Abstract
Background:
Pharmacologic adherence measures were critical to the interpretation of the tenofovir (TFV)-disoproxil-fumarate/emtricitabine (TDF/FTC) PrEP trials. These measures are being incorporated into PrEP demonstration projects, but currently-available metrics in plasma, cells, hair or urine involve expensive and time-intensive mass-spectrometry (MS)-based methods. No point-of-care method to assess PrEP adherence in real-time has yet been implemented. Antibody-based tests allow for low-cost, easy-to-perform, point-of-care drug detection. In this study, we developed an antibody-based TFV immunoassay and evaluated its test characteristics among individuals taking TDF/FTC.
Methods:
We synthesized possible immunogens based on TFV’s molecular structure, injected rabbits with the conjugated derivatives, and bled them monthly for subsequent ELISA-testing for TFV-specific antibodies. We purified an antibody with specific TFV binding and created dose–response curves for ELISA-quantification. We then quantified TFV in urine from human participants not taking TDF/FTC and from individuals taking daily TDF/FTC 300 mg/200 mg for 7 days with a 7-day washout period using ELISA with this TFV-specific antibody. ELISA results were compared with the gold-standard test for TFV detection/quantification using liquid-chromatography-tandem-MS (LC–MS/MS).
Findings:
None of the urine samples from 115 participants not taking TDF/FTC showed ELISA- reactivity, indicating 100% specificity (95% CI 97–100%) of the immunoassay. Among participants taking TDF/FTC, 67 of 70 samples positive by LC–MS/MS were positive by the ELISA-immunoassay for an estimated diagnostic sensitivity of 96% (95% CI 88–99%). The precision of the assay was high (coefficient of variationb15%). The rank correlation between ELISA and LC–MS/MS values in the 70 quantitative urine TFV levels positive by LC–MS/MS across a wide range of concentrations among participants on TDF/FTC was high (r = 0.96).
Interpretation:
Our antibody-based immunoassay for measuring TFV in urine performed well compared to the gold-standard of LC–MS/MS among individuals taking TDF/FTC. A sensitive and specific immunoassay paves the way for real-time monitoring/feedback on recent adherence to TFV-based regimens, which should optimize interpretation and outcomes during PrEP and ART roll-out.
Keywords: Antiretroviral adherence, Tenofovir, Immunoassay, Antibody, PrEP, Antiretroviral treatment, Real-time, Point-of-care, Urine, Test characteristics
1. Introduction
The profound utility of assessing antiretroviral (ARV) adherence via pharmacologic metrics was shown for the first time in the placebo-controlled pre-exposure prophylaxis (PrEP) trials [1]. PrEP with oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) was demonstrated to be effective in preventing HIV acquisition among at-risk individuals who take the drug, but objective measures of adherence were critical to interpreting the initial trial results [2]. Although PrEP was effective among men-who-have-sex-with-men (MSM) [3] and men and women in serodiscordant couples [4], there was no efficacy of oral PrEP observed in two large trials (FEM-PrEP [5] and VOICE [6]) conducted among young sexually active women in Africa. Measurement of plasma tenofovir (TFV) levels were key in resolving this discrepancy in results across trials: in the two trials among women, despite self-reporting N90% adherence to study product, fewer than 30% of active-arm participants had detectable TFV levels [5,6], thereby explaining the lack of efficacy. Furthermore, even in the first PrEP trial (iPrEx), the efficacy of TDF/FTC rose from 44% to an estimated 92% among those with detectable blood drug levels [3].
Pharmacologic measures examine drug concentrations in a biomatrix such as plasma [7], peripheral blood mononuclear cells (PBMCs) [1], hair [8], or dried blood spots (DBS) [9]. Discordance between self-reported measures of adherence and pharmacologic metrics, even in the PrEP open label studies and demonstration projects, where adherence was higher than in placebo-controlled trials, has highlighted the importance of incorporating objective measures of adherence during PrEP roll-out. As PrEP enters an exciting phase of global implementation, assessing adherence via objective metrics will aid in defining the real-world effectiveness of this important prevention HIV strategy.
Beyond study interpretation, assessing adherence objectively and providing feedback to patients may improve subsequent adherence and, ultimately, the effectiveness of PrEP. Real-time monitoring of adherence, accompanied by feedback to patients, has led to improved adherence in a variety of disease states, as evidenced by increases in drug levels and better outcomes [10,11]. In FEM-PrEP [5] and VOICE [6], access to a real-time metric of PrEP adherence could have revealed low adherence and the potential impact of that early on. Qualitative work to understand non-adherence in VOICE [6,12] revealed that participants, including those with low, intermediate or high adherence as adjudicated by plasma drug level data, felt that real-time monitoring of adherence and feedback in future trials would be acceptable and improve adherence [12]. In the open-label extension (OLE) study of iPrEx [13], results of plasma PrEP drug levels collected at prior visits were shared with individuals at a later visit. Receiving these results was highly acceptable [13,14] and, for those who were not adherent, motivating to improve subsequent adherence [14].
