Abstract
Background:
The role of antiretroviral therapy (ART) in reducing or contributing to liver fibrosis in persons with HIV is unclear. We evaluated participants in the Strategic Timing of AntiRetroviral Treatment trial (START) for liver fibrosis using the AST to platelet ratio index (APRI) and Fibrosis-4 Index (FIB-4), and assessed for a benefit of early versus delayed antiretroviral therapy (ART) on liver fibrosis progression.
Design and Intervention:
ART-naïve persons with high CD4 counts (>500 cells/μL) from 222 clinical sites in 35 countries were randomized to receive ART either at study enrolment (immediate treatment arm) or when their CD4 count fell below 350 cells/μL (deferred treatment arm). The following outcomes were evaluated: fibrosis (APRI>0.5 or FIB-4>1.45), significant fibrosis (APRI>1.5 or FIB-4>3.25), hepatic flare, and resolution of elevated APRI and FIB-4 scores.
Results:
Of the 4684 enrolled into the START study, 104 did not have APRI or FIB-4 results and were excluded. Among 4580 participants (2273 immediate treatment; 2307 deferred treatment), the median age was 36 years, 26.9% were female, and 30.4% were black. Three percent had an alcoholism or substance abuse history, 6.4% had hepatitis, and 1.1% had significant fibrosis at baseline. The median CD4 count was 651 and 5.3% had HIV RNA≤200. Immediate arm participants were at lower risk of developing increased fibrosis scores than deferred arm participants (hazard ratio [HR]=0.66; 95% confidence interval [CI]=0.57—0.78; p<0.001), and more likely to have resolution of elevated baseline scores (HR 1.6; 95% CI 1.3—1.9; p<0.001).
Conclusions:
Significant liver fibrosis was rare among ART-naïve HIV-positive persons with high CD4 counts. Our findings suggest a benefit of early ART in preventing the development of liver fibrosis.
Keywords: liver disease, non-invasive markers, APRI, FIB-4
Introduction
Liver disease remains a major cause of morbidity and mortality in HIV-infected individuals globally (1–3) and is highest among those with concurrent alcohol use and/or Hepatitis B and C virus coinfection (3–5). However, non-alcoholic fatty liver disease (NAFLD), which is frequently associated with metabolic syndrome (6), has an increased prevalence among HIV-monoinfected individuals as well (7, 8). While NAFLD most commonly causes simple steatosis, it can also progress to non-alcoholic steatohepatitis, fibrosis and/or cirrhosis (6). A recent metaanalysis reported that the prevalence of NAFLD (by imaging) was 35% (7), compared to 25% in the general population (9). Similarly, the prevalence of non-alcoholic steatohepatitis and fibrosis diagnosed by biopsy was 42% and 22%, respectively (7), compared to the general population prevalence of 1.5–6.45% by biopsy (9) and 2.8% by non-invasive fibrotest (10), respectively.
The role of ART in reducing or contributing to liver fibrosis progression in HIV-infected individuals is unclear. Studies have highlighted that traditional metabolic risk factors such as obesity, diabetes and dyslipidemia (7, 11, 12) are important risk factors for NAFLD and that HIV-specific risk factors such as low CD4 count, high HIV viral load, and exposure to ART are associated with elevated liver enzymes (8) and fibrosis (13, 14). In addition, specific ART agents have been shown to be associated with mitochondrial toxicity and insulin resistance (15, 16), as well as hepatotoxicity or drug-induced liver injury (8, 15, 17). As the majority of these participants in these studies were taking ART prior to study entry, there is insufficient evidence to support or reject earlier commencement of ART to specifically prevent liver disease.
The START (Strategic Timing of Antiretroviral Treatment) study (18, 19) was a randomized controlled study that enrolled ART-naïve HIV-positive adults with high CD4 counts (>500 cells/μL) and randomized them to receive ART at study enrollment (immediate treatment arm) or defer therapy until their CD4 counts fell below 350 cells/μL (deferred treatment arm). One of the aims of the START study was to evaluate liver disease among study participants at baseline and follow-up, using non-invasive markers of liver function such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase, bilirubin and platelet count to assess for fibrosis. These measures can be used individually or in validated composite scores such as the AST to Platelet Ratio Index (APRI) (20) and Fibrosis-4 Index (FIB-4) (21) which have been used as non-invasive markers of liver fibrosis in various populations (14, 22–26). In a substudy of 221 START trial participants with transient elastography results, 7.8% had significant liver fibrosis, and higher ALT, higher HIV RNA, and Hispanic/Latino ethnicity were associated with higher elastography scores (27). In this study our objective was to use APRI and FIB-4 to determine the prevalence of and risk factors for liver fibrosis among START study participants at baseline and follow-up and to assess for an effect of early versus delayed initiation of ART on progression of liver fibrosis over time.
Methods
START Study Population and Procedures
Participants were enrolled from April 2009 – December 2013, at 222 clinical sites in 35 countries (18). Study visits were conducted at baseline, one month, four months and every four months thereafter. The choice of ART regimen was determined by the clinician and the subject prior to randomization, and was specified to include two nucleoside reverse transcriptase inhibitors plus either a non-nucleoside reverse transcriptase inhibitor, integrase strand transfer inhibitor or a ritonavir-boosted protease inhibitor.
At baseline and follow-up, laboratory measures of HIV infection (CD4 count and HIV RNA) were collected and participants were asked about alcohol intake, current smoking, ART use, whether they had any history of comorbidities such as cirrhosis, hepatic steatosis, diabetes or alcohol and substance abuse, and whether they were using any hepatotoxic medications. All participants were tested for Hepatitis B and C co-infections at baseline using Hepatitis B surface antigen and Hepatitis C antibody screening tests; diagnoses of Hepatitis B and C during follow-up were reported. Serum glucose and measures of liver function including AST, ALT, total bilirubin, alkaline phosphatase, platelet count, albumin, and lipids were collected annually. Details on the START study design, randomization procedures, data collection and sample size calculations have been previously published (18, 19).
Fibrosis Scores
Liver fibrosis was assessed using annual biomarkers to calculate two composite scores. The APRI (20) score is calculated using AST and platelet counts in the formula: APRI = [AST level (IU/L) / AST upper limit of normal (IU/L) / Platelet count (109/L)] × 100. The APRI has a lower cut-off of ≤0.5 for predicting the absence of significant fibrosis (Ishak 0–2) and a higher cut-off of >1.5 for predicting the presence of significant fibrosis (Ishak 3–6). The FIB-4 score (21) is calculated using age, AST, ALT and platelet count in the formula: FIB-4 = [Age (years) x AST (IU/L)] / [Platelets (109/L) x ALT (IU/L)]. The FIB-4 also has a lower cut-off of ≤1.45 for predicting the absence of advanced fibrosis (Ishak 0–3) and a higher cut-off of >3.25 (Ishak 4–6) for predicting the presence of advanced fibrosis.
Statistical Analysis
All HIV-positive START participants with available serum markers were included in this analysis. The association of baseline factors and fibrosis at baseline was examined using logistic regression models. All factors significant (p<0.05) in univariate models were included in multivariate analyses. Even if not significant on univariate analysis, age, race and gender were included in the multivariate models because these variables have been found to be important determinants of liver fibrosis in other studies (5, 8, 14, 28).
Analyses examining predictors of fibrosis during follow-up included participants without fibrosis at baseline and with at least one follow-up measurement available. Follow-up was censored at the last available measurement prior to May 15, 2015, when an interim analysis led the data and safety monitoring board to recommend that the primary question of START had been addressed. Logistic regression models were used to examine predictors of fibrosis during follow-up, stratified by the number of measurements available per participant. In multivariate analyses for follow-up fibrosis, treatment group and all variables that were significant on univariate analysis for either treatment group or for the treatment groups combined were included.
Because the majority of our study participants had no evidence of significant fibrosis at baseline (i.e. most had APRI≤0.5 and FIB-4≤1.45), and because these scores have shown higher accuracy at ruling out significant fibrosis using the lower cut-offs rather than ruling in significant fibrosis using the higher cut-offs (23, 24), we report primarily on associations with a lack of significant fibrosis using the lower cut-offs. Therefore, we refer to scores of APRI>0.5 and FIB-4>1.45 as being associated with an increased risk of fibrosis, rather than confirmed fibrosis. Results of associations with the higher cut-offs (APRI>1.5 and FIB-4>3.25) are reported in the Supplementary Material.
