Abstract
Importance:
Retention in care for individuals with opioid use disorder (OUD) is one of the single greatest predictors of reduced mortality. Although clinical trials support use of OUD medications among adolescents and young adults (“youth”), data on timely receipt of buprenorphine, naltrexone, and methadone and its association with retention in care in real-world treatment settings are lacking.
Objective:
To identify the proportion of youth who receive timely addiction treatment, and to determine whether timely receipt of OUD medications is associated with retention in care.
Design:
Retrospective cohort.
Setting:
Enrollment and complete health insurance claims of 2.4 million Medicaid-enrolled youth from 11 states, January 1, 2014 to December 31, 2015.
Participants:
Youth of age 13–22 years diagnosed with OUD.
Exposures:
Receipt of OUD medication (buprenorphine, naltrexone, or methadone) within three months of diagnosis, compared to receipt of behavioral health services alone.
Main Outcome and Measures:
Retention in care, with attrition defined as ≥60 days without any treatment-related claims.
Results:
Among 4,837 youth diagnosed with OUD, 56.9% were female and 76.0% were non-Hispanic white. Median age was 20 years (interquartile range [IQR], 19–22). Overall, 3,654 (75.5%) youth received any treatment within three months. Most received only behavioral health services (n=2,515; 52.0%), with fewer receiving OUD medications (n=1,139; 23.5%). Only 4.7% (95% confidence interval [CI], 3.1–6.2%) of adolescents <18 years and 24.7% (95% CI, 23.4–26.0%) of young adults ≥18 years received timely OUD medications. Median retention in care among youth who received timely buprenorphine, naltrexone, or methadone was 123 days (IQR, 33–434), 150 days (IQR, 50–670), and 324 days (IQR, 115–670), respectively, compared to 67 days (IQR, 14–206) among youth who received only behavioral health services. Timely receipt of buprenorphine (adjusted hazard ratio [aHR], 0.58; 95% CI, 0.52–0.64), naltrexone (aHR, 0.54; 95% CI, 0.43–0.69), and methadone (aHR, 0.32; 95% CI, 0.22–0.47) were each independently associated with lower attrition from treatment compared to receiving behavioral health services alone.
Conclusions and Relevance:
Timely receipt of buprenorphine, naltrexone, or methadone is associated with greater retention in care among youth with OUD. Strategies to address the underutilization of evidence-based medications for youth are urgently needed.
Keywords: buprenorphine, naltrexone, methadone, adolescent, young adult, opioid-related disorders
INTRODUCTION
As the US confronts the opioid crisis, morbidity and mortality for adolescents and young adults (collectively, “youth”) continues to rise. Overdose deaths, hospitalizations for nonfatal overdose, and diagnoses of opioid use disorder (OUD) among youth have increased sharply since the early 2000s.1–4 Multiple major professional organizations and government bodies recommend providing youth of any age effective treatment as early as possible, including use of OUD medications buprenorphine, naltrexone, or methadone.5–8 Despite these evidence-based recommendations, youth are only one-tenth as likely as adults to receive medication,1,9 likely owing to poor availability of pediatric prescribers, provider discomfort with medications, and stigma surrounding medication treatment.10,11 Even when youth receive medications, it is often only after providers have first exhausted other non-pharmacologic treatment options, such as behavioral health services.1,9,11
Ensuring timely treatment with OUD medications may be especially important in light of data showing that adults who receive medications are more likely to be retained in addiction treatment.12–14 Since living with addiction can be a lifelong process that involves cycles of relapse and recovery, maximizing retention in care is a central strategy in the pursuit of decreased mortality among individuals with OUD,.15,16 In longitudinal cohort studies of adults who have initiated OUD treatment, all-cause mortality when an individual is in treatment is less than half that observed after discontinuing treatment.15 Since attrition from OUD treatment is greater for youth than it is for adults, strategies to prevent youth from leaving care are urgently needed.17–20 Small randomized controlled trials (RCTs) show that, under experimental conditions, youth who receive medication treatment are more likely to be retained in treatment up to 12 weeks.20–22 However, we know of no large studies with follow-up beyond this timeframe, or studies that have used data from real-world treatment settings.
Using an 11-state sample of Medicaid-enrolled youth with OUD, we sought to identify: (1) the frequency with which youth who present to care for OUD receive timely addiction treatment, including behavioral health services and/or OUD medications; and (2) the association between timely receipt of OUD medications and subsequent retention in care. We hypothesized that timely receipt of buprenorphine, naltrexone, or methadone would predict longer retention in addiction treatment.
METHODS
Study Design and Sample
A retrospective cohort study was conducted using the 2014–2015 Truven-IBM Watson Health MarketScan Medicaid Database. These data included 2,490,114 youth of age 13 to 22 years with at least six months of continuous enrollment and all associated inpatient, outpatient, emergency department, behavioral health, and retail prescription drug claims between January 1, 2014 and December 31, 2015. Data were collected from 11 de-identified states representing all census regions of the US. The study was not considered human subjects research by the Boston University School of Medicine Institutional Review Board.