Urine may be a particularly suitable matrix for real-time adherence monitoring, because urine collection is noninvasive and TFV levels in urine correlate with TDF adherence [15]. However, the current techniques to measure PrEP or PrEP metabolites in urine [15], as well as other biological matrices (e.g. plasma [7], PBMCs [1], DBS [9], and hair[8]) all require expensive liquid chromatography–tandem mass spec-trometry (LC–MS/MS) or other spectrometry-based machines. Moreover, the techniques to extract drug and assess levels via spectrometry-based methods are laborious and require specialized personnel. None of these methods, therefore, presently allow for point-of-care monitoring of ARV adherence.
Antibody-based tests (when packaged into lateral flow immunoas-says) are low-cost, enable drug detection to occur within minutes, and can be performed by non-trained personnel [16]. However, no antibody-based test for PrEP adherence has yet been assessed among individuals taking TDF/FTC. This paper describes the development of an antibody-based (immunoassay) for TFV with high specificity, sensitivity, precision and correlation with the gold standard metric of LC–MS/MS among individuals on TDF/FTC. Since TFV-based regimens (TDF or tenofovir alafenamide (TAF)) are also the backbone of antiretroviral treatment (ART), this immunoassay would have additional utility in monitoring recent adherence during treatment, where consistent drug-taking is important to prevent virologic failure and viral resistance. The packaging of this antibody into a low-cost point-of-care (POC) assay has the potential to aid both interpretation and optimization of outcomes during PrEP and ART roll-out worldwide.
2. Methods
2.1. Development of a TFV-specific Antibody for the Immunoassay
For the development of a TFV-specific immunoassay (Fig. 1 illustrates a schema of immunoassay development) [17], the UCSF Hair Analytical Laboratory (HAL), with expertise in LC/MS–MS and analyzing ARV concentrations in hair in the context of HIV treatment and prevention [18,19], formed a collaboration with Alere™ Rapid Diagnostics, a company with expertise in developing POC immunoassays [20,21]. Scientists from the UCSF HAL and Alere™ scrutinized the molecular structure of TFV to identify unique derivatives with structural distinction from endogenous nucleotides. Of note, small molecules like TFV do not generally elicit an immune response in animals and typically require conjugation to a large molecular weight carrier protein for immunogenicity. Therefore, following the multi-step chemical synthesis of the small molecule TFV derivatives (haptens), which involved attachment of suitable linkers, each was then conjugated to bovine thyroglobulin (BTG) to prepare the immunogens. Three rabbits were then immunized per immunogen. The rabbits were injected with boosters of the immunogens and bled monthly. The rabbit sera were purified by affinity chromatography using a protein G column to obtain the immunoglobulin G (IgG) fraction. The TFV haptens were also conjugated to horseradish peroxidase (HRP) to prepare the matched enzyme conjugate required to generate a signal in the immunoassay. The TFV IgGs were immobilized on 96-well microtiter plates and screened for specific antibody binding activity using enzyme linked immunosorbent assays (ELISA). The analyte of interest (TFV) is then added to the antibody–antigen mix to obtain inhibition of signal and create a dose–response curve. The y-axis value is plotted as the percent of the sample bound to the antibody divided by the maximal possible binding to the antibody (%B/B0).
Fig. 1.
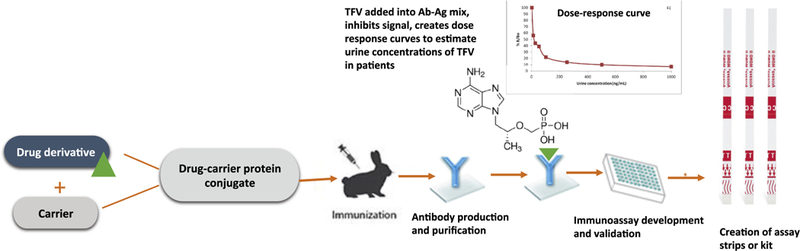
Schema of immunoassay development.
