Abstract
Although weight loss improves blood pressure (BP), its association with mortality remains unclear. In the Trials of Hypertension Prevention (TOHP) individuals aged 30-54 years with high normal BP were randomized to weight loss, usual care or other intervention over 18 months (TOHP I) or 3-4 years (TOHP II), with average 23-year mortality follow-up. We examined mortality and 1) randomized weight loss and 2) observed weight change among all with high baseline weight. Among 2,964 randomized participants, 227 deaths occurred, with no intervention difference (hazard ratio (HR)=0.97, 95% confidence interval (CI)=0.75-1.26, p=0.84). Among 3,828 high-weight participants, weight change was directly related to mortality (HR=1.14 per 5% change, 95% CI=1.02-1.28, p=0.019). During the trial 15% lost > 5% (HR=0.82), 29% lost 0-<=5% (HR=0.94), 41% gained 0-< 5% (reference), and 16% gained > 5% (HR=1.29) (p-trend = 0.046). This is consistent with a long-term beneficial effect of presumed intentional weight loss on mortality.
Keywords: Weight loss, mortality, blood pressure, trials
1. INTRODUCTION
Observational studies have consistently confirmed a direct association of weight and weight gain with blood pressure even within the normal weight range,1 and randomized trials have confirmed the effects of weight loss on improving glycemic control2 and reducing blood pressure in normotensive adults.3–7 However, long-term beneficial effects of intentional weight loss on hard clinical outcomes, including total mortality, remain unproven. While there are strong theoretical grounds to expect favorable effects of weight loss, the Look AHEAD trial found no difference in cardiovascular disease incidence by weight loss intervention among adults with type 2 diabetes. In addition, some observational studies have suggested an increased mortality risk among those at both higher and lower weight levels, with the lowest rates among those with BMI between 25 and 30 kg/m2,8,9 or with BMI between 20 and 25 kg/m2,10,11 though the latter was attenuated after longer follow-up and among never smokers. Controversy remains regarding reverse causation, particularly illness-related weight loss, and adequacy of attempts to control for confounding in these studies of weight at a single point in time.12,13
In this context, studies of weight change can be particularly informative. While weight loss in observational studies may reflect underlying change in health status, studies primarily involving intentional weight loss may be less prone to such potential bias. The objectives of this paper are to assess the effect of randomized weight loss interventions during the Trials of Hypertension Prevention (TOHP) on subsequent mortality during long-term follow-up and to assess the relation of observed changes in weight, presumably intentional, with subsequent long-term mortality among all participants at higher baseline weight levels in TOHP.
2. METHODS
2.1. TOHP Trials
The TOHP Follow-up Study is an observational follow-up of Phases I and II of TOHP, and has been described previously.14,15 Phase I of TOHP (TOHP I) took place from 1987-1990, and evaluated effects on blood pressure over 18 months of four supplement and three lifestyle interventions, including weight loss and sodium reduction.6 Participants included 2,182 men and women aged 30-54 years with diastolic blood pressure between 80-89 mm Hg. Eligible persons for the weight loss intervention or its control group were in the high-weight stratum, defined as body mass index (BMI) of 26.1 to 36.1 kg/m2 for men and 24.3 to 36.1kg/m2 for women, representing approximately 115% to 165% of desirable body weight.16 A total of 308 participants were randomized to the active weight loss group, with 256 in their usual care comparison group (eFigure 1A). The net weight change in the weight loss intervention group was −8.6 lbs (−3.9 kg) at 18 months.6
In Phase II of TOHP (TOHP II), which took place from 1990-1995, a 2×2 factorial design was used to assess effects of sodium reduction and weight loss on blood pressure in 2,382 men and women aged 30-54 years with diastolic blood pressure of 83-89 who were followed for 3-4 years.7 Eligible participant had a BMI in the range 26.1 to 37.4 kg/m2 for men and 24.4 to 37.4 kg/m2 for women. A total of 1,192 participants were randomized to the active weight loss intervention, and 1,190 to its control group (eFigure 1B). The net weight change in the weight loss intervention group was −4.4 lbs (−2.0 kg) at 36 months.
