Abstract
Studies over the last decade have decisively demonstrated that innate immune natural killer (NK) cells exhibit enhanced long-lasting functional responses following a single activation event. With the increased recognition of memory and memory-like properties of NK cells, questions have arisen with regards to their ability to effectively mediate vaccination responses in humans. Moreover, recently discovered innate lymphoid cells (ILC) could also potentially exhibit memory-like functions. Here we review different forms of NK cell memory, and speculate about the ability of these cells and ILCs to meaningfully contribute to vaccination responses.
Introduction
Natural Killer (NK) cells and innate lymphoid cells (ILCs) derive from the common lymphoid progenitor (CLP) and depend on the interleukin-2 receptor common gamma chain (IL-2rg, γc) for development (Diefenbach et al., 2014; Eberl et al., 2015). These cells are distinct from T and B lymphocytes for their lack of recombined antigen-specific receptors. While seemingly limited in their ability to recognize antigens, a fundamental attribute of T and B cell immunologic memory, over the past decade it has become clear that NK cells also possess features of memory, and have the capacity to learn from prior experiences. Whether ILCs have attributes of memory is not known. In the following paragraphs, we will discuss different forms of NK cell memory, speculate about the ability of ILCs to learn from experience, and propose potentially relevant vaccination strategies based on NK and ILC memory.
Different forms of NK cell memory
NK cells have classically been characterized as innate immune lymphocytes capable to kill target cells without prior sensitization or MHC restriction (Yokoyama, 2013). NK cells express a genetically-determined repertoire of germline-encoded activating and inhibitory receptors that control NK cell education and target cell recognition and activation/killing. Despite their lack of antigen-specific receptors, over the past decade it has become clear that NK cells also possess features of memory, and have the capacity to learn from prior experiences. Three primary routes of memory NK cell differentiation have been described thus far, antigen-specific liver-resident NK cell memory, cytomegalovirus (CMV)-driven memory, and cytokine-induced memory.
The first evidence of NK cell memory came from the Von Andrian laboratory with the finding that a subset of NK cells can mediate hapten-specific contact hypersensitivity (CHS) (O’Leary et al., 2006; Paust et al., 2010). Hapten-specific NK cell memory is limited to a specific subset of liver-resident cells in the mouse with the phenotype NK1.1+DX5-CXCR6+CD49a+ (O’Leary et al., 2006; Paust et al., 2010; Peng et al., 2013). Liver-resident NK cells demonstrate specific memory against a variety of haptens and other antigens, including virus-like particles (VLPs), through an unidentified recognition mechanism (Paust et al., 2010). In addition to antigen, cytokine signals, including IL-12, IFN-γ, IFN-α, and IL-18 are required for development of memory (Majewska-Szczepanik et al., 2013; van den Boorn et al., 2016). It is unknown whether a similar subset of liver-resident memory cells exists in humans, but a study in rhesus macaques suggest antigen-specific NK cells may also exist in primates (Reeves et al., 2015).
NK cells also have the capacity to alter their function following infection with CMV. Half of NK cells in C57BL/6 mice express the activating receptor Ly49H, which specifically recognizes the murine CMV (MCMV) ligand m157, likely as the result of positive selection of this receptor recognizing a nearly-ubiquitous pathogen (O’Sullivan et al., 2015). During MCMV infection, Ly49H+ NK cells specifically proliferate and are required for survival (Dokun et al., 2001). The Lanier laboratory first demonstrated that Ly49H+ cells have enhanced functionality following MCMV infection, can be detected months after infection, and provide superior protection in a neonatal infection model compared to naïve Ly49H+ NK cells (Sun et al., 2009). MCMV-induced memory NK cells appear specific, and did not have enhanced functionality against influenza or Listeria infection (Min-Oo and Lanier, 2014).