2.2. Methods to Measure Urine Concentrations of TFV via the Immunoassay and LC–MS/MS
2.2.1. LC–MS/MS Concentrations
Using previously described methods [8], the UCSF HAL, a National In stitute of Allergy and Infectious Diseases (NIAID) Division of AIDS’ Clinical Pharmacology and Quality Assurance Program (CPQA) [22]-certified laboratory, measured TFV levels in urine via LC–MS/MS. TFV is separated via reverse-phase high performance chromatography (LC) and quantified by MS/MS using electrospray positive ionization in multiple reaction monitoring mode (TFV, 287·9/175·9 (Q1/Q3)). A prespecified urine TFV concentration ≥500 ng/ml was considered a positive test based on prior LC–MS/MS-based studies of TFV in urine from individuals taking TDF/FTC [15].
2.2.2. ELISA-immunoassay Concentrations
TFV levels were estimated in urine samples from individuals taking TDF/FTC or not taking TDF/FTC via the TFV-specific antibody using ELISA. Working solutions of TFV of known concentrations were prepared. Calibrators or different concentrations of TFV were incubated on a microtiter plate with the hapten to generate a dose response curve. An ELISA plate reader extrapolates the concentration of TFV in the unknown specimen based on the calibration curve. Any detectable TFV concentration above the limit of detection (≥1000 ng/mL) was considered a positive test.
2.3. Human Study Participants for Testing Performance Characteristics of the Immunoassay
To assess the performance of the immunoassay, urine samples were tested from two sources: 1) banked urine samples from healthy human volunteers not taking TDF/FTC and 2) urine collected prospectively from healthy volunteers recruited to take TDF/FTC as part of the UCSF Point-of-Care Urine Monitoring for Adherence (PUMA) study. The PUMA study recruited a convenience sample of ten healthy, non-pregnant adult volunteers at UCSF to take TDF/FTC 300/200 mg at the same time each day for 7 days. TDF/FTC tablets were provided by the study. Participants collected a urine sample prior to TDF/FTC dosing, took TDF/FTC at the same time every day for 7 days and recorded times of drug-taking on a study-provided spreadsheet, then collected urine approximately every three hours in the first 12 h of washout after the last dose, and then every 24 h after the last dose for the next 7 days. A total of 102 timed samples were collected from 10 participants (n = 102) in the PUMA study. The UCSF Committee on Health Research (CHR) approved the protocol for this study and written informed consent from all participants was obtained.
2.4. Data analysis
2.4.1. Specificity
To determine the specificity of the immunoassay, we screened banked urine samples at Alere from 115 healthy volunteers not taking TDF/FTC via ELISA using the TFV-specific antibody. We also screened 32 pre-and post-dosing urine samples by the immunoassay which were negative by the LC–MS/MS assay from participants in the PUMA study.
2.4.2. Sensitivity
To examine the sensitivity of the immunoassay, we analyzed urine samples from the 10 participants taking TDF/FTC in the PUMA study. Urine samples were split and analyzed by both ELISA and LC–MS/MS (with the latter serving as the gold standard). Of note, since TFV concentrates in urine, and to compare TFV levels with those in the literature [15], we diluted the urine samples 1:1000 prior to analysis. Investigators performing the assays were blinded to all participant information, including dosing, and to the results of the other assay (LC–MS/MS or ELISA-immunoassay) (i.e., each sample was labeled with a randomly-generated number).
2.4.3. Precision
The ELISA-immunoassay was challenged using 5 non-blinded urine specimens from each of the 10 volunteers (n = 50) in the PUMA study at different portions of the dose–response curve over multiple runs. Timepoints were chosen to represent a range of values across the concentration range and data from each individual were analyzed separately. The percent coefficient of variation (% CV) of the assay was assessed to determine precision.
2.4.4. Agreement and Correlation of Urine TFV Concentrations Measured via the Immunoassay with Those Measured Using LC–MS/MS
Split urine specimens from the 10 volunteers who collected urine after controlled dosing of TDF/FTC in PUMA were used for this analysis. Agreement between the quantitative urine TFV levels positive both by the ELISA-immunoassay and by LC–MS/MS was calculated using Bland–Altman methods [23] after logarithmic transformation in order to focus on relative errors, which are likely to be more clinically relevant than raw errors. We also calculated the rank correlation, using all the observations that were positive by LC–MS/MS. We separately addressed sensitivity and specificity by cross-tabulating detectable vs undetectable TFV concentrations by the two different assays, with confidence intervals calculated from the binomial distribution. This separate assessment of undetectable specimens ensured that the correlation and agreement calculations would not be inflated by inclusion of specimens without drug.
