Abstract
The goal of this study was to test the hypothesis that stem cells, as a response to valve-specific extracellular matrix “niches” and mechanical stimuli, would differentiate into valvular interstitial cells (VICs). Porcine aortic root scaffolds were prepared by decellularization. After verifying that roots exhibited adequate hemodynamics in vitro, we seeded human adipose-derived stem cells (hADSCs) within the interstitium of the cusps and subjected the valves to in vitro pulsatile bioreactor testing in pulmonary pressures and flow conditions. As controls we incubated cell-seeded valves in a rotator device which allowed fluid to flow through the valves ensuring gas and nutrient exchange without subjecting the cusps to significant stress. After 24 days of conditioning, valves were analyzed for cell phenotype using immunohistochemistry for vimentin, alpha-smooth muscle cell actin (SMA) and prolyl-hydroxylase (PHA). Fresh native valves were used as immunohistochemistry controls. Analysis of bioreactor-conditioned valves showed that almost all seeded cells had died and large islands of cell debris were found within each cusp. Remnants of cells were positive for vimentin. Cell seeded controls, which were only rotated slowly to ensure gas and nutrient exchange, maintained about 50% of cells alive; these cells were positive for vimentin and negative for alpha-SMA and PHA, similar to native VICs. These results highlight for the first time the extreme vulnerability of hADSCs to valve-specific mechanical forces and also suggest that careful, progressive mechanical adaptation to valve-specific forces might encourage stem cell differentiation towards the VIC phenotype.
Keywords: Scaffolds, Heart valves, Stem cells, Bioreactor
INTRODUCTION
Aortic valve pathology is an important chapter of cardiovascular diseases, affecting millions of patients of all ages worldwide [1–7]. Currently, the only therapy for aortic valve disease is their surgical repair or replacement with artificial devices such as mechanical valves or glutaraldehyde-treated, devitalized animal valves that mimic the function, but not the biology of the native valve. Artificial valves fail within 15–20 years after implantation and repeated open-heart surgery to replace defective implants is undesirable. The most physiologic valve replacements are considered the living, pulmonary autografts and cryopreserved aortic allografts. The latter exhibit good hemodynamics and durability, but are not readily available and eventually fail in the long term due to lack of living cells. Thus, there is a great need for living aortic valve replacements with extended durability [8]. Such constructs could be built by tissue engineering methods, taking into account the complex biology, biochemistry and biomechanics of the valve.
The aortic valve leaflets are exposed to a unique hemodynamic environment, characterized by area-specific shear stress, leaflet bending forces and strains in circumferential and radial directions that vary in intensity and direction during each cardiac cycle [8]. To respond to these challenges, valve leaflets have a trilayered (fibrosa, spongiosa, ventricularis) and anisotropic structure (stiffer in the circumferential axis vs. radial axis), which is essential for mechanical durability and stress distribution [9,10]. This active dynamism is maintained by cells which respond to mechanical stimuli and matrix remodeling and repair by area-specific cells as a response to micromechanical forces [8]. Cells have mechano-sensitive receptors and channels and provide paracrine mediators, extracellular matrix (ECM) proteins and ECM-degrading enzymes and their inhibitors to maintain tissue integrity [8]. ECM homeostasis in the aortic valve, maintained by valvular interstitial cells (VICs), is the essential mechanism which allows valves to withstand billions of biomechanical cycles without significant long-term damage [11].
Current tissue engineering approaches comprise implantation of acellular scaffolds derived from porcine roots or from root-shaped synthetic or natural polymers. Despite good hemodynamics, these scaffolds are invariably and undesirably infiltrated by host myofibroblasts which assume fibrotic phenotypes and lead to chronic valve stenosis [12].
Our hypothesis states that the most logical approach to heart valve regeneration is to generate acellular roots which preserve the biological “niche” of the valve, seed them with stem cells and encourage cell differentiation and maturation in a heart valve bioreactor before implantation.
To test this hypothesis, we generated decellularized aortic roots and treated the scaffolds with penta-galloyl glucose (PGG) for tissue stabilization, to reduce premature degradation, stenosis and calcification [13–15]. The cusps were then seeded internally with human adipose-tissue derived stem cells (hADSCs). We chose hADSCs because they are clinically relevant and because of their plasticity and potential ability to differentiate into valve-like cells [10,16]. We then exposed seeded valve roots to bioreactor mechanical conditioning and analyzed cells for expression of VIC-specific markers. In current study we reveal previously unreported vulnerability of hADSCs to valve-specific mechanical forces and also suggest that careful, progressive mechanical adaptation to valve-specific forces might encourage stem cell differentiation towards the VIC phenotype.
