Abstract
Osteoarthritis is a common chronic disease that can be better treated with the help of self-management interventions. Mobile health (mHealth) technologies are becoming a popular means to deliver such interventions. We reviewed the current state of research and development of mHealth technologies for osteoarthritis self-management to determine gaps future research could address. We conducted a systematic review of English articles and a survey of apps available in the marketplace as of 2016. Among 117 unique articles identified, 25 articles that met our inclusion criteria were reviewed in-depth. The app search identified 23 relevant apps for osteoarthritis self-management. Through the synthesis of three research themes (osteoarthritis assessment tools, osteoarthritis measurement tools, and osteoarthritis motion monitoring tools) that emerged from the current knowledge base, we provide a design framework to guide the development of more comprehensive osteoarthritis mHealth apps that facilitate self-management, decision support, and shared decision-making.
Keywords: arthritis, mHealth, mobile health, smartphone app, osteoarthritis of knee, self-management
Introduction
Mobile health applications (mHealth apps) enable delivery of health services through mobile devices such as smartphones.1 mHealth apps can support patients’ self-management of their health conditions, especially chronic diseases that require a long period of supervision, observation, care, and special training of the patient for rehabilitation and may leave residual disability,2 such as diabetes, asthma, depression, and bipolar disorder.3–6 In light of the increasing ownership of smart-phones—68 percent of American adults owned a smartphone as of 2015 compared to 35 percent in 20117—mHealth apps running on smartphones have the potential to play an important role in supporting personal health management (i.e. self-management) by providing up-to-date information on health topics and encouraging proactive health behaviors.8,9 mHealth apps also have the potential to increase access to healthcare services and reduce cost if the solutions are designed to address the needs of all stakeholders and deployed effectively.10
Osteoarthritis (OA) is a common chronic disease that occurs in the joints of the knees, hips, hands, and spine, causing pain, stiffness, and reduced motion.11 Statistics reveal that OA causes considerable burden in terms of patients’ quality of life as well as costs for medical treatments including both direct costs (e.g. drugs and hospitalizations) and indirect costs (e.g. lost time doing chores including paid help and time lost from work).12 Knee and hip replacements were the most common inpatient procedures with almost 1 million surgeries performed in the United States in 2012.13 In particular, knee OA was one of the leading causes of disability that limited the patients’ daily activities such as stair climbing, walking a mile, housekeeping, and carrying bundles.14
OA-related pain and disability can be improved through appropriate treatment such as exercise, weight control, rest and joint care, medications, and surgery.15,16 In particular, when physical therapy and medication no longer relieve arthritis pain, total joint replacement (TJR) surgery is the most common treatment.17 However, many patients do not seek help for their arthritis-related symptoms until these symptoms become unbearable due to misunderstanding of their condition and lack of accurate information about the surgery.18 Thus, it is critical for patients to be able to recognize the signs of early-to-moderate OA and seek the appropriate treatments for the condition, including TJR surgery.
Self-management of OA can enable patients to be better informed about their OA-related symptoms. Informed patients are better equipped to play a more active role in the shared decision-making process during which patients and clinicians discuss treatment alternatives and make decisions based on mutual agreement.19 Clinical guidelines for OA have endorsed that self-management can improve outcomes of OA treatment when it is used as a supplemental tool with medical care, especially for adequately informed patients seeking OA treatments, including TJR surgery, in a long-term plan.15,16 Effective use of OA self-management tools, therefore, can facilitate not only the intervention programs targeted at patient education and behavioral modification20,21 but also the interactions between the patients and clinicians throughout the OA treatment, that is, shared decision-making.22 Considering that the mode of self-management intervention delivery used in previous studies, so far, has been limited to face-to-face, Internet, and telephone,20 there is room for utilizing mHealth technologies, especially mobile apps, as an effective means of intervention delivery to enhance the effectiveness of OA self-management.
There have been reviews on mHealth apps as an effective means to deliver health interventions in different domains such as mental health disorders (e.g. depression, stress, and bipolar disorder)23 and the most prevalent health conditions selected by the World Health Organization (WHO) Global Burden of Disease24 (e.g. diabetes, asthma, hearing loss, and migraine).4,25 To the best of our knowledge, however, there is no review focused particularly on patients’ use of mHealth technology for OA, a significant medical condition that warrants a review. In this article, we systematically review articles, using different study designs such as randomized trials, cohort studies, and case reports that develop and/or evaluate mHealth technologies to help patients self-manage OA and make shared decisions about choosing the ‘right’ treatment methods including TJR surgery with their clinicians. We also survey major app stores to analyze the main functions of existing mHealth apps for OA management available in the marketplace. The goal of this systematic review is, therefore, to advance our understanding of currently available mHealth technologies that can be used for OA management, specifically targeting the most common joints (i.e. knees and hips) that affect a large population, as well as to identify the gap to be addressed in future research.
Materials and methods
Literature search strategy
We developed our own literature review protocol to pre-specify and standardize the article search process (Table 1). We used PubMed, Web of Science, ScienceDirect, Association for Computing Machinery (ACM) Digital Library, and Institute of Electrical and Electronics Engineering (IEEE) Xplore Digital Library for our literature search to capture the interdisciplinary domain of mHealth. Using the Medical Subject Headings (MeSH), we identified seven relevant search terms regarding OA, in general, and OA of knee and hip joints, in particular, as they are the most common joints where OA occurs and are the major cause of disability, especially among older adults:13,14 (a) osteo-arthritis, (b) knee joint, (c) hip joint, (d) arthroplasty, (e) total joint replacement, (f) total knee replacement, and (g) total hip replacement. In addition, we identified seven terms regarding mHealth technology: (a) mobile health, (b) mhealth, (c) m-health, (d) mobile application, (e) mobile app, (f) smartphone, and (g) mobile phone. Using these terms, we formulated search queries, which were then used in the online database search (Table 1). We limited the search results to original papers in English published in peer-reviewed journals and conference proceedings between 2007 (start of the smartphone era) and June 2016.
Table 1.