2.2. Definition of Weight Change
Analyses of the randomized weight loss intervention compared all those randomized to weight or its usual care comparison group in both phases. In each study, weight was measured to the nearest 0.2 kg (0.5 lb) using a calibrated balance-beam scale; participants wore indoor clothing without shoes. Observational analyses of weight change included all those in a high-weight stratum, defined as BMI of at least 26 kg/m2 for men and 24 kg/m2 for women, which comprised 1,481 individuals in TOHP I and all 2382 individuals in TOHP II.
Weight change during the trial periods was defined in terms of percent change in weight from baseline (randomization) to the end of the trial period at either 18 months (TOHP I) or 3-4 years (TOHP II). Change was treated as both a continuous variable and in categories defined as weight loss >5%, loss <=5% (lost 0-5%), gain <5% (gained 0-5%), and gain >5%. Those gaining <5% served as the reference group.
For descriptive purposes, self-reported weight was assessed by questionnaire in follow-ups conducted between 2001 and 2004. Longer-term change in weight was defined as the change from trial entry to the first self-reported weight, occurring a median of 11.8 years from baseline (25th, 75th percentiles = 9.6, 13.1 years).
2.3. Mortality Follow-up
We previously conducted an active mail-based follow-up of TOHP participants for cardiovascular disease endpoints through early 2005, including a search of the National Death Index (NDI) for deaths through December 2003.14,15 We conducted an additional search of the NDI to ascertain deaths through December 2013. In the analysis of the randomized weight loss intervention, all-cause mortality from the time of randomization was included, with up to 26 years of follow-up in TOHP I (median = 25.7; 25th, 75th percentiles = 25.5, 26.0) and up to 23 years in TOHP II (median = 22.3; 25th, 75th percentiles = 22.0, 22.6). The median follow-up for both phases was 22.5 years (25th, 75th percentiles = 22.0, 22.8). Observational analyses of weight change during the trial periods examined mortality following the trials, representing a median of 23.9 years for TOHP I (25th, 75th percentiles = 23.9, 23.9) and 18.8 years for TOHP II (25th, 75th percentiles = 18.8, 18.8), with an overall median of 18.8 years (25th, 75th percentiles = 18.8, 23.9). Observational analyses of weight change excluded 35 individuals who died or experienced a cardiovascular event during the trial periods (eFigure 2).
2.4. Statistical Analysis
We first compared individuals randomized to the weight loss intervention vs. usual care groups in TOHP I and II. Baseline trial characteristics by randomized intervention have previously been reported.6,7,14 Analysis of the effects on total mortality was based on the intention-to-treat principle. The hazard ratio (HR) was estimated using Cox regression analysis stratified by trial phase and adjusting for clinic, age, race, and sex, and randomization to the sodium reduction intervention in TOHP II. Cumulative incidence curves, adjusted for the same factors, were estimated and plotted for each trial. We also estimated the cumulative HRs over time in five-year follow-up periods.
In the observational analysis of usual intake, weight change from randomization to the end to the trial periods was determined as described above. Baseline characteristics by gender were expressed as percentages or means, and were tested for trend over weight change categories using chi-square statistics or regression analysis. We also examined self-reported long-term weight change by category of short-term weight loss during the trial.
Cox regression analysis was used to estimate the association of mortality with weight change in a continuous fashion as well as after grouping into the previously described categories. Continuous weight change was winsorized at 3 standard deviations to reduce the influence of extreme values in linear models. Models were adjusted for clinic, age, sex, race/ethnicity, baseline weight and other treatment assignments (model 1), and additionally for education, alcohol use and amount, smoking, exercise, family history of cardiovascular disease, and sodium and potassium excretion and their interaction with the sodium intervention (model 2). Interactions of weight change with time, phase, gender, age, smoking, and the weight loss intervention were examined. Penalized splines with four degrees of freedom were used to examine linearity of effect.
In secondary analyses similar models were fit for baseline weight and BMI among those who were not in a weight loss intervention. All analyses were conducted in SAS 9.2 except for the cumulative incidence plots and spline analyses which were conducted in R using the coxph function.