To generate the study sample, the following eligibility criteria were applied to identify all youth initiating a new episode of care for OUD: (i) primary or secondary diagnosis of OUD using International Classification of Diseases, Ninth Revision (ICD-9) codes 304.0x (“Opioid type dependence”) and 304.7x (“Combinations of opioid type drug with any other drug dependence”) in at least one inpatient or emergency department claim or two outpatient claims;1,23 (ii) prior to diagnosis, a 60-day period without another OUD diagnosis or receipt of buprenorphine, methadone, or naltrexone;1,23,24 and (iii) at least three months of enrollment data after diagnosis (eFigure 1).1,23 We defined the date of the first observed OUD diagnosis as the start of the episode of care. Data from the first observed episode of care for OUD were included in analyses; any subsequent episodes of care were excluded.
Variables
Timely addiction treatment was defined as receipt of (i) behavioral health services and/or (ii) OUD medication (buprenorphine, naltrexone, or methadone) within three months of diagnosis. The three-month window was selected based on prior research;1,23 sensitivity analyses also examined receipt of addiction treatment within one, two, six, nine, and 12 months of diagnosis. Behavioral health services were identified using claims for individual outpatient, group outpatient, intensive outpatient, partial hospitalization, residential, and inpatient treatment based on Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes (eTable 1).25,26 Receipt of each of the three OUD medications was identified as follows: buprenorphine, using pharmacy claims that included a National Drug Code (NDC) for sublingual buprenorphine or buprenorphine/naloxone;1,23 methadone, using HCPCS code H0020 (“Methadone administration and/or service”);27,28 and naltrexone, using pharmacy claims that included an NDC for oral or long-acting injectable naltrexone and using HCPCS code J2315 (“Naltrexone, depot form”) (eTable 2).1
Retention in care was defined as time from receipt of first addiction treatment (either behavioral health services or OUD medication) to time of treatment discontinuation. An individual was considered to have discontinued treatment if at least 60 days passed without a claim for behavioral health services or OUD medication. The date of treatment discontinuation was defined as the last date of any qualifying claim. Youth were censored if they disenrolled from their insurance plan.
Sociodemographic covariates included: age of diagnosis, sex, race/ethnicity, and Medicaid eligibility (disability or income). Clinical covariates included (at the time of diagnosis or during the preceding three months): pregnancy, depression, anxiety disorder, attention deficit hyperactivity disorder (ADHD), alcohol use disorder, other substance use disorder, acute pain condition, or chronic pain condition based on ICD-9 diagnosis codes (eTable 3).29–32 Covariates were selected based on their established association with OUD and potential influences on treatment and retention in care.1,5,6,9,33
Statistical Analysis
Sociodemographic and clinical characteristics associated with receipt of any timely addiction treatment were identified using multivariable logistic regression. Among youth who received timely addiction treatment, a subsequent model examined characteristics associated with receipt of OUD medication (with or without behavioral health services) compared to receipt of behavioral health services alone. Multivariable models included all sociodemographic and clinical covariates. Characteristics of youth receiving each of the three OUD medications were compared using chi-square tests or Fisher’s exact test.
The Kaplan-Meier method was then used to measure retention in care among youth who received timely OUD medications as compared to youth who received only behavioral health services. Since the exposure of interest (timely receipt of OUD medication) was a subset of the outcome (ongoing receipt of behavioral health services and/or OUD medication), a separate Kaplan-Meier curve examined the outcome of retention in behavioral health services alone. Multivariable Cox proportional hazards regression was then used to identify the association of receiving timely OUD medications with retention in care. Some youth had an initial claim for addiction treatment but did not receive any subsequent services, which resulted in violation of the proportional hazards assumption of Cox regression.34 The analysis was therefore limited to youth who had at least one subsequent claim after their initial claim; potential differences in sociodemographic and clinical characteristics between youth with and without subsequent claims were identified using multivariable logistic regression. Models examined retention in care in relation to the initial OUD medication used (buprenorphine, naltrexone, methadone, or none), and were adjusted for receipt of higher levels of behavioral health care (intensive outpatient treatment, partial hospitalization, residential care, or inpatient care),25 as well as all sociodemographic and clinical characteristics.
Analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC). All statistical tests were two-sided and considered significant at p<0.05.
RESULTS
Sample
The data included 2,483,250 Medicaid-enrolled youth of age 13–22 years, among whom 4,837 (0.2%) initiated a new episode of care for OUD and thus met sample inclusion criteria. Median age of diagnosis was 20 (interquartile range [IQR], 19–21) years. Youth were 56.9% female and 76.0% non-Hispanic white. Females comprised 40.6% (n=67) of 13- to 15-year-olds with OUD, 41.4% (n=233) of 16- and 17-year-olds with OUD, 55.8% (n=1,030) of 18- to 20-year-olds with OUD; and 62.8% (n=1,422) of 21- and 22-year-olds with OUD. Overall, 28.0% (n=773) of all females were pregnant at the time of OUD diagnosis or in the preceding three months.