2.5. Role of The Funding Source
The National Institutes of Health provided funding for this study but played no role in the collection, analysis, or interpretation of data.
3. Results
3.1. Development of a TFV-specific Antibody for the Immunoassay
The rabbits were injected with boosters and blood was collected monthly, with subsequent testing of serum by ELISA. After 5 months of monthly blood collection, each of the three rabbits injected with one of the immunogens developed a polyclonal antibody demonstrating very specific binding to TFV by ELISA. Inhibition of the signal of the antibody–antigen mix was demonstrated with the addition of increasing concentrations of TFV to create dose response curves (Fig. 2). The strong inverse relationship between signal and TFV urine concentrations seen with this antibody demonstrated its suitability for further testing among individuals on TDF/FTC as a TFV immunoassay.
Fig. 2.
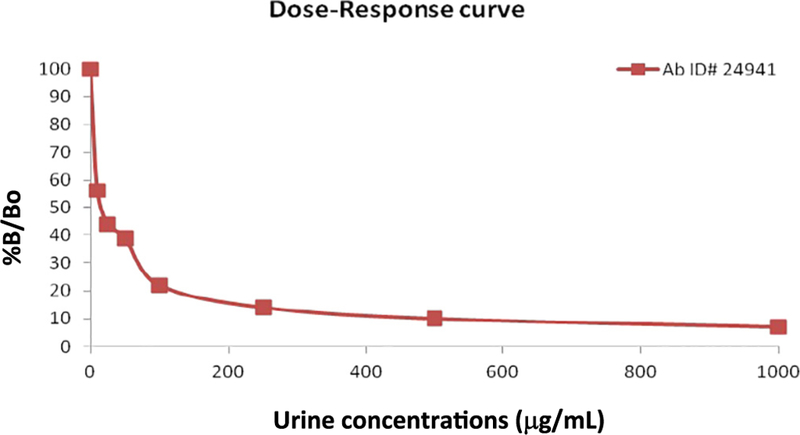
Dose–response curve for quantification of tenofovir concentrations in urine by immunoassay. %B/Bo represents the percent of the sample bound to the antibody divided by the maximal possible binding to the antibody. The dose–response shows a strong inverse relationship between signal and TFV urinary concentrations with this antibody.
3.2. Study Participants
The 115 urine samples to determine the specificity of the assay are from frozen urine stocks from volunteers not taking any concomitant medications. Of the 10 healthy volunteers enrolled in the PUMA study who ingested daily oral TDF/FTC, 5 were female; none were on concomitant medications; the median age was 40 years (range 33–49); and the race/ethnicity was 60% White, 20% South Asian, 20% East Asian.
3.3. Test Characteristics of the Immunoassay (Fig. 3)
Fig. 3.
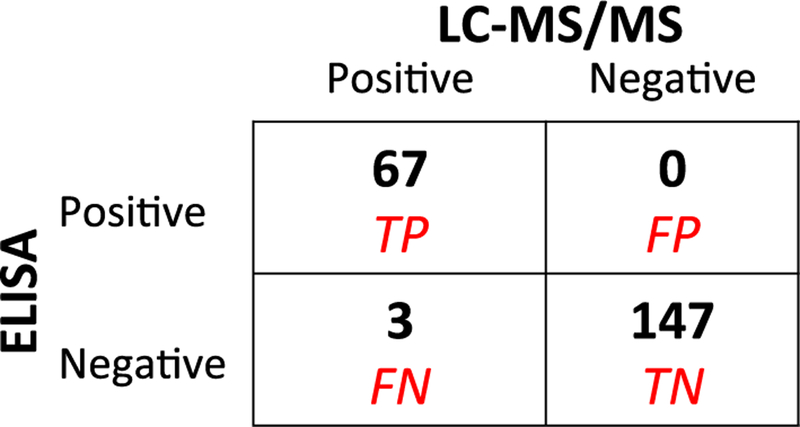
2 × 2 table of LC–MS/MS vs ELISA immunoassay results from banked negative urine samples and participants in PUMA study. 115 banked negative samples +102 urine samples from patients taking TDF/FTC in PUMA study; LC–MS/MS gold standard. TP = True positive; FP = false positive; FN = false negative; TN = true negative.
3.3.1. Specificity
None of the urine samples from the 115 healthy volunteers not taking TDF/FTC showed ELISA reactivity with the isolated antibody, indicating 100% specificity (95% confidence interval (CI) 97–100%) of the TFV immunoassay. Moreover, of the 102 urine samples from the 10 participants recruited in PUMA, 32 (collected prior to dosing and late in the washout period) were negative for TFV by LC–MS/MS. All 32 of these LC–MS/MS-negative samples were also negative by the ELISA immuno-assay, additionally confirming 100% specificity of the assay. Of note, TFV resembles adenine, an endogenous nucleotide in the human body, but the high specificity of the immunoassay confirms that the antibody does not cross-react with endogenous compounds.
