Abstract
Background:
Early diagnosis and therapy improves outcomes in heart failure with severely reduced left ventricular ejection fraction (LVEF ≤35%), but some patients may remain undiagnosed. We hypothesized that a combination of ECG markers may identify individuals with severely reduced LVEF.
Methods:
From a community-based study in the Northwest US (the Oregon Sudden Unexpected Death Study), we evaluated the prevalence of conventional ECG markers by LVEF. We then evaluated the association of 9 additional ECG markers and LVEF. We validated the correlation of these ECG markers and LVEF in a separate, large health system in Los Angeles, California.
Results:
In the discovery population (n=1047), patients with LVEF ≤35% were twice as likely as those with LVEF >35% to have ≥1 conventional ECG abnormality. In the subset without conventional ECG abnormalities, ≥4 abnormal ECG markers from the expanded panel were found in 12% vs. 1% of patients with LVEF ≤35% and >35%, respectively. In the validation population (n=9742), 44% with LVEF ≤35% and 17% with LVEF >35% had ≥1 conventional ECG abnormality. In patients without conventional ECG abnormalities (n=7601), 40% with LVEF ≤35% and 5% with LVEF >35% had ≥4 abnormal ECG markers from the expanded panel. Each additional abnormal ECG marker from the expanded panel (range 0 to ≥4) more than doubled the odds of LVEF ≤35%.
Conclusions:
An expanded panel of easily obtained ECG markers correlated strongly with severely reduced LVEF in two separate populations. This electrical surrogate score could facilitate diagnosis of severely reduced LVEF, and warrants prospective evaluation.
Keywords: Electrocardiography (ECG), heart failure, cardiomyopathy, echocardiography, left ventricular ejection fraction, left ventricular systolic dysfunction
1. INTRODUCTION
Heart failure with severely reduced LV ejection fraction (LVEF ≤35%)1 remains a major public health problem in the US, with an average 5-year mortality of 50% due to pump failure or sudden cardiac death (SCD).2
Early diagnosis and initiation of pharmacologic therapy for left ventricular systolic dysfunction reduces morbidity and increases survival.3–5 Severely reduced LVEF is detected by cardiac imaging, mostly echocardiography, but due to practical and cost-effectiveness considerations, broad deployment of imaging tools for screening of asymptomatic patients in the community is not viable. Myocardial electrical remodeling is a consistent feature of the HF syndrome and manifests as abnormalities in the 12-lead electrocardiogram (ECG), reported in a variety of patient populations.6–14 However, published studies of the association between electrical remodeling with LV dysfunction are relatively small, and have focused mainly on a limited number of individual ECG variables, largely atrial fibrillation, left bundle branch block (LBBB) and ventricular pacing.6–14 We hypothesized that a panel of additional specific, easily obtained ECG markers could identify a larger proportion of patients with severely reduced LVEF, and also distinguish these patients from those with preserved LVEF.
We evaluated the correlation of nine expanded ECG markers with LVEF ≤35% from the ongoing community-based Oregon Sudden Unexpected Death Study (Oregon SUDS) based in Portland, Oregon. Subsequently, we evaluated this association in a separate population from the Cedars-Sinai Health System in Los Angeles, California.
2. METHODS
2.1. Discovery Population
We examined the association of ECG risk markers with echocardiographically assessed LV function among participants in the ongoing Oregon Sudden Unexpected Death Study (SUDS). The methods and rationale of the Oregon SUDS have been described previously.15, 16 Briefly, the study prospectively identifies out-of-hospital sudden cardiac arrest (SCA) cases occurring in the Portland, OR metro area, and conducts comparisons with control subjects from the same geographic area. Clinical history for study participants, including cardiac tests and imaging, is obtained from available medical records. For this analysis, cardiac arrest cases and control subjects enrolled from 2002 to 2015 were pooled and included if they had LVEF assessed by echocardiography and an ECG available in existing medical records; if >1 echocardiogram or ECG was available, the one closest to arrest / ascertainment was obtained. For SCA cases, the ECG and echocardiogram were required to have been performed prior, and unrelated to the cardiac arrest event.
2.2. Validation Population
The association of LVEF ≤35% with myocardial electrical remodeling as measured by the expanded ECG panel was validated in a large, separate patient population from the Cedars-Sinai Health System (Los Angeles, CA). Patients with a transthoracic echocardiogram performed from Jan. 1 – Dec. 31, 2015 were retrospectively identified from the hospital’s echocardiography laboratory database, and the most recent test was analyzed. LVEF was calculated using the biplane method of disk summation (modified Simpson’s rule) based on left ventricular end-diastolic and end-systolic volumes measured in the apical 2- and 4-chamber views.17 Patients with LVEF ≤35% were considered to have severely reduced LV systolic function.
Subsequently, the MUSE (GE Healthcare, Milwaukee, WI) electrocardiographic database management system and archive of all the ECGs recorded at the medical center was queried to obtain the digital ECG closest to the echocardiogram of each patient. Patients were included in this analysis if they were over 16 years old and had a resting 12-lead ECG performed within 14 days of the echocardiogram (67% of all patients with an echo had an ECG within 14 days).
The study was approved by the Institutional Review Boards of Cedars-Sinai Medical Center, Oregon Health and Science University, and all participating hospitals and health systems, and subjects provided informed consent as directed by these boards.
2.3. ECG Markers
The 9 parameters of the expanded ECG panel were the following: heart rate, P-wave duration, PR interval, QRS duration, QTc interval (Bazett’s correction), frontal QRS-T angle (calculated as the absolute difference between the frontal QRS axis and T-wave axis with values 0° – 180°), delayed QRS transition zone (R-wave amplitude less than S-wave amplitude in lead V4), delayed intrinsicoid deflection (defined as R-peak time ≥50ms in lead V5 or V6), and LVH (by Cornell voltage or Sokolow-Lyon criteria). In addition, the ECG was evaluated for rhythm, presence of left bundle branch block (LBBB) and acute ST-elevation myocardial infarction (MI). In the Oregon SUDS, we analyzed archived resting 12-lead ECGs with paper speed of 25mm/s and calibration of 10mm/mV, as previously described.18, 19 QRS transition zone, intrinsicoid deflection, LVH, LBBB, and acute ST elevation MI were determined by manual review. At Cedars-Sinai, computerized measurements of all parameters were available from the digital ECG reports. Clinically over-read diagnoses were used to identify paced ECG rhythm, atrial fibrillation / flutter (AF), LVH, LBBB, and acute ST-elevation MI. The reliability, sensitivity and specificity of these diagnostic algorithms has been previously demonstrated.20 From the total ECGs available, we excluded ECGs with evidence of acute ST-elevation MI.
2.4. Statistical Methods
As a first step, we evaluated the association of ECG findings conventionally associated with LVEF that included ventricular pacing, atrial fibrillation/flutter, or presence of LBBB. In Oregon SUDS, we excluded ventricular paced ECGs a priori, and therefore, ventricular paced rhythms were not included in analyses of conventional ECG abnormalities. Subsequently, we analyzed the remaining larger subset of patients without conventional ECG abnormalities, to examine associations between the expanded panel of 9 ECG markers and LVEF. We used chi-square tests for univariate associations, and automated stepwise logistic regression to test the multivariable-adjusted association of each ECG marker, with LVEF ≤