Timely Addiction Treatment
Overall, 3,654 (75.5%) youth received any timely addiction treatment (Table 1). The percentage receiving any treatment did not differ significantly between adolescents <18 years (76.1%; 95% confidence interval [CI], 73.0%−79.2%) and young adults ≥18 years (73.7%; 95% CI, 72.4%−75.1%). The majority of the 3,654 youth receiving timely addiction treatment received behavioral health services (n=3,238; 88.6%), either alone or in combination with OUD medications. Of youth receiving any behavioral health services, 872 (26.9%) received intensive outpatient treatment or partial hospitalization, and 1,664 (51.4%) received residential or inpatient care. The remaining 702 (21.7%) received outpatient care only.
Table 1.
Receipt of addiction treatmenta within 3 months of diagnosis among 4,837 Medicaid-enrolled youth with OUD.
| Received Any Timely Treatmenta (N = 3,654) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total, N |
N (%) | Adjustedb OR (95% CI) |
||||
| Age of diagnosis | |||||||
| 21–22 years | 2,263 | 1,550 (68.5) | Reference | ||||
| 18–20 years | 1,846 | 1,479 (80.1) | 1.09 (0.94 − 1.26) | ||||
| 16–17 years | 563 | 432 (76.7) | 1.24 (0.97 − 1.58) | ||||
| 13–15 years | 165 | 122 (74.0) | 0.68 (0.47 − 0.98) | ||||
| Sex | |||||||
| Male | 2,085 | 1,590 (76.3) | Reference | ||||
| Female | 2,752 | 2,064 (75.0) | 1.02 (0.87 − 1.18) | ||||
| Race/ethnicity | |||||||
| White non-Hispanic | 3,677 | 2,876 (78.2) | Reference | ||||
| Black non-Hispanic | 388 | 242 (62.4) | 0.51 (0.41 − 0.65) | ||||
| Hispanic | 55 | 41 (74.5) | 0.83 (0.44 − 1.54) | ||||
| Other | 717 | 495 (69.0) | 0.64 (0.54 − 0.77) | ||||
| Medicaid-eligible due to disability | |||||||
| No | 4,571 | 3,510 (76.8) | Reference | ||||
| Yes | 266 | 144 (54.1) | 0.41 (0.32 − 0.54) | ||||
| Pregnancyc | |||||||
| No | 4,064 | 3,109 (76.5) | Reference | ||||
| Yes | 773 | 545 (70.5) | 0.73 (0.60 − 0.88) | ||||
| Depressionc | |||||||
| No | 3,227 | 2,415 (74.8) | Reference | ||||
| Yes | 1,610 | 1,239 (77.0) | 1.28 (1.08 − 1.51) | ||||
| Anxiety disorderc | |||||||
| No | 3,439 | 2,616 (76.1) | Reference | ||||
| Yes | 1,398 | 1,038 (74.2) | 0.84 (0.71 − 1.00) | ||||
| Attention deficit hyperactivity disorderc | |||||||
| No | 4,244 | 3,191 (75.2) | Reference | ||||
| Yes | 593 | 463 (78.1) | 1.21 (0.97 − 1.53) | ||||
| Alcohol use disorderc | |||||||
| No | 4,138 | 3,082 (74.5) | Reference | ||||
| Yes | 699 | 572 (81.8) | 1.43 (1.15 − 1.78) | ||||
| Other substance use disorderc | |||||||
| No | 2,307 | 1,740 (75.4) | Reference | ||||
| Yes | 2,530 | 1,914 (75.7) | 0.94 (0.82 − 1.08) | ||||
| Acute pain conditionc | |||||||
| No | 3,282 | 2,505 (76.3) | Reference | ||||
| Yes | 1,555 | 1,149 (73.9) | 1.16 (0.98 − 1.38) | ||||
| Chronic pain conditionc | |||||||
| No | 3,253 | 2,555 (78.5) | Reference | ||||
| Yes | 1,584 | 1,099 (69.4) | 0.59 (0.50 − 0.69) | ||||
Had a claim for either behavioral health services or medication treatment within 3 months of diagnosis
Adjusted for all other covariates listed in the table
At or during the three months prior to receiving OUD diagnosis
Only a minority of youth received timely buprenorphine, naltrexone, or methadone (n=1,139; 23.5%) (Table 2). Overall, 4.7% (95% CI, 3.1–6.2%) of adolescents received OUD medications as compared to 24.7% (95% CI, 23.4–26.0%) of young adults. Most of the 1,139 youth who received medication also received concurrent behavioral health services (n=723; 63.5%). Most youth who received an OUD medication received it within one month of diagnosis (eTable 4).