We used time-to-event methods, including Kaplan-Meier survival curves and Cox proportional-hazards models, to compare the two treatment groups for the following outcomes: increased fibrosis (APRI>0.5 or FIB-4>1.45), confirmed significant fibrosis (APRI>1.5 or FIB-4>3.25), hepatic flare, hepatitis, and normalization of elevated APRI and FIB-4 scores. Hepatic flare was defined as an ALT greater than five times the upper limit of normal (30 for men and 19 for women). Change in APRI and FIB-4 over follow-up by treatment group and pre-specified ART was examined using longitudinal mixed models with random intercepts including the following in the model: treatment group; visit; baseline value; an indicator for pre-specified protease inhibitor vs. non-nucleoside reverse transcriptase inhibitor; and an interaction term for treatment group and pre-specified ART.
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc, Cary NC), and figures were created using R software, version 3.2.
Human Subjects
Informed consent was obtained in writing from each participant prior to study enrolment. The study protocol and associated documents were reviewed and approved by the appropriate Institutional Review Board at each recruitment site.
Results
Baseline Characteristics of Study Participants
In total, 4684 HIV+ participants were enrolled into the START study (18). Of these, 104 did not have APRI or FIB-4 results at study entry, resulting in 4580 participants that were included (2273 in the immediate arm and 2307 in the deferred arm). The median age was 36 years, 26.9% of participants were female, 43.9% were white, 30.4% were black, and the median body mass index was 25 (Table 1). Forty-five percent reported current or prior smoking, and 31.5% reported drinking one or more days per week, but only 3.3% had a diagnosed history of alcoholism or substance dependence. Few participants were co-infected with Hepatitis C (3.7%) or Hepatitis B (2.8%), and very few had known chronic liver disease (0.3%), hepatic steatosis (0.2%) or diabetes (2.6%). Just over 2% were on HMG-CoA reductase inhibitors (statins) and 1.3% were on anti-tuberculosis medications.
Table 1.
Baseline demographic, clinical and laboratory characteristics of study participants
| Demographic Characteristics | Immediate Therapy
Arm N=2,273 % (unless otherwise noted) |
Deferred Therapy Arm N=2,307 % (unless otherwise noted) |
Total N=4,580 % (unless otherwise noted) |
|---|---|---|---|
| Age (years median, IQR) | 36 (29, 44) | 36 (29, 44) | 36 (29, 44) |
| Female gender | 26.7 | 27.1 | 26.9 |
| Race | |||
| White | 43.3 | 44.5 | 43.9 |
| Black | 30.3 | 30.4 | 30.4 |
| Latino/Hispanic | 13.9 | 13.7 | 13.8 |
| Asian | 8.6 | 8.2 | 8.4 |
| Other | 3.9 | 3.2 | 3.5 |
| Geographic Region | |||
| Africa | 21.6 | 21.6 | 21.6 |
| Asia | 7.9 | 7.6 | 7.8 |
| Australia | 2.4 | 2.3 | 2.3 |
| Europe and Israel | 31.9 | 31.7 | 31.8 |
| United States | 10.9 | 11.2 | 11.0 |
| Latin America | 25.4 | 25.6 | 25.5 |
| Clinical Characteristics and Medical History | |||
| BMI (median, IQR) | 25 (22, 28) | 24 (22, 28) | 25 (22, 28) |
| Smoking Status | |||
| Current | 31.3 | 32.6 | 32.0 |
| Former | 13.2 | 12.5 | 12.8 |
| Never | 55.4 | 54.9 | 55.2 |
| Alcohol Consumption | |||
| Days per Week | |||
| 0 | 37.0 | 39.4 | 38.2 |
| < 1 | 30.8 | 30.0 | 30.4 |
| 1 – 3 | 24.3 | 22.1 | 23.2 |
| 4 – 7 | 8.0 | 8.5 | 8.3 |
| Drinks at a Time | |||
| 0 | 29.2 | 28.3 | 28.7 |
| 1 | 19.4 | 19.4 | 19.4 |
| 2 – 4 | 38.9 | 39.4 | 39.2 |
| 5 – 7 | 9.2 | 8.8 | 9.0 |
| 8+ | 3.3 | 4.1 | 3.7 |
| Medical History | |||
| Alcoholism or substance dependence | 3.3 | 3.3 | 3.3 |
| Hepatitis B | 2.8 | 2.9 | 2.8 |
| Hepatitis C | 4.0 | 3.5 | 3.7 |
| Cirrhosis | 0.0 | 0.0 | 0.0 |
| Chronic liver disease | 0.3 | 0.4 | 0.3 |
| Hepatic steatosis | 0.3 | 0.1 | 0.2 |
| Diabetes mellitus | 2.6 | 2.7 | 2.6 |
| Concomitant Treatments at Baseline | |||
| Fibric acid | 0.4 | 0.3 | 0.3 |
| HMG-CoA reductase inhibitors (statins) | 2.2 | 2.3 | 2.2 |
| Anti-tuberculosis drugs | 1.3 | 1.3 | 1.3 |
| Drug treatment for hepatitis C | 0.0 | 0.0 | 0.0 |
| Anabolic steroids | 0.1 | 0.1 | 0.1 |
| Systemic corticosteroids | 0.4 | 0.6 | 0.5 |
| Systemic antifungal drugs | 0.7 | 0.4 | 0.6 |
| Laboratory Results | |||
| CD4 count (cells/mm3; median, IQR) | 652 (586, 765) | 651 (581, 764) | 651 (584, 765) |
| Nadir CD4 count (cells/mm3; median, IQR) | 553 (486, 660) | 554 (491, 650) | 553 (489, 654) |
| HIV RNA (log10copies/mL; median, IQR) | 4.1 (3.5, 4.6) | 4.1 (3.5, 4.6) | 4.1 (3.5, 4.6) |
| HIV RNA, undetectable (with RNA ≤ 200 copies/mL) | 5.3 | 5.3 | 5.3 |
| ALT (SGPT) (U/L; median, IQR) | 24 (17, 34) | 24 (17, 34) | 24 (17, 34) |
| No elevation | 73.6 | 73.8 | 73.7 |
| Grade 1 elevation (1.25–2.4 × ULN1) | 21.4 | 21.5 | 21.5 |
| Grade 2 elevation (2.5–4.9 × ULN1) | 4.3 | 4.0 | 4.1 |
| Grade 3 elevation (5.0–9.9 × ULN1) | 0.6 | 0.7 | 0.6 |
| Grade 4 elevation (≥10 × ULN1) | 0.1 | 0.0 (N=1) | 0.1 |
| AST (SGOT) (U/L; median, IQR) | 25 (20, 31) | 25 (21, 32) | 25 (20, 31) |
| No elevation | 94.5 | 93.5 | 94.0 |
| Grade 1 elevation (1.25–2.4 × ULN2) | 5.0 | 5.6 | 5.3 |
| Grade 2 elevation (2.5–4.9 × ULN2) | 0.4 | 0.8 | 0.6 |
| Grade 3 elevation (5.0–9.9 × ULN2) | 0.1 | 0.1 | 0.1 |
| Grade 4 elevation (≥10 × ULN2) | 0.0 (N=1) | 0.0 (N=1) | 0.0 (N=2) |
| Platelets (x109/L; median, IQR) | 233 (196, 273) | 230 (195, 274) | 231 (195,273) |
| Normal (>1253) | 97.9 | 98.3 | 98.1 |
| Grade 1 thrombocytopenia (100–125) | 1.4 | 0.8 | 1.1 |
| Grade 2 thrombocytopenia (50–99) | 0.6 | 0.8 | 0.7 |
| Grade 3 thrombocytopenia (25–49) | 0.1 | 0.0 (N=1) | 0.1 |
| Grade 4 thrombocytopenia (<25) | 0.0 (N=1) | 0.0 (N=1) | 0.0 (N=2) |
| Albumin (mg/dL median, IQR) | 4.4 (4.1, 4.6) | 4.4 (4.2, 4.6) | 4.4 (4.1, 4.6) |
| Normal | 99.6 | 99.3 | 99.4 |
| Grade 1 hypoalbuminemia (3.0 to <LLN4) | 0.3 | 0.5 | 0.4 |
| Grade 2 hypoalbuminemia (2.0–2.9) | 0.1 | 0.2 | 0.2 |
| Grade 3 hypoalbuminemia (<2.0) | 0.0 (N=0) | 0.0 (N=0) | 0.0 (N=0) |
| Total Bilirubin (mg/dL; median, IQR) | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.7) |
| No elevation | 93.9 | 93.2 | 93.6 |
| Grade 1 elevation (1.1–1.5 × ULN5) | 4.9 | 5.6 | 5.3 |
| Grade 2 elevation (1.6–2.5 × ULN5) | 1.0 | 1.0 | 1.0 |
| Grade 3 elevation (2.6–4.9 × ULN5) | 0.2 | 0.1 | 0.2 |
| Grade 4 elevation (≥5 × ULN5) | 0.0 (N=0) | 0.0 (N=0) | 0.0 (N=0) |
| Alkaline Phosphatase | 74 (59, 96) | 74 (60, 95) | 74 (60, 95) |
| No elevation | 88.4 | 88.0 | 88.2 |
| Grade 1 elevation (1.25–2.4 × ULN6) | 11.0 | 11.4 | 11.2 |
| Grade 2 elevation (2.5–4.9 × ULN6) | 0.6 | 0.6 | 0.6 |
| Grade 3 elevation (5.0–9.9 × ULN6) | 0.0 (N=1) | 0.0 (N=0) | 0.0 (N=1) |
| Grade 4 elevation (≥10.0 × ULN6) | 0.0 (N=0) | 0.0 (N=0) | 0.0 (N=0) |
| Glucose (mg/dL; median, IQR) | 85 (79, 92) | 85 (79, 92) | 85 (79, 92) |
| Total cholesterol (mg/dL; median, IQR) | 166 (143, 193) | 169 (143, 197) | 168 (143, 194) |
| LDL cholesterol (mg/dL; median, IQR) | 101 (82, 124) | 102 (83, 124) | 102 (82, 124) |
| HDL cholesterol (mg/dL; median, IQR) | 42 (35, 50) | 41 (35, 50) | 41 (35, 50) |
| Triglycerides (mg/dL; median, IQR) | 97 (71, 142) | 97 (71, 142) | 97 (71, 142) |
| FIB-4 (median, IQR) | 0.78 (0.58, 1.07) | 0.80 (0.58, 1.08) | 0.79 (0.58, 1.07) |
| APRI score (median, IQR) | 0.27 (0.21, 0.37) | 0.27 (0.21, 0.37) | 0.27 (0.21, 0.37) |
IQR=interquartile ratio; ALT= alanine aminotransferase; ULN=upper limit of normal; AST= aspartate aminotransferase; LLN=lower limit of normal; FIB-4=Fibrosis-4 Index; APRI=AST to Platelet Ratio Index
ALT ULN is 30 for men, 19 for women.
