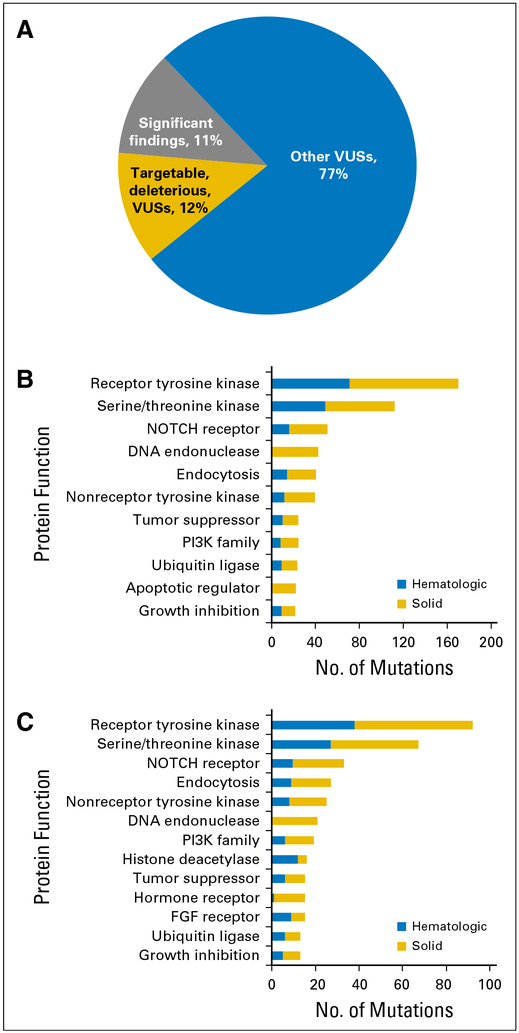
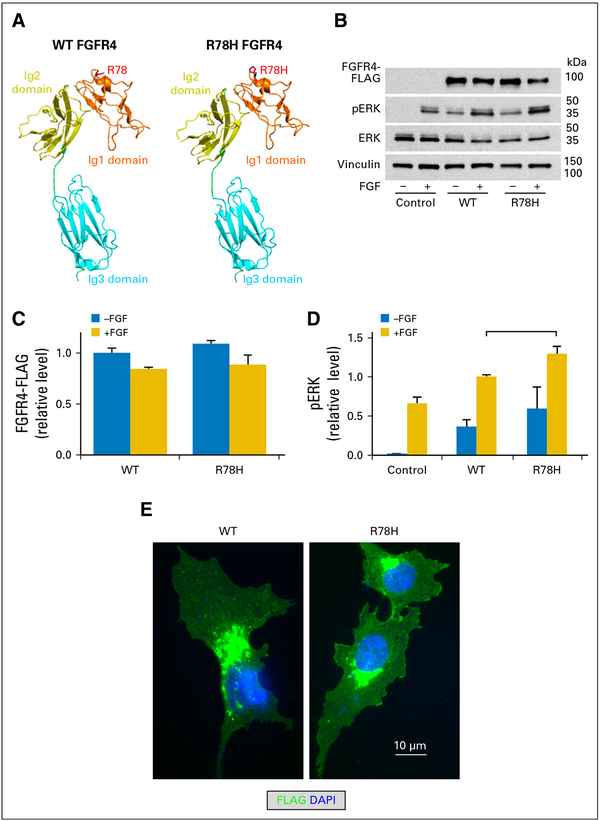
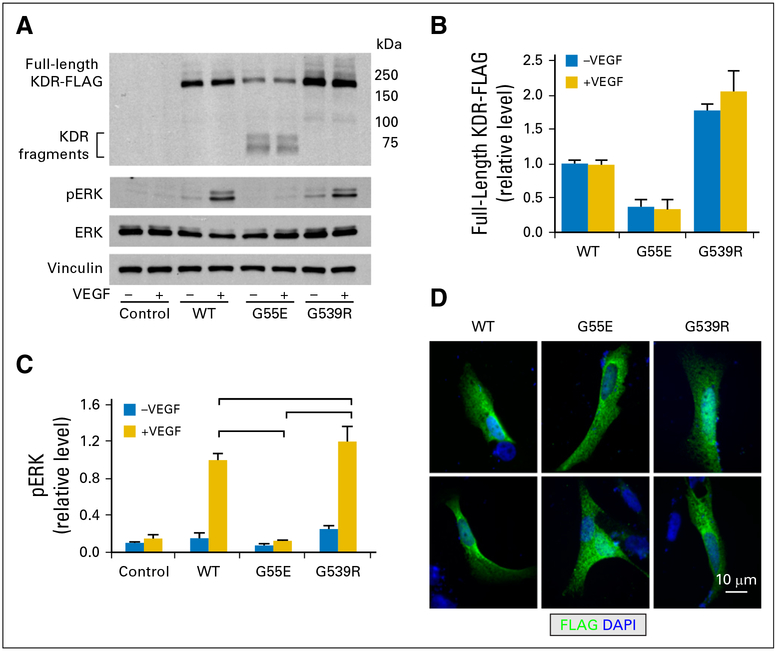
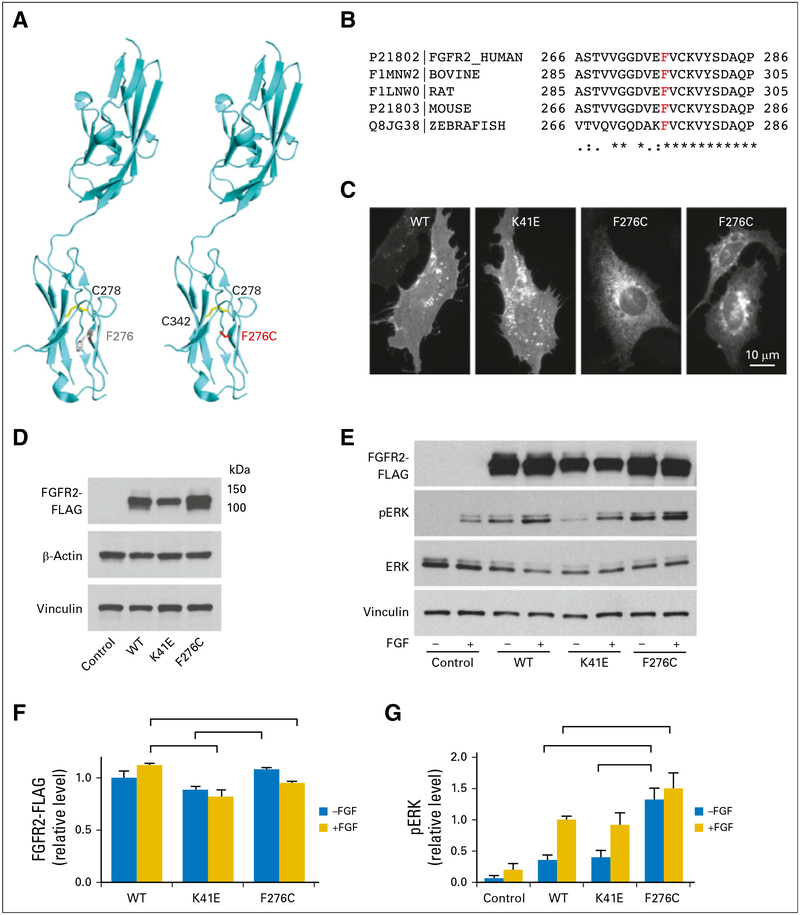
Jan B Egan
Jan B Egan
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
David L Marks
David L Marks
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Tara L Hogenson
Tara L Hogenson
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Anne M Vrabel
Anne M Vrabel
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Ashley N Sigafoos
Ashley N Sigafoos
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Ezequiel J Tolosa
Ezequiel J Tolosa
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Ryan M Carr
Ryan M Carr
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Stephanie L Safgren
Stephanie L Safgren
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Elisa Enriquez Hesles
Elisa Enriquez Hesles
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Luciana L Almada
Luciana L Almada
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Paola A Romecin-Duran
Paola A Romecin-Duran
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Eriko Iguchi
Eriko Iguchi
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Aryan Ala’Aldeen
Aryan Ala’Aldeen
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Jean-Pierre A Kocher
Jean-Pierre A Kocher
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Gavin R Oliver
Gavin R Oliver
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Naresh Prodduturi
Naresh Prodduturi
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
David W Mead
David W Mead
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Asif Hossain
Asif Hossain
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Norine E Huneke
Norine E Huneke
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Colleen M Tagtow
Colleen M Tagtow
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Sikander Ailawadhi
Sikander Ailawadhi
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Stephen M Ansell
Stephen M Ansell
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Michaela S Banck
Michaela S Banck
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Alan H Bryce
Alan H Bryce
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Estrella M Carballido
Estrella M Carballido
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Asher A Chanan-Khan