Table 2.
Type of addiction care received within 3 months of diagnosis among 3,654 Medicaid-enrolled youth who received any treatment for OUD.
| Characteristic | Behavioral Health Services Only (N = 2,515), N (%) |
Received OUD Medicationa (N = 1,139), N (%) |
Adjustedb OR for Receipt of OUD Medication (95% CI) |
|
|---|---|---|---|---|
| Age of diagnosis | ||||
| 21–22 years | 889 (57.3) | 661 (42.7) | Reference | |
| 18–20 years | 1,035 (70.0) | 444 (30.0) | 0.78 (0.67 − 0.91) | |
| 16–17 years | 402 (93.1) | 30 (6.9) | 0.16 (0.11 − 0.24) | |
| 13–15 years | 118 (96.7) | 4 (3.3) | 0.08 (0.03 − 0.23) | |
| Sex | ||||
| Male | 1,175 (73.9) | 415 (26.1) | Reference | |
| Female | 1,340 (64.9) | 724 (35.1) | 1.06 (0.90 − 1.26) | |
| Race/ethnicity | ||||
| White non-Hispanic | 1,948 (67.7) | 928 (32.3) | Reference | |
| Black non-Hispanic | 206 (85.1) | 36 (14.9) | 0.48 (0.33 − 0.70) | |
| Hispanic | 31 (75.6) | 10 (24.4) | 0.68 (0.32 − 1.45) | |
| Other | 330 (66.7) | 165 (33.3) | 1.01 (0.82 − 1.26) | |
| Medicaid-eligible due to disability | ||||
| No | 2,391 (68.1) | 1,119 (31.9) | Reference | |
| Yes | 124 (86.1) | 20 (13.9) | 0.47 (0.28 − 0.77) | |
| Pregnancyc | ||||
| No | 2,219 (71.4) | 890 (28.6) | Reference | |
| Yes | 296 (54.3) | 249 (45.7) | 1.62 (1.31 − 2.00) | |
| Depressionc | ||||
| No | 1,562 (64.7) | 853 (35.3) | Reference | |
| Yes | 953 (76.9) | 286 (23.1) | 0.81 (0.67 − 0.98) | |
| Anxiety disorderc | ||||
| No | 1,733 (66.2) | 883 (33.8) | Reference | |
| Yes | 782 (75.3) | 256 (24.7) | 0.89 (0.72 − 1.09) | |
| Attention deficit hyperactivity disorderc | ||||
| No | 2,135 (66.9) | 1,056 (33.1) | Reference | |
| Yes | 380 (82.1) | 83 (17.9) | 0.91 (0.69 − 1.21) | |
| Alcohol use disorderc | ||||
| No | 2,020 (65.5) | 1,062 (34.5) | Reference | |
| Yes | 495 (86.5) | 77 (13.5) | 0.43 (0.33 − 0.56) | |
| Other substance use disorderc | ||||
| No | 1,045 (60.1) | 695 (39.9) | Reference | |
| Yes | 1,470 (76.8) | 444 (23.2) | 0.60 (0.52 − 0.70) | |
| Acute pain conditionc | ||||
| No | 1,685 (67.3) | 820 (32.7) | Reference | |
| Yes | 830 (72.2) | 319 (27.8) | 0.91 (0.74 − 1.10) | |
| Chronic pain conditionc | ||||
| No | 1,755 (68.7) | 800 (31.3) | Reference | |
| Yes | 760 (69.2) | 339 (30.8) | 1.30 (1.07 − 1.59) | |
Includes youth who did and did not receive concurrent behavioral health services
Adjusted for all other covariates listed in the table
At or during the three months prior to receiving OUD diagnosis
Of the 1,139 youth who received timely OUD medications, 936 (82.1%) received buprenorphine, 135 (11.9%) received naltrexone, and 68 (6.0%) received methadone (eTable 5). Adolescents were more likely to receive naltrexone than young adults (35.3% vs. 11.1%; p<0.001 for group difference). No adolescents received methadone.
Retention in Care
The 3,654 youth who received any treatment contributed 13,185 person-months of follow-up. Overall, 2,575 (70.5%) youth discontinued treatment (crude incidence density, 19.5 discontinuing events per 100 person-months). Youth who received timely OUD medications were more likely to be retained in any addiction treatment and more likely to be retained in behavioral health services (Figure 1).
Figure 1:
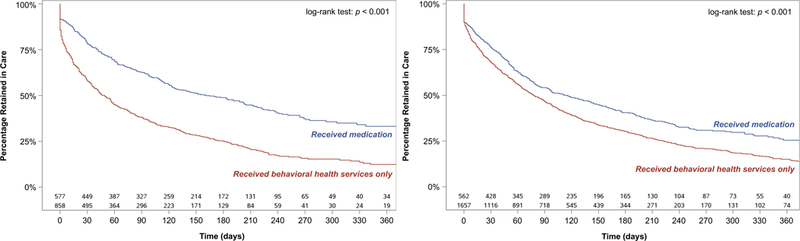
Retention in care according to timely receipt of OUD medication (buprenorphine, naltrexone, or methadone) within 3 months of diagnosis among youth (A) receiving any addiction treatment, and (B) behavioral health services.