AST ULN is 40 for men, 32 for women.
43 (0.9%) of participants had platelets >450×109/L at baseline
Albumin LLN is 3.3.
Total bilirubin ULN is 1.0.
Alkaline phosphatase ULN is 350 for ages < 20, 115 for men ≥ 20, 100 for women ≥ 20.
The median CD4 count was 651 cells/mm3 and 5.3% of participants had HIV RNA≤200 copies/ml prior to ART initiation. Median AST and ALT were 25 (interquartile range 20, 31) and 24 (interquartile range 17, 34), respectively (Table 1). Grade 1 or higher abnormal laboratory values were found in 26.3% for ALT, 6.0% for AST, 1.9% for platelets, 0.6% for albumin, and 6.5% for bilirubin. Median FIB-4 score was 0.79 (interquartile range 0.58, 1.07) and median APRI was 0.27 (0.21, 0.37). Overall, 84.4% of participants had no significant fibrosis by either FIB-4 or APRI score and 94.4% of participants had no significant fibrosis by both FIB-4 and APRI scores (Figure 1). Of note, low proportions of participants had intermediate scores (FIB-4>1.45 and ≤3.45; APRI >0.5 and≤ 1.5), by either APRI or FIB-4 (14.5%) or both APRI and FIB-4 (5.3%), and a very small proportion had confirmed significant fibrosis (APRI>1.5 and FIB-4>3.25) by either APRI or FIB-4 (1.1%) or both APRI and FIB-4 (0.3%). There were no significant differences in baseline APRI or FIB-4 scores between participants randomized to the immediate treatment arm compared with the deferred treatment arm (data not shown).
Figure 1. Proportion of participants with baseline scores indicating no significant fibrosis, intermediate scores and scores indicating significant fibrosis.
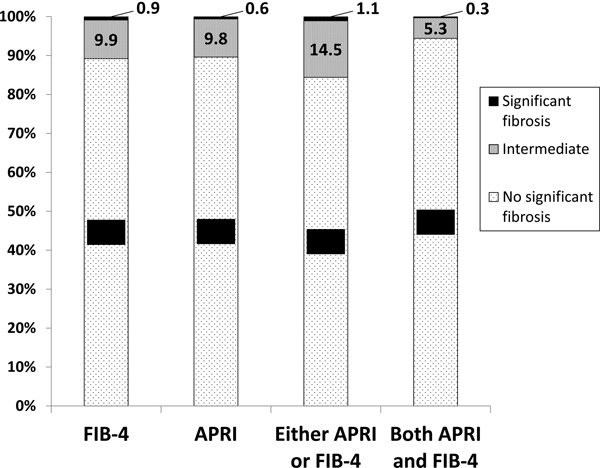
FIB-4=Fibrosis-4 Index; APRI=AST to Platelet Ratio Index FIB-4 cut-offs: No significant fibrosis (FIB-4≤1.45); Intermediate (FIB-4>1.45 but≤3.25); Significant fibrosis (FIB-4>3.25) APRI cut-offs: No significant fibrosis (APRI≤0.5); Intermediate (APRI>0.5 but≤1.5); Significant fibrosis (APRI>1.5) Either APRI or FIB-4 cut-offs: No significant fibrosis (FIB-4≤1.45 or APRI≤0.5); Intermediate (1.45< FIB-4≤ 3.45 or 0.5<APRI≤1.5); Significant fibrosis (FIB-4>3.25 or APRI>1.5) Both APRI and FIB-4: No significant fibrosis (FIB-4≤1.45 and APRI≤0.5); Intermediate (1.45<FIB-4≤3.45 and 0.5<APRI≤1.5); Significant fibrosis (FIB-4>3.25 and APRI>1.5)
Factors Associated with Having Fibrosis at Baseline
Univariate and multivariate analyses of factors associated with having an APRI>0.5 or FIB-4>1.45 at baseline are shown in Table 2a and 2b, respectively (factors associated with having an APRI>1.5 or FIB-4>3.25 are shown in the Supplementary Tables 1a and 1b, respectively). On multivariate analysis, using a cut-off of APRI>0.5 or FIB-4>1.45, black race, being from a high-income country, co-infection with hepatitis, lower CD4 count, and history of liver disease were significantly associated with having an increased risk of fibrosis at baseline by both measures. Older age, having higher ALT, and having lower LDL were associated with having an increased risk of fibrosis by APRI only, and male gender, having lower albumin, having higher total cholesterol, and having higher HDL were associated with having an increased risk by FIB-4 only.
Table 2.