3.3.2. Sensitivity
Of the 102 urine samples collected in the PUMA study, 70 were positive (as defined by ≥500 ng/ml) for TFV by LC–MS/MS. All but 3 of these 70 urine samples were also positive by the ELISA-immunoassay, indicating 96% sensitivity (95% CI 88–99%) of the TFV immunoassay relative to LC–MS/MS. Of note, all 3 of the specimens that were negative by ELISA but positive by LC- MS/MS were collected from participants late in the washout period and demonstrated TFV levels b3200 ng/mL.
3.3.3. Precision
The ELISA-immunoassay was challenged using five non-blinded specimens from each of the 10 participants in PUMA at different portions of the dose–response curve over multiple runs. The %CV of the assay for these 50 urine specimens was b15%, demonstrating high precision.
3.4. Agreement and Correlation of Urine TFV Concentrations via the Two Methods
Split urine specimens from the 10 participants in PUMA were analyzed for TFV using LC–MS/MS and the ELISA-immunoassay. Natural log transformed values for the 67 observations positive by both assays had an average difference of 0.12 (standard deviation (SD) 0.40), corresponding to an average relative difference of 12% and suggesting that 95% of immunoassay values would fall within 51% and 247% of the LC–MS/MS value [23]. The rank correlation of urine TFV levels by each assay between all 70 urine TFV levels positive by LC–MS/MS across a wide range of TFV concentrations was high (r = 0.96)(Fig. 4).
Fig. 4.
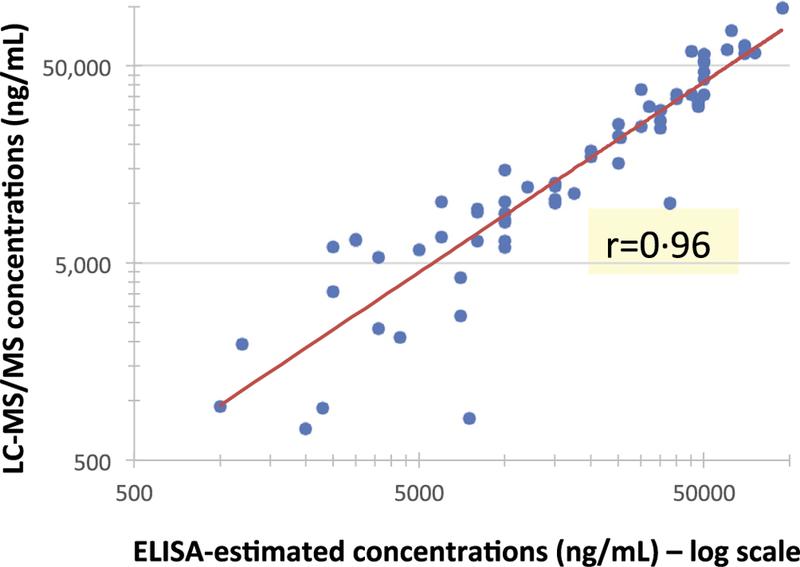
Scatterplot of TFV concentrations in urine measured via ELISA immunoassay vs LCMS/MS Red line indicates the linear regression line between the two sets of values; the rank correlation coefficient is 0.96; the median (range) of TFV concentrations in urine in the 70 samples from the PUMA participants’ positive by LC–MS/MS was 12,450 (723–98,100) ng/mL.
4. Discussion
In this paper, we describe the development an antibody-based assay to measure TFV in urine quantitatively using ELISA-based methods. The antibody-based TFV immunoassay described here was highly specific (100%), sensitive (96%) and estimates TFV levels in urine that correlate strongly with those measured via LC–MS/MS (r = 0.96) among participants on TDF/FTC. This antibody-based test, given its excellent performance characteristics in clinical samples, paves the way for POC adherence testing to tenofovir-based regimens.
Although pharmacologic adherence measures of PrEP using the expensive and labor-intensive modality of LC–MS/MS are available, no POC method to assess adherence to PrEP has yet been implemented. This study shows the sensitivity, specificity, precision and correlation of an antibody-based immunoassay using samples from individuals taking TDF/FTC for the first time. The next step in the development of a TFV immunoassay with these test characteristics will be to package it into an easy-to-use, clinically-useful, low-cost tool to characterize adherence patterns over the past 7 days using interpretative cut-offs for the assay established by a completed directly-observed therapy (DOT) study of TDF/FTC [24]. The data from the DOT study of 2, 4 and 7 doses a week will allow us to determine the appropriate cut-off concentrations of TFV to create the fully-packaged POC test with three test lines indicating no/low, moderate, or high recent adherence, respectively.