Median retention in care among youth who received only behavioral health services was shorter (67 days; IQR, 14–206) than among youth who received timely buprenorphine (123 days; IQR, 33–434), naltrexone (150 days; IQR, 50–670), or methadone (324 days; IQR, 115–670). Similarly, median duration of behavioral health services among youth who did not receive timely OUD medications was shorter (65 days; IQR, 13–204) than among youth who received timely buprenorphine (108 days; IQR, 34–290), naltrexone (152 days; IQR, 55–670), or methadone (217 days; IQR, 41–354).
Of the 3,654 youth who received any timely addiction treatment, 3,247 (88.9%) met criteria for inclusion in the Cox regression analysis of retention in care (i.e., had at least one subsequent claim for behavioral health services or an OUD medication); the remaining 407 (11.1%) youth were excluded to satisfy the proportional hazards assumption of Cox regression. Included youth were more likely than excluded youth to be pregnant (aOR, 1.55; 95% CI, 1.10–2.18); have received intensive outpatient treatment, partial hospitalization, residential, or inpatient behavioral health services (aOR, 2.39; 95% CI, 1.86–3.08); have received buprenorphine (aOR, 1.35; 95% CI, 1.04–1.74); or have received methadone (aOR, 5.22; 95% CI, 1.26–21.5). Of included youth, 97 (2.9%) youth had claims for detoxification services. Of the 3,238 youth who had an initial behavioral health claim, 2,885 (89.1%) had a subsequent claim.
Compared to youth who received only behavioral health services, youth who received timely buprenorphine, naltrexone, and methadone were 42% (95% CI, 36–48%), 46% (95% CI, 31–57%), and 68% (95% CI, 53–78%) less likely, respectively, to discontinue addiction treatment, and were 27% (95% CI, 18–36%), 43% (95% CI, 27–56%), and 53% (95% CI, 32–67%) less likely, respectively, to discontinue behavioral health services (Table 3).
Table 3.
Retention in care among youth with at least two claims for addiction treatment.
| Characteristic | Adjusteda HR (95% CI) |
|
|---|---|---|
| Attrition from Any Addiction Treatmentb,c (n = 3,247) |
Attrition from Behavioral Health Servicesb (n = 2,885) |
|
| Sociodemographic characteristics | ||
| Age ≥21 years | 1.63 (1.45 – 1.83) | 1.59 (1.41 – 1.80) |
| Male sex | 1.13 (1.03 – 1.24) | 1.13 (1.02 – 1.24) |
| Non-Hispanic white | 1.03 (0.93 – 1.14) | 1.05 (0.94 – 1.18) |
| Medicaid-eligible due to disability | 0.63 (0.49 – 0.80) | 0.60 (0.46 – 0.78) |
| Clinical characteristics | ||
| Pregnancyd | 0.87 (0.75 – 0.99) | 0.90 (0.77 – 1.04) |
| Comorbid behavioral health diagnosis (depression, anxiety, or attention deficit hyperactivity disorder)d |
1.04 (0.95 – 1.14) | 1.01 (0.92 – 1.11) |
| Comorbid alcohol or other substance use disorderd | 1.02 (0.93 – 1.12) | 1.00 (0.91 – 1.10) |
| Acute or chronic pain conditiond | 0.92 (0.84 – 1.01) | 0.92 (0.83 – 1.01) |
| Treatment received | ||
| Higher level of behavioral health servicese within 3 months of initiating episode of care |
0.94 (0.85 – 1.02) | 0.92 (0.83 – 1.01) |
| Received timely OUD medication within 3 months of diagnosis |
||
| No medication | Reference | Reference |
| Buprenorphine | 0.58 (0.52 – 0.64) | 0.73 (0.64 – 0.82) |
| Naltrexone | 0.54 (0.43 – 0.69) | 0.57 (0.44 – 0.73) |
| Methadone | 0.32 (0.22 – 0.47) | 0.47 (0.33 – 0.68) |
Multivariable models included all covariates listed in table
Attrition defined as ≥60 days without any claims for services
Includes receipt of any behavioral health services or OUD medications
At or during the three months prior to receiving OUD diagnosis
Includes intensive outpatient treatment, partial hospitalization, residential care, or inpatient care
DISCUSSION
In this multistate study of addiction treatment and retention in care, we found that three-quarters of youth diagnosed with OUD received timely treatment within three months. However, most treatment included behavioral health services only, and fewer than one in four received timely buprenorphine, naltrexone, or methadone. A marked difference was observed by age, with only 4.7% of adolescents receiving an OUD medication, compared to 24.7% of young adults. Receipt of each of the three OUD medications was independently associated with enhanced retention. Youth receiving buprenorphine, naltrexone, and methadone were 42%, 46%, and 68% less likely, respectively, to discontinue treatment during the follow-up period compared to youth who received only behavioral health services.