MATERIALS AND METHODS
Preparation of valve scaffolds
Porcine hearts were obtained from a local slaughter house and transported on ice to the laboratory. During harvesting of the aortic roots, we preserved about 2 cm of aorta above the sinus of Valsalva and 4 to 5 cm of endocardial tissue (thinned to 2–3 mm) and the anterior mitral leaflet. The coronary arteries were ligated using 2.0 TiCron suture and valves were immersion-decellularized with detergents and enzymes and sterilized according to published protocols [17]. To stabilize the valve ECM, the acellular roots were treated with 0.1% PGG, a collagen binding polyphenol [17]. Valves were stored in sterile PBS until use. Extent of decellularization was confirmed by histology using hematoxylin and eosin (H&E) stain and fluorescent DAPI stain for nuclei (n=4 valves). DNA was extracted and purified from tissue samples (n=4) with the DNeasy Blood & Tissue Kit (Qiagen Inc., Valencia, CA, USA) before analysis by Ethidium Bromide agarose gel electrophoresis and quantitative spectro photometry on the NanoDrop 2000c (Thermo Fisher Scientific Inc., Waltham, MA, USA). Quantities of DNA were normalized to dry tissue weight and expressed as ng/mg dry tissue.
Resistance to collagenase
To evaluate stabilization of collagen, PGG-treated acellular cusp scaffolds and non-treated acellular cusp scaffolds were lyophilized and dry samples (25–30 mg) were incubated (n=8 per group) with 10 U of collagenase (type I, Sigma-Aldrich Inc., St. Louis, MO, USA,) at 37°C with agitation. After 3 days, samples were rinsed, lyophilized, and % mass loss calculated. To assess the nature of PGG-scaffold interactions, dry samples of PGG-treated acellular cusp scaffolds (n=8 per group) were incubated at room temperature with 100% DMSO to break hydrophobic bonds and separately with 4 M guanidine-HCl to break hydrogen bonds. After 24 hours, samples were rinsed, lyophilized, subjected to collagenase as above and % mass loss calculated.
Valve hemodynamics
The previously described pulsatile heart valve bioreactor [17,18] was upgraded and adapted for valve functionality testing in pulmonary conditions set at 70.58 bpm, 25/10 mm Hg pressures and 70 mL stroke volume (HBVR2000-P, patent pending, Aptus, LLC, Central, SC, USA). In a first series of experiments, the bioreactor was validated with 19 mm diameter mechanical heart valves (St Jude Medical Inc., St. Paul, MN, USA) and bio-prosthetic (pericardial) heart valves (Edwards, Lifesciences Corp., Irvine, CA, USA). After validation, fresh aortic roots, acellular roots and PGG-treated acellular roots were mounted into metal mounting rings (Aptus, LLC) which are compatible with the rotator conditioning chambers as well as with the heart valve bio-reactor, allowing “no-touch” handling and transfer of tissues between devices. Detailed aspects of valve mounting have been published earlier [17]. Valves (n=4 per valve type) were tested at pulmonic conditions (25/10 mm Hg, 70 mL ejection fraction, 65 bpm) and videos were obtained at 240 fps using a high speed camera and software (Free Studio v. 6.4) was used to convert each video frame to a jpg file. Image J (NIH freeware) was then used for measurements of geometric orifice areas (GOA) for each frame of three cycles of open and close per valve. Since the valves were of varying diameters, GOA data was normalized to diameter for each valve and averages calculated for each point. Data sets were plotted as GOA as a function of time.
Seeding of stem cells
Human adipose-derived stem cells (hADSCs, Life Technologies Inc., Carlsbad, CA, USA) were cultured for 3–4 passages in MesenPRO RS medium to preserve stemness. Before seeding, the valves underwent a neutralization step where roots were in cubated in rotating chambers filled with 50% FBS in DMEM for 22 hours at 37°C. For seeding, hADSCs at a cell density of 8–10 million cells per mL were prepared. To facilitate seeding, we first adapted an air pump with a sterile inline filter and injected 1 mL of sterile air inside each cusp base through a 33 G×1¼ inch needle. Cell suspension was then injected into the inflated portions of each cusp (4 million cells per cusp). Seeded valves were then randomly divided into two groups: 1) pulsatile bioreactor conditioning and 2) 3D rotator conditioning.