The translation of the microplate-based immunoassay (ELISA) to a lateral flow immunoassay (LFIA) is a well-established process [17]. The LFA components include an adsorbent pad onto which the test sample (e.g. urine) is applied; a conjugate pad where the analyte-specific antibodies are conjugated to colored particles such as colloidal gold; a cellulose membrane where a test line will form if the analyte is present; and a wicking pad designed to draw the sample across the reaction membrane by capillary action [17]. These components are fixed to an inert backing material and packaged as a strip test, or within a plastic casing. Since the lateral flow technology is a mature, commoditized platform, any current good manufacturing practices (cGMP) production facility should easily be able to support manufacturing for resource-limited settings. The final price of the LFA (which should be packaged within the next 6 months) is expected to be low (b$2·00/test, for example, for point-of-care HIV serologic testing), and its use will be familiar to health workers already using LFAs to diagnose malaria, HIV, or TB, as well as clinicians who use the urine toxicology screen to determine opioid consumption and substance use [25].
A TFV urine-based immunoassay has the potential to monitor and improve adherence in the context of both HIV prevention and treatment. Since TFV-based regimens (TDF or TAF) are the backbone of anti-retroviral treatment (ART) for HIV infection worldwide, providing a low-cost tool to monitor TFV drug-taking behavior at the clinical point-of-care should also be useful for adherence interpretation during treatment. Indeed, a low-cost POC assay may help avert virologic failure and drug resistance by providing monitoring between less frequent and more expensive viral load measurements on ART [26]. Moreover, in many settings, the return of viral load results from public health laboratories requires a substantial turnaround time, thus making a POC metric particularly valuable. Among HIV-negative individuals on PrEP, in whom viral loads cannot be measured, pharmacologic measures can be used to objectively assess adherence. For either PrEP or ART, not only can real-time feedback on adherence itself improve adherence, but providing practitioners real-time knowledge on how a patient is adhering to ARVs can allow for interventions to be triggered before prevention or treatment effectiveness is compromised. Finally, the integration of POC objective adherence metrics into adherence promotion interventions with known efficacy should be evaluated, both for interpretation and for real-time modification of the intervention when ineffective.
4.1. Limitations
Although other immunoassays [27] or aptamer-based assays [28] for TFV are in development, this is the first study to examine a selective TFV antibody to measure urine TFV levels among individuals actually taking TDF/FTC (demonstrating high specificity, sensitivity, precision and correlation with LC–MS/MS across a wide range of urine TFV concentrations), moving this product close to development into a suitable lateral flow immunoassay. A rapid lateral flow strip test is likely to be the most cost effective POC test for use in resource-limited settings[16], and we have experience in developing rapid strip LFAs from suitable antibodies, but the POC test still requires packaging and marketing. The high specificity of the immunoassay indicates no cross-reactivity of the antibody with endogenous compounds but, since none of our study volunteers were on concomitant medications, this study cannot comment on interactions with other drugs. Finally, while substantial relative differences occurred between this immunoassay and LC–MS/MS on split specimens, the ELISA-immunoassay is not meant to replace LC–MS/MS-based testing, but to allow for the POC testing (which is not yet possible with LC–MS/MS). The high sensitivity, specificity, and correlation of this novel assay suggest strong promise for provision of real-time adherence feedback using the POC immunoassay.
Although urine is a useful, noninvasive matrix for POC testing of ARV adherence, a major limitation of urine-based assays is that urine levels reflect plasma levels [29], which measure only short-term adherence and can be susceptible to temporary increases in adherence prior to visits (e.g. “white coat adherence”). Despite this theoretical concern, this phenomenon has been observed only very infrequently in PrEP. Plasma levels of TFV/FTC served as the “gold standard” adherence metric in all of the placebo-controlled trials of TDF/FTC-based PrEP. Across these trials, plasma PrEP levels provided robust and critical information for interpreting trial results. Indeed, our group found no evidence of white-coat adherence when examining a combination of short and long-term adherence metrics (hair and plasma levels) in VOICE [30]. To address this limitation, however, a combination of short-and long-term metrics may provide the most complete detail to examine patterns of PrEP adherence in roll-out programs [30]. Another way to minimize this concern in future studies or the clinic is to perform unannounced urine assays [31]. Individuals with adherence challenges could then be followed more closely (via differentiated service delivery) by collecting both additional urine and longer-term PrEP adherence testing (e.g. hair, DBS), while implementing adherence-promoting interventions. Finally, self-monitoring of easy-to-perform urine TFV tests, once a protective threshold is established, may provide immediate feedback and motivation to patients on PrEP between clinic visits.