3. RESULTS
3.1. Randomized analysis of weight loss intervention
Detailed comparisons of baseline characteristics by randomized interventions in TOHP I and II are provided in eTables 1 (by phase) and 2 (by phase and gender) in the Appendix. Average age was 42.8 and 43.6, with 32% and 34% female in TOHP I and II, respectively. The average weight at randomization was 209 lbs (95 kg) in men and 174 lbs (79 kg) in women in TOHP I and 218 lbs (99 kg) in men and 184 lbs (83 kg) in women in TOHP II. There were net weight reductions in the weight loss group during the TOHP I (−8.6 lbs [−3.9 kg]) and TOHP II (−5.1 lbs [−2.3 kg]) intervention periods, but substantial variation in weight change within randomized groups. In TOHP I the median (25th to 75th percentiles) weight change was −7 (−16 to 0) lbs in the weight loss group and 0 (−4 to 5) lbs in the usual care group. In TOHP II these were 0 (−7 to 7) lbs in the active group and 4 (−2 to 10) lbs in the usual care group.
Self-reported weight was provided in 2001-2004 by 1,971 participants without intervening cardiovascular disease (eTable 3), with recidivism over time. Those in the active intervention group gained a net 5.6 lbs (2.5 kg) over an average 11.6 years in TOHP I and 2.4 lbs (1.1 kg) over an average 6.3 years in TOHP II following the trial periods. There remained a net lower weight loss of 3.4 lbs (1.5 kg; p=0.12) with the weight loss intervention in TOHP I and 2.8 lbs (1.3 kg; p=0.005) in TOHP II during long-term follow-up.
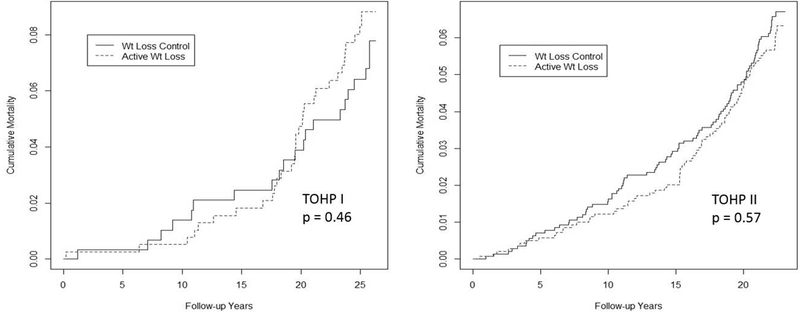
Over up to 26 years of follow-up, 227 deaths occurred, with no apparent differences by randomized intervention (Table 1). There was some indication of divergence over time (Figure 1), but no significant interaction with time either by phase or overall. In analyses of cumulative incidence by follow-up time, there was a suggestive 31% reduction in mortality at 15 years (HR=0.69, 95% CI=0.44-1.06, p=0.093), but this did not persist in later follow-up (eFigure 2).
Table 1.
Total mortality from randomization in the Trials of Hypertension Prevention through 2013 by weight loss intervention group.
| Number of Deaths/ Total (%) | |||||
|---|---|---|---|---|---|
| Phase | Weight Loss | Usual Care | HR* | 95% CI | p |
| TOHP I alone | 33/308 (10.7) | 20/256 (7.8) | 1.24 | 0.70-2.17 | 0.46 |
| TOHP II alone | 83/1192 (7.0) | 91/1190 (7.6) | 0.92 | 0.68-1.23 | 0.57 |
| Combined | 116/1500 (7.7) | 111/1446 (7.7) | 0.97 | 0.75-1.26 | 0.84 |
From Cox regression analysis stratified by trial and adjusted for clinic, age, race, sex, and sodium reduction intervention. HR, hazard ratio; CI, confidence interval.
Figure 1.