Factors associated with having a risk of liver fibrosis at baseline (APRI>0.5 or FIB-4>1.45)
Table 2a.APRI>0.5 vs. ≤0.5
Table 2b.FIB-4>1.45 vs. ≤1.45
| Baseline predictor | APRI>0.5 (N=476) |
APRI≤0.5 (N=4104) |
Univariate OR (95% CI) |
Univariate p-value | Multivariate OR (95% CI) |
Multivariate p-value |
|---|---|---|---|---|---|---|
| Age (median, IQR; OR per 10 years older) | 39 (31 – 47) | 35 (28 – 43) | 1.25 (1.15 – 1.37) | <0.001 | 1.36 (1.20 – 1.53) | <0.001 |
| Gender (% female) | 16.8 | 28.1 | 0.52 (0.40 – 0.66) | <0.001 | 0.89 (0.63 – 1.25) | 0.507 |
| Race (% black) | 26.1 | 30.8 | 0.79 (0.64 – 0.98) | 0.031 | 1.45 (1.08 – 1.95) | 0.013 |
| Low income country (%) | 46.4 | 55.8 | 0.69 (0.57 – 0.83) | <0.001 | 0.72 (0.56 – 0.93) | 0.012 |
| BMI (median, IQR; OR per 5 kg/m2) | 25.1 (22.7 – 28.4) | 24.5 (22.0 – 27.8) | 1.10 (1.01 – 1.19) | 0.033 | 0.95 (0.84 – 1.08) | 0.435 |
| Current smoker (%) | 36.8 | 31.4 | 1.27 (1.04 – 1.55) | 0.018 | 1.22 (0.94 – 1.57) | 0.132 |
| Hepatitis co-infected (%) | 18.0 | 5.1 | 4.08 (3.10 – 5.36) | <0.001 | 1.92 (1.30 – 2.82) | <0.001 |
| Nadir CD4 count (median, IQR; OR per 100 cells) | 529 (461 – 603) | 556 (492 – 661) | 0.83 (0.77 – 0.88) | <0.001 | * | |
| CD4 (median, IQR; OR per 100 cells) | 631 (572 – 727) | 654 (585 – 768) | 0.89 (0.83 – 0.95) | <0.001 | 0.89 (0.82 – 0.96) | 0.004 |
| HIV RNA (median, IQR; OR per log10copies/mL) | 4.3 (3.8 – 4.8) | 4.1 (3.5 – 4.6) | 1.31 (1.17 – 1.47) | <0.001 | 1.12 (0.97 – 1.29) | 0.122 |
| Albumin (median, IQR; OR per 1 mg/dL) | 4.4 (4.1 – 4.6) | 4.4 (4.2 – 4.6) | 0.83 (0.65 – 1.06) | 0.138 | ||
| Bilirubin (median, IQR; OR per 1 mg/dL) | 0.5 (0.4 – 0.7) | 0.5 (0.4 – 0.7) | 1.49 (1.13 – 1.97) | 0.004 | 1.41 (0.99 – 2.01) | 0.056 |
| Glucose (median, IQR; OR per 10 mg/dL) | 86 (80 – 94) | 85 (79 – 92) | 1.04 (1.01 – 1.07) | 0.015 | 1.00 (0.95 – 1.06) | 0. 980 |
| Total cholesterol (median, IQR; OR per 10mg/dL) | 168 (142 – 197) | 168 (144 – 194) | 1.00 (0.98 – 1.03) | 0.821 | ||
| LDL (median, IQR; OR per 10 mg/dL) | 99 (77 – 123) | 102 (83 – 124) | 0.97 (0.94 – 1.00) | 0.044 | 0.92 (0.88 – 0.95) | <0.001 |
| HDL (median, IQR; OR per 10 mg/dL) | 40 (33 – 49) | 42 (35 – 50) | 0.96 (0.89 – 1.03) | 0.268 | ||
| Triglycerides (median, IQR; OR per 10mg/dL) | 106 (77 – 165) | 97 (71 – 139) | 1.02 (1.02 −1.03) | <0.001 | 1.01 (0.99 – 1.02) | 0.296 |
| History of Diabetes (%) | 4.2 | 2.5 | 1.74 (1.07 – 2.84) | 0.027 | 0.89 (0.40 – 1.98) | 0.780 |
| History of liver disease** (%) | 2.3 | 0.1 | 16.16 (5.95 – 43.89) | <0.001 | 6.43 (1.73 – 23.95) | 0.006 |
| History of hepatic steatosis (%) | 1.1 | 0.1 | 10.88 (2.91 – 40.65) | <0.001 | 1.69 (0.28 – 10.13) | 0.566 |
| Alcoholism/other substance abuse (%) | 6.9 | 2.9 | 2.52 (1.69 – 3.75) | <0.001 | 0.83 (0.45 – 1.51) | 0.531 |
| ALT (SGPT) (median, IQR; OR per 10 U/L) | 48 (32 – 75) | 22 (17 – 31) | 1.75 (1.67 – 1.84) | <0.001 | 1.75 (1.66 – 1.85) | <0.001 |
| Baseline predictor | FIB-4>1.45 (N=497) |
FIB-4≤1.45 (N=4083) |
Univariate OR (95% CI) |
Univariate p-value | Multivariate OR (95% CI) |
Multivariate p-value |
|---|---|---|---|---|---|---|
| Gender (% female) | 27.8 | 26.8 | 1.05 (0.85 – 1.29) | 0.653 | 0.74 (0.57 – 0.97) | 0.030 |
| Race (% black) | 38.4 | 29.4 | 1.50 (1.24 – 1.82) | <0.001 | 1.46 (1.14 – 1.86) | 0.002 |
| Low income country (%) | 45.1 | 56.0 | 0.64 (0.53 – 0.78) | <0.001 | 0.65 (0.52 – 0.81) | <0.001 |
| BMI (median, IQR; OR per 5 kg/m2) | 25.2 (22.5 – 28.6) | 24.5 (22.0 – 27.8) | 1.09 (1.01 – 1.19) | 0.034 | 1.00 (0.91 – 1.11) | 0.953 |
| Current smoker (%) | 33.8 | 31.7 | 1.10 (0.90 – 1.34) | 0.352 | ||
| Hepatitis co-infected (%) | 14.9 | 5.4 | 3.05 (2.30 – 4.05) | <0.001 | 2.35 (1.72 – 3.22) | <0.001 |
| Nadir CD4 count (median, IQR; OR per 100 cells) | 527 (459 – 613) | 556 (493 – 660) | 0.86 (0.80 – 0.92) | <0.001 | * | |
| CD4 (median, IQR; OR per 100 cells) | 638 (573 – 740) | 653 (585 – 768) | 0.92 (0.86 – 0.98) | 0.007 | 0.92 (0.86 – 0.98) | 0.008 |
| HIV RNA (median, IQR; OR per log10copies/mL) | 4.2 (3.6 – 4.7) | 4.1 (3.5 – 4.6) | 1.07 (0.96 −1.19) | 0.198 | ||
| Albumin (median, IQR; OR per 1 mg/dL) | 4.3 (4.0 – 4.5) | 4.4 (4.2 – 4.6) | 0.38 (0.30 – 0.48) | <0.001 | 0.36 (0.27 – 0.47) | <0.001 |
| Bilirubin (median, IQR; OR per 1 mg/dL) | 0.5 (0.4 – 0.7) | 0.5 (0.4 – 0.7) | 0.89 (0.65 – 1.22) | 0.464 | ||
| Glucose (median, IQR; OR per 10 mg/dL) | 86 (79 – 94) | 85 (79 – 92) | 1.05 (1.01 – 1.08) | 0.005 | 1.00 (0.96 – 1.05) | 0.875 |
| Total cholesterol (median, IQR; OR per 10mg/dL) | 172 (147 – 200) | 167 (143 – 194) | 1.03 (1.00 – 1.05) | 0.038 | 1.03 (1.00 – 1.06) | 0.026 |
| LDL (median, IQR; OR per 10 mg/dL) | 103 (81 – 125) | 101 (83 – 124) | 1.00 (0.97 – 1.03) | 0.929 | ||
| HDL (median, IQR; OR per 10 mg/dL) | 42 (35 – 53) | 41 (35 – 50) | 1.12 (1.05 – 1.20) | 0.001 | 1.14 (1.05 – 1.24) | 0.001 |
| Triglycerides (median, IQR) | 99 (75 – 148) | 97 (71 – 142) | 1.01 (1.00 – 1.02) | 0.072 | ||
| History of Diabetes (%) | 5.4 | 2.3 | 2.44 (1.57 – 3.78) | <0.001 | 1.68 (0.91 – 3.10) | 0.095 |
| History of liver disease* (%) | 2.0 | 0.2 | 11.94 (4.53 – 31.52) | <0.001 | 4.20 (1.43 – 12.29) | 0.009 |
| History of hepatic steatosis (%) | 0.6 | 0.1 | 4.13 (1.03 – 16.55) | 0.045 | 1.01 (0.15 – 6.90) | 0.993 |
| Alcoholism/other substance abuse (%) | 6.8 | 2.9 | 2.49 (1.68 – 3.69) | <0.001 | 1.24 (0.78 – 1.97) | 0.354 |
OR=odds ratio; CI=confidence interval; IQR=interquartile ratio; BMI=body mass index; ALT=alanine aminotransferase
Nadir CD4 count was not included in the multivariable models because it was highly correlated with baseline CD4 count
Cirrhosis or chronic liver disease
OR=odds ratio; CI=confidence interval; BMI=body mass index; IQR=interquartile ratio
Nadir CD4 count was not included in the multivariable models because it was highly correlated with baseline CD4 count
Cirrhosis or chronic liver disease
Development of Increased Risk of Fibrosis over Time
An increased risk of fibrosis during study follow-up, defined as developing either APRI>0.5 or FIB-4>1.45 after having no fibrosis by either marker at baseline, was analysed among 1,836 participants in the immediate ART arm and 1,853 participants in the deferred ART arm (Figure 2, Panel A). Seven hundred and sixteen participants were excluded because they already had increased fibrosis scores at baseline and 175 were excluded because they did not have any follow-up APRI or FIB-4 results available. Participants in the immediate ART arm were at lower risk of developing increased fibrosis scores (APRI>0.5 or FIB-4>1.45) than those in the deferred arm (hazard ratio [HR]=0.66; 95% confidence interval [CI]=0.57—0.78; p<0.001). This difference was also significant for each individual marker type (Figure 3, Panel A). Because some participants in the deferred therapy arm started ART after enrolment, we also analysed the risk of developing increased fibrosis scores (APRI>0.5 or FIB-4>1.45) while censoring deferred therapy arm participants when they started ART, as a sensitivity analysis, and found no significant change in our results (OR 0.62, 95% CI 0.52–0.74, p<0.0001). The proportion of participants who developed significant fibrosis (APRI>1.5 or FIB-4>3.25) during follow-up is shown in the Supplementary Table 2.
Figure 2. Cumulative proportion of patients with liver-related outcomes over time from randomization by treatment arm.