Human CMV (HCMV) infection is associated with the expansion of unique subsets of human NK cells, primarily marked by a mature phenotype (CD56dimCD57+NKG2A+) and high expression of the activating receptor CD95/NKG2C, which recognizes the non-classical MHC molecule HLA-E (Foley et al., 2012; Guma et al., 2004; Rolle and Brodin, 2016). The emergence and long-term maintenance of NKG2C-high NK cells in individuals with a history of CMV, and their expansion during acute infection with HCMV and other viruses (in HCMV-experienced individuals) has led to their description as HCMV-adaptive or “memory-like” cells (Holmes and Bryceson, 2016; Rolle and Brodin, 2016). While most HCMV-adaptive NK cells express high levels of NKG2C, this activating receptor is not known to specifically recognize an HCMV antigen, and HCMV-adaptive NK cells were also recently identified in individuals genetically deficient in NKG2C (Liu et al., 2016; Wagner and Fehniger, 2016). Recently, human NK cells lacking the intracellular signaling adapter FcεRγ were identified as HCMV-adaptive NK cells (Hwang et al., 2012; Zhang et al., 2013), and may represent a broader population of HCMV-adapted NK cells. FcεRγ-deficient NK cells have a mature phenotype, variable but higher expression of NKG2C, and exhibit robust antibody-mediated expansion and cytokine production driven by FcγRIIIa (CD16) (Lee et al., 2015; Liu et al., 2016; Rolle and Brodin, 2016; Schlums et al., 2015). Epigenetic changes in these cells leading to alter intracellular signaling proteins and transcription factors provide a molecular basis for altered function in HCMV-adapted NK cells (Lee et al., 2015; Schlums et al., 2015).
The Yokoyama laboratory first demonstrated that cytokine stimulation alone can lead to long-term enhanced NK cell responses. Using an adoptive transfer system, splenic murine NK cells were stimulated with a combination of IL-12/15/18 and transferred into naïve hosts. NK cells with a prior history of cytokine activation had enhanced IFN-γ responses to activating receptors, cytokines, and tumor targets weeks to months later (Cooper et al., 2009; Keppel et al., 2013). The Cerwenka laboratory has shown that murine NK cells pre-activated with IL-12/15/18 had enhanced IFN-γ and perforin-dependent anti-tumor activity in vivo, and were dependent on CD4+ T cells, macrophages, and IL-2 for proliferation and long-term function (Ni et al., 2016; Ni et al., 2012). A similar form of cytokine-induced memory was demonstrated in humans, using an in vitro culture system. Human NK cells, pre-activated with cytokines (IL-12/15/18) and maintained in vitro with low-dose IL-15, displayed enhanced IFN-γ and TNF production in response to re-stimulation with cytokines and primary acute myeloid leukemia (AML) blasts, and had enhanced killing of K562 tumor targets in vitro (Romee et al., 2016; Romee et al., 2012). These findings led us to carry out a first-in-human clinical trial of adoptive immunotherapy with IL-12/15/18 activated haploidentical NK cells in patients with relapsed/refractory AML. Preliminary results from this clinical trial demonstrate that NK cells expand and are detectable in the blood and bone marrow of patients, have enhanced functional activity compared to the recipient’s own NK cells, and five of nine patients had clinical responses, with four having complete remissions (Romee et al., 2016).
Together, these studies demonstrate memory and memory-like properties of mouse and human NK cells induced by different mechanisms, including antigen-specific (haptens, MCMV, VLPs), viral-driven (HCMV), and antigen-independent (cytokine-induced) forms of memory.
Can NK cell memory play a role in vaccination?
Knowing that NK cells can alter their function based on prior activation, should NK cells be targeted for vaccination? Current vaccination strategies target the ability of T and B lymphocytes to form long-lasting specific memory, protecting the host years after a single vaccination series. The discovery of memory in NK cells brings to question whether they should also be targeted for vaccination. There are a number of factors to consider with regards to harnessing NK cell memory for vaccination. What type of NK cell memory should be targeted for vaccination? What vaccination strategy would best target NK cells? What is the lifespan and durability of memory NK cells? What is the correct model organism for pre-clinical testing of vaccination strategies?