Cumulative mortality curves for TOHP I (left), and TOHP II (right) by randomized weight loss intervention group from Cox regression analysis stratified by trial and adjusted for clinic, age, race, sex, and sodium reduction intervention
3.2. Observational analysis of weight change
There were 1477 participants meeting the TOHP definition of high weight in TOHP I and 2351 in TOHP II, of whom 21% and 50%, respectively, were assigned to a weight loss intervention. This included 2,539 men and 1,289 women with a mean weight change during the trial of −1.8 lbs (−0.8% of baseline body weight) over 1.5 years in TOHP I and 1.6 lbs (0.8%) over 3-4 years in TOHP II. Of these, 556 (15%) lost >5%, 1,101 (29%) lost 0-<=5%, 1,567 (41%) gained 0-5%, and 604 (16%) gained >5% in body weight. The average difference in weight change between the highest and lowest weight loss group was 37.3 lbs (16.9) in men and 34.2 lbs (15.5 kg) in women. While all in TOHP II were assigned to a weight loss intervention or usual care, some in TOHP I were assigned to other interventions, other usual care control groups, or placebo. Their baseline characteristics were similar to those in the randomized usual care group (eTable 1).
There were several baseline differences by weight loss group (Table 2). Those who lost >5% were more likely to be in an active weight loss intervention and in Phase I of TOHP. They were slightly older, less likely to be black, and consumed less sodium and more potassium. There were small differences in baseline weight among men only. Differences were somewhat more pronounced in TOHP II (eTable 4).
Table 2.
Baseline characteristics among participants with extended follow-up in the Trials of Hypertension Prevention by categories of % weight change during the intervention period.
| % Weight Change | P for Trend | ||||
|---|---|---|---|---|---|
| Lost > 5% | Lost 0-<= 5% | Gained 0-<5% | Gained 5%+ | ||
| MEN | |||||
| N | 372 | 788 | 1050 | 329 | |
| Change in weight during trial (lbs) | −20.4 | −4.4 | 4.6 | 16.9 | - |
| Baseline weight (lbs) | 213.9 | 212.0 | 214.4 | 216.7 | 0.042 |
| Active weight loss intervention (%) | 64.5 | 43.5 | 27.7 | 33.7 | <0.0001 |
| Active Na reduction intervention (%) | 31.4 | 34.5 | 36.5 | 39.8 | 0.014 |
| TOHP II (%) | 53.5 | 56.4 | 61.0 | 79.0 | <0.0001 |
| Age (yrs) | 43.4 | 43.8 | 43.1 | 41.6 | <0.0001 |
| Black (%) | 7.5 | 9.6 | 12.3 | 14.3 | 0.0007 |
| College (%) | 68.8 | 64.6 | 67.3 | 66.3 | 0.91 |
| Current smoker (%) | 8.6 | 9.1 | 9.7 | 9.7 | 0.50 |
| Past smoker (%) | 40.0 | 42.6 | 38.1 | 41.6 | 0.57 |
| Alcohol (% drinker) | 44.4 | 47.1 | 48.5 | 41.0 | 0.76 |
| Alcohol (drinks/wk among drinkers) | 6.7 | 7.4 | 7.2 | 7.2 | 0.61 |
| Exercise (% ≥once/wk) | 72.4 | 69.0 | 70.5 | 76.2 | 0.26 |
| SBP (mmHg) | 126.3 | 126.1 | 126.2 | 126.0 | 0.63 |
| DBP (mmHg) | 84.9 | 85.2 | 85.3 | 85.3 | 0.021 |
| Sodium (mmol/24hr) | 169.8 | 172.6 | 175.9 | 186.1 | 0.0002 |
| Potassium (mmol/24hr) | 70.1 | 67.3 | 65.5 | 67.1 | 0.006 |
| WOMEN | |||||
| N | 184 | 313 | 517 | 275 | |
| Change in weight during trial (lbs) | −18.0 | −4.0 | 3.8 | 16.2 | - |
| Baseline weight (lbs) | 180.1 | 181.7 | 179.4 | 179.5 | 0.44 |
| Active weight loss intervention (%) | 50.0 | 40.6 | 32.7 | 40.4 | 0.011 |
| Active Na reduction intervention (%) | 32.6 | 37.4 | 34.6 | 41.4 | 0.13 |
| TOHP II (%) | 58.7 | 63.6 | 56.9 | 74.9 | 0.007 |
| Age (yrs) | 44.2 | 44.7 | 43.9 | 43.3 | 0.028 |
| Black (%) | 22.3 | 31.0 | 33.1 | 32.0 | 0.033 |
| College (%) | 45.1 | 48.2 | 44.1 | 44.0 | 0.47 |
| Current smoker (%) | 11.4 | 13.1 | 10.2 | 10.9 | 0.50 |
| Past smoker (%) | 28.3 | 25.9 | 25.2 | 27.6 | 0.88 |
| Alcohol (% drinker) | 26.6 | 24.3 | 23.0 | 22.2 | 0.25 |
| Alcohol (drinks/wk among drinkers) | 5.1 | 5.3 | 5.2 | 5.4 | 0.72 |
| Exercise (% ≥once/wk) | 62.8 | 55.8 | 56.3 | 58.4 | 0.50 |
| SBP (mmHg) | 126.7 | 127.2 | 127.4 | 126.8 | 0.84 |
| DBP (mmHg) | 85.0 | 84.9 | 85.1 | 85.3 | 0.097 |
| Sodium (mmol/24hr) | 130.5 | 128.9 | 138.0 | 143.4 | <0.0001 |
| Potassium (mmol/24hr) | 53.7 | 50.0 | 49.4 | 51.7 | 0.25 |
Data represent means or percentages. SBP indicates systolic blood pressure; DBP, diastolic blood pressure; Na, sodium.