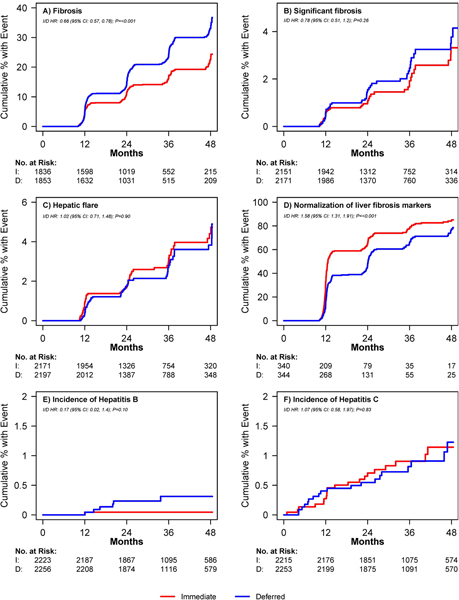
I=immediate treatment arm; D=deferred treatment arm; HR=hazard ratio; CI=confidence interval; No.=number; FIB-4=Fibrosis-4 Index; APRI=AST to Platelet Ratio Index Panel A. Proportion of participants developing elevated fibrosis score (APRI>0.5 or FIB-4>1.45) over time. Number at risk = those with no fibrosis at baseline based on availability of measurements at visits. Panel B. Proportion of participants developing significant fibrosis (APRI>1.5 or FIB-4>3.25) over time. Number at risk = those with no significant fibrosis at baseline based on availability of measurements at visits. Panel C. Proportion of participants developing hepatic flare over time. Hepatic flare was defined as ALT greater than five times the upper limit of normal (30 for men and 19 for women). Number at risk = those with no hepatic flare at baseline and follow-up ALT measurements available. Panel D. Proportion of participants with normalization of abnormal baseline APRI and FIB-4 scores (i.e. APRI>0.5 or FIB-4>1.45 normalizing to APRI≤0.5 and FIB-4≤1.45) over time. Number at risk = those with abnormal scores at baseline and availability of at least one follow-up measurement. Panel E. Proportion of participants developing Hepatitis. Number at risk = number who tested negative for Hepatitis B at baseline. Panel F. Proportion of participants developing Hepatitis C over time. Number at risk = number who tested negative for Hepatitis C at baseline.
Figure 3. Cumulative proportion of patients with liver-related outcomes over time from randomization by treatment arm and score type (APRI, FIB-4, and APRI or FIB-4).
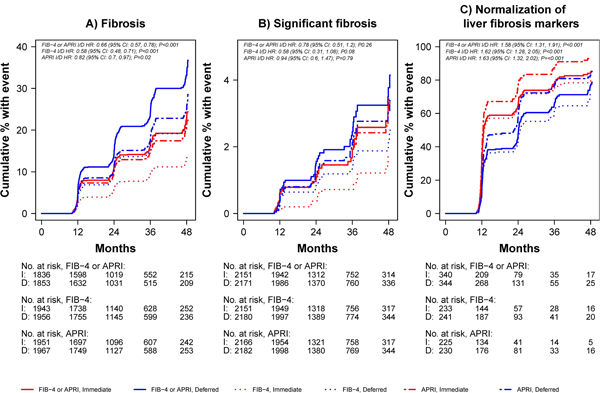
I=immediate treatment arm; D=deferred treatment arm; HR=hazard ratio; CI=confidence interval; No.=number; FIB-4=Fibrosis-4 Index; APRI=AST to Platelet Ratio Index Panel A. Cumulative proportion of participants with elevated fibrosis scores (APRI>0.5 or FIB-4>1.45) Panel B. Cumulative proportion of patients with significant fibrosis (APRI>1.5 or FIB-4>3.25) Panel C. Cumulative proportion of patients with normalization of abnormal fibrosis scores (i.e. APRI>0.5 or FIB-4>1.45 normalizing to APRI≤0.5 and FIB-4≤1.45) over time
Univariate and multivariate analyses of predictors of the development of increased risk of fibrosis during follow-up are shown by treatment arm and by treatment groups combined in Tables 3a (APRI>0.5) and 3b (FIB-4>1.45). Factors associated with significant fibrosis are shown in the Supplementary Table 3a (APRI>1.5) and 3b (FIB-4>3.25). On multivariate analysis using a cut-off of either APRI>0.5 or FIB-4>1.45, an increased risk of fibrosis on follow-up was significantly associated with deferred ART, male gender and hepatitis co-infection. Having a history of alcoholism or other substance abuse and having a higher ALT (SGPT) were associated with increased risk by APRI only, and being from a high-income country, having lower albumin, having higher total cholesterol and having higher triglycerides were associated with increased risk by FIB-4 only.
Table 3.
Baseline predictors of developing a risk of liver fibrosis during follow up, by treatment arm and treatment groups combined
Table 3a.Predictors based on APRI>0.5 vs. ≤0.5
Table 3b.Predictors based on FIB-4>1.45 vs. ≤1.45
| Baseline predictor | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Immediate ART | Deferred ART | Combined Groups | Combined Groups | |||||
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | OR (95% CI)* | p-value | OR (95% CI)* | p-value | |
| Treatment group (Imm. vs Def.) | -- | -- | -- | -- | 0.78 (0.65 – 0.93) | 0.006 | 0.78 (0.65 – 0.95) | 0.011 |
| Age (per 10 years older) | 1.09 (0.96 – 1.24) | 0.207 | 0.87 (0.77 – 0.99) | 0.034 | 0.97 (0.89 – 1.06) | 0.490 | 0.94 (0.85 – 1.03) | 0.200 |
| Gender (female vs. male) | 0.47 (0.33 – 0.67) | <0.001 | 0.34 (0.24 – 0.48) | <0.001 | 0.39 (0.31 – 0.51) | <0.001 | 0.45 (0.34 – 0.60) | <0.001 |
| Race (black vs. other) | 0.81 (0.60 – 1.10) | 0.180 | 0.61 (0.46 – 0.83) | 0.001 | 0.70 (0.57 – 0.87) | 0.001 | 1.06 (0.83 – 1.35) | 0.644 |
| Low income country | 1.00 (0.77 – 1.32) | 0.979 | 0.89 (0.69 – 1.15) | 0.368 | 0.94 (0.78 – 1.13) | 0.509 | ||
| BMI (per 5 kg/m2) | 0.97 (0.86 – 1.10) | 0.679 | 0.98 (0.87 – 1.10) | 0.705 | 0.97 (0.89 – 1.06) | 0.512 | ||
| Current smoker | 0.92 (0.69 – 1.22) | 0.550 | 1.20 (0.93 – 1.55) | 0.155 | 1.06 (0.88 – 1.29) | 0.527 | ||
| Hepatitis co-infected | 2.01 (1.23 – 3.28) | 0.005 | 2.10 (1.31 – 3.36) | 0.002 | 2.05 (1.46 – 2.88) | <0.001 | 2.03 (1.42 – 2.89) | <0.001 |
| CD4 (per 100 cells) | 1.00 (0.93 – 1.08) | 0.989 | 0.91 (0.84 – 0.99) | 0.022 | 0.95 (0.90 – 1.01) | 0.089 | ||
| Nadir CD4 count (per 100 cells) | 0.97 (0.89 – 1.05) | 0.476 | 0.91 (0.84 – 0.99) | 0.029 | 0.94 (0.89 – 1.00) | 0.036 | 0.96 (0.90 – 1.02) | 0.173 |
| HIV RNA (per log10copies/mL) | 0.99 (0.85 – 1.14) | 0.850 | 1.11 (0.96 – 1.28) | 0.158 | 1.05 (0.95 – 1.17) | 0.349 | ||
| Albumin (per 1 mg/dL) | 1.14 (0.81 – 1.60) | 0.460 | 1.60 (1.16 – 2.19) | 0.004 | 1.36 (1.08 – 1.71) | 0.009 | 1.06 (0.82 – 1.37) | 0.660 |
| Bilirubin (per 1 mg/dL) | 1.26 (0.85 – 1.87) | 0.246 | 1.50 (1.05 – 2.15) | 0.027 | 1.40 (1.08 – 1.82) | 0.012 | 1.10 (0.82 – 1.47) | 0.525 |
| Glucose (per 10 mg/dL) | 1.02 (0.97 – 1.07) | 0.540 | 0.99 (0.94 – 1.05) | 0.836 | 1.01 (0.97 – 1.04) | 0.724 | ||
| Total chol. (per 10mg/dL) | 1.02 (0.98 – 1.05) | 0.398 | 1.01 (0.98 – 1.05) | 0.381 | 1.01 (0.99 – 1.04) | 0.226 | ||
| LDL (per 10 mg/dL) | 0.99 (0.95 – 1.04) | 0.755 | 1.01 (0.97 – 1.05) | 0.517 | 1.00 (0.98 – 1.03) | 0.779 | ||
| HDL (per 10 mg/dL) | 1.06 (0.95 – 1.17) | 0.306 | 0.93 (0.84 – 1.03) | 0.151 | 0.99 (0.92 – 1.06) | 0.703 | ||