Online database search results by search queries (searched on 9 June 2016).
| Search queries | Search results (by database) | ||||
|---|---|---|---|---|---|
| PM | WoS | SD | ACM | IEEE | |
| osteoarthritis AND (“mobile applications” OR “mobile application” OR “mobile apps” OR “mobile app”) | 3 | 17 | 4 | 4 | 2 |
| osteoarthritis AND (“mobile health” OR “mhealth” OR “m-health”) | 8 | 1 | 4 | 4 | 0 |
| osteoarthritis AND (“mobile phone” OR smartphone) | 3 | 17 | 2 | 4 | 1 |
| (“hip joint”) AND (“mobile applications” OR “mobile application” OR “mobile apps” OR “mobile app”) | 2 | 0 | 0 | 5 | 0 |
| (“hip joint”) AND (“mobile health” OR “mhealth” OR “m-health”) | 0 | 0 | 0 | 5 | 0 |
| (“hip joint”) AND (“mobile phone” OR smartphone) | 5 | 1 | 1 | 5 | 1 |
| “knee joint” AND (“mobile applications” OR “mobile application” OR “mobile apps” OR “mobile app”) | 4 | 6 | 0 | 16 | 0 |
| “knee joint” AND (“mobile health” OR “mhealth” OR “m-health”) | 2 | 1 | 0 | 16 | 0 |
| “knee joint” AND (“mobile phone” OR smartphone) | 12 | 5 | 1 | 16 | 0 |
| “total joint replacement” AND (“mobile applications” OR “mobile application” OR “mobile apps” OR “mobile app”) | 0 | 0 | 0 | 0 | 0 |
| “total joint replacement” AND (“mobile health” OR “mhealth” OR “m-health”) | 0 | 0 | 0 | 0 | 0 |
| “total joint replacement” AND (“mobile phone” OR smartphone) | 0 | 0 | 1 | 0 | 0 |
| arthroplasty AND (“mobile applications” OR “mobile application” OR “mobile apps” OR “mobile app”) | 3 | 1 | 0 | 3 | 0 |
| arthroplasty AND (“mobile health” OR “mhealth” OR “m-health”) | 5 | 0 | 5 | 3 | 0 |
| arthroplasty AND (“mobile phone” OR smartphone) | 6 | 3 | 0 | 3 | 0 |
| (“total knee replacement” OR “total hip replacement”) AND (“mobile applications” OR “mobile application” OR “mobile apps” OR “mobile app”) | 0 | 0 | 0 | 2 | 0 |
| (“total knee replacement” OR “total hip replacement”) AND (“mobile health” OR “mhealth” OR “m-health”) | 0 | 0 | 0 | 2 | 0 |
| (“total knee replacement” OR “total hip replacement”) AND (“mobile phone” OR “smartphone”) | 0 | 0 | 1 | 2 | 0 |
| Total | 53 | 52 | 19 | 90 | 4 |
PM: PubMed; WoS: Web of Science; SD: ScienceDirect; ACM: Association for Computing Machinery Digital Library; IEEE: Institute of Electrical and Electronics Engineering Xplore Digital Library.
Inclusion and exclusion criteria
Two of the authors together evaluated the titles and abstracts, and in some cases the full text, of the retrieved articles against our inclusion/exclusion criteria. At this initial stage, full text of the articles was only reviewed when an inclusion/exclusion decision could not be made through abstract reviews. We included articles that mentioned (a) the development of mHealth technologies to monitor and manage OA-related pains and symptoms or to provide educational content, (b) the development of mHealth technologies to analyze human gestures and motions that were considered to be useful for OA management, (c) the use of mHealth technologies to deliver clinical interventions to OA patients in both pre- and post-surgery phases, and (d) the use of mHealth technologies to provide decision support related to OA management.
We excluded articles that did not focus on OA (or related joints such as knees and hips) or those that did not involve any type of mHealth technologies. Thus, articles that used mobile technologies in different contexts, such as video game development, simulation, and virtual augmented reality, which were not directly related to OA management, were excluded. Furthermore, articles that briefly mentioned OA in the background or related work sections of the papers were also excluded—these articles usually mentioned OA as an example of common diseases for adults rather than the main focus of research. In the same context, articles that used mobile technologies as an auxiliary tool for data collection (e.g. text messages to engage the participants in the study) were excluded. Finally, we excluded articles that focused primarily on the effects of clinical interventions for OA treatment without specific involvement of mHealth technologies in the intervention process.
We conducted an in-depth review of included articles to identify the research themes addressed in each article and categorized research findings to determine the state of knowledge. Specifically, we identified the research questions addressed in the study, the type and purpose of mobile technologies used, target joints examined, findings that are relevant to use of mHealth technologies for OA management, and for papers that mentioned the use of human subjects, the characteristics of participants. Due to the inclusive nature of the review purpose (i.e. to explore the current state of research on the use of mHealth technologies for OA self-management), the design of the included studies varied. Thus, we did not use a specific measure to assess the quality of the included studies.
mHealth app review
We followed the search strategy and selection criteria used in a previous app review paper that investigated existing mHealth apps for OA and other diseases.25 We searched for “osteoarthritis” in five online app stores: Google Play,26 Apple iTunes app Store,27 BlackBerry World,28 Microsoft Store,29 and Opera Mobile Store.30 Of the 147 apps identified by the app search, we included apps if they were (a) categorized in the health, fitness, or medical categories; (b) centered on OA; (c) developed for humans; and (d) written in English. We excluded apps that were in the games or entertainment categories, built for animals, and provided access to journals or magazines. We categorized remaining apps by main features they provide: provides educational contents or allows users to keep track of OA-related pains and symptoms or both.
Results
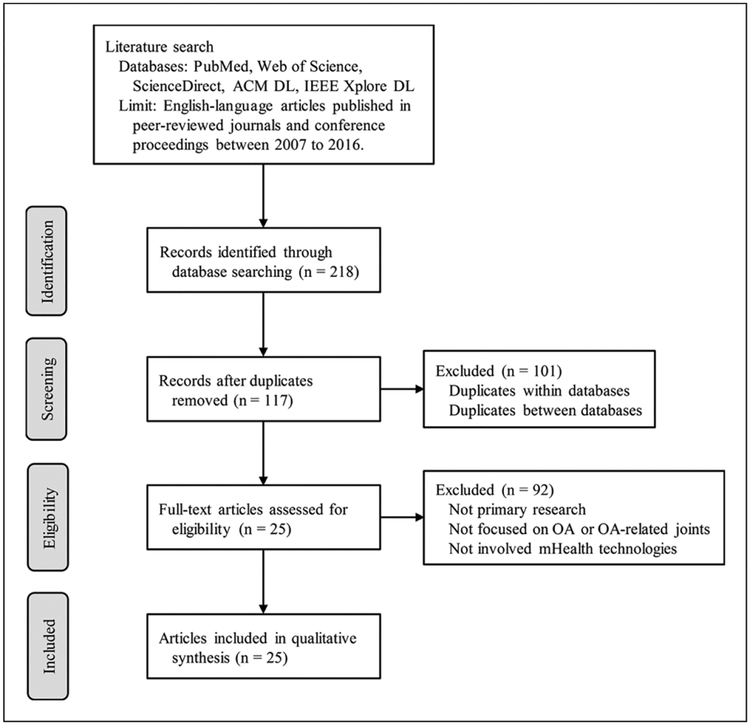
We identified 218 articles from the online database search; after adjusting for duplicates across different databases, 117 unique articles remained. Of these, 25 articles that met our inclusion criteria remained for in-depth review (Figure 1).
Figure 1.
Flow diagram of article selection process.