When long-term self-reported weight was examined, there was recidivism over time (eTable 5). For example, men who lost >5% of weight during the trial regained about the same amount over the long-term for a net loss of 0.03 lbs; those who gained >=5% continued to gain some weight, with a net gain of 20.6 lbs from baseline. Similar results were seen in women. The net differences between weight change groups remained sizeable with a difference between extreme groups of 20.6 pounds (9.3 kg) in men and 22.6 lbs (10.3 kg) in women (p<0.0001).
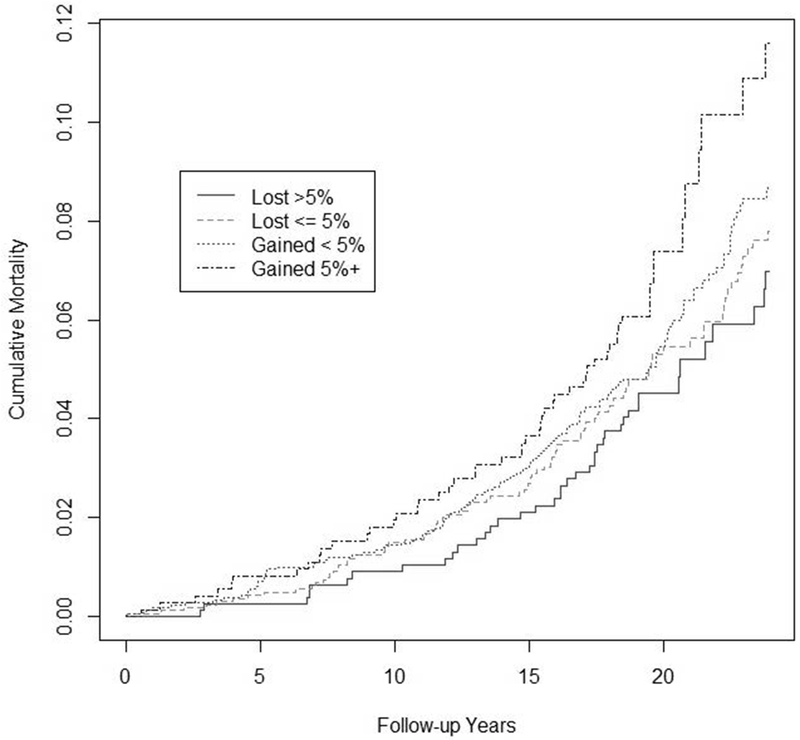
Those achieving a larger weight loss experienced lower mortality following the trials, with p-trend over categories = 0.046 (Table 3). Compared to those who gained 0-<5% of body weight, those who lost >5% had an 18% lower mortality (HR=0.82, 95% CI=0.57-1.18, p=0.29) after adjustment for baseline characteristics. Compared to those who gained ≥ 5% body weight, those who lost >5% had a 36% reduction in risk (HR=0.64, 95% CI =0.41-0.98, p=0.041). There was no interaction with time, and there appeared to be a consistent relation with total mortality through follow-up (Figure 2).
Table 3.