While all of these questions have scientific merit, perhaps the most important question when considering investment of resources to targeting NK cell memory is whether, in immunocompetent individuals, there is any advantage to targeting NK cells versus adaptive lymphocytes?
The best evidence for NK cell memory responses during vaccination comes from studies of liver-resident memory cells. Paust et al. demonstrated that vaccination of T and B cell Rag-deficient mice with UV-irradiated vesicular stomatitis virus (VSV) provided protection against subsequent local infection with VSV, which was otherwise fatal in half of unimmunized mice (Paust et al., 2010). Using adoptive transfer models, they also demonstrated that purified liver NK cells from Rag-deficient mice immunized three months prior with influenza VLPs conferred a survival advantage to influenza A infection in T/B/NK-deficient (Rag2−/−IL2rg−/−) hosts, although transferred NK cells did provide a long-term survival benefit. Interestingly, they also demonstrated liver-resident NK cell mediated CHS in response to HIV-I containing VLPs, a virus for which murine NK cells would not be expected to have evolved a recognition mechanism. A recent study from van den Boom et al. demonstrated anti-tumor liver-resident memory NK cell responses (van den Boorn et al., 2016). Sensitization with monobenzone led to haptenization of melanocyte antigens, with development of liver-resident memory NK cells capable of monobenzone-specific CHS and cytotoxic activity against pigmented cells. T and B cell-deficient mice sensitized with monobenzone had enhanced anti-tumor response to in vivo challenge with a melanoma tumor, correlating with increased activated NK cells present in tumors. Liver-resident NK cells from monobenzone-sensitized mice had enhanced cytotoxicity in vitro against melanoma cells or a syngeneic melanoma cell line, presumably due to a shared melanocyte antigen. NK cell protection in this model was antigen-specific, as there was no enhanced response to a different tumor (sarcoma challenge) (van den Boorn et al., 2016).
Thus, murine studies demonstrate that vaccination with VLPs, irradiated virus, and haptens confer liver-resident NK-mediated immunity to those antigens. However, a number of questions still exist with regards to this system. First, the mechanism by which liver-resident NK cells recognize a wide array of antigens is unknown, and suggests that they may have the potential to somatically re-arrange their activating receptors for specific recognition. Without a basis for antigen-recognition, it is challenging to design antigens and develop rationale vaccine strategies. Second, it is unknown whether human liver-resident NK cells (or other tissue-specific NK cell subset) have a similar capacity to specifically recognize a wide array of antigens. Preliminary evidence in rhesus macaques suggest a similar type of NK cell memory in primates (Reeves et al., 2015). However, since these studies were performed in immunocompetent organisms that produce antibodies, it cannot be excluded that NK cell memory is mediated by specific antibodies that engage and activate Fc receptors on NK cells. Moreover, further investigation in humans is necessary.
There is no direct evidence for vaccination strategies with CMV- or cytokine-induced NK cell memory. NK cell memory observed after MCMV infection is specific to a model system in which half of the NK cells express a germline-encoded receptor recognize a specific virally-encoded antigen, and no such situation is known to exist in humans. One possibility this system does suggest with regards to vaccination, is that NK cell stimulation via a specific activating receptor might lead to enhanced functional responses when that receptor is triggered in the future. Indeed, the Lanier laboratory also demonstrated that in the context of an inflammatory challenge (low-dose m157-deficient MCMV infection), stimulation of murine NK cells via Ly49D, a cell-surface activating receptor that recognizes a MHC class I molecule (H2-Dd), led to a similar form of NK cell memory as the Ly49H-m157 MCMV system (Nabekura and Lanier, 2014). Ly49D-sensitized NK cells had enhanced degranulation and IFN-γ production in response to H2-Dd positive tumors. These findings suggest the potential for a vaccination strategy targeting activating receptors on NK cells. For example, NKG2D is an activating receptor present on the majority of mouse and human NK cells (and T cells). NKG2D recognizes several self-encoded stress ligands upregulated on tumor and infected cells. If NKG2D-bearing NK cells were targeted with vaccination, it is possible that those cells would have enhanced tumor immunosurveillance and responses to “stressed” cells, including virally-infected cells.