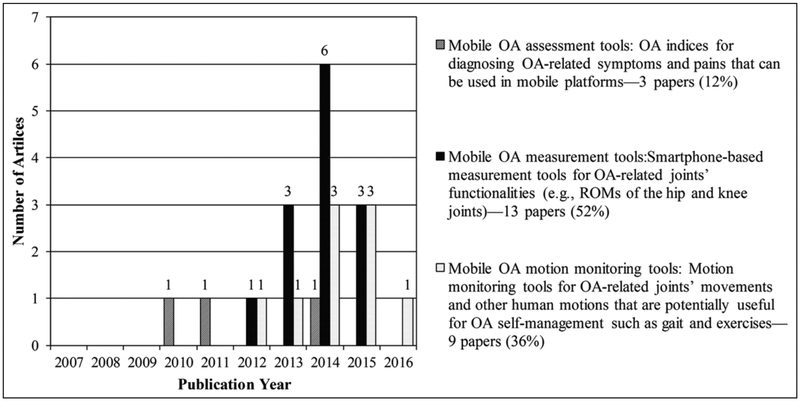
Three main research themes emerged from our analysis of included articles: (a) mobile OA assessment tools—articles that address OA indices to diagnose OA-related symptoms and pains that can be used in mobile platforms; (b) mobile OA measurement tools—articles that examine the applicability of smartphones as measurement tools for OA-related joints such as knees, hips, and ankles; and (c) mobile OA motion monitoring tools—articles that analyze motions that are directly related to OA or potentially useful to support OA management such as gait. Figure 2 illustrates the distributions of the included articles by publication year and research theme.
Figure 2.
Themes of included articles by publication year.
Mobile OA assessment tools
Articles included in this category present the development and validation of two OA assessment tools—Mobile Western Ontario and McMaster Index (m-WOMAC)31,32 and Appropriate Use Criteria for Osteoarthritis of the Knee (AUC OAK)33—which are proven to be effective in diagnosing OA-related symptoms and pains in mobile platforms. The m-WOMAC demonstrated good validity, reliability, and responsiveness, compatible with the original paper-based WOMAC (p-WOMAC); OA patients participated in the study comparing the m-WOMAC versus p-WOMAC did not present any mode preference.32 Moreover, patients were able to successfully complete the m-WOMAC index survey independently and transmit the data to the remote server,31 which indicates that the WOMAC can be delivered effectively through mobile apps.
The AUC OAK developed by the American Academic Orthopaedic Surgeons (AAOS)34 includes eight criteria and guidelines for knee OA diagnosis and assessment. The AUC OAK is available on the AAOS website,34 and as an mHealth app named OrthoGuidelines, which runs on both Android35 and iOS36 devices. Table 2 lists the criteria included in the two OA assessment tools, which are based on objective measurements of OA-related joints (e.g. ROM extension/flexion), as well as more subjective, patient-reported outcomes (PROs) measures (e.g. pain and stiffness).
Table 2.
Summary of OA assessment tools reviewed.
| Tool | Developer | Assessment criteria | Mobile platform |
|---|---|---|---|
| m-WOMAC | Western Ontario and McMaster Universities | Pain Stiffness Function Total index score |
The original paper-based WOMAC has been tested in mobile platforms31,32 |
| AUC OAK | The American Academy of Orthopaedic Surgeons (AAOS) | Function-limiting pain ROM extension/flexion Age |
The AUC OAK33 is available as an app named OrthoGuidelines for Android35 and iOS36 devices |
Although the evidence is limited to only three studies, the use of mobile technologies for delivering assessment tools is a promising area of research, as proven by the fact that these professional societies are exploring how to effectively integrate mobile OA assessment tools into clinical practice. mHealth tools that allow patients access to OA assessment results can empower them to have a better understanding of their symptoms and get involved in the shared decision-making process, which is an important part of OA self-management with positive effects on medical outcomes.15,16,22
Mobile OA measurement tools
Articles grouped in the second category develop smartphone-based measurement tools for OA-related joints.37–49 As Table 3 shows, 8 articles (out of 13; 61.5%) focus on knee joints;39,41,43–47,49 3 articles (23.1%) focus on hip joints;37,42,48 1 article (7.7%) focuses on ankle joints;38 and 1 (7.7%) addresses OA-related joints in general.40 Articles in this group examine whether smartphone-based tools can measure the target joint’s functionality as accurately as the standard/conventional tools. Most of the articles calculate intra- and/or inter-rater reliabilities and concurrent validity of the smartphone-based tools against their standard/conventional counterparts. Two articles investigate the effect of rater’s expertise on the accuracy of measurements, comparing the measurement results between the less trained examiners (e.g. first-year physiotherapy students) and experienced examiners (e.g. expert physiotherapists).38,43 Overall, the studies show promising results and conclude that smartphone-based tools are reliable and valid for OA-related measurements.
Table 3.
Summary of articles on mobile OA measurement tools.
| Reference | Raters and subjects | Joint | Measures | Mobile devices | Results |
|---|---|---|---|---|---|
| Ockendon and Gilbert41 | Raters: 2 experienced raters Subjects: 5 healthy adults (all males, aged 30–40 years) | Knee | Flexion | Apple iPhone 3GS (Knee Goniometer) Telescopic-armed goniometer (Lafayette Instrument) | Intra-rater reliability of smartphone: Pearson’s r = 0.982, 95% CI = 0.962–0.991 Inter-rater reliability of smartphone between two raters: r = 0.994, 95% CI = 0.986–0.997 Concurrent validity of smartphone against goniometer: r = 0.947, 95% CI = 0.913–0.968 |
| Ferriero et al.47 | Raters: 5 PTs and 5 physicians Subjects: 35 pictures of knees from 10 healthy adults | Knee | Flexion Extension | Apple iPhone (Dr. Goniometer) Universal goniometer | Intra-rater reliability of smartphone: ICC = 0.996, 95% CI = 0.995–0.997 Inter-rater reliability: ICC = 0.994, 95% CI = 0.991–0.996 Agreement between smartphone and universal goniometer: 95% LoA = 14.1 (−6.6 to + 7.5) |
| Jenny46 | Raters: 1 operating surgeon and 1 assistive surgeon Subjects: 10 OA patients (4 males and 6 females; mean age = 69 years) | Knee | Flexion | Apple iPhone (Angle) Navigation system (OrthoPilot) | Intra-rater reliability of smartphone by operating surgeon: ICC = 0.81 Inter-rater reliability between operating surgeon and assistant surgeon: ICC = 0.79 Concurrent validity of smartphone against navigating system: r2 = 0.88–0.99, p = 0.01–0.001 |