5. Conclusion
In conclusion, we have developed and validated an immunoassay to detect TFV in urine as an objective adherence monitoring tool among individuals taking TDF/FTC. Our antibody-based immunoassay is highly sensitive, specific and precise, measuring concentrations of TFV in urine that correlate strongly with those derived from the gold standard LC–MS/MS-based method among participants on TDF/FTC. Further work to package this immunoassay into a low-cost POC adherence-monitoring tool and testing of its potential to improve adherence to PrEP in at-risk populations is underway. Moreover, we hope that this work will spur a new field of investigation on POC metrics for adherence to other agents for PrEP (e.g. the dapivirine vaginal ring) or ART. A tool to monitor TFV drug-taking behavior at the clinical point-of-care should improve adherence interpretation and launch an entirely new field of adherence intervention to maximize the promise of antiretroviral medications for HIV prevention and treatment worldwide.
Research in context
Evidence before this study
The efficacy of tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC)-based pre-exposure prophylaxis (PrEP) for the prevention of HIV acquisition is reliant on adequate adherence. Pharmacologic measures of adherence were critical to the interpretation of the placebo-controlled PrEP trials and remain important tools in the interpretation of PrEP effectiveness during global PrEP roll-out. Although PrEP drug levels can be measured in plasma, dried blood spots (DBS), peripheral blood mononuclear cells (PBMCs), urine and hair, all of the assays implemented and scaled to date require expensive, specialized and time-intensive mass-spectrometry (MS)-based methods. A point-of-care (POC) metric to determine PrEP adherence in real time has the potential to both motivate adherence by providing immediate adherence feedback to the patient and trigger adherence-promoting interventions.
Antibody-based tests (lateral flow immunoassays) are low-cost, enable drug detection to occur within minutes, and can be performed by non-trained personnel. Urine is a particularly suitable matrix for POC testing since its collection is noninvasive. To find other reports of pharmacologic measures used for PrEP monitoring, we searched PubMed and abstracts from the Conference on Retroviruses and Opportunistic Infections (CROI) since 2009 with the search terms “Pre-exposure prophylaxis”, “PrEP”, “adherence”, “pharmacologic measures” “objective measures”, “point-of-care”, “spectrometry”, “immunoassay”, “antibody”, “tenofovir”, measures of adherence using “urine”, “plasma”, “PBMCs”, “hair”, or “DBS or dried blood spots”. A large number of studies have examined pharmacologic measures of adherence using spectrometry-based methods in a number of biomatrices in the context of PrEP. Several studies have examined liquid chromatography/tandem mass spectrometry (LC–MS/MS) based methods to analyze TFV in urine among individuals on TDF/FTC. One recent study describes the development of an immunoassay for TFV with testing performed only in blank urine samples spiked with TFV; no validation of antibody specificity or sensitivity in urine from patients taking TDF/FTC in this study was performed. No study prior to this report has developed and characterized the test performance of a TFV-based immunoassay in real urine samples across a wide range of concentrations from individuals who have consumed TDF/FTC daily to achieve steady-state concentrations after dosing and during washout.
Added value of this study
We have developed a highly suitable antibody using rabbit serum to measure TFV concentrations in urine semi-quantitatively via an immunoassay with a strong selectivity for TFV, strong inhibition by added TFV into the antigen–antibody mix, and a dose– response curve showing a strong inverse relationship. Furthermore, we describe the testing of a TFV immunoassay for the first time using urine from individuals taking TDF/FTC. Our antibody-based TFV immunoassay is highly specific (100%), sensitive (96%) and estimates quantitative TFV levels in urine that correlate strongly with those measured via the gold standard of LC–MS/MS (r = 0.96) among participants on TDF/FTC. The translation of the plate-based immunoassay to a lateral flow immunoassay (LFA) is a well-established process and we have experience in developing low-cost POC assays.