In vitro conditioning
For pulsatile bioreactor conditioning, valves (n=3) were mounted in the sterile heart valve bioreactor systems [17] in DMEM/10% FBS with 1% antibiotic/antimycotic and pressures progressively increased from 10 mm Hg to 25 mm Hg over 14 days to reach pulmonic conditions (25/10 mm Hg) at 70 mL ejection fraction and 65 bpm. Valves were kept at these conditions for 10 more days and the study concluded after 24 total days within the bioreactor. Media was changed every 3–4 days. Video monitoring ensured that valves functioned properly throughout the study.
For rotator conditioning, we mounted valves (n=3) in sterile purpose-designed acrylic containers adapted with gas-permeable filters and set them up in a computer-controlled 3D rotator which combined horizontal with orbital movements. To achieve these movements we built and mounted two aluminum frames to an orbital shaker. The frames were connected by two plates capable of rotating vertically, each adapted with three holes which allow for securing of the valve containers in place. Rotation of the plates was ensured by a motor controlled by LabView. Speed was progressively increased from 2.5 rpm orbital horizontal rotation and 5 rpm vertical rotation over 10 days until 10 rpm horizontal×4 rpm vertical rotations (total 40 rpm) were achieved. The remaining 14 days of the study the valves remained at these conditions, and on day 24 the study was concluded. Media was changed every 3–4 days. This regimen allowed for media flow through the valve and around it, without subjecting cusps to any pressure, but inducing slight movement of the cusps. Tissues collected from each valve group were fixed in 10% formalin, embedded in paraffin, sectioned at 5 μm and stained with H&E, DAPI for nuclei and immunohistochemistry for vimentin, alpha-smooth muscle cell actin (SMA) and prolylhydroxylase (PHA) an enzyme involved in collagen synthesis, as described previously [14] using rabbit anti-human antibodies (Abcam Inc., Cambridge, MA, USA) followed by detection with the rabbit ABC system (Vector Laboratories Inc., Burlingame, CA, USA).
RESULTS
Valve properties
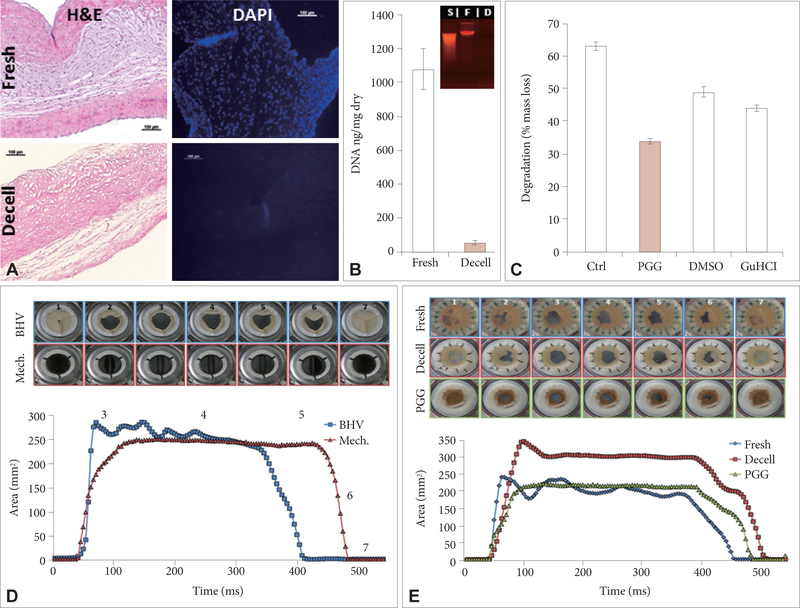
In vitro characterization of the xenografts showed that porcine valve cusps were fully decellularized as demonstrated by histology using H&E, DAPI nuclear stain and agarose gel electrophoresis (Fig. 1A and B). Quantitative measurements showed more than 95% reduction in cusp DNA content similar to values reported in the literature for fully decellularized scaffolds [19–21]. PGG-treatment increased the cusps’ resistance to colla genase digestion by about 50% (Fig. 1C). PGG-collagen interactions were partially disrupted by 100% DMSO and separately by 4 M GuHCl, indicating that PGG binds to collagen by multiple hydrogen bonds as well as hydrophobic interactions (Fig. 1C).
