets, and then monodispersely emulsifying it through a hierarchical splitting device by hand-injection with a syringe, taking a few seconds (Fig. 1b). The emulsion is incubated for 16 hours at 30°C to allow φ29 to amplify the single-molecule templates in the droplets. The droplets are chemically ruptured, pooling their contents, which are then processed for sequencing. When we start with a single-cell genome of ~6 picograms, ddMDA amplifies this to over a microgram, providing ample DNA for library preparation, sequencing, and CNV measurements (Fig. 1c).
Fig. 1. Rapid emulsification ddMDA workflow.
(a) Single cells are isolated by FACS into each well. Alkaline lysis at high temperature induces cell lysis, and DNA denaturation, and fragmentation. (b) The sample is then rapidly emulsified through a microfluidic splitter, and incubated for amplification. The amplified DNA is recovered from the droplets and sequenced, (c).
Our rapid emulsification device is based on geometrically-mediated droplet breakup23, consisting of a sequence of channel bifurcations (Fig. 2a). It improves upon the premix emulsification method24,25, where preformed emulsions are dispersed further by flowing through a porous membrane. At sufficiently high flow rate, the final droplet size asymptotes to the dimensions of the smallest channel in the hierarchy, such that initially large droplets split more than small droplets, yielding a uniform emulsion. Thus, an advantage of this approach is that the final droplet size is insensitive to flow rate, allowing injection to be performed by hand, and obviating the need for microfluidic expertise or specialized pumps. The device also runs at high speeds (>10,000 μL/hour), generating millions of droplets in a few seconds. Combined, these features make rapid emulsification ddMDA especially valuable for applications that require processing multiple samples.
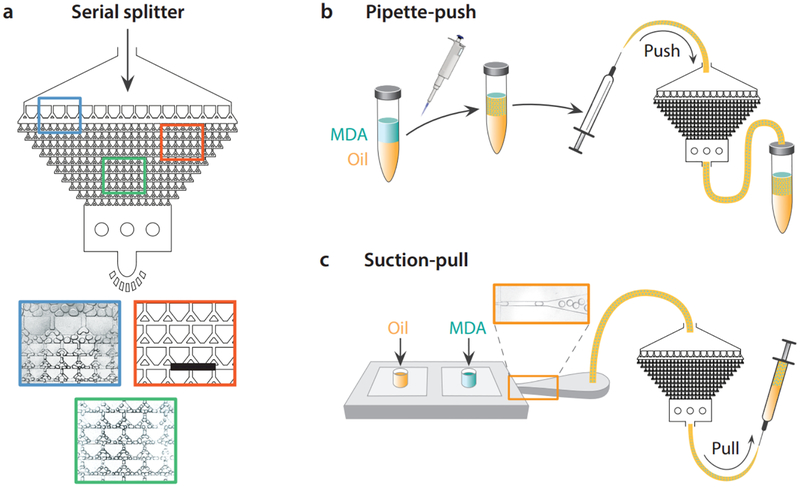
Fig. 2. Methods for rapidly generating uniform emulsions for ddMDA.
A pre-formed emulsion comprising large, polydisperse droplets is introduced into the splitter device inlet, and split to final droplets ~40 μm in diameter (colored panels). The droplets can be generated either in positive pressure (b) or negative pressure (c) modes. In positive pressure mode, a coarse emulsion of the MDA sample is generated via pipetting, loaded into a syringe, and injected through the splitter. In negative pressure mode, the sample is loaded with oil into the inlets of a large droplet generator connected in series with the splitter, and the fluids are drawn through both devices by applying syringe suction. While the suction method generates more uniform emulsions, the positive pressure method is faster, emulsifying a sample of 50 μL in a few seconds. Scale bar in (a) is 600 μm.
We present two methods for rapidly preparing droplets for ddMDA. In the pipette-push method (Fig. 2b), pipetting by hand generates large polydisperse droplets with a broad size distribution. The polydisperse emulsion is then processed through the splitter, generating a uniform emulsion with rare instances of large droplets. Injecting a pipetted emulsion through the splitter is easy, fast, and yields reasonably uniform droplets for ddMDA. However, the starting pipetted emulsion may vary between users. Moreover, occasional very large droplets are not completely fragmented to the final size, resulting in some final polydispersity. Hence, as an alternative approach, we also emulsify the sample using a tandem device consisting of a droplet generator and a splitter, operated by a hand-held syringe (Fig. 2c). The ddMDA reagents are loaded into the inlets of the droplet generator connected to the splitter through a tube. A syringe is connected to the splitter outlet to generate a vacuum, providing suction that draws the fluids through the droplet generator and splitter. Because the droplet generator forms monodisperse large droplets (Fig. 2c, orange panel) and the splitter operates at reasonably constant flow rates, these emulsions are even more uniform than the ones formed by the pipette-push method. However, in return for these benefits, the suction-pull method is slower, requiring tens of seconds to emulsify a 50 μL sample.