The viral trigger driving the differentiation of HCMV-adapted NK cells is unknown, thus presenting a challenge to vaccination strategies targeting these cells. The utility of inducing HCMV-adapted NK cells by vaccination is unclear, since most individuals acquire HCMV infection early during life, generating HCMV-adapted NK cells in situ. In addition, it is unknown whether the presence of HCMV-adapted NK cells benefits the host. Interestingly, there was a case report of an infant with a T cell deficiency who contracted HCMV and demonstrated massive expansion of NKG2C+ NK cells, with apparent control of infection prior to initiation of antivirals (Kuijpers et al., 2008). This suggests that HCMV-adapted NK cells may have an anti-viral effect in patients. HCMV-adapted NK cells also expand during several other viral infections (in HCMV-seropositive individuals), and have enhanced antibody-dependent FcγRIIIa-mediated responses, suggesting that they have a functional role in human health (Rolle and Brodin, 2016). Thus, vaccination strategies targeted at differentiating HCMV-adapted NK cells might be a rationale strategy in individuals for whom a natural infection could be lethal, such as neonates and HCMV-negative pregnant women, immunocompromised individuals, and patients undergoing hematopoietic cell transplantation (HCT). Development of such a vaccine will require more knowledge of the factors driving differentiation and maintenance of HCMV-adapted NK cells.
Cytokine-induced memory-like NK cells arise after a brief exposure to IL-12, IL-18, and IL-15, with similar biology between murine models and humans. Several properties suggest that this type of pre-activation that results in NK cell memory-like differentiation may be harnessed utilizing vaccine-type approaches. First, memory-like NK cells are long-lived, and pass on their enhanced functionality to daughter cells after cell division, evidenced by detection 4 months after adoptive transfer in syngeneic mice (Cooper et al., 2009). Second, the stimulatory pro-inflammatory cytokines are well characterized and conventional naïve NK cells constitutively express their receptors. Third, these cytokines are produced by dendritic cells and macrophages, and in theory cytokine-induced memory-like NK cells differentiate after an encounter with a matured, activated dendritic cell at the site of infection or other robust pro-inflammatory signal. Systemic administration of IL-12, IL-15 and IL-18 is not feasible, as these results in a toxic-shock response (Carson et al., 2000). However, local provision or induction may be a viable, non-toxic strategy. For example, injection of TLR agonists, which are being explored in clinical investigations in several types of immunotherapy (Hammerich et al., 2016; Shirota and Klinman, 2014), induces a self-limited pro-inflammatory cytokine cascade that may result in NK cell memory-like responses. In theory, local injection of recombinant IL-12, IL-15, and IL-18 may also be feasible, for example, at the site of a tumor or within a single lymph node. With recent progress in protein engineering, it may be feasible to target a heterocytokine complex to a specific target cell or location. For example, using a single-chain variable fragment (scFv) fusion protein to target IL-12, IL-15, and IL-18 simultaneously to the site of a tumor. While these strategies are supported by the activity of IL-12/15/18-induced memory-like NK cells, and are translatable, initial feasibility studies in mice, followed by careful in human studies will be required to test their practicality and effectiveness. To this end, further study of memory-like NK cell biology, including the ability to identify and track these cells in vivo in mice and humans will be a critical area of study.
ILCs: potential for memory functions and impact in vaccination.