| Triglycerides (per 10mg/dL) | 1.01 (1.00 – 1.03) | 0.137 | 1.01 (1.00 – 1.03) | 0.041 | 1.01 (1.00 – 1.02) | 0.013 | 1.01 (1.00 – 1.02) | 0.251 |
| History of Diabetes | 1.37 (0.63 – 2.99) | 0.423 | 0.90 (0.42 – 1.96) | 0.796 | 1.13 (0.65 – 1.96) | 0.662 | ||
| History of liver disease** | 6.87 (0.96 – 48.99) | 0.054 | NA;*** | NA;*** | 2.90 (0.53 – 15.98) | 0.221 | ||
| Alcoholism/other substance abuse | 2.24 (1.22 – 4.12) | 0.009 | 1.73 (0.92 – 3.25) | 0.090 | 1.96 (1.26 – 3.03) | 0.003 | 1.72 (1.08 – 2.71) | 0.021 |
| ALT (SGPT) (per 10 U/L) | 1.17 (1.09 – 1.27) | <0.001 | 1.24 (1.15 – 1.34) | <0.001 | 1.21 (1.14 – 1.27) | <0.001 | 1.16 (1.09 – 1.23) | <0.001 |
| Baseline predictor | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Immediate ART | Deferred ART | Combined Groups | Combined Groups | |||||
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | OR (95% CI)* | p-value | OR (95% CI)* | p-value | |
| Treatment group (Imm. vs Def.) | -- | -- | -- | -- | 0.55 (0.44 – 0.68) | <0.001 | 0.55 (0.44 – 0.68) | <0.001 |
| Gender (female vs. male) | 0.55 (0.35 – 0.86) | 0.009 | 0.89 (0.65 – 1.23) | 0.478 | 0.75 (0.58 – 0.96) | 0.025 | 0.72 (0.53 – 0.97) | 0.033 |
| Race (black vs. other) | 0.93 (0.64 – 1.37) | 0.730 | 1.05 (0.77 – 1.42) | 0.771 | 1.00 (0.79 – 1.26) | 0.992 | 1.18 (0.90 – 1.54) | 0.243 |
| Low income country | 0.77 (0.55 – 1.07) | 0.122 | 0.65 (0.50 – 0.86) | 0.002 | 0.70 (0.56 – 0.86) | <0.001 | 0.75 (0.60 – 0.95) | 0.015 |
| BMI (per 5 kg/m2) | 1.01 (0.87 – 1.18) | 0.881 | 0.97 (0.85 – 1.11) | 0.664 | 0.98 (0.89 – 1.08) | 0.722 | ||
| Current smoker | 1.16 (0.82 – 1.64) | 0.400 | 0.87 (0.65 – 1.16) | 0.339 | 0.98 (0.79 – 1.22) | 0.885 | ||
| Hepatitis co-infected | 2.03 (1.16 – 3.57) | 0.013 | 1.52 (0.90 – 2.57) | 0.121 | 1.72 (1.17 – 2.51) | 0.006 | 1.70 (1.15 – 2.52) | 0.008 |
| CD4 (per 100 cells) | 1.05 (0.96 – 1.14) | 0.331 | 0.91 (0.84 – 1.00) | 0.049 | 0.97 (0.91 – 1.03) | 0.345 | ||
| Nadir CD4 count (per 100 cells) | 1.04 (0.95 – 1.15) | 0.388 | 0.88 (0.80 – 0.97) | 0.008 | 0.95 (0.89 – 1.02) | 0.136 | 0.95 (0.89 – 1.02) | 0.165 |
| HIV RNA (per log10copies/mL) | 0.91 (0.75 – 1.09) | 0.297 | 1.12 (0.95 – 1.31) | 0.169 | 1.03 (0.91 – 1.16) | 0.675 | ||
| Albumin (per 1 mg/dL) | 0.76 (0.50 – 1.17) | 0.215 | 0.75 (0.53 – 1.05) | 0.093 | 0.75 (0.57 – 0.97) | 0.032 | 0.69 (0.52 – 0.92) | 0.011 |
| Bilirubin (per 1 mg/dL) | 1.31 (0.80 – 2.13) | 0.286 | 0.87 (0.57 – 1.33) | 0.519 | 1.05 (0.76 – 1.45) | 0.751 | ||
| Glucose (per 10 mg/dL) | 1.02 (0.96 – 1.08) | 0.523 | 1.05 (1.00 – 1.10) | 0.036 | 1.04 (1.00 – 1.08) | 0.026 | 1.01 (0.96 – 1.06) | 0.606 |
| Total chol. (per 10mg/dL) | 1.05 (1.01 – 1.10) | 0.025 | 1.05 (1.01 – 1.08) | 0.008 | 1.05 (1.02 – 1.08) | <0.001 | 1.04 (1.00 – 1.07) | 0.023 |
| LDL (per 10 mg/dL) | 1.02 (0.97 – 1.07) | 0.432 | 1.02 (0.98 – 1.06) | 0.335 | 1.02 (0.99 – 1.05) | 0.194 | ||
| HDL (per 10 mg/dL) | 1.02 (0.89 – 1.16) | 0.781 | 1.01 (0.91 – 1.13) | 0.789 | 1.01 (0.93 – 1.10) | 0.775 | ||
| Triglycerides (per 10mg/dL) | 1.03 (1.01 – 1.04) | 0.003 | 1.02 (1.01 – 1.03) | 0.002 | 1.02 (1.01 – 1.03) | <0.001 | 1.02 (1.01 – 1.03) | 0.005 |
| History of Diabetes | 1.77 (0.74 – 4.26) | 0.202 | 1.86 (0.89 – 3.88) | 0.096 | 1.85 (1.06 – 3.23) | 0.030 | 1.36 (0.65 – 2.86) | 0.416 |
| History of liver disease** | 4.78 (0.49 – 46.20) | 0.177 | 3.37 (0.30 – 37.77) | 0.325 | 3.79 (0.73 – 19.69) | 0.113 | ||
| Alcoholism/other substance abuse | 2.58 (1.27 – 5.24) | 0.009 | 1.42 (0.69 – 2.90) | 0.339 | 1.85 (1.12 – 3.06) | 0.016 | 1.43 (0.85 – 2.43) | 0.180 |
OR=odds ratio; CI=confidence interval; BMI=body mass index; ALT=alanine aminotransferase
OR for APRI > 0.5 at any point during follow-up, stratified by the number of follow-up measurements available
Cirrhosis or chronic liver disease (history of hepatic steatosis was not included in the follow-up analyses because no one with FIB-4>1.45 only 1 person with APRI>0.5 had hepatic steatosis)
In the Deferred ART group, 0 participants with a history of liver disease had a follow-up APRI > 0.5
OR=odds ratio; CI=confidence interval; BMI=body mass index
OR for APRI > 0.5 at any point during follow-up, stratified by the number of follow-up measurements available
Cirrhosis or chronic liver disease (history of hepatic steatosis was not included in the follow-up analyses because no one with FIB-4>1.45 only 1 person with APRI>0.5 had hepatic steatosis)
We found no difference in the development of fibrosis during follow-up stratified by baseline HIV RNA level (<100,000 vs. ≥100,000) (interaction p-value=0.68 for APRI and 0.25 for FIB4; Supplementary Material Table 4). We also evaluated HIV RNA suppression (<200 copies/mL vs. ≥200 copies/mL) as a time-updated variable and found no interaction between treatment group and HIV suppression. Using APRI, the <200 copies/mL vs. ≥200 copies/mL time-updated OR (95% CI) for fibrosis in the immediate arm was 0.86 (0.52 – 1.43), p-value=0.57 and the deferred arm OR (95% CI) was 0.76 (0.58 – 1.00); p=0.051, with an interaction p-value=0.81. Using FIB-4, the <200 copies/mL vs. ≥200 copies/mL time-updated OR (95% CI) for fibrosis in the immediate arm was 0.66 (0.37 – 1.19); p=0.17 and the deferred arm OR (95% CI) was 0.60 (0.44, 0.81); p<0.001, with an interaction p-value=0.87.
There was no difference in changes in APRI or FIB-4 from baseline by pre-specified ART regimens that included a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor (p-value for interaction between treatment arm and ART group=0.32 for FIB-4 and 0.53 for APRI) (Supplementary Material Table 5). The number and proportion of patients assigned to each pre-specified regimen is shown in Supplementary Material Table 6.