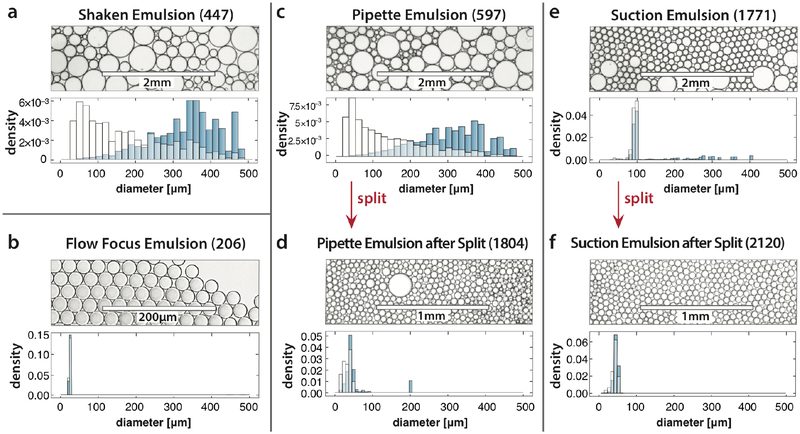
To assess the effectiveness of these methods for generating emulsions, we compare size distributions of the resultant droplets (Fig. 3). The simplest and fastest way to generate an emulsion for ddMDA is to vortex the ddMDA mix with oil and surfactant; however, the resultant droplets are extremely polydisperse, as shown in Fig. 3a and, consequently, result in some bias6. In contrast, a flow focus microfluidic droplet generator operated at controlled flow rates using syringe pumps can form exquisitely monodisperse emulsions (Fig. 3b) that yield ideal data4–6; however, the requirement of pumps and slow rate of formation limit this method’s broad adoption. Indeed, the sequencing process itself introduces bias from multiple sources, including from the MDA reactions preference for amplifying certain sequences, limited cycle PCR during library preparation, and systematic errors produced by the sequencer itself26. Consequently, such extreme monodispersity may not be necessary to produce the best possible data and, rather, the optimal method is one that obtains this data in the most convenient and fastest protocol possible.
Fig. 3. Assessment of droplet quality prepared by different methods.
Shaken emulsions are easy and fast to make, but polydisperse, resulting in bias (a). Microfluidic flow focusing, by contrast, requires specialized pumps and is relatively slow (>10 min for a 50 μL sample), but provides exceedingly uniform droplets, and superbly uniform sequencing data. Handinjection of a polydisperse emulsion generated by pipetting (c) yields reasonably uniform droplets (d) with greater polydispersity than flow focusing but much less than shaking. Negative pressure generation in a tandem flow focus (e) and splitter (f) yields even more uniform emulsions, although sacrifices some speed. The volume-weighted histogram (blue bars) illustrates expected amplification bias resulting from polydispersity, since product numbers scale with encapsulating droplet volume. Numbers in parentheses are counts of analyzed droplets.
Rapid emulsification ddMDA accomplishes this by trading impeccable monodispersity for a simplified workflow and markedly faster emulsification. The sample is first coarsely emulsified by hand using a pipette, generating large, polydisperse droplets with a broad size distribution (Fig. 3c). The polydisperse emulsion is then processed through the splitter, generating a uniform emulsion with rare instances of large droplets (Fig. 3d). The remaining polydispersity can be traced to extremely large droplets in the pipetted emulsion, with diameters >350 μm. Our splitter contains 11 sequential splits, yielding a reduction in volume of 211 and in diameter of ~13-fold. Hence, droplets larger than 350 μm do not reduce to the final ~30 μm size, resulting in some large droplets. Nevertheless, rare droplets are massively outnumbered by correctly sized droplets and, thus, do not significantly affect data quality. When the suction-pull method is used, the droplets processed through the splitter almost never exceed the maximum size (Fig. 3e), resulting in an even more uniform final emulsion (Fig. 3f).
An important area in which accurate and quantitative sequencing of low-input DNA is necessary is single cancer cell genomics. Solid tumors shed cells into a patient’s blood stream, called circulating tumor cells (CTCs). Many technologies are available for enriching CTCs and can recover cells from patients with metastases for many different types of cancers27–29. Moreover, because CTCs originate from a tumor, they may share similar genetic and phenotypic characteristics, affording the potential to obtain detailed information about the tumor without having to procure tissue biopsies that are rarely performed due to difficulty, cost and morbidity30. A particularly important genomic feature of many cancers is CNV, in which certain regions of the genome are duplicated or deleted. CNV is important because edits of the genome that change the counts of sequences are thought to more likely yield selectable phenotypes than mutations that alter gene sequences31. Additionally, CNV has been shown to correlate with the metastatic and evolutionary potential of numerous cancers, making it a potentially valuable biomarker for cancer diagnostics32. However, measuring CNV is challenging because the single-cell genome must be massively amplified to yield sufficient DNA for sequencing, often destroying the valuable CNV information. Here, again, ddMDA’s ability to uniformly amplify minute quantities of DNA enables accurate CNV measurements of single cancer cells4.