| Jenny et al.45 | Raters: n/a Subjects: 10 OA patients (5 males and 5 females; mean age = 69 years) | Knee | Flexion | Apple iPhone (Goniometer Pro) Apple iPhone (Dr. Goniometer) Navigation system (OrthoPilot) | Paired difference between OrthoPilot and Goniometer Pro: Mean = 7.5°, SD = 5.3° Paired difference between OrthoPilot and Dr. Goniometer: Mean = 0.7°, SD = 1.5° |
| Andrea et al.49 | Raters: 2 orthopedists Subjects: 35 patients with ACL-deficient knees | Knee | ATT in ACL- deficient knee | Apple iPhone and Android phone (SmartJoint) Mechanical device (KT 1000) | Inter-rater reliability between two raters: ICC = 0.989, 95% CI = 0.981–0.994 Concurrent validity of smartphone against mechanical device: ICC = 0.981, 95% CI = 0.981–0.991 |
| Milanese et al.43 | Raters: 3 PTs and 3 adults in physiotherapy Subjects: 6 healthy adults (3 males and 3 females) | Knee | Flexion Extension | Apple iPhone Universal goniometer (Chattanooga) | Intra-rater reliability of smartphone: CCCExpert = 0.996, SEMExpert = 0.79; CCCNovice = 0.998, SEMNovice = 0.55 Concurrent validity of smartphone against universal goniometer: CCC = 0.991 |
| Salehi et al.39 | Raters: n/a Subject: 1 adult | Knee | Flexion Extension | Optical tracking system Wearable pants (STants) | For right knee angles: RMSE = 6.65, correlation coefficient = 0.987 For left knee angles: RMSE = 4.46, correlation coefficient = 0.991 |
| Jones et al.44 | Raters: 1 PT and 1 exercise physiologist Subjects: 36 adults (8 males and 28 females; mean age = 60.6 years) | Knee | Flexion Extension | Apple iPhone 3GS (Simple Goniometer) Universal goniometer (Chattanooga) | Inter-rater reliability of smartphone: ICC = 0.93–0.97 Concurrent validity of smartphone against universal goniometer: Pearson’s r = 0.96–0.98 |
| Niijima et al.42 | Rater: n/a Subjects: 14 young adults (7 males and 7 females; aged 18–22) and 8 older adults (4 males and 4 females; aged 64–80 years) | Hip | Flexion Extension | Samsung Galaxy S4 3D system (MA-8000) | Concurrent validity of smartphone against 3D system: R2 = 0.7–0.9; RMSE = 4–6 |
| Yoon et al.37 | Raters: 2 PTs Subjects: 10 healthy adults (5 males and 5 females; mean age = 22.2 years) | Hip | Proximal femoral neck axis Distal femoral condylar axis | Apple iPhone (IntegraSoftHN) Digital inclinometer (GemRed DBB) | Intra-rater reliability of smartphone: ICCRater1 = 0.95, SEMRater1 = 2.20; ICCRater2 = 0.95, SEMRater2 = 1.9 Concurrent validity of smartphone against digital inclinometer: ICC = 0.85, SEM = 4.1 |
| Charlton et al.48 | Rater: 1 PT Subjects: 20 healthy adults (all males; mean age = 23.8 years) | Hip | Flexion Abduction Adduction Supine IR Supine ER Sitting IR Sitting ER | Samsung Galaxy S2 Bubble inclinometer 3D system (Vicon) | Intra-rater reliability of smartphone: flexion (ICC = 0.86, SEM = 2.3); abduction (ICC = 0.68, SEM = 4.6); adduction (ICC = 0.68, SEM = 2.5); supine IR (ICC = 0.94, SEM = 3.2); supine ER (ICC = 0.87, SEM = 2.6); sitting IR (ICC = 0.84, SEM = 3.4); sitting ER (ICC = 0.63; SEM = 2.8) Concurrent validity of smartphone against 3D system: flexion (ICC = 0.92); abduction (ICC = 0.98); adduction (ICC = 0.91); supine IR (ICC = 0.88); supine ER (ICC = 0.71); sitting IR (ICC = 0.92); sitting ER (ICC = 0.90) |
| Williams et al.38 | Raters: 1 podiatrist with 2 years clinical experience and 1 podiatrist with 17 years experience Subjects: 20 healthy adults (4 males and 16 females; mean age = 40 years) | Ankle | Ankle weight-bearing lunge test at the positions: Leg straight Knee bent | Apple iPhone (TiltMeter app) Digital inclinometer | Intra-rater reliability of smartphone: ICCStraight = 0.81, SEM = 0.08; ICCBent = 0.85, SEM = 0.06 Inter-rater reliability of smartphone between novice and experienced raters: ICCStraight = 0.80; ICCBent = 0.96 Agreement between smartphone and digital inclinometer: 95% LoA = 9.1 (−4.064 to + 5.025) |
| Park et al.40 | Rater: the authors Subjects: n/a | All joints | u-CTX-II | LG-F320L | Variation of the u-CTX-II assay across the repeated measures: about 5% Correlation coefficient: R2 = 0.98 |
ATT in ACL-deficient knees: anterior tibial translation in anterior cruciate ligament-deficient knees; ER: external rotation; IR: internal rotation; u-CTX-II: urinary C-terminal telopeptide fragment of type II collagen; CCI: concordance correlation coefficient; CI: confidence interval; ICC: intra-class correlation coefficient; LoA: limits of agreement; RMSE: root mean square error; SD: standard deviation; SEM: standard error of measurement.
Mobile OA motion monitoring tools
Articles in this group use mHealth technologies to monitor the movements of OA-related joints and other human motions, including gait, that have the potential to support OA management.50–58 These motion-monitoring tools can help OA patients monitor not only the standardized physical therapy exercises suggested by their physicians but also other motions in their everyday lives that may affect their OA management. Patients then can share this data with their clinicians to support shared decision-making. In total, 4 articles (out of 9; 44.4%) analyze gait patterns to determine whether mHealth technology embedded in smartphones, such as accelerometer sensing, can successfully detect differing human gaits and distinguish among different types of motions such as running and biking;51–53,55 2 articles (22.2%) focus particularly on monitoring the movements and behaviors of patients who are in the pre- or post-surgery phases;54,56 and 3 (33.3%) use various sensing technologies to monitor human motions such as swinging and walking in a golf game and other home exercises.50,57,58 These studies highlight that using sensing technologies not only enable clinicians to remotely monitor patients’ motions but also motivate patients to get involved in exercises that can facilitate the rehabilitation process. Table 4 provides more details about the study designs and findings presented in these articles.
Table 4.