Figure 1.
Properties of porcine aortic root scaffolds. (A) Representative histology of fresh and decellularized cusps. Scale bars are 100 microns in all histology images. (B) DNA analysis using NanoDrop and ethidium bromide agarose gel electrophoresis (insert) of fresh and decellularized cusps. (C) Collagenase degradation of untreated acellular cusps, cusps after PGG treatment and PGG-treated acellular cusps further treated with 100% DMSO to break hydrophobic bonds and separately with 4 M GuHCl to break hydrogen bonds. (D) Hemodynamic validation of the bioreactor system by testing pericardial bioprosthetic heart valves and mechanical heart valves. GOA is shown as a function of time for a typical cycle (500 ms). (E) Hemodynamics and GOA values as a function of time for native porcine aortic roots, acellular roots and PGG-treated acellular roots. Inserts in (D) and (E) show representative top view (arterial) macro images of valves in closed positions (1 and 7), midway through opening (2) and closing (6), maximally open (3), midway through the opening phase (4) and just before starting to close (5). Decell: decellularized, H&E: hematoxylin and eosin, DAPI: 4’,6-diamino-2-phenylindole, S: standard, F: fresh, D: decellularized, Ctrl: controls, PGG: penta-galloyl glucose, DMSO: dimethylsufoxide, GuHCl: guanidine HCl, BHV: bioprosthetic heart valve, Mech: mechanical valve, GOA: geometric orifice area.
To evaluate valve functionality, we first validated the bioreactor system with pericardial bioprosthetic valves and mechanical heart valves tested in pulmonary conditions of flow and pressures (Fig. 1D). Video analysis showed that both types of valves functioned well without regurgitation and their function did not change with time over multiple cycles. Maximum GOA reached about 250 mm2 for the 19 mm diameter valves, which is almost 90% of calculated maximal GOA. The almost symmetrical shape of the open/close curves for the artificial valves exemplified their adequate functionality. Moreover, slight fluttering in the tissue valve was observed during the open segment, as one cusp appeared to be a bit ‘lazy’, lagging behind the other two (Fig. 1D, between areas 3 and 4). Next we pursued functional analysis of native, acellular and PGG-treated porcine acellular roots (Fig. 1E). All valves opened maximally reaching 200–300 mm2 GOA for the 19–21 mm diameter roots. They opened very quickly reaching maximum GOA after 40–50 milliseconds and remained open for about 300–350 milliseconds after which they closed within 50–100 milliseconds, without regurgitation. The only noticeable differences between the three valve groups were fluttering of the cusps in the native roots, and slight delay in closure of the PGG-treated cusps (Fig. 1E, between areas 5 and 7).
In vitro tests
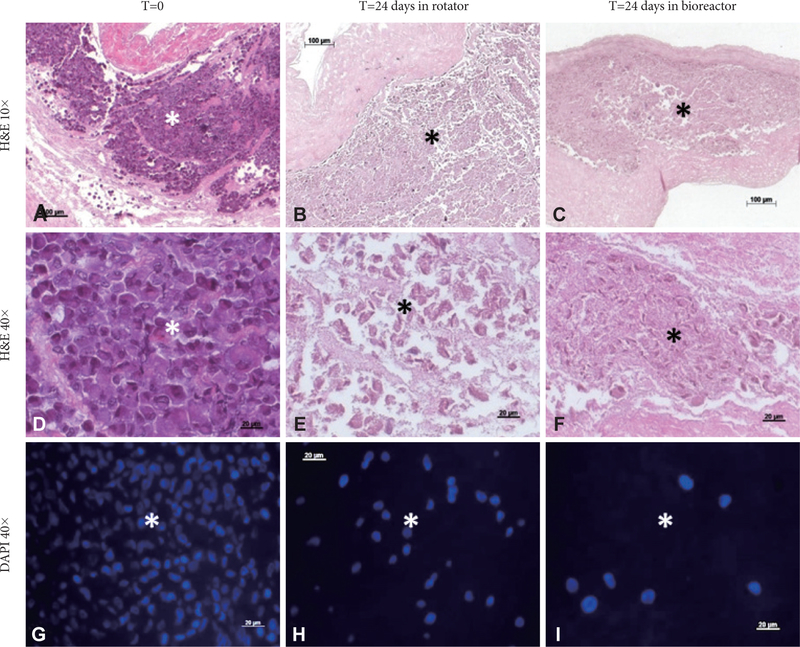
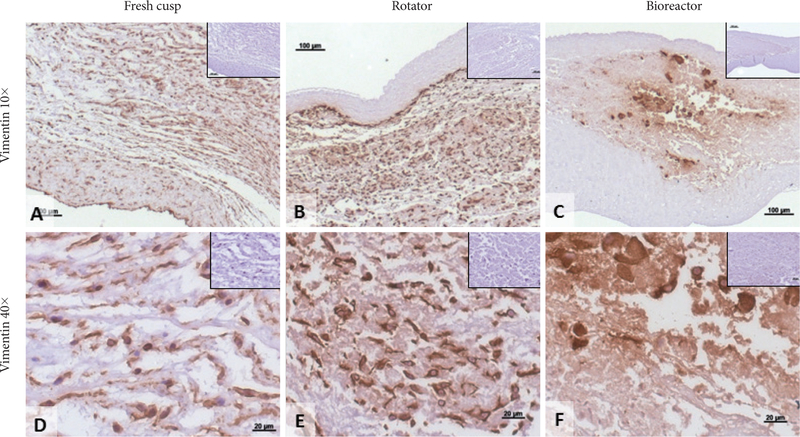
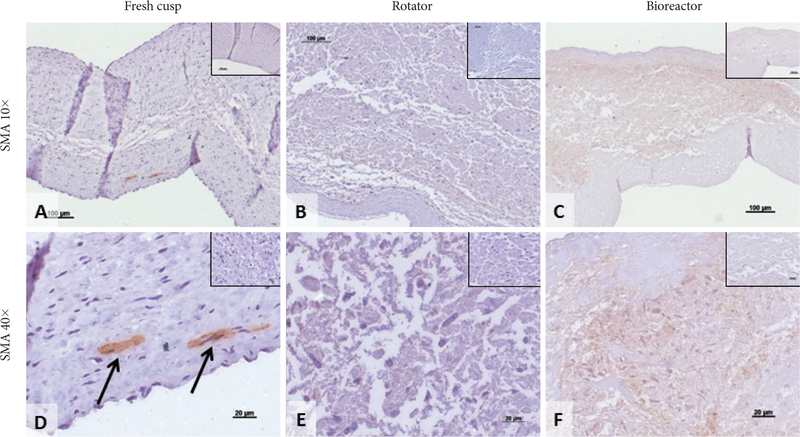
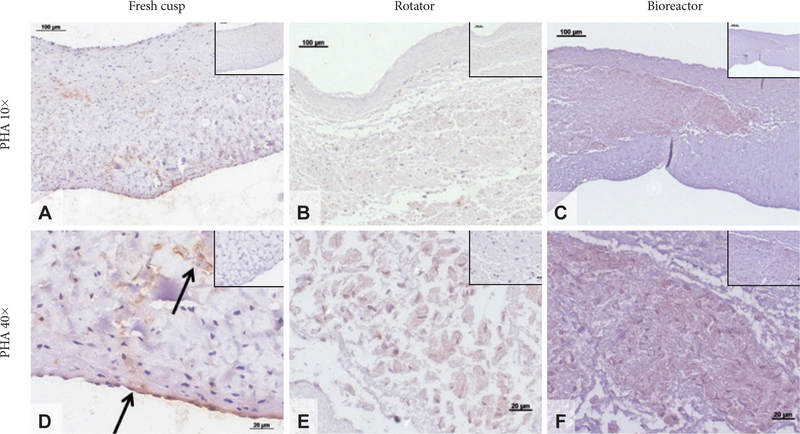
To evaluate the effects of mechanical conditioning on stem cells, we seeded PGG-treated acellular valves with hADSCs and mounted them inside pulsatile heart valve bioreactors (to mimic implantation) and subjected them to physiologic pulmonary flow and pressure conditions for up to 24 days. Controls consisted of hADSC-seeded valves maintained in a rotator system which allowed fluid to flow through the valve and around it, thus ensuring gas and nutrient exchange without subjecting the cusps to significant stresses. Valves mounted in the bioreactor functioned well by opening and closing (Fig. 2). Valves mounted in the rotator could be seen to open and close slowly as a result of media flow through the valves (not shown). Histology using DAPI nuclear stain, H&E and immunohistochemistry for vimentin showed that the majority of cells (>95%) subjected to the pulsatile bioreactor conditions were destroyed or devitalized, leaving behind only cell debris (Fig. 3). Cusps subjected to the rotator system lost about 50% of cells when compared to t=0 cell seeded cusps, but also contained 4–5 times more living cells when compared to the bioreactor-conditioned cells. Most of surviving cells in the rotator samples expressed vimentin (Fig. 4) but were not positive to SMA or PHA (Figs. 5 and 6).