Retention in care is critical to successful addiction treatment and is increasingly being adopted as a quality measure.26,35–40 In treatment protocols and clinical trials, eliminating or reducing substance use has traditionally been the primary endpoint.41 However, the rise in overdose mortality and the recognition that addiction is a chronic, relapsing condition have prompted clinicians, researchers, and policymakers to increasingly focus on retention in care.6,41 Even when patients do not reduce their substance use, individuals engaged and retained in care can receive harm reduction services and treatment of comorbid medical and psychiatric conditions.42–44 The advantage of this approach is supported by a recent large meta-analysis, which found that among adults, remaining in treatment is associated with substantially reduced all-cause and overdose mortality.15
Our findings reveal a critical gap in quality of care for youth, with only a minority who come to medical attention receiving the evidence-based OUD medications recommended by multiple national treatment guidelines.5–8 Furthermore, this poor deployment of timely OUD medication may place youth at risk for early treatment discontinuation. Our results build on the findings of the only three RCTs of OUD medications among youth conducted to date. These RCTs showed improved treatment outcomes – including short-term enhanced retention in care – among youth who received buprenorphine under experimental conditions.20–22 We found that not only buprenorphine, but also naltrexone and methadone, when provided in real-world treatment settings, are associated with enhanced retention in care compared to behavioral health services alone. Since clinical follow-up in youth RCTs to date has only ranged from 28 days to 12 weeks,20,21 our results also suggest that OUD medications may contribute to enhanced retention in care persisting well beyond the timeframes previously studied.
Strategies to enhance access to OUD medications for youth are urgently needed.1,9,45 Our study sample included only youth who presented to medical attention, a group comprising only a minority of the true population of youth with OUD.6 Youth encounter substantial barriers to accessing OUD medications, including insufficient pediatric prescribers, poor familiarity with medications among providers, systemic barriers to accessing methadone, and stigma surrounding medication use.1,9–11 At many treatment programs, youth may be denied OUD medications due to their younger age, or paradoxically if they are receiving an OUD medication prescribed elsewhere, use of medications may preclude entry into treatment.11 As of January 2018, the Substance Abuse and Mental Health Services Administration Treatment Locator lists 1,765 addiction treatment programs for adolescents or young adults, among which only 37% prescribe OUD medications – and of the remaining programs, 43% deny admission to youth receiving medications prescribed elsewhere.46 Our findings suggest that the practice of limiting access to OUD medications for youth may be detrimental for addiction treatment programs, since receipt of medication is associated with enhanced retention in care.
Critically, we observed differences by race in access to OUD medications. In one recent study of youth with private health insurance, black youth were 42% less likely to receive buprenorphine or naltrexone for OUD compared to white youth,1 which is consistent with our finding that black youth were 59% less likely to receive OUD medications. Importantly, we did not observe any differences in retention in care according to race after controlling for receipt of medications. Therefore, amidst national efforts to expand access to evidence-based treatment, it is crucial that policymakers address the national treatment gap in a way that improves, rather than exacerbates, the disparities that we observed.
There are several limitations to this study. First, due to the observational nature of our study, we are unable to conclusively determine whether the enhanced retention in care we observed was due to the OUD medications themselves, or to the clinical systems in which they were provided. For example, methadone is typically administered in a setting with strict rules and regulations that may promote greater adherence for patients who receive such treatment.47 Similarly, provision of evidence-based medications such as buprenorphine or naltrexone could be more common in treatment centers with higher quality standards.38,39 Second, we found that behavioral health services alone were associated with poorer retention in care than OUD medication treatment, but the behavioral health services we included were diverse. Although we adjusted for level of care, we cannot exclude the possibility that some behavioral health services may have been highly effective but were categorized with less effective behavioral health services.
Third, although we adjusted for sociodemographic and clinical characteristics of youth, we cannot exclude the potential influence of unmeasured confounders. In particular, given the administrative nature of the data, we were unable to adjust for OUD disease severity, and youth with more severe OUD may have been more likely to receive medication as well as added resources to maximize retention. Fourth, we were unable to identify buprenorphine used in detoxification settings, which, when rapidly tapered, may be associated with poorer retention in care compared to longer term buprenorphine maintenance treatment.20 Since only 2.9% of youth received detoxification services in the study sample, this potential limitation is unlikely to have had a substantial impact on the effect sizes we observed.