Summary of articles on mobile OA motion monitoring tools.
| Reference | Subjects | Data collection settings | Monitored objects | Measures | Mobile devices | Results |
|---|---|---|---|---|---|---|
| Li et al.54 | 20 trainers | Experiments under the guidance of doctors (controlled) | Non-standard knee movements recognition rate | Insufficient holding time Bent-leg raise Rapid leg movement | Acceleration sensors (MMA7361) Android smartphone (KOA) | 89% for insufficient holding time 89.7% for bent-leg raise 89.4% for rapid leg movement |
| Kim et al.56 | 13 TKA patients | Manual data input by patients using the app (uncontrolled): Pre-surgery (14 days) Post-surgery (30 days) | Adherence rate | Pre-surgery: educational class; medication and activity protocols Post-surgery: quality-of-life questions; physical therapy exercises | Apple iPad mini (iGetBetter) | Pre-surgery: 3.54 out of 6 occasions (59%) on average; ranged 0–6 occasions Post-surgery: 17.77 out of 30 days (59.2%) on average; ranged 4–30 days |
| Majumder et al.52 | 15 adults (aged 20–35 years) | Lab environment (controlled) | Gait recognition rate | Normal walking Standing still Simulated peg leg Simulated leg length discrepancy | smartPrediction system using: Apple iPhone (prototype app) Smartshoe | 91% for all the four movement patterns |
| Lu et al.53 | 47 adults for walking detector training 12 adults for supervised data 8 adults for unsupervised data | Supervised activity sessions (controlled) Daily life activities (uncontrolled) | Gait recognition from other activities | Stationary Biking Running Vehicle Random movements | Android smartphones: Samsung Galaxy S3 and 4 Google Nexus 5 Intel Xolo | Accuracy was improved by increasing training data Accuracy was higher (at least 5%) with the supervised data than the unsupervised data Accuracy was higher when placed in pant pocket than in hands |
| Mazilu et al.51 | 9 Parkinson’s disease patients (6 males & 3 females; mean age = 68.3 years) | Gait-training exercise (controlled) Daily life activities (uncontrolled)—data collected from 5 out of 9 patients. | Gait | User satisfaction FoG duration | GaitAssist system using: Wearable sensors attached to ankles Smartphone (GaitAssist) Earphones | User ratings (5-point scale) on average: system operation = 4; wearability = 4; exercise content = 3.8; subjective opinions = 3.8 FoG duration was decreased: in the gait-training session (3 out of 5 patients); in daily life setting (4 out of 5 patients) |
| LeMoyne et al.55 | 1 subject with trans-tibial amputation | Gait analysis in an indoor environment (controlled) | Gait | Stance to stance temporal disparity Time-averaged acceleration from stance to stance | Apple iPhone (accelerometer app) 3D printed adapter | Measures were consistent: Temporal disparity measure, mean = 1.10 (seconds), SD = 0.02 with a 96% CI Acceleration measure, mean = 1.47 (g’s), SD = 0.02 with a 96% CI |
| Shin and Wuensche50 | 10 adults (8 males and 2 females) 10 golf players (3 males and 7 females; mean age = 37.7 years) | Golf shots measuring session (controlled) Virtual 3-hole golf course in an outdoor golf club (uncontrolled) | Golf game-related movements | Driving distance Angular velocity User perception s of usability, enjoyment, effectiveness, realism |
Android smartphones (G-Swing app): Huawei U8100 Samsung s5620 and I9000 HTC One X809 Motorola Droid 2 | Formula for short distance estimation: (a) Driver = 15.2 × angular velocity – 19.1; (b) Iron = 10.7 × angular velocity – 6.0 The game concept, the required motions, and the duration of the game were perceived as suitable for arthritis patients |
| Chandra et al.58 | 3 physicians (mean age = 30 years) 11 patients (age ranged 16–75 years) | Interview | Prescribed exercise at home: Comments during interviews | N/A | Design guidelines identified: Target patients’ understanding of exercises and the correctness of their performance Present simple visualizations Focus on accurate and informative depiction of data Include reminder and scheduling systems for exercise Enhance communication channels between patients and therapists Keep patients’ data private |
mHealth apps for OA management in the marketplace
We found 27 relevant apps for OA as of June 2016. After removing the overlapping apps across the stores, 23 unique apps remained. Table 5 presents our app search results and the results presented in a study published in 2013.25 Each cell reports the number of relevant apps out of the total number of apps. It is important to note that we identified four overlapping apps available in multiple mobile operating systems (OSs) providing the same content regardless of OS type.
Table 5.
mHealth apps for OA available in app stores in 2013 and 2016; number of relevant apps/total number of apps.a
| Search year | Google Play26 | Apple iTunes27 | BlackBerry World28 | Microsoft Store29 | Opera Mobile Store30 | Total |
|---|---|---|---|---|---|---|
| 2013 | 16/46 | 5/16 | 0/0 | 2/2 | 1/1 | 24/65 |
| 2016 | 14/115 | 11/30 | 0/0 | 2/2 | 0/0 | 27/147 |
The commercial app reviewed in the original paper in 201325—Osteoarthritis of Knee—was not available in our search in 2016. When we used the link provided by the authors, it returned an error message notifying that the app is not available in the United States. Based on the authors’ affiliation information, we assume that they had access to app stores available in Europe where as we were able to search app stores available in the United States.
Of the 23 unique apps, 14 (60.9%) were developed to provide educational content regarding OA; 2 (8.7%) allowed users to record and keep track of OA-related symptoms and pain levels; and 7 (30.4%) provided both the educational content and the pain diary features.
Discussion
Summary of evidence
The purpose of this systematic review was to investigate empirical applications of the state-of-theart mHealth technologies, especially mHealth apps, that can support self-management of OA and decision-making related to OA including TJR surgery. Based on our in-depth review of included articles, we were able to organize the research in this field under three main themes, namely, mobile OA assessment tools, mobile OA measurement tools, and mobile OA motion-monitoring tools. Although the articles in each category showed promising results for the use of mHealth technology in OA management, we were not able to find evidence of research on developing evidence-based mHealth apps focused on OA and investigating their effectiveness in OA management.
Our app review revealed that the lack of research on the development and evaluation of mHealth apps for OA management was reflected in the marketplace. As shown in Table 2, even though the total number of apps identified by the search term “osteoarthritis” in the app stores doubled over the past few years, the number of apps relevant for OA management increased only by 12 percent, a minimal change since 2013 when a review on existing mHealth apps for the eight most prevalent health conditions by WHO found only 24 apps focused on OA in the app stores.25 Furthermore, looking into the main features provided by the existing apps for OA management as of June 2016, the majority of them (60.9%) only focus on providing educational information about OA, such as definition of OA, common symptoms, or instructions on how to do activities and exercises that are known to be helpful in managing OA-related pain and symptoms.
Given the lack of apps in the current app markets that actually allow patients to quantify and keep track of their OA-related pain and symptoms and share the data with their clinicians to make more informed decisions as they collectively manage the disease, the mHealth technologies covered in our review hold potential to fill the gap. Specifically, the two evidence-based OA assessment tools reviewed—m-WOMAC31,32 and AUC OAK33—can be used in OA mHealth apps to effectively measure OA-related symptoms and pain progress. In particular, the WOMAC index, which has been used and validated by previous studies,59–61 can be used as an accurate and reliable PRO measurement index for OA. Although the AUC OAK is designed to assist clinical decisions of OA treatment, it can also be useful for clinicians to interpret the collected data by their patients. The AUC OAK is also useful to guide the data collection of OA-related symptoms in patient-facing mHealth apps. Since the AUC OAK includes objective measures of physical functioning such as ROM extension/flexion, there is room for integrating the evidence-based mHealth technologies such as measurement tools, as reviewed in Table 3.