To test whether re-ddMDA enables single-cell CNV measurements, we apply it to cancer cells from the VCaP cell line. As a control, we collect total DNA from 5 million VCaP cells and perform array-based comparative genomic hybridization (aCGH), the gold standard in characterizing CNV for cultivable cancer cells (Fig. 4a). The aCGH array provides CNV measurements with a theoretical resolution of ~100 kb, estimated from the median distance of 13 kb between each hybridization probe on the 4×180K array.
To confirm that similar measurements can be obtained from sequencing data, we apply standard microfluidic ddMDA with monodisperse 26-μm droplets to gDNA from 50 VCaP cells. Sequence amplifications (blue), deletions (red) and long-range dropouts (grey) are marked for the aCGH and ddMDA data. As expected, we find excellent correspondence between the ddMDA sequence data and aCGH reference (Fig. 4b), illustrating the power of ddMDA with uniform droplets. A powerful feature of ddMDA is that it allows accurate sequencing of single cells. To confirm this, we repeat the measurement on a single VCaP cell isolated by FACS, again marking copy number signatures, and find excellent correspondence with the 50- and 5 million-cell data (Fig 4c). To determine whether our re-ddMDA approach provides the uniformity necessary to obtain accurate CNV measurements, we also apply it to a single VCaP cell (Fig. 4d). Again, we find excellent agreement with the control samples, illustrating re-ddMDA’s effectiveness for measuring CNVs in single cells. As expected, the data from vortexed emulsions exhibit larger variation and noise in the read depth profile, resulting in poorer CNV detection (Fig. 4e). We also compute the Pearson correlation coefficients against the aCGH data to quantitatively assess the similarities between each measurement (Table S2). While the 50-cell ddMDA data show the highest correlation with the aCGH data (r = 0.67), the single-cell ddMDA data (r = 0.66) and the single-cell re-ddMDA data (r = 0.52) yield similar correlation coefficients, confirming the consistency of copy number information between the methods. The vortexed emulsion data yields the lowest value (r = 0.31). The correlation between ddMDA and re-ddMDA is higher, as indicated by the correlation coefficients against the 50-cell ddMDA data: 0.96 and 0.82 for 1-cell ddMDA and 1-cell re-ddMDA, respectively. The sequencing data are obtained at very low coverage (~0.1×) and are not processed further for bias correction or normalization. The correspondence between aCGH and the ddMDA methods may improve with greater sequencing coverage or by employing more sophisticated CNV detection algorithms such as GC bias correction and segmentation with variable bin size33,34.
Conclusions
Uniform amplification of low-input DNA is important for a variety of applications, including hybridization array analysis and next generation sequencing. Existing ddMDA methods require microfluidic expertise and are limited in speed. Here, we have shown that emulsification of samples with hand-operated syringes and a simple microfluidic droplet splitter can generate emulsions that yield data of similar quality. In addition to being simpler to adopt, our approach can emulsify samples in a few seconds, making it valuable for preparing multiple samples. While our approach requires access to a microfluidic device consisting of a bifurcating channel network, the device is simple to fabricate and could easily be constructed and purchased from existing commercial vendors. While we use hand-held syringes to operate the device, it should also be possible to do so using a pipette by integrating the device into a disposable pipette tip.
Our data on a metastatic cancer cell line provides initial support for application of this approach to conducting CNV measurements of single cells. Further studies using CTCs from patient samples will provide valuable genomic information for medical treatment. Our method should be useful for applications requiring uniform sequence data from minimal starting material, but also in which speed and convenience are important factors.
Supplementary Material
Acknowledgments
We thank Angus Sidore and Freeman Lan for helpful scientific discussions. This work was supported by the UCSF Division of Hematology-Oncology Perkins Philanthropy (PLP); the National Science Foundation CAREER Award (Grant Number DBI-1253293); the National Institutes of Health (NIH) (Grant Numbers HG007233–01, R01-EB019453–01 and DP2-AR068129–01); and the Defense Advanced Research Projects Agency Living Foundries Program (Contract Numbers HR0011–12-C-0065, N66001–12-C-4211 and HR0011–12-C-0066) and Fold F(x) Program (Contract Number DE-AC02–05CH11231).
Footnotes
Conflict of interests
The authors declare no conflict of interest.