Total mortality following the Trials of Hypertension Prevention through 2013 by categories of weight change, among 3828 participants fulfilling eligibility criteria for TOHP weight loss intervention (high-weight stratum).*
| % Weight Change | P for Trend | HR per 5% change† | P Value | ||||
|---|---|---|---|---|---|---|---|
| Loss > 5% | Loss 0-<= 5% | Gain 0-< 5% | Gain 5%+ | ||||
| Deaths/ Total (%) | |||||||
| TOHP I | 22/249 (8.8) | 44/458 (9.6) | 73/632 (11.6) | 16/138 (11.6) | 0.18 | ||
| TOHP II | 19/307 (6.2) | 49/643 (7.6) | 55/935 (5.9) | 35/466 (7.5) | 0.92 | ||
| Model 1 | |||||||
| HR | 0.86 | 0.95 | 1.00 | 1.34 | 0.052 | 1.14 | 0.018 |
| 95%CI | 0.60-1.23 | 0.72-1.24 | (Reference) | 0.96-1.86 | 1.02-1.27 | ||
| Model 2 | |||||||
| HR | 0.82 | 0.94 | 1.00 | 1.29 | 0.046 | 1.14 | 0.019 |
| 95%CI | 0.57-1.18 | 0.72-1.23 | (Reference) | 0.92-1.80 | 1.02-1.28 | ||
From Cox proportional hazards regression models stratified by trial phase and adjusted as follows: Model 1 (age, sex, race/ethnicity, clinic, baseline weight, and treatment assignments), Model 2 (Model 1 variables plus education status, alcohol use, smoking, exercise, sodium and potassium excretion, family history of cardiovascular disease, and interactions of sodium intervention with sodium and potassium). HR, hazard ratio; CI, confidence interval.
Weight change is winsorized at 3 standard deviation.
Figure 2.
Cumulative incidence of total mortality by percent weight change categories in observational analysis of all participants in the high weight stratum of TOHP I and II, from Cox regression models adjusted for clinic, age, sex, race/ethnicity, baseline weight and other treatment assignments, education status, alcohol use, smoking, exercise, sodium and potassium excretion, family history of cardiovascular disease, and interactions of sodium intervention with sodium and potassium.
When considered as a continuous variable, the HR was 1.14 (95% CI=1.02-1.28, p=0.019) per 5% increase in weight. No differences by subgroups defined by phase, gender, age, smoking or weight loss intervention were apparent (eTable 6). In spline analysis (eFigure 3) there appeared to be a direct relation of percent weight change to mortality. In contrast, among those not in a weight loss intervention, there was no apparent effect of baseline weight (HR=1.04 per 10 lbs, 95% CI=0.98-1.10, p=0.21) or baseline BMI (HR=1.03 per kg/m2, 95% CI=0.98-1.08, p=0.31) on mortality (eFigures 4–5).
4. DISCUSSION
Observational analysis of the TOHP follow-up found a direct linear relationship of percent change in weight during the trial periods with later mortality persisting throughout the 19-24 year post-trial follow-up. Since these participants were healthy at baseline and included in a weight loss, another lifestyle, or a nutritional supplement trial, the weight loss experienced was presumably intentional. The persistence of group differences over decades argues against reverse causation due to underlying illness.
In contrast, we found no sustained difference in mortality by the randomized weight loss intervention over the full follow-up of 22-26 years. Despite a suggested beneficial effect at 10-15 years post-trial, none was seen during longer follow-up. There was recidivism in weight loss, however, leading to attenuation over time, with a net difference of 2-3 lbs remaining in self-reported weight after a median 12 years after trial end. This likely diminished further over longer follow-up. This small difference between randomized groups is in contrast to the much larger over 30-lb difference in weight between observational weight change categories, and likely led to smaller effects for the randomized comparison.
Few weight loss trials have reported long-term effects of the trial intervention (randomized analyses) and of weight change (observational analyses). The Look AHEAD weight loss trial in type 2 diabetics,2 while attaining a large short-term weight difference, had only a 2.6 kg (5.7 lb) between-group difference after 10 years. Similar to our trial analyses, there was no overall difference in their cardiovascular endpoint or in total mortality, though there was some reduction in cardiovascular risk in primary prevention only (HR=0.86, 95%CI=0.72-1.02). Observational analyses of the Look AHEAD trial, however, found an association between the magnitude of weight loss over the first year and incidence of cardiovascular disease over a median 10 years among those with type 2 diabetes.17 In TOHP, such differences extended to total mortality over 20 years.