To our knowledge, this is the first large study to examine receipt of each of the three evidence-based OUD medications among youth. We are also unaware of other large studies that have examined retention in care in relation to timely OUD medication treatment for youth. The finding that medications were provided to only approximately one in four youth presenting for care overall – including only one in 21 adolescents – highlights a crucial potential opportunity to improve OUD care and enhance retention in treatment. As overdose deaths mount among US youth, it is vital that clinicians, researchers and policymakers ensure that access to evidence-based OUD medications for young people remains a national priority.
Supplementary Material
KEY POINTS.
Question: What percentage of youth receive timely medications for opioid use disorder (OUD), and are youth who receive timely medications more likely to remain in care?
Findings: In this multistate cohort of 4,837 youth with OUD, one in 21 adolescents <18 years and one in four young adults 18–22 years received medication within three months of diagnosis. Youth who received buprenorphine, naltrexone, or methadone were 42%, 46%, and 68% less likely, respectively, to discontinue treatment.
Meaning: Pharmacotherapy, a critical evidence-based intervention to address OUD, is underutilized in youth. Youth who receive timely medications are more likely to remain in care.
Acknowledgements
We would like to thank Dr. Jason Vassy, MD, MPH, SM for his review of the manuscript.
Role of Funding Source
Dr. Hadland was supported by the Thrasher Research Fund Early Career Award, the Academic Pediatric Association Young Investigator Award, and L40 DA042434 (NIH/NIDA). Dr. Bagley was supported by K23 DA044324 (NIH/NIDA). Dr. Silverstein was supported by K24 HD081057 (NIH/NICHD). Dr. Larochelle was supported by K23 DA042168 (NIH/NIDA). Dr. Samet was supported by R25 DA13582 (NIH/NIDA). Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in Receipt of Buprenorphine and Naltrexone for Opioid Use Disorder Among Adolescents and Young Adults, 2001–2014. JAMA Pediatr 2017;171(8):747–755. 10.1001/jamapediatrics.2017.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaither JR, Leventhal JM, Ryan SA, Camenga DR. National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr 2016;170(12):1195–1201. 10.1001/jamapediatrics.2016.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtin SC, Tejada-Vera B, Warner M. Drug Overdose Deaths Among Adolescents Aged 15–19 in the United States: 1999–2015. NCHS Data Brief, No 282 Hyattsville, MD; 2017. [PubMed] [Google Scholar]
- 4.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Substance Use and Prevention. Medication-assisted treatment of adolescents with opioid use disorders. Pediatrics 2016;138(3):e20161893 10.1542/peds.2016-1893. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services (HHS) Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health Washington, DC; 2016. [PubMed] [Google Scholar]
- 7.Center for Substance Abuse Treatment. Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series, No. 40 Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [Google Scholar]
- 8.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med 2015;9(5):358–367. 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feder KA, Krawczyk N, Saloner B. Medication-Assisted Treatment for Adolescents in Specialty Treatment for Opioid Use Disorder. J Adolesc Heal 2017;60(6):747–750. 10.1016/j.jadohealth.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblatt RA, Andrilla CHA, Catlin M, Larson EH. Geographic and Specialty Distribution of US Physicians Trained to Treat Opioid Use Disorder. Ann Fam Med 2015;13(1):23–26. 10.1370/afm.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagley SM, Hadland SE, Carney BL, Saitz R. Addressing Stigma in Medication Treatment of Adolescents With Opioid Use Disorder. J Addict Med 2017;11(6):415–416. 10.1097/ADM.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 12.Thomas CP, Fullerton CA, Kim M, et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv 2014;65(2):158–170. 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- 13.Krupitsky E, Nunes E V, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet 2011;377(9776):1506–1513. 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 14.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction 2009;104(7):1193–1200. 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- 15.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupouy J, Palmaro A, Fatséas M, et al. Mortality Associated With Time in and Out of Buprenorphine Treatment in French Office-Based General Practice: A 7-Year Cohort Study. Ann Fam Med 2017;15(4):355–358. 10.1370/afm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuman-Olivier Z, Weiss RD, Hoeppner BB, Borodovsky J, Albanese MJ. Emerging adult age status predicts poor buprenorphine treatment retention. J Subst Abuse Treat 2016;47(3):202–212. 10.1016/j.jsat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein ZM, Kim HW, Cheng DM, et al. Long-term retention in Office Based Opioid Treatment with buprenorphine. J Subst Abuse Treat 2017;74:65–70. 10.1016/j.jsat.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreifuss JA, Griffin ML, Frost K, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug Alcohol Depend 2013;131(1–2):112–118. 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsch LA, Moore SK, Borodovsky JT, et al. A randomized controlled trial of buprenorphine taper duration among opioid-dependent adolescents and young adults. Addiction 2016;111(8):1406–1415. 10.1111/add.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA 2008;300(17):2003–2011. 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsch LA, Bickel WK, Badger GJ, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch Gen Psychiatry 2005;62(10):1157–1164. 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- 23.Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend 2012;123(1–3):72–78. 10.1016/j.drugalcdep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Garnick DW, Lee MT, O’Brien PL, et al. The Washington circle engagement performance measures’ association with adolescent treatment outcomes. Drug Alcohol Depend 2012;124(3):250–258. 10.1016/j.drugalcdep.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Society of Addiction Medicine. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions 3rd ed. (Mee-Lee D, ed.). Chevy Chase, MD: American Society of Addiction Medicine; 2013. [Google Scholar]
- 26.Harris AHS, Ellerbe L, Phelps TE, et al. Examining the Specification Validity of the HEDIS Quality Measures for Substance Use Disorders. J Subst Abuse Treat 2015;53:16–21. 10.1016/j.jsat.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Mohlman MK, Tanzman B, Finison K, Pinette M, Jones C. Impact of Medication-Assisted Treatment for Opioid Addiction on Medicaid Expenditures and Health Services Utilization Rates in Vermont. J Subst Abuse Treat 2016;67:9–14. 10.1016/j.jsat.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Frazier W, Cochran G, Lo-Ciganic W- H, et al. Medication-Assisted Treatment and Opioid Use Before and After Overdose in Pennsylvania Medicaid. JAMA 2017;318(8):750–752. 10.1001/jama.2017.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-9-CM https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp#overview. Published 2017. Accessed August 17, 2017.