Asher A Chanan-Khan
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Kelly K Curtis
Kelly K Curtis
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Ernesto Resnik
Ernesto Resnik
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Chelsea D Gawryletz
Chelsea D Gawryletz
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Ronald S Go
Ronald S Go
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Thorvardur R Halfdanarson
Thorvardur R Halfdanarson
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Thai H Ho
Thai H Ho
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Richard W Joseph
Richard W Joseph
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Prashant Kapoor
Prashant Kapoor
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Aaron S Mansfield
Aaron S Mansfield
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Nathalie Meurice
Nathalie Meurice
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Amulya A Nageswara Rao
Amulya A Nageswara Rao
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Grzegorz S Nowakowski
Grzegorz S Nowakowski
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Animesh Pardanani
Animesh Pardanani
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Sameer A Parikh
Sameer A Parikh
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
John C Cheville
John C Cheville
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Andrew L Feldman
Andrew L Feldman
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Ramesh K Ramanathan
Ramesh K Ramanathan
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Steven I Robinson
Steven I Robinson
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Raoul Tibes
Raoul Tibes
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Heidi D Finnes
Heidi D Finnes
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Jennifer B McCormick
Jennifer B McCormick
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Robert R McWilliams
Robert R McWilliams
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Aminah Jatoi
Aminah Jatoi
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Mrinal M Patnaik
Mrinal M Patnaik
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Alvin C Silva
Alvin C Silva
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Eric D Wieben
Eric D Wieben
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Tammy M McAllister