Several observational studies have suggested a U- or J-shaped relationship of weight and mortality, including a meta-analysis of 97 studies,9 an analysis of three large cohorts,18 and an individual participant meta-analysis from the Global BMI Mortality Collaboration.19 Another meta-analysis,20 however, found that an apparent J-shaped curve was attenuated in never smokers and was largely absent among healthy never smokers. The association was affected by the length of follow-up, suggesting some confounding by pre-diagnostic disease, and the J disappeared after 20+ years.
Fewer studies have looked at the impact of weight change and later mortality. In the Chicago Western Electric Company Study, both weight loss and weight gain were associated with increased cardiovascular and all-cause mortality within 15-years of follow-up, but not in the 16-25 year follow-up period,21 suggesting some early reverse causation. Song et al22 examined weight trajectories over the life course and found that those maintaining a stable lean body shape had the lowest mortality over 15-16 years. A review and meta-analysis of weight changes found increased risk of mortality among those experiencing weight loss as well as weight gain.23 However, a recent analysis of data from large-scale cohorts found a monotonically increased risk of major chronic diseases with weight change from early to middle adulthood.24 There was a slight increase in mortality risk among women who lost more than 2.5 kg, but this was erased among never smokers and not seen among men.
Most observational studies cannot distinguish between intentional weight loss and that associated with long-term preexisting conditions. In studies that did attempt to examine intentional weight loss, findings have been mixed.25–27 Using data from the National Health Interview Survey, Gregg et al28 assessed both intentional and unintentional weight change and found lower mortality among those trying to lose weight, regardless of actual weight change, suggesting an impact of healthy behavior and motivation rather than actual weight loss. Data from the Atherosclerosis Risk in Communities Study (ARIC)29 found that short-term 3-year weight loss led to increased immediate risk of coronary heart disease and stroke, but longer-term weight loss from age 25 had little effect on these outcomes, suggesting that short-term unintentional weight loss may indicate increased risk. However, distinguishing deliberate from non-intentional weight loss is difficult in observational studies. The TOHP cohorts offered a unique opportunity to prospectively evaluate the long-term impact of presumably intentional weight loss in a healthy population interested in lifestyle interventions.
Some limitations of this analysis should be noted. First, the TOHP population was healthy, though pre-hypertensive, and relatively young at 30-54 years at entry. Though likely generalizable, the size of effects may not apply to others. Second, the observed benefit of weight change might have resulted from changes in other risk factors and behaviors as well as change in weight. It is possible that those losing the most weight were more motivated to pursue other healthy interventions, including exercise, healthy diet or changes in other risk factors and health behaviors. Such data are unavailable and therefore not included in our models. Third, the self-reported weight obtained in our later follow-up may have been biased due to differential follow-up response or misreporting. However, these reflect the expected recidivism occurring about a decade after an active intervention ended. Finally, we used the NDI only rather than active follow-up to obtain information on deaths from 2005 through 2013, which may have led to some degree of under-ascertainment, though we would expect little bias.
These results have research and policy implications. First, weight loss studies, even those with short-term interventions of just 18 months, should be designed with the potential to collect long-term mortality data. Second, it is important to analyze weight loss trials not just by randomized group but also by level of achieved weight loss, especially given the broad range of weight loss that occurs in both active and comparison arms. Third, the potential benefit of short-term weight loss on mortality supports reimbursement of weight loss interventions.
Note that while the participants in these trials were described as having high normal blood pressure, they would now be classified as having stage I hypertension according to the 2017 guidelines.30 Weight loss is a class I recommendation based on high quality evidence from randomized trials for such patients who are overweight or obese. We found that, besides lowering blood pressure, intentional weight loss of 5% or more is associated with lower mortality, even with recidivism. This should provide some encouragement to patients, even if their weight eventually returns to baseline, since they may be preventing further weight gain over time.