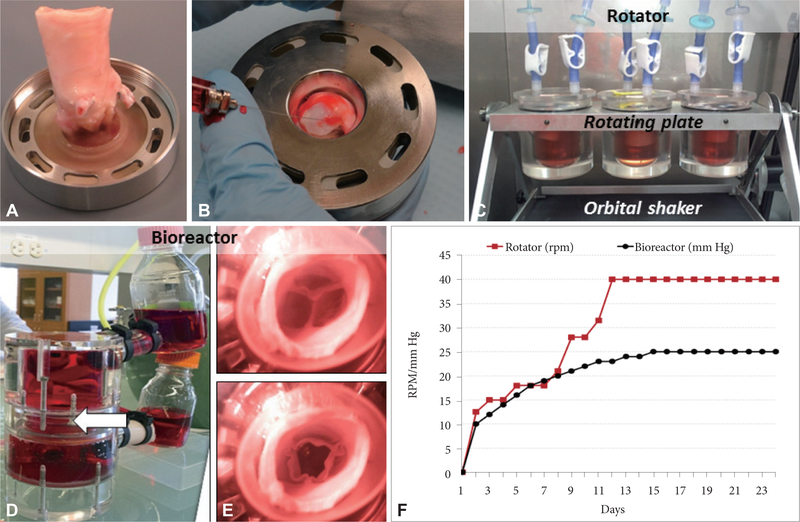
Figure 2.
Aortic valve scaffold seeding and conditioning. (A) Representative aortic root mounted in the universal valve mount. (B) Injection of hADSCs into each cusp through the bottom part of the mount (valve ventricularis layer). (C) Three seeded roots are shown in culture media (red) in individual jars, adapted with sterile air filters, and mounted in the rotator within a cell culture incubator. The plates holding the jars rotate in a vertical plane and the frame to which the plate is attached is mounted onto a horizontal orbital shaker. (D) Overview of the heart valve bioreactor filled with culture media. The valve is mounted in the middle section (arrow). (E) Representative top images of hADSC-seeded valves in the bioreactor confirming adequate closing and opening. (F) Progressive conditioning regimens used for rotated valves (red) which combined vertical and horizontal rotations (expressed as rotations per minute, RPM) and for bioreactor conditioning in pulmonary conditions (black) expressed as mm Hg. hADSCs: human adipose-tissue derived stem cells.
Figure 3.
Cell retention in seeded cusps. (A, D, and G) Representative images of cusps immediately after seeding with hADSCs (T=0). (B, E, and H), 24 days after rotator conditioning and (C, F, and I), 24 days after bioreactor conditioning. Samples were stained with hematoxylin and eosin (H&E) and DAPI (nuclei, blue). Scale bars are 100 microns in the 10× images and 20 microns in the 40× images.*injected cell islands. hADSCs: human adipose-tissue derived stem cells, DAPI: 4’,6-Diamino-2-phenylindole.
Figure 4.
Cell phenotypes in seeded cusps. Representative sections showing immunohistochemical (IHC) staining for vimentin (positive, brown) in fresh native cusps (A and D), hADSC-seeded cusps after 24 days in the rotator system (B and E) and hADSC-seeded cusps after 24 days in the bioreactor (C and F). Scale bars are 100 microns in the 10× images and 20 microns in the 40× images. Inserts represent corresponding IHC negative controls. hADSCs: human adipose-tissue derived stem cells.
Figure 5.
Smooth muscle cell actin staining in seeded cusps. Representative sections showing immunohistochemical (IHC) staining for alpha-smooth muscle actin (SMA, positive, brown) in fresh native cusps (A and D), hADSC-seeded cusps after 24 days in the rotator system (B and E) and hADSC-seeded cusps after 24 days in the bioreactor (C and F). Scale bars are 100 microns in the 10× images and 20 microns in the 40× images. Inserts represent corresponding IHC negative controls. Arrows in (D) point to SMA-positive cells in fresh cusps. hADSC: human adipose-tissue derived stem cells.
Figure 6.