Another important finding regarding self-measurement of OA joints is that people were able to accurately and reliably measure ROMs of OA joints using mHealth apps with a certain amount of education. As mentioned above, the studies that compared the measurement results by experts (e.g. experienced physicians) and novices (e.g. college students) found no significant difference between the two groups.38,43 The results, therefore, support the idea of OA self-measurement that laypersons can utilize mHealth apps as a handy, yet accurate and reliable tool to record functional performances of their OA joints on a daily basis. The data collected by such apps could be shared with their clinicians, which would help them make evidence-based decisions about choosing the ‘right’ treatment for OA, including TJR surgery.
Findings from the articles analyzing OA-related movements and other human motions such as gait and exercises50–53,55 may also be useful in developing mHealth apps for OA. For instance, some of the exercises that are clinically proven to be helpful for OA patients, such as walking, biking, and golfing, could be monitored by mHealth apps. By doing so, patients could quantify and log their daily activity and have a better understanding of their readiness for OA treatments including TJR surgery. Such apps could also play a role as a motivator by visualizing patients’ activity data and setting goals customized for individual users. For those who are in the exercise therapy, maximizing the adherence to their therapy is a key factor that leads to a successful treatment.62 A recent study reported that supervised exercise sessions followed by home exercises could enhance patients’ adherence.63 Mobile motion-monitoring tools, therefore, have great potential to help OA patients work on their exercise sessions at home while being connected with their therapists. Furthermore, mHealth apps can provide instructional audios or videos of the exercises to help patients’ self-exercise sessions at home, as many of the existing OA apps do.
A framework of mHealth apps for OA self-management and shared decision-making
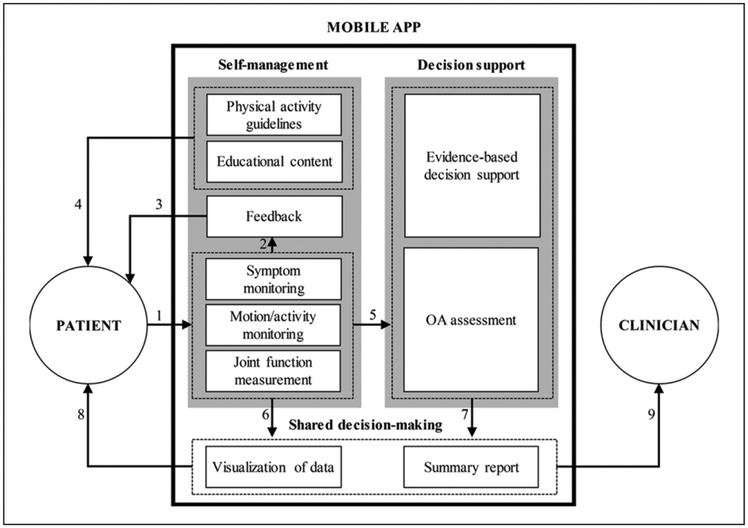
OA self-management is a behavioral intervention that improves patients’ understanding of disease symptoms and encourages them to actively participate in the treatment process by monitoring OA-related pain, communicating with healthcare providers about their symptoms, and making informed/shared decisions about appropriate treatment options including TJR surgery.9,21 Based on the promising potential of self-management and shared decision-making in chronic disease management as well as our findings, we propose a design framework that identifies desired components of mHealth apps for OA and articulates how these components can work together to support OA patients’ self-management and shared decision-making about treatment options with their clinicians. Overall, the proposed framework, illustrated in Figure 3, focuses on patient-facing mobile apps, as opposed to a clinician-facing tool, that enable patients to get involved in the process of OA treatment with their clinicians. The three main modules that should be included in the mobile apps are (a) self-management, (b) decision support, and (c) shared decision-making.
Figure 3.
A framework for developing mHealth apps fro OA management.
The self-management module provides patients with the ability to monitor their disease progress and be better informed about OA including its symptoms and available treatment options. The patients can quantify and log pain levels, limitations in joint motions (e.g. ROMs of knee joints) and limitations in activities performed (e.g. climbing stairs) using the app on a regular basis (link 1). The mHealth technologies focused on OA measurement tools (Table 3) and OA motion-monitoring tools (Table 4) can be integrated into the app to enable comprehensive self-monitoring functionality. The patient-reported data then should be used to generate concise, yet useful feedback messages for patients (link 2). The main intent of generating feedback is to inform patients about their progress in OA self-management over a certain period of time (e.g. weekly and monthly) and motivate them to continue entering their data into the app (link 3). In addition, the app should provide educational content to improve patients’ understanding of OA in general and address potential questions patients might have regarding their symptoms and how to manage them. Providing guidelines for physical activity will be useful for improving management of symptoms, especially for those who are in the preoperative or postoperative phases (link 4).
The decision support module is the backend of the app where the expert knowledge and computational power should come into play to suggest evidence-based, data-driven treatment options for patients. This module should use patient-reported data fed by the self-management module of the app (link 5) and patient-centered research outcomes reported in the literature to assess patient’s status from the medical treatment perspective. In particular, this data can be used to calculate the OA indices reviewed in this article such as m-WOMAC and AUC OAK (Table 2). Furthermore, this module can assess patient’s readiness for more advanced treatment options, especially TJR surgery when it becomes unavoidable as the OA symptoms progress, using predictive algorithms to determine the optimum timing of TJR surgery based on clinical evidence.
The shared decision-making module allows patients and clinicians to share data collected/generated using the self-management module (link 6) and decision support module (link 7) and to choose the ideal treatment together. To facilitate the shared decision-making process, the app should visualize patients’ trends of symptoms, motion/activity, and joint functions, as well as clinical assessment indices over a period of time and present these results in detailed and summary forms targeting the needs of two user types (patients and clinicians). The app, therefore, can provide the patients with the opportunity to access their detailed OA assessment results (link 8) and allow the clinicians to review assessment summary reports with their patients and involve them in the decision-making process (link 9). Given the versatility of the two key concepts of the framework—self-management and shared decision-making—we expect that our framework may be applicable to developing mHealth apps for other medical conditions of which self-management and shared decision-making are proven to be helpful to improve clinical treatment outcomes.
Finally, we note that regulatory bodies such as the US Food and Drug Administration64 are interested in ensuring the safety in using mHealth technologies to deliver interventions to patients. Considering that regulations are changing and may vary by domain, designers should take the current and relevant regulations for the given domain into consideration when they use our framework to develop an mHealth app.
Conclusion
The current systematic review identified a gap in the literature on the potential impact of using mHealth apps for OA management. Specifically, mHealth research addressing OA to date has focused more on OA measurement and education, and less on OA self-management—as shown in Figure 1, the majority of included articles develop and test measurement tools for OA-related joints such as knees and hips. Future work, therefore, should focus on designing comprehensive mHealth apps dedicated to OA by combining the relevant research evidence from the previous studies, such as mobile OA assessment tools, measurement tools, and motion-tracking technologies. The framework suggested in this article (Figure 3) can guide the design of such apps. Given that the main user group of such apps will be older adults, who are relatively less familiar with mHealth technologies, it is critical to explore their expectations and requirements in the early stage of the app development. Finally, the app should be tested for its usability, as well as the effectiveness in OA self-management from the patient’s perspective, as well as in clinical management of OA from the clinician’s perspective.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by grant number R21HS024003 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Wonchan Choi, Worcester Polytechnic Institute, USA.