While NK cells can be considered the innate counterparts of CD8+ cytotoxic T cells, ILCs resemble CD4+ T helper cell subsets (Diefenbach et al., 2014; Eberl et al., 2015). ILCs include three major classes - ILC1s, ILC2s and ILC3s - which are defined based on the signature cytokines they produce: ILC1s secrete IFNγ; ILC2s produce IL-5 and IL-13; and ILC3s secrete IL-22 and IL-17. Thus, ILCs can be considered the innate counterparts of Th1, Th2 and Th17 cells, as they share functional modules that encompass cytokine secretion as well as transcription factors that underpin cytokine specialization (Diefenbach et al., 2014; Eberl et al., 2015).
ILCs lack the antigen-specific receptors of CD4+ T cells and do not express receptors analogous to the CMV receptors of NK cells; what triggers their activation? In fact, ILCs respond directly to cytokine signals in the microenvironment. Specificity is controlled by the cytokine receptors that ILCs express, which allows distinct combinations of myeloid and/or tissue-produced cytokines to activate each ILC class. IL-12, IL-18 and IL-15 trigger ILC1s; IL-25, thymic stromal lymphopoietin (TSLP) and IL-33 stimulate ILC2s; and IL-23 and IL-1β prompt ILC3s (Diefenbach et al., 2014; Eberl et al., 2015).
To date, there is no evidence that ILCs are capable of memory responses. Given that cytokine receptors are the major trigger of ILC activation and cytokine production, it is likely that cytokine stimulation alone may lead to enhanced long-term ILC responses, as is observed for NK cells. Supporting this hypothesis, recent epigenetic studies have shown that the regulatory circuitries that control ILC effector functions contain active enhancers as well as poised enhancers, which can be further activated by cytokine stimulation (Koues et al., 2016; Shih et al., 2016). Thus, repeated cytokine stimulation is likely to enhance and sustain ILC responses over time, resulting in the generation of ILC memory-like cells.
Given that ILC1s have been identified in tumors (Dadi et al., 2016) and that intestinal ILC3s and ILC1s have been shown to augment host resistance to viral and bacterial infections (Abt et al., 2015; Hernandez et al., 2015), memory-like ILC1s and ILC3s could be harnessed for vaccine-type approaches. For example, administration of stimuli for ILC1s, such as IL-12 and IL-15, at the site of a tumor may enhance tumor immunosurveillance; similarly, introducing IL-23 or other stimuli for ILC3s into the intestinal tract may reinforce pathogen immunosurveillance. A subset of ILC3s corresponds to lymphoid tissue inducer cells, which are endowed with the capacity to induce lymphoid organs (Bar-Ephraim and Mebius, 2016) and produce B cell stimulatory factors in humans (Cella et al., 2010); given this, ILC3s might also be exploited to boost adaptive responses, particularly antibody responses (Reboldi et al., 2016).
However, recent studies on the function of ILCs in the context of immunocompetent mice have shown a considerable redundancy between ILCs and CD4+ T helper cell subsets (Rankin et al., 2016; Song et al., 2015). Thus, it remains to be tested what role, if any, ILC memory cells may have in the protection from pathogens or tumors in immunocompetent organisms.
The three most important questions for the field to move forward with NK cell and ILC-based vaccination strategies
While research over the past decade has led to the discovery of NK cell memory and ILC subsets, three questions remain open: What is the longevity of memory NK cells and ILCs? How do studies in model organisms translate to humans? Would targeting NK cells and ILCs provide any advantage to immunocompetent patients? Adressing these fundamental questions will be essetial to decide whether NK cells and ILCs can be harnessed for vaccination strategies.
Pathways for generation of memory NK cells and possibly memory ILCs.