- 30.Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics 2014;133(4):602–609. 10.1542/peds.2013-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Prescription Drug Overdose Data & Statistics https://www.cdc.gov/drugoverdose/pdf/pdo_guide_to_icd-9-cm_and_icd-10_codes-a.pdf. Published 2013. Accessed August 17, 2017. [Google Scholar]
- 32.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in Opioid Prescribing by Race/Ethnicity for Patients Seeking Care in US Emergency Departments. JAMA 2008;299(1):431–438. 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 33.Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA psychiatry 2016;73(1):39–47. 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption. In: Survival Analysis New York, NY: Springer; 2012:161–200. [Google Scholar]
- 35.Harris AHS, Humphreys K, Bowe T, Tiet Q, Finney JW. Does meeting the HEDIS substance abuse treatment engagement criterion predict patient outcomes? J Behav Health Serv Res 2010;37(1):25–39. 10.1007/s11414-008-9142-2. [DOI] [PubMed] [Google Scholar]
- 36.Watkins KE, Ober AJ, Lamp K, et al. Collaborative Care for Opioid and Alcohol Use Disorders in Primary Care. JAMA Intern Med August 2017. 10.1001/jamainternmed.2017.3947. [DOI] [PMC free article] [PubMed]
- 37.Chou R, Korthuis PT, Weimer M, et al. Medication-Assisted Treatment Models of Care for Opioid Use Disorder in Primary Care Settings. Technical Brief No. 28. (Prepared by the Pacific Northwest Evidence-Based Practice Center under Contract No. 290–2015-00009-I.) AHRQ Publication No. 16(17)-EHC0 Rockville, MD: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 38.Carroll KM, Weiss RD. The Role of Behavioral Interventions in Buprenorphine Maintenance Treatment: A Review. Am J Psychiatry 2017;174(8):738–747. 10.1176/appi.ajp.2016.16070792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Society for Addiction Medicine. The ASAM Performance Measures For the Addiction Specialist Physician Chevy Chase, MD: American Society for Addiction Medicine; 2014. [Google Scholar]
- 40.New York State Department of Health. Medicaid Redesign Team (MRT) Behavioral Health Reform Work Group Final Recommendations https://www.health.ny.gov/health_care/medicaid/redesign/docs/mrt_behavioral_health_reform_recommend.pdf. Published 2011. Accessed December 27, 2017.
- 41.Uchtenhagen A Commentary on Metrebian et al (2015): What is addiction treatment research about? Some comments on the secondary outcomes of the Randomized Injectable Opioid Treatment Trial. Addiction 2015;110(3):491–493. 10.1111/add.12821. [DOI] [PubMed] [Google Scholar]
- 42.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med 2011;171(5):425–431. 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): Statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat 2016;60:6–13. 10.1016/j.jsat.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakeman SE. Another Senseless Death - The Case for Supervised Injection Facilities. N Engl J Med 2017;376(11):1011–1013. 10.1056/NEJMp1613651. [DOI] [PubMed] [Google Scholar]
- 45.Saloner B, Feder KA, Krawczyk N. Closing the Medication-Assisted Treatment Gap for Youth With Opioid Use Disorder. JAMA Pediatr 2017;171(8):729–731. 10.1001/jamapediatrics.2017.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SAMHSA Behavioral Health Treatment Services Locator. Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 47.Wachino V, Hyde PS. Coverage of Behavioral Health Services for Youth with Substance Use Disorders https://www.medicaid.gov/federal-policy-guidance/downloads/cib-01-26-2015.pdf. Accessed December 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