References
- 1.Shapiro E, Biezuner T & Linnarsson S Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet 14, 618–630 (2013). [DOI] [PubMed] [Google Scholar]
- 2.van Dijk EL, Auger H, Jaszczyszyn Y & Thermes C Ten years of next-generation sequencing technology. Trends Genet 30, 418–426 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Shendure J & Ji H Next-generation DNA sequencing. Nat. Biotechnol 26, 1135–1145 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Fu Y et al. Uniform and accurate single-cell sequencing based on emulsion whole-genome amplification. Proc. Natl. Acad. Sci 112, 11923–11928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa Y et al. Monodisperse Picoliter Droplets for Low-Bias and Contamination-Free Reactions in Single-Cell Whole Genome Amplification. PLOS ONE 10, e0138733 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidore AM, Lan F, Lim SW & Abate AR Enhanced sequencing coverage with digital droplet multiple displacement amplification. Nucleic Acids Res 44, e66–e66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee M, Light YK, Meagher RJ & Singh AK Digital Droplet Multiple Displacement Amplification (ddMDA) for Whole Genome Sequencing of Limited DNA Samples. PLOS ONE 11, e0153699 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blainey PC & Quake SR Digital MDA for enumeration of total nucleic acid contamination. Nucleic Acids Res 39, e19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romanowsky MB, Abate AR, Rotem A, Holtze C & Weitz DA High throughput production of single core double emulsions in a parallelized microfluidic device. Lab. Chip 12, 802–807 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Lim J et al. Parallelized ultra-high throughput microfluidic emulsifier for multiplex kinetic assays. Biomicrofluidics 9, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conchouso D, Castro D, Khan SA & Foulds IG Three-dimensional parallelization of microfluidic droplet generators for a litre per hour volume production of single emulsions. Lab Chip 14, 3011–3020 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Nisisako T & Torii T Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab. Chip 8, 287–293 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Blanco L et al. Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J. Biol. Chem 264, 8935–8940 (1989). [PubMed] [Google Scholar]
- 14.Dean FB, Nelson JR, Giesler TL & Lasken RS Rapid Amplification of Plasmid and Phage DNA Using Phi29 DNA Polymerase and Multiply-Primed Rolling Circle Amplification. Genome Res 11, 1095–1099 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binga EK, Lasken RS & Neufeld JD Something from (almost) nothing: the impact of multiple displacement amplification on microbial ecology. ISME J 2, 233–241 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Lasken RS Genomic sequencing of uncultured microorganisms from single cells. Nat. Rev. Microbiol 10, 631–640 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Raghunathan A et al. Genomic DNA Amplification from a Single Bacterium. Appl. Environ. Microbiol 71, 3342–3347 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcy Y et al. Nanoliter Reactors Improve Multiple Displacement Amplification of Genomes from Single Cells. PLoS Genet 3, e155 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcy Y et al. Dissecting biological ‘dark matter’ with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc. Natl. Acad. Sci. U. S. A 104, 11889–11894 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung K et al. Robust high-performance nanoliter-volume single-cell multiple displacement amplification on planar substrates. Proc. Natl. Acad. Sci 201520964 (2016). doi: 10.1073/pnas.1520964113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y & Hang J Fragmentation of Genomic DNA using Microwave Irradiation. J. Biomol. Tech. JBT jbt.13-2402-005 (2013). doi: 10.7171/jbt.13-2402-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Li X, Greenspoon SA, Scherer JR & Mathies RA Integrated DNA purification, PCR, sample cleanup, and capillary electrophoresis microchip for forensic human identification. Lab. Chip 11, 1041 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Link DR, Anna SL, Weitz DA & Stone HA Geometrically Mediated Breakup of Drops in Microfluidic Devices. Phys. Rev. Lett 92, 054503 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Nazir A, Boom RM & Schroën K Droplet break-up mechanism in premix emulsification using packed beds. Chem. Eng. Sci 92, 190–197 (2013). [Google Scholar]
- 25.Hornig N & Fritsching U Liquid dispersion in premix emulsification within porous membrane structures. J. Membr. Sci 514, 574–585 (2016). [Google Scholar]
- 26.Aird D et al. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol 12, R18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedlander TW, Premasekharan G & Paris PL Looking back, to the future of circulating tumor cells. Pharmacol. Ther 142, 271–280 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Yu M, Stott S, Toner M, Maheswaran S & Haber DA Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol 192, 373–382 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alunni-Fabbroni M & Sandri MT Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods San Diego Calif 50, 289–297 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Maheswaran S & Haber DA Circulating Tumor Cells: a window into cancer biology and metastasis. Curr. Opin. Genet. Dev 20, 96–99 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlien A & Malkin D Copy number variations and cancer. Genome Med 1, 62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zack TI et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet 45, 1134–1140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abyzov A, Urban AE, Snyder M & Gerstein M CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res 21, 974–984 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garvin T et al. Interactive analysis and assessment of single-cell copy-number variations. Nat. Methods 12, 1058–1060 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.