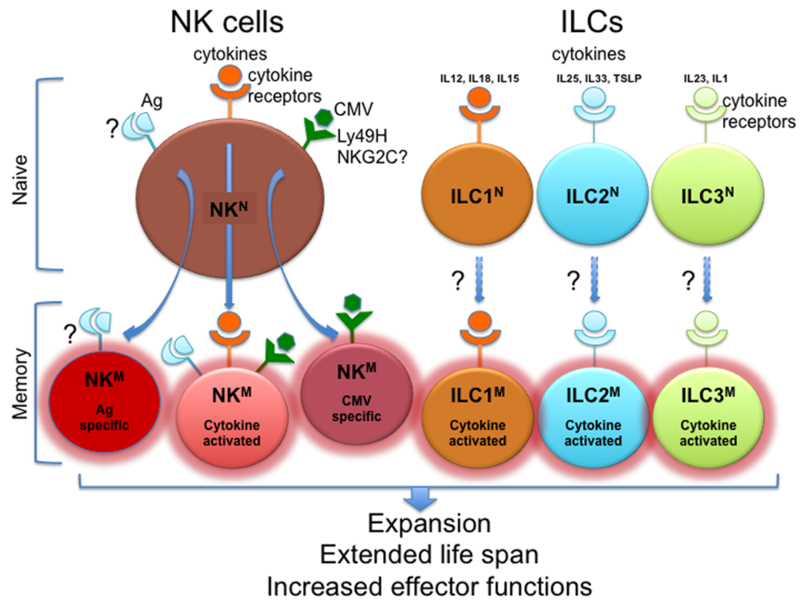
Naïve NK cells can differentiate and expand into memory NK cells with increased lifespan and effector functions through three primary routes: antigen-specific stimulation through yet undefined receptors; cytomegalovirus (CMV)-driven stimulation through Ly-49H in mouse and, possibly, NKG2C in human; cytokine-induced stimulation. Memory ILCs have not been reported. However, since they lack antigen-specific receptors and do not express receptors analogous to the CMV receptors of NK cells, they may acquire memory-like functions only through stimulation of cytokine receptors.
Acknowledgments
We thank Susan Gilfillan for critical comments.
References
- Abt MC, Lewis BB, Caballero S, Xiong H, Carter RA, Susac B, Ling L, Leiner I, and Pamer EG 2015. Innate Immune Defenses Mediated by Two ILC Subsets Are Critical for Protection against Acute Clostridium difficile Infection. Cell Host Microbe 18: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ephraim YE, and Mebius RE 2016. Innate lymphoid cells in secondary lymphoid organs. Immunol Rev 271: 185–199. [DOI] [PubMed] [Google Scholar]
- Carson WE, Dierksheide JE, Jabbour S, Anghelina M, Bouchard P, Ku G, Yu H, Baumann H, Shah MH, Cooper MA, Durbin J, and Caligiuri MA 2000. Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-gamma production and STAT-mediated signal transduction. Blood 96: 1465–1473. [PubMed] [Google Scholar]
- Cella M, Otero K, and Colonna M 2010. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A 107: 10961–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, and Yokoyama WM 2009. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 106: 1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, Toure A, Pritykin Y, Huse M, Leslie CS, and Li MO 2016. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 164: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Colonna M, and Koyasu S 2014. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41: 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, and Yokoyama WM 2001. Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2: 951–956. [DOI] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, and McKenzie AN 2015. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348: aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, and Miller JS 2012. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 119: 2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, and Lopez-Botet M 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104: 3664–3671. [DOI] [PubMed] [Google Scholar]
- Hammerich L, Bhardwaj N, Kohrt HE, and Brody JD 2016. In situ vaccination for the treatment of cancer. Immunotherapy 8: 315–330. [DOI] [PubMed] [Google Scholar]
- Hernandez PP, Mahlakoiv T, Yang I, Schwierzeck V, Nguyen N, Guendel F, Gronke K, Ryffel B, Holscher C, Dumoutier L, Renauld JC, Suerbaum S, Staeheli P, and Diefenbach A 2015. Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol 16: 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TD, and Bryceson YT 2016. Natural killer cell memory in context. Semin Immunol. [DOI] [PubMed] [Google Scholar]
- Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, and Kim S 2012. Identification of human NK cells that are deficient for signaling adaptor FcRgamma and specialized for antibody-dependent immune functions. Int Immunol 24: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel MP, Yang L, and Cooper MA 2013. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immunol 190: 4754–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koues OI, Collins PL, Cella M, Robinette ML, Porter SI, Pyfrom SC, Payton JE, Colonna M, and Oltz EM 2016. Distinct Gene Regulatory Pathways for Human Innate versus Adaptive Lymphoid Cells. Cell 165: 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, and Roosnek E 2008. Human NK cells can control CMV infection in the absence of T cells. Blood 112: 914–915. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, and Kim S 2015. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, Hammer Q, Goodridge JP, Larsson S, Jayaraman J, Oei VY, Schaffer M, Tasken K, Ljunggren HG, Romagnani C, Trowsdale J, Malmberg KJ, and Beziat V 2016. Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep 15: 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska-Szczepanik M, Paust S, von Andrian UH, Askenase PW, and Szczepanik M 2013. Natural killer cell-mediated contact sensitivity develops rapidly and depends on interferon-alpha, interferon-gamma and interleukin-12. Immunology 140: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Oo G, and Lanier LL 2014. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J Exp Med 211: 2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura T, and Lanier LL 2014. Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J Exp Med 211: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Holsken O, Miller M, Hammer Q, Luetke-Eversloh M, Romagnani C, and Cerwenka A 2016. Adoptively transferred natural killer cells maintain long-term anti-tumor activity by epigenetic imprinting and CD4+ T cell help. Oncoimmunology Published online 05 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Miller M, Stojanovic A, Garbi N, and Cerwenka A 2012. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 209: 2351–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, and von Andrian UH 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7: 507–516. [DOI] [PubMed] [Google Scholar]
- O’Sullivan TE, Sun JC, and Lanier LL 2015. Natural Killer Cell Memory. Immunity 43: 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, and von Andrian UH 2010. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, and Tian Z 2013. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest 123: 1444–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin LC, Girard-Madoux MJ, Seillet C, Mielke LA, Kerdiles Y, Fenis A, Wieduwild E, Putoczki T, Mondot S, Lantz O, Demon D, Papenfuss AT, Smyth GK, Lamkanfi M, Carotta S, Renauld JC, Shi W, Carpentier S, Soos T, Arendt C, Ugolini S, Huntington ND, Belz GT, and Vivier E 2016. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol 17: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, and Cyster JG 2016. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352: aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, von Andrian UH, and Barouch DH 2015. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol 16: 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle A, and Brodin P 2016. Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends Immunol 37: 233–243. [DOI] [PubMed] [Google Scholar]
- Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee Y-S, Mulder A, Claas F, Cooper MA, and Fehniger TA 2016. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia Science Translational Medicine In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, and Fehniger TA 2012. Cytokine activation induces human memory-like NK cells. Blood 120: 4751–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, Larsson S, Schaffer M, Malmberg KJ, Ljunggren HG, Miller JS, and Bryceson YT 2015. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HY, Sciume G, Mikami Y, Guo L, Sun HW, Brooks SR, Urban JF Jr., Davis FP, Kanno Y, and O’Shea JJ 2016. Developmental Acquisition of Regulomes Underlies Innate Lymphoid Cell Functionality. Cell 165: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota H, and Klinman DM 2014. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines 13: 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, Mack M, Cella M, and Colonna M 2015. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med 212: 1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, and Lanier LL 2009. Adaptive immune features of natural killer cells. Nature 457: 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boorn JG, Jakobs C, Hagen C, Renn M, Luiten RM, Melief CJ, Tuting T, Garbi N, Hartmann G, and Hornung V 2016. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity 44: 1406–1421. [DOI] [PubMed] [Google Scholar]
- Wagner JA, and Fehniger TA 2016. Human Adaptive Natural Killer Cells: Beyond NKG2C. Trends Immunol 37: 351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM (2013). Natural Killer Cells In Fundamental Immunology, Paul W, ed. (Philadelphia: Lippincott, Williams &Wilkins; ), pp. 395–430. [Google Scholar]
- Zhang T, Scott JM, Hwang I, and Kim S 2013. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 190: 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]