Development of Other Liver-related Events over Time
Significant fibrosis (APRI>1.5 or FIB-4>3.25) was analysed among 4322 participants (2151 in the immediate arm, 2171 in the deferred arm); 258 were excluded because 52 had significant fibrosis at baseline and 206 did not have follow-up APRI or FIB-4 results (Figure 2, Panel B). There was no difference in the incidence of significant fibrosis during follow-up between the two groups (HR 0.78; 95% CI 0.51–1.20; p=0.26); this was similar when analysed by individual marker type (Figure 3, Panel B).
Hepatic flare was analysed among 4368 participants (2171 in the immediate arm, 2197 in the deferred arm); 182 were excluded because they did not have a follow-up ALT and 30 were excluded because they had an ALT greater than five times the upper limit of normal at baseline (Figure 2, Panel C). The incidence of hepatic flare during follow-up did not differ between the immediate therapy and the deferred therapy arm (both arms had 57 events at a rate of 9.9/1000 person years; HR=1.02; 95% CI=0.71—1.48; p=0.90). In univariate analysis, baseline risk factors associated with an increased risk of hepatic flare over time included female gender (HR 1.63; 95% CI 1.09—2.44; p=0.02), being co-infected with hepatitis (HR 2.18; 95% CI 1.27—3.75; p=0.005), and elevated triglycerides (per 10mg/dL increase, HR 1.01; 95% CI 1.00—1.03; p=0.02). On multivariate analysis (model also included age and race), the following remained significant: female gender (HR 1.72; 95% CI 1.10—2.69; p=0.02), being co-infected with hepatitis (HR 2.34; 95% CI 1.35—4.05; p=0.002), and elevated triglycerides (per 10mg/dL increase, HR 1.02; 95% CI 1.01—1.03; p=0.004).
Resolution of abnormal baseline APRI and FIB-4 scores over time was evaluated among participants with APRI>0.5 or FIB-4>1.45 at baseline and at least one follow-up measure available (340 in the immediate arm, 344 in the deferred arm) (Figure 2, Panel D). By both APRI and FIB-4, liver fibrosis markers normalised faster among those in the immediate ART group vs. the deferred therapy ART group (HR 1.58; 95% CI 1.31—1.91; p<0.001). This difference was also significant when analysed by marker type (Figure 3, Panel C). Among participants with APRI>0.5, normalization occurred at a rate of 56.6 per 100 person-years in the immediate arm compared with 40.9 per 100 person-years in the deferred arm (HR 1.63; 95% CI 1.32—2.02; p<0.001). Among participants with FIB-4>1.45, normalization occurred at a rate of 40.6 per 100 person-years in the immediate arm compared with 27.1 per 100 person-years in the deferred arm (HR 1.62; 95% CI 1.28—2.05; p<0.001).
The incidence of Hepatitis B and C over time was evaluated among participants who tested negative at baseline (2,122 participants in the immediate arm and 2,166 participants in the deferred arm) (Figure 2, Panels E and F). There was no difference in the development of Hepatitis B or C during follow-up (HR 0.89; 95% CI 0.50–1.59; p=0.70). Hepatitis B incidence was 0.1 per 1000 person-years in the immediate arm compared with 0.8 per 1000-person-years in the deferred arm (HR 0.2; 95% CI 0.02—1.4; p=0.10) and the incidence of Hepatitis C was 3.0 per 1000 person-years in the immediate arm compared with 2.8 per 1000 person-years in the deferred arm (HR 1.07; 95% CI 0.6—2.0; p=0.83).
Overall, there were few other liver-related clinical events reported over follow-up with only one case of hepatocellular carcinoma and no deaths due to liver disease.
Discussion
In this large cohort of subjects with HIV and high CD4 counts, we found that approximately 84–94% of patients had APRI or FIB-4 scores at baseline that ruled out significant fibrosis, indicating overall low rates of liver fibrosis in our study population. In addition, over the long period of follow-up in the START study, very few patients developed indicators of worsening liver disease such as the development of increased fibrosis scores (APRI>0.5 or FIB-4>1.45), significant fibrosis (APRI>1.5 or FIB-4>3.25), hepatic flare, or infection with Hepatitis B or C. Further, our data suggest that early initiation of ART may improve, rather than worsen liver health outcomes in persons with HIV, as measured by non-invasive fibrosis scores.
Non-invasive biomarkers have been used to measure hepatic fibrosis in patients with HIV monoinfection (14, 22). Our results are likely in line with other published studies, though our patient population consisted of ART-naïve patients with or without liver disease at baseline, which is different from other studies. Several studies have selected populations of HIV monoinfected patients with abnormal liver function tests (22, 29), resulting in increased prevalence of fibrosis in the study population. In addition, many studies report data on liver health among HIV populations with significant exposure to ART (5, 8, 14, 22, 28, 30, 31).
Most studies have identified traditional risk factors for abnormal liver enzymes in patients with HIV mono-infection such as metabolic syndrome (7, 32) and some have found an association with HIV-related factors such as increased HIV viral load and exposure to certain antiretrovirals (8, 33). While the APRI and FIB-4 were not always in agreement, our baseline analysis identified key variables known to be associated with an increased risk of liver fibrosis, including older age, male gender, black race, abnormal ALT, co-infection with hepatitis and a history of known liver disease. Interestingly, our results with regard to markers of metabolic syndrome were mixed. While having higher total cholesterol and being from a high-income country (consistent with metabolic syndrome) were associated with higher risk of fibrosis, having higher HDL and lower albumin and lower LDL were also associated with a higher risk of fibrosis using these non-invasive markers. Further a lower CD4 at baseline was associated with greater likelihood of abnormal fibrosis scores. One possible explanation is that patients with advanced immunodeficiency (lower weight, lower CD4) had lower platelet counts that falsely elevated fibrosis scores. However, the median platelet count at baseline was 232 with a very low proportion having Grade1 or higher abnormalities. Factors predicting development of abnormal fibrosis scores over follow-up were more traditional with male gender, hepatitis co-infection, history of alcoholism or other substance abuse, being from a high-income country, and having higher ALT, total cholesterol and triglycerides all being associated with a higher risk, though having lower albumin was also found to be associated with higher risk. It is important to note that APRI and FIB-4 did not always identify the same variables; therefore, the use of serum biomarkers to predict or exclude fibrosis in a specific clinical setting should be done based on validation of that particular algorithm within the setting of interest, as performance may vary depending on population characteristics.
Our finding that early initiation of ART improved liver fibrosis scores is worth further exploration. Older ART agents have been shown to cause direct hepatocellular damage, mitochondrial toxicity and insulin resistance, as well as be associated with liver disease (5, 8, 15, 17, 28, 30). However, uncontrolled HIV viremia has also been shown to be associated with liver fibrosis (12, 13, 30, 31), suggesting that ART, particularly modern agents, may be protective against developing liver fibrosis. Relative to other studies in people with HIV, all of our participants were ART-naïve and had high CD4 counts at baseline. Therefore, our study population is likely to have had HIV for a shorter period of time than other study populations, and as a result, shorter cumulative exposure to HIV viremia, immunodeficiency and immune activation, and as a result, possibly less HIV-associated liver fibrosis. In addition, our study population was not exposed to older non-nucleoside reverse transcriptase inhibitors and protease inhibitors that had higher risks of hepatotoxicity, and the newer pre-specified regimens used in our study likely had less hepatotoxicity than regimens used by participants in other studies who were more likely to have been exposed to older agents.
Our findings are subject to several limitations. Our study population only included people with HIV with CD4 counts greater than 500 and the majority of our study population was HIV-monoinfected. Therefore, our findings cannot be generalized to persons with lower CD4 counts or persons with viral hepatitis co-infection. Low numbers of patients with significant fibrosis limits our ability to evaluate for factors associated with significant liver fibrosis or whether there is a benefit of early ART initiation in reducing its development in this patient population. There were few patients in the cohort with other significant liver health outcomes, such as hepatocellular carcinoma or liver-related deaths, so we were unable to evaluate the effects of ART on these uncommon outcomes. In addition, our results are limited by a relatively short duration of follow-up as the trial was stopped early and as a result, our results cannot necessarily be applied to persons on ART for longer durations of time.
A major limitation to our findings is the utility of the non-invasive markers as measures of liver fibrosis. Although APRI and FIB-4 are two widely used and accepted non-invasive fibrosis scores(6, 14, 22), interpretation of their results must be made with caution. Neither is considered to be as accurate as liver biopsy or transient elastography(6, 25), and both APRI and FIB-4 have “intermediate” scoring ranges that cannot be interpreted to either rule in or out liver fibrosis (20, 21). In addition, these composite scores depend on their individual components, such as AST, ALT, and platelets. When combined in algorithms these components have clearly been shown to correlate with fibrosis on more traditional measures such as liver biopsy, however they may be affected individually by other processes, including the introduction of ART and subsequent changes in viral load and inflammation. As a result using longitudinal changes in these scores to confirm true hepatic fibrosis development or regression should be done with caution. Supporting this is the fact that changes in fibrosis scores in our study were observed at relatively early time points during the study (as early as 12–24 months) when true fibrosis may take longer to develop. Unfortunately the START study was stopped early and longer follow-up to see if these changes were maintained was not possible. Early termination of the study is also the reason why the numbers reduced significantly over follow-up. However, despite numbers reducing over time the difference between the arms was maintained, even though statistical significance was lost.