In conclusion, among healthy individuals taking part in lifestyle and nutrition supplement trials, weight change during the trial was directly associated with mortality over two decades later. These results are consistent with a long-term beneficial effect of presumed intentional weight loss on total mortality.
Supplementary Material
ACKNOWLEGDEMENTS
Funding Sources: TOHP I and II were supported by cooperative agreements HL37849, HL37852, HL37853, HL37854, HL37872, HL37884, HL37899, HL37904, HL37906, HL37907, and HL37924, all from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. The TOHP Follow- up Study was supported by grant HL57915 from the NHLBI and award 14GRNT18440013 from the American Heart Association (AHA). The NHLBI and AHA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: The authors declare no conflict of interest
REFERENCES
- 1.Stamler J Epidemiologic findings on body mass and blood pressure in adults. Ann Epidemiol. 1991;1:347–362. [DOI] [PubMed] [Google Scholar]
- 2.Look ARG, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. July 11 2013;369(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hypertension Prevention Trial Research Group. The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Arch Intern Med. 1990;150:153–162. [PubMed] [Google Scholar]
- 4.Stamler R, Stamler J, Gosch FC, et al. Primary Prevention of Hypertension by Nutritional-Hygienic Means: final report of a randomized controlled trial. J Amer Med Assoc. 1989;262:1801–1807. [PubMed] [Google Scholar]
- 5.Wood PD, Stefanik ML, Dreon DM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. New Engl J Med. 1988;319(18):1173–1179. [DOI] [PubMed] [Google Scholar]
- 6.The Trials of Hypertension Prevention Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. J Amer Med Assoc. 1992;267(9):1213–1220. [DOI] [PubMed] [Google Scholar]
- 7.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high- normal blood pressure. The Trials of Hypertension Prevention, Phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157(6):657–667. [PubMed] [Google Scholar]
- 8.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. New Engl J Med. 2010;363(23):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. N Engl J Med. 2011;365:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol. 2011;173:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. Estimating the number of deaths due to obesity: Can the divergent findings be reconciled? J Women’s Health. 2007;16(2):168–176. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR, Cutler JA, Obarzanek E, et al. The long-term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the Trials of Hypertension Prevention. BMJ. 2007;334:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook NR, Obarzanek E, Cutler JA, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The Trials of Hypertension Prevention (TOHP) Follow-up Study. Arch Intern Med. 2009;169(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metropolitan Life Insurance Co. 1983 Metropolitan height and weight tables. Statistical Bulletin. 1983;64:4. [PubMed] [Google Scholar]
- 17.Look Ahead Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. The lancet. Diabetes & endocrinology. November 2016;4(11):913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu E, Ley SH, Manson JE, et al. Weight History and All-Cause and Cause-Specific Mortality in Three Prospective Cohort Studies. Ann Intern Med. May 02 2017;166(9):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. Aug 20 2016;388(10046):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 2000;152(4):324–333. [DOI] [PubMed] [Google Scholar]
- 22.Song M, Hu FB, Wu K, et al. Trajectory of body shape in early and middle life and all cause and cause specific mortality: results from two prospective US cohort studies. BMJ. 2016;353:i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karahalios A, English DR, Simpson JA. Change in body size and mortality: a systematic review and meta-analysis. International journal of epidemiology. 2017;46(2):526–546. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Manson JE, Yuan C, et al. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA. Jul 18 2017;318(3):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women’s Health Study. Am J Epidemiol. 1999;149(6):504–514. [DOI] [PubMed] [Google Scholar]
- 26.Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diab Care. 2000;23(10):1499–1504. [DOI] [PubMed] [Google Scholar]
- 27.Yaari S, Goldbourt U. Voluntary and involuntary weight loss: associations with long term mortality in 9,228 middle-aged and elderly men. Am J Epidemiol. 1998;148(6):546–555. [DOI] [PubMed] [Google Scholar]
- 28.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138(5):383–389. [DOI] [PubMed] [Google Scholar]
- 29.Stevens J, Erber E, Truesdale KP, Wang CH, Cai J. Long- and short-term weight change and incident coronary heart disease and ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. July 15 2013;178(2):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. May 15 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.