Hua Zheng, University of Massachusetts Medical School, USA.
Patricia Franklin, University of Massachusetts Medical School, USA.
Bengisu Tulu, Worcester Polytechnic Institute, USA.
References
- 1.Medical Subject Headings. Mobile health, https://meshb.nlm.nih.gov/record/ui?ui=D017216 (2017, accessed 12 October 2017).
- 2.Medical Subject Headings. Chronic disease. https://meshb.nlm.nih.gov/record/ui?ui=D002908 (2017, accessed 12 October 2017).
- 3.Mamykina L, Heitkemper EM, Smaldone AM, et al. Structured scaffolding for reflection and problem solving in diabetes self-management: qualitative study of mobile diabetes detective. J Am Med Inform Assoc 2016; 23: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui CY, Walton R, McKinstry B, et al. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc 2016; 24: 619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BinDhim NF, Shaman AM, Trevena L, et al. Depression screening via a smartphone app: cross-country user characteristics and feasibility. J Am Med Inform Assoc 2015; 22: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murnane EL, Cosley D, Chang P, et al. Self-monitoring practices, attitudes, and needs of individuals with bipolar disorder: implications for the design of technologies to manage mental health. J Am Med Inform Assoc 2016; 23: 477–484. [DOI] [PubMed] [Google Scholar]
- 7.Anderson M Technology device ownership: 2015. Pew Research Center, 29 October 2015. http://www.pewinternet.org/2015/10/29/technology-device-ownership-2015/ (accessed 12 October 2017).
- 8.Wantland DJ, Portillo CJ, Holzemer WL, et al. The effectiveness of Web-based vs. non-Web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res 2004; 6: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker C, Swerissen H and Belfrage J. Self-management: its place in the management of chronic illnesses. Aust Health Rev 2003; 26: 34–42. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). mHealth: new horizons for health through mobile technologies: based on the findings of the second global survey on eHealth. Geneva: WHO, 2011. [Google Scholar]
- 11.National Institute of Arthritis and Musculoskeletal and Skin Diseases. What is osteoarthritis? http://www.niams.nih.gov/health_info/Osteoarthritis/osteoarthritis_ff.asp (2014, accessed 14 October 2016).
- 12.Maetzel A, Li LC, Pencharz J, et al. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis 2004; 63: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fingar KR, Stocks C, Weiss AJ, et al. Most frequent operating room procedures performed in U.S. Hospitals, 2003–2012: Statistical Brief #186 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality, December 2014. https://www.ncbi.nlm.nih.gov/books/NBK274246/ (accessed 12 October 2017). [Google Scholar]
- 14.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 1994; 84: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guideline for care and management in adults. London: Royal College of Physicians, 2008. [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (NICE). Osteoarthritis: care and management, https://www.nice.org.uk/guidance/cg177/resources/osteoarthritis-care-and-management-35109757272517 (2014, accessed 14 October 2016). [PubMed]
- 17.Rankin EA, Alarcon GS, Chang RW, et al. NIH Consensus statement on total knee replacement: December 8–10, 2003. J Bone Joint Surg Am 2004; 86: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 18.Franklin PD, Li W and Ayers DC. Functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res 2008; 466: 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles C, Gafni A and Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997; 44: 681–692. [DOI] [PubMed] [Google Scholar]
- 20.Kroon FP, van der Burg LR, Buchbinder R, et al. Self-management education programmes for osteo-arthritis. Cochrane Database Syst Rev. Epub ahead of print 15 January 2014. DOI: 10.1002/14651858.CD008963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toomey E, Currie-Murphy L, Matthews J, et al. The effectiveness of physiotherapist-delivered group education and exercise interventions to promote self-management for people with osteoarthritis and chronic low back pain: a rapid review part I. Man Ther 2015; 20: 265–286. [DOI] [PubMed] [Google Scholar]
- 22.Joosten EA, DeFuentes-Merillas L, De Weert GH, et al. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom 2008; 77: 219–226. [DOI] [PubMed] [Google Scholar]
- 23.Donker T, Petrie K, Proudfoot J, et al. Smartphones for smarter delivery of mental health programs: a systematic review. J Med Internet Res 2013; 15: e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathers C, Fat DM and Boerma The global burden of disease: 2004 update. Geneva: WHO, 2008. [Google Scholar]
- 25.Martinez-Perez B, de la Torre-Diez I and Lopez-Coronado M. Mobile health applications for the most prevalent conditions by the World Health Organization: review and analysis. J Med Internet Res 2013; 15: e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Google. Google Play, https://play.google.com/store/apps (accessed 1 June 2016).
- 27.Apple. iTunes App Store, http://www.apple.com/itunes/ (accessed 1 June 2016).
- 28.BlackBerry. BlackBerry World, https://appworld.blackberry.com/webstore/product/1/?lang=en&countrycode=US (accessed 12 October 2017).
- 29.Microsoft. Microsoft Store: Windows Phone Apps, https://www.microsoft.com/en-us/store/apps/windows-phone?icid=en_US_Store_UH_apps_WinPho (accessed 1 June 2016).
- 30.Opera Software. Opera Mobile Store, http://ovi.sigma.apps.opera.com/ (accessed 1 June 2016).
- 31.Bellamy N, Patel B, Davis T, et al. Electronic data capture using the Womac NRS 3.1 Index (m-Womac): a pilot study of repeated independent remote data capture in OA. Inflammopharmacology 2010; 18: 107–111. [DOI] [PubMed] [Google Scholar]
- 32.Bellamy N, Wilson C, Hendrikz J, et al. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J Clin Epidemiol 2011; 64: 182–190. [DOI] [PubMed] [Google Scholar]
- 33.Sanders J, Murray J and Gross L. Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Am Acad Orthop Surg 2014; 22: 256–260. [DOI] [PubMed] [Google Scholar]
- 34.American Academic Orthopaedic Surgeons (AAOS). Appropriate use criteria, http://www.aaos.org/aucapp/ (accessed 15 May 2016).
- 35.American Academic Orthopaedic Surgeons (AAOS; Google Play). OrthoGuidelines, https://play.google.com/store/apps/details?id=com.ionicframework.orthoguidelines408928 (accessed 31 May 2016).
- 36.American Academic Orthopaedic Surgeons (AAOS; Apple iTunes App Store). OrthoGuidelines, https://itunes.apple.com/WebObjects/MZStore.woa/wa/viewSoftware?id=1040658933&mt=8 (accessed 31 May 2016).