Prolyl-hydroxylase (PHA) staining in seeded cusps. Representative sections showing immunohistochemical (IHC) staining for PHA (positive, brown) in fresh native cusps (A and D), hADSC-seeded cusps after 24 days in the rotator system (B and E) and hADSC-seeded cusps after 24 days in the bioreactor (C and F). Scale bars are 100 microns in the 10× images and 20 microns in the 40× images. Inserts represent corresponding IHC negative controls. Arrows in (D) point to areas rich in PHA expression. hADSCs: human adipose-tissue derived stem cells.
DISCUSSION
To test our approach, we completely decellularized porcine aortic valves using and treated them with PGG. This resulted in functional aortic roots with preserved matrix and hemodynamic properties. In previous studies we showed that treatment with PGG, a non-cytotoxic collagen and elastin binding polyphenol reduced susceptibility to collagenase, thus increasing the durability of matrix scaffolds upon implantation [10,15,22,23]. In current study we also showed that PGG interacts with collagen via multiple hydrogen bonds and hydrophobic interactions; this may explain its extended durability in vivo [10,17,22]. In an attempt to re-cellularize the aortic roots, we seeded hADSCs into each of the three cusps by injection. To our knowledge this is among the first attempts to revitalize the cusp interstitium with stem cells. The injection created a single bolus of cells; we hypothesized that these cells would diffuse out and spread throughout the porous cusp interstitium and possibly differentiate into VICs as a response to physiological mechanical stimuli. We then subjected a group of seeded valves to pulmonary conditions of flow, pressures and mechanical stress; a second group of seeded valves were subjected to a rotational regime which served as low stress controls.
Afer 24 days in the bioreactor, most cells had died and large islands of cell debris were found within each cusp. Controls, which were only rotated slowly to ensure gas and nutrient exchange, maintained about 50% of cells alive. These results suggested that immediate exposure of hADSC-seeded valves to pulmonary conditions of pressure and mechanical stress leads to cell demise. This is, to our knowledge the first report on the vulnerability of adult stem cells to mechanical stresses typical of heart valves. Colazzo et al. [16,24] have exposed hADSC mono-layers to 10% equibiaxial stretch in FlexerCell systems to study their synthetic properties and those particular conditions did not damage the cells. Comparison of these results raise the question whether the bending, shearing, compression and recoil motions typical of valve cusp tissues [25–28] were responsible for the in vitro cell death in our study.
Notably, cells seeded within valves and exposed to the rotation conditions exhibited improved survival and were positive for vimentin, and negative for SMA and PHA. These markers could be taken as indicative of in vitro stem cell differentiation into quiescent VICs [8].
In conclusion, PGG-stabilized acellular aortic roots are adequate valve scaffolds for tissue engineering; however hADSC-seeded valves could not support viability of interstitial cells when exposed to pulmonary conditions. These results highlight for the first time the extreme vulnerability of hADSCs to valve-specific mechanical forces and also suggest that careful, progressive mechanical adaptation to valve-specific forces might encourage stem cell differentiation towards the VIC phenotype in vitro.
Acknowledgements
This project was funded in part by NHLBI of the National Institutes of Health under award number RO1HL093399, by NIGMS of the National Institutes of Health under award number 5P20GM103444-07, by the Harriet and Jerry Dempsey Associate Professorship in Bioengineering Award and by a grant from the Romanian National Authority for Scientific Research, CNCS-UEFISCDI, project number PNII-ID-PCCE- 2011-2-0036.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
Ethical Statement
There are no animal experiments carried out for this article.