Implications of all the available evidence
Our study, in the context of existing evidence, provides further evidence that PrEP drug concentrations in a biomatrix measure adherence to TDF/FTC consumption. No true POC metric to assess adherence to TDF/FTC has been implemented to date, although antibody-based immunoassays have promise. The antibody-based TFV immunoassay described here is highly sensitive, specific and precise, measuring concentrations of TFV in urine that correlate strongly with those derived from the gold standard of LC–MS/MS across a wide range of values among individuals taking TDF/FTC. In the context of PrEP roll-out, a packaged POC metric using this highly-characterized immunoassay will be useful for both interpreting and optimizing outcomes. Since TFV-based antiretrovirals are used for both HIV prevention and treatment, a POC assay to measure TFV adherence in real-time can also benefit antiretroviral treatment (ART) monitoring. A low-cost POC assay may help avert virologic failure and drug resistance in patients on ART between more expensive viral load measurements. This study has important implications for the incorporation of POC adherence metrics into novel adherence-promoting interventions to maximize the promise of PrEP and ART during global roll-out.
Acknowledgments
The authors thank the participants of the PUMA study, as well as other members of the UCSF Hair Analytical Laboratory, including Karen Kuncze, Nhi Phung, Alexander Louie, Shirley Yee, and Jamie Shum.
Role of Funding Source
The National Institutes of Health (NIAID/NIH 2R01AI098472, P.I. Gandhi) provided funding for this study but played no role in the collection, analysis, or interpretation of data.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interests to report.
Ethics Committee Approval
The UCSF Committee on Human Research approved the study (UCSF Institutional Review Board study #17–21617).
References
- [1].Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012;4(151) (151ra25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van der Straten A, Brown ER, Marrazzo JM, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc 2016;19(1):20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012;367(5):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of Tenofovir-Emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014;9(1):e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018:62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burke LE, Zheng Y, Ma Q, et al. The SMARTER pilot study: testing feasibility of real-time feedback for dietary self-monitoring. Prev Med Rep 2017;6:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS 2015;29(16):2161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koester KA, Liu A, Eden C, et al. Acceptability of drug detection monitoring among participants in an open-label pre-exposure prophylaxis study. AIDS Care 2015;27 (10):1199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koenig HC, Mounzer K, Daughtridge GW, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med 2017;18(6):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pecoraro V, Banfi G, Germagnoli L, Trenti T. A systematic evaluation of immunoassay point-of-care testing to define impact on patients’ outcomes. Ann Clin Biochem 2017;54(4):420–31. [DOI] [PubMed] [Google Scholar]
- [17].Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem 2016;60(1):111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Phung N, Kuncze K, Okochi H, et al. Development and validation of an assay to analyze atazanavir in human hair via liquid chromatography-tandem mass spectrometry (LC-MS/MS). Rapid Commun Mass Spectrom 2018;32(5):431–41 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011;52(10): 1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Patel K, Nagel M, Wesolowski M, et al. Evaluation of a urine-based rapid molecular diagnostic test with potential to be used at point-of-care for pulmonary tuberculosis: Cape Town cohort. J Mol Diagn 2018;20(2):215–24. [DOI] [PubMed] [Google Scholar]
- [21].Chang M, Steinmetzer K, Raugi DN, et al. Detection and differentiation of HIV-2 using the point-of-care Alere q HIV-1/2 detect nucleic acid test. J Clin Virol 2017;97:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Difrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther 2013;93 (6):479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307–10. [PubMed] [Google Scholar]
- [24].Cressey TR, Siriprakaisil O, Klinbuayaem V, et al. A randomized clinical pharmacokinetic trial of Tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis 2017;17(1):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bang HI, Jang MA, Lee YW. Evaluation of the triage TOX drug screen assay for detection of 11 drugs of abuse and therapeutic drugs. Ann Lab Med 2017;37(6):522–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nachega JB, Sam-Agudu NA, Mofenson LM, Schechter M, Mellors JW. Achieving viral suppression in 90% of people living with HIV on antiretroviral therapy in low- and middle-income countries: progress, challenges, and opportunities. Clin Infect Dis 2018. 10.1093/cid/ciy008 Jan 8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pratt GW, Fan A, Melakeberhan B, Klapperich CM. A competitive lateral flow assay for the detection of tenofovir. Anal Chim Acta 2018;1017:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aliakbarinodehi N, Jolly P, Bhalla N, et al. Aptamer-based field-effect biosensor for Tenofovir detection. Sci Rep 2017;7:44409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 2001;45(10): 2733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koss CA, Bacchetti P, Hillier SL, et al. Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses 2017. 10.1089/aid.2016.0202 Mar 02 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fredericksen R, Feldman BJ, Brown T, et al. Unannounced telephone-based pill counts: a valid and feasible method for monitoring adherence. AIDS Behav 2014; 18(12):2265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