- 37.Yoon T-L, Park K-M, Choi S-A, et al. A comparison of the reliability of the trochanteric prominence angle test and the alternative method in healthy subjects. Man Ther 2014; 19: 97–101. [DOI] [PubMed] [Google Scholar]
- 38.Williams CM, Caserta AJ and Haines TP. The TiltMeter app is a novel and accurate measurement tool for the weight bearing lunge test. J Sci Med Sport 2013; 16: 392–395. [DOI] [PubMed] [Google Scholar]
- 39.Salehi S, Bleser G and Stricker D. Design and development of low-cost smart training pants (STants). In: 4th international conference on wireless mobile communication and healthcare (Mobihealth), Athens, 3–5 November 2014, pp. 51–4. New York: IEEE. [Google Scholar]
- 40.Park YM, Han YD, Kim KR, et al. An immunoblot-based optical biosensor for screening of osteoarthritis using a smartphone-embedded illuminometer. Anal Methods 2015; 7: 6437–6442. [Google Scholar]
- 41.Ockendon M and Gilbert RE. Validation of a novel smartphone accelerometer-based knee goniometer. J Knee Surg 2012; 25: 341–345. [DOI] [PubMed] [Google Scholar]
- 42.Niijima A, Mizuno O and Tanaka T. A study of gait analysis with a smartphone for measurement of hip joint angle. In: 2014 annual summit and conference on Asia-pacific Signal and Information Processing Association (APSIPA), Siem Reap, Cambodia, 9–12 December 2014, pp. 1–4. New York: IEEE. [Google Scholar]
- 43.Milanese S, Gordon S, Buettner P, et al. Reliability and concurrent validity of knee angle measurement: smart phone app versus universal goniometer used by experienced and novice clinicians. Man Ther 2014; 19: 569–574. [DOI] [PubMed] [Google Scholar]
- 44.Jones A, Sealey R, Crowe M, et al. Concurrent validity and reliability of the Simple Goniometer iPhone app compared with the Universal Goniometer. Physiother Theory Pract 2014; 30: 512–516. [DOI] [PubMed] [Google Scholar]
- 45.Jenny JY, Bureggah A and Diesinger Y. Measurement of the knee flexion angle with smartphone applications: which technology is better? Knee Surg Sports Traumatol Arthrosc 2016; 24: 2874–2877. [DOI] [PubMed] [Google Scholar]
- 46.Jenny JY. Measurement of the knee flexion angle with a smartphone-application is precise and accurate. J Arthroplasty 2013; 28: 784–787. [DOI] [PubMed] [Google Scholar]
- 47.Ferriero G, Vercelli S, Sartorio F, et al. Reliability of a smartphone-based goniometer for knee joint goniometry. Int J Rehabil Res 2013; 36: 146–151. [DOI] [PubMed] [Google Scholar]
- 48.Charlton PC, Mentiplay BF, Pua Y-H, et al. Reliability and concurrent validity of a Smartphone, bubble inclinometer and motion analysis system for measurement of hip joint range of motion. J Sci Med Sport 2015; 18: 262–267. [DOI] [PubMed] [Google Scholar]
- 49.Andrea F, Luigi V, Daniele M, et al. Smartphone versus knee ligament arthrometer when size does not matter. Int Orthop 2014; 38: 2197–2199. [DOI] [PubMed] [Google Scholar]
- 50.Shin Y and Wunsche BC. A Smartphone-based golf simulation exercise game for supporting arthritis patients. In: 28th international conference on image and vision computing New Zealand (eds Rhee T, Rayudu R, Hollitt C, et al. ), Wellington, New Zealand, 27–29 November 2013, pp. 459–464. New York: IEEE. [Google Scholar]
- 51.Mazilu S, Blanke U, Dorfman M, et al. A wearable assistant for gait training for Parkinson’s disease with freezing of gait in out-of-the-lab environments. ACM T Interact Intell Syst 2015; 5: 5. [Google Scholar]
- 52.Majumder AJA, Zerin I, Ahamed SI, et al. A multi-sensor approach for fall risk prediction and prevention in elderly. Appl Comput Rev 2014; 14: 41–52. [Google Scholar]
- 53.Lu H, Huang J, Saha T, et al. Unobtrusive gait verification for mobile phones. In: 2014 ACM international symposium on wearable computers (ISWC’14), Seattle, WA, 13–17 September 2014, pp. 91–98. New York: ACM. [Google Scholar]
- 54.Li YR, Ye B, Zeng NY, et al. A novel movement monitoring system of knee osteoarthritis using the Android system. J Med Imag Health In 2015; 5: 1575–1579. [Google Scholar]
- 55.LeMoyne R, Mastroianni T, Montoya K, et al. Implementation of a smartphone for evaluating gait characteristics of a trans-tibial prosthesis. In: 2014 36th annual international conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, 26–30 August 2014, pp. 3674–3677. New York: IEEE. [DOI] [PubMed] [Google Scholar]
- 56.Kim K, Pham D and Schwarzkopf R. Mobile application use in monitoring patient adherence to perioperative total knee arthroplasty protocols. Surg Technol Int 2016; 28: 253–260. [PubMed] [Google Scholar]
- 57.Deen MJ. Information and communications technologies for elderly ubiquitous healthcare in a smart home. Pers Ubiquitous Comput 2015; 19: 573–599. [Google Scholar]
- 58.Chandra H, Oakley I and Silva H. Designing to support prescribed home exercises: understanding the needs of physiotherapy patients. In: 7th Nordic conference on human-computer interaction: making sense through design (Nordichi’12), Copenhagen, 14–17 October 2012, pp. 607–616. New York: ACM. [Google Scholar]
- 59.Marsh JD, Bryant DM, MacDonald SJ, et al. Patients respond similarly to paper and electronic versions of the WOMAC and SF-12 following total joint Arthroplasty. J Arthroplasty 2014; 29: 670–673. [DOI] [PubMed] [Google Scholar]
- 60.Dworkin RH, Peirce-Sandner S, Turk DC, et al. Outcome measures in placebo-controlled trials of osteo-arthritis: responsiveness to treatment effects in the REPORT database. Osteoarthritis Cartilage 2011; 19: 483–492. [DOI] [PubMed] [Google Scholar]
- 61.Gandek B Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis index: a systematic review. Arthritis Care Res (Hoboken) 2015; 67: 216–229. [DOI] [PubMed] [Google Scholar]
- 62.Jan MH, Tang PF, Lin JJ, et al. Efficacy of a target-matching foot-stepping exercise on proprioception and function in patients with knee osteoarthritis. J Orthop Sports Phys Ther 2008; 38: 19–25. [DOI] [PubMed] [Google Scholar]
- 63.Bennell KL and Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport 2011; 14: 4–9. [DOI] [PubMed] [Google Scholar]
- 64.US Food and Drug Administration. Mobile medical applications, https://www.fda.gov/MedicalDevices/DigitalHealth/MobileMedicalApplications/default.htm (2017, accessed 10 July 2017).