REFERENCES
- 1.Wirrig EE, Yutzey KE. Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler Thromb Vasc Biol 2014;34:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathieu P, Boulanger MC. Basic mechanisms of calcific aortic valve disease. Can J Cardiol 2014;30:982–993. [DOI] [PubMed] [Google Scholar]
- 3.Weiss RM, Miller JD, Heistad DD. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res 2013;113:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towler DA. Molecular and cellular aspects of calcific aortic valve disease. Circ Res 2013;113:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akerström F, Barderas MG, Rodríguez-Padial L. Aortic stenosis: a general overview of clinical, pathophysiological and therapeutic aspects. Expert Rev Cardiovasc Ther 2013;11:239–250. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos A, Kaoukis A, Papadaki H, Pyrgakis V. Pathophysiologic mechanisms of calcific aortic stenosis. Ther Adv Cardiovasc Dis 2012;6:71–80. [DOI] [PubMed] [Google Scholar]
- 7.Ladich E, Nakano M, Carter-Monroe N, Virmani R. Pathology of calcific aortic stenosis. Future Cardiol 2011;7:629–642. [DOI] [PubMed] [Google Scholar]
- 8.Chester AH, El-Hamamsy I, Butcher JT, Latif N, Bertazzo S, Yacoub MH. The living aortic valve: from molecules to function. Glob Cardiol Sci Pract 2014;2014:52–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simionescu DT, Chen J, Jaeggli M, Wang B, Liao J. Form follows function: advances in trilayered structure replication for aortic heart valve tissue engineering. J Healthc Eng 2012;3:179–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedder ME, Simionescu A, Chen J, Liao J, Simionescu DT. Assembly and testing of stem cell-seeded layered collagen constructs for heart valve tissue engineering. Tissue Eng Part A 2011;17:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoen FJ. Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol 2011;22:698–705. [DOI] [PubMed] [Google Scholar]
- 12.Voges I, Bräsen JH, Entenmann A, Scheid M, Scheewe J, Fischer G, et al. Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur J Cardiothorac Surg 2013;44:e272–e279. [DOI] [PubMed] [Google Scholar]
- 13.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 2007;115: 1729–1737. [DOI] [PubMed] [Google Scholar]
- 14.Chow JP, Simionescu DT, Warner H, Wang B, Patnaik SS, Liao J, et al. Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol. Biomaterials 2013;34: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennel T, Fercana G, Bezuidenhout D, Simionescu A, Chuang TH, Zilla P, et al. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. Biomaterials 2014;35:6311–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colazzo F, Alrashed F, Saratchandra P, Carubelli I, Chester AH, Yacoub MH, et al. Shear stress and VEGF enhance endothelial differentiation of human adipose-derived stem cells. Growth Factors 2014;32:139–149. [DOI] [PubMed] [Google Scholar]
- 17.Sierad LN, Simionescu A, Albers C, Chen J, Maivelett J, Tedder ME, et al. Design and testing of a pulsatile conditioning system for dynamic endothelialization of polyphenol-stabilized tissue engineered heart valves. Cardiovasc Eng Technol 2010;1:138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sierad LN, Shaw EL, Launius R, McBride S, Storholt C, Poole R, et al. Toward an endothelial-cell covered mechanical valve; surface re-engineering and bioreactor testing of mechanical heart valves. Chall Regen Med 2014;1:22–34. [Google Scholar]
- 19.Londono R, Badylak SF. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann Biomed Eng 2015;43:577–592. [DOI] [PubMed] [Google Scholar]
- 20.Keane TJ, Badylak SF. The host response to allogeneic and xenogeneic biological scaffold materials. J Tissue Eng Regen Med 2015;9:504–511. [DOI] [PubMed] [Google Scholar]
- 21.Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaf-fold for regenerative medicine: factors that influence the host response. Ann Biomed Eng 2014;42:1517–1527. [DOI] [PubMed] [Google Scholar]
- 22.Tedder ME, Liao J, Weed B, Stabler C, Zhang H, Simionescu A, et al. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A 2009;15:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang TH, Stabler C, Simionescu A, Simionescu DT. Polyphenol-stabilized tubular elastin scaffolds for tissue engineered vascular grafts. Tissue Eng Part A 2009;15:2837–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colazzo F, Chester AH, Taylor PM, Yacoub MH. Induction of mesenchymal to endothelial transformation of adipose-derived stem cells. J Heart Valve Dis 2010;19:736–744. [PubMed] [Google Scholar]
- 25.Schoen FJ, Hobson CE. Anatomic analysis of removed prosthetic heart valves: causes of failure of 33 mechanical valves and 58 bioprostheses, 1980 to 1983. Hum Pathol 1985;16:549–559. [DOI] [PubMed] [Google Scholar]
- 26.Schoen FJ. Pathologic findings in explanted clinical bioprosthetic valves fabricated from photooxidized bovine pericardium. J Heart Valve Dis 1998;7:174–179. [PubMed] [Google Scholar]
- 27.Rajamannan NM. The role of Lrp5/6 in cardiac valve disease: LDL-density-pressure theory. J Cell Biochem 2011;112:2222–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajamannan NM. Bicuspid aortic valve disease: the role of oxidative stress in Lrp5 bone formation. Cardiovasc Pathol 2011;20:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]