Tammy M McAllister
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Kandelaria M Rumilla
Kandelaria M Rumilla
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Sarah E Kerr
Sarah E Kerr
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Konstantinos N Lazaridis
Konstantinos N Lazaridis
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Gianrico Farrugia
Gianrico Farrugia
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
A Keith Stewart
A Keith Stewart
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Karl J Clark
Karl J Clark
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Eileen J Kennedy
Eileen J Kennedy
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Eric W Klee
Eric W Klee
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Mitesh J Borad
Mitesh J Borad
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1,
Martin E Fernandez-Zapico
Martin E Fernandez-Zapico
1David L. Marks, Tara L. Hogenson, Anne M. Vrabel, Ashley N. Sigafoos, Ezequiel J. Tolosa, Ryan M. Carr, Stephanie L. Safgren, Elisa Enriquez Hesles, Luciana L. Almada, Paola A. Romecin-Duran, Eriko Iguchi, Aryan Ala’Aldeen, Jean-Pierre A. Kocher, Gavin R. Oliver, Naresh Prodduturi, David W. Mead, Asif Hossain, Norine E. Huneke, Colleen M. Tagtow, Sikander Ailawadhi, Stephen M. Ansell, Michaela S. Banck, Asher A. Chanan-Khan, Ronald S. Go, Thorvardur R. Halfdanarson, Richard W. Joseph, Prashant Kapoor, Aaron S. Mansfield, Amulya A. Nageswara Rao, Grzegorz S. Nowakowski, Animesh Pardanani, Sameer A. Parikh, John C. Cheville, Andrew L. Feldman, Ramesh K. Ramanathan, Steven I. Robinson, Heidi D. Finnes, Jennifer B. McCormick, Robert R. McWilliams, Aminah Jatoi, Mrinal M. Patnaik, Eric D. Wieben, Tammy M. McAllister, Kandelaria M. Rumilla, Sarah E. Kerr, Konstantinos N. Lazaridis, Gianrico Farrugia, Karl J. Clark, Eric W. Klee, and Martin E. Fernandez-Zapico, Mayo Clinic, Rochester; Ernesto Resnik, Bio-Techne, Minneapolis, MN; Sikander Ailawadhi, Asher A. Chanan-Khan, and Richard W. Joseph, Mayo Clinic, Jacksonville, FL; Jan B. Egan, Alan H. Bryce, Estrella M. Carballido, KellyK. Curtis, Chelsea D. Gawryletz, Thai H. Ho,Nathalie Meurice, Ramesh K. Ramanathan, Raoul Tibes, Alvin C. Silva, A. Keith Stewart, and Mitesh J. Borad, Mayo Clinic, Phoenix, AZ; and Eileen J. Kennedy, University of Georgia, Athens, GA.
1