Despite their limitations, APRI and FIB-4 scores have been widely validated in other settings and at baseline are likely to truly reflect a lack of significant fibrosis in this population. This is supported by the identification of factors known to be associated with liver disease in our study. Whether the subsequent changes truly reflect hepatic fibrosis development, or some improvement in inflammation linked to ART commencement is unclear. While ART is known to improve HIV-induced thrombocytopenia, only 1.9% of our study participants had a Grade 1 or higher platelet abnormality indicating that any effect of ART on thrombocytopenia in our study population would have been small. We also examined the results by HIV RNA suppression but found no effect, suggesting that if the benefit is related to HIV control, it is more complex than just RNA suppression. Irrespective of the cause however, our findings confirm a benefit to starting ART on these markers, and therefore on potential subsequent fibrosis development.
Conclusions
In conclusion, we report data on liver fibrosis as measured by non-invasive liver fibrosis scores in previously untreated persons with HIV and high CD4 counts. In this largest study of its kind in predominantly HIV-monoinfected individuals, we show firstly that significant liver fibrosis was rare and both fibrosis and other liver related events continued to be uncommon over 14,379 person year of follow-up. We also found a potential benefit in reducing markers of liver fibrosis among persons immediately treated with ART and confirmed the lack of hepatotoxicity of the ART regimens used in this study. Our results support current guidance to initiate ART in all patients with HIV regardless of CD4 count, particularly considering that modern ART regimens have lower rates of hepatotoxicity than older regimens. Patients with Hepatitis B and C co-infection, as well as those with NAFLD and existing steatosis, should be particularly prioritized for treatment to possibly reduce progression of liver fibrosis in such patients with elevated risk and to preserve overall liver health.
Supplementary Material
Acknowledgements
The authors would like to thank all of the patients that participated in this study. See N Engl J Med 2015; 373:795–807 for the complete list of START investigators.
Financial Support
Supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health Clinical Center, National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundes ministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom), and University of Minnesota. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
Supported by NIH Grants UM1-AI068641 and UM1-AI120197. Dr. Dharan has received support through an Australian Government Research Training Program Scholarship.
List of abbreviations
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- ART
antiretroviral therapy
- FIB-4
fibrosis-4 Index
- HIV
human immunodeficiency virus
- NAFLD
non-alcoholic fatty liver disease
- START
Strategic Timing of Antiretroviral Treatment
Footnotes
Disclaimer
The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The Burnet Institute receives funding from the Victorian Operational Infrastructure Support Program. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health (award R01DA015999), Janssen-Cilag Pty Ltd and Gilead Sciences Inc as investigator-initiated studies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Australian Government.
References:
- 1.Macias J, Pineda JA, Real LM. Non-Alcoholic Fatty Liver Disease in HIV Infection. AIDS Rev 2017;19:35–46. [PubMed] [Google Scholar]
- 2.Rockstroh JK. Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) in HIV. Curr HIV/AIDS Rep 2017;14:47–53. [DOI] [PubMed] [Google Scholar]
- 3.Data Collection on Adverse Events of Anti HIVdSG, Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010;24:1537–1548. [DOI] [PubMed] [Google Scholar]
- 4.Jaquet A, Wandeler G, Nouaman M, Ekouevi DK, Tine J, Patassi A, Coffie PA, et al. Alcohol use, viral hepatitis and liver fibrosis among HIV-positive persons in West Africa: a cross-sectional study. J Int AIDS Soc 2017;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco F, Barreiro P, Ryan P, Vispo E, Martin-Carbonero L, Tuma P, Labarga P, et al. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat 2011;18:11–16. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 7.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017;31:1621–1632. [DOI] [PubMed] [Google Scholar]
- 8.Kovari H, Ledergerber B, Battegay M, Rauch A, Hirschel B, Foguena AK, Vernazza P, et al. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin Infect Dis 2010;50:502–511. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 10.Poynard T, Lebray P, Ingiliz P, Varaut A, Varsat B, Ngo Y, Norha P, et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol 2010;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishijima T, Gatanaga H, Shimbo T, Komatsu H, Nozaki Y, Nagata N, Kikuchi Y, et al. Traditional but not HIV-related factors are associated with nonalcoholic fatty liver disease in Asian patients with HIV-1 infection. PLoS One 2014;9:e87596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohr R, Schierwagen R, Schwarze-Zander C, Boesecke C, Wasmuth JC, Trebicka J, Rockstroh JK. Liver Fibrosis in HIV Patients Receiving a Modern cART: Which Factors Play a Role? Medicine (Baltimore) 2015;94:e2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HN, Nance R, Van Rompaey S, Delaney JC, Crane HM, Cachay ER, Geng E, et al. Poorly Controlled HIV Infection: An Independent Risk Factor for Liver Fibrosis. J Acquir Immune Defic Syndr 2016;72:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendeni M, Foca E, Gotti D, Ladisa N, Angarano G, Albini L, Castelnuovo F, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis 2011;52:1164–1173. [DOI] [PubMed] [Google Scholar]
- 15.Nunez M Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol 2006;44:S132–139. [DOI] [PubMed] [Google Scholar]
- 16.Foca E, Fabbiani M, Prosperi M, Quiros Roldan E, Castelli F, Maggiolo F, Di Filippo E, et al. Liver fibrosis progression and clinical outcomes are intertwined: role of CD4+ T-cell count and NRTI exposure from a large cohort of HIV/HCV-coinfected patients with detectable HCV-RNA: A MASTER cohort study. Medicine (Baltimore) 2016;95:e4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuille-Lessard E, Lebouche B, Lennox L, Routy JP, Costiniuk CT, Pexos C, Giannakis A, et al. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016;30:2635–2643. [DOI] [PubMed] [Google Scholar]
- 18.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babiker AG, Emery S, Fatkenheuer G, Gordin FM, Grund B, Lundgren JD, Neaton JD, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013;10:S5–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–526. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi R, Lever R, Smith C, Marshall N, Rodger A, Bhagani S, Tsochatzis E. Liver test abnormalities in patients with HIV mono-infection: assessment with simple noninvasive fibrosis markers. Ann Gastroenterol 2017;30:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallet V, Dhalluin-Venier V, Roussin C, Bourliere M, Pettinelli ME, Giry C, Vallet-Pichard A, et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2009;29:409–415. [DOI] [PubMed] [Google Scholar]
- 24.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–36. [DOI] [PubMed] [Google Scholar]
- 25.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol 2018;68:305–315. [DOI] [PubMed] [Google Scholar]
- 26.Kilonzo SB, Gunda DW, Kashasha F, Mpondo BC. Liver Fibrosis and Hepatitis B Coinfection among ART Naive HIV-Infected Patients at a Tertiary Level Hospital in Northwestern Tanzania: A Cross-Sectional Study. J Trop Med 2017;2017:5629130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews GV, Neuhaus J, Bhagani S, Mehta SH, Vlahakis E, Doroana M, Naggie S, et al. Baseline prevalence and predictors of liver fibrosis among HIV-positive individuals: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015;16 Suppl 1:129–136. [DOI] [PubMed] [Google Scholar]
- 28.Merchante N, Perez-Camacho I, Mira JA, Rivero A, Macias J, Camacho A, Gomez-Mateos J, et al. Prevalence and risk factors for abnormal liver stiffness in HIV-infected patients without viral hepatitis coinfection: role of didanosine. Antivir Ther 2010;15:753–763. [DOI] [PubMed] [Google Scholar]
- 29.Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M, Hale B, et al. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol 2010;8:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anadol E, Lust K, Boesecke C, Schwarze-Zander C, Mohr R, Wasmuth JC, Rockstroh JK, et al. Exposure to previous cART is associated with significant liver fibrosis and cirrhosis in human immunodeficiency virus-infected patients. PLoS One 2018;13:e0191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackard JT, Welge JA, Taylor LE, Mayer KH, Klein RS, Celentano DD, Jamieson DJ, et al. HIV mono-infection is associated with FIB-4 - A noninvasive index of liver fibrosis - in women. Clin Infect Dis 2011;52:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardi R, Sambatakou H, Mariolis I, Cokkinos D, Papatheodoridis GV, Tsochatzis EA. Prevalence and predictors of liver steatosis and fibrosis in unselected patients with HIV mono-infection. Dig Liver Dis 2016;48:1471–1477. [DOI] [PubMed] [Google Scholar]
- 33.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci 2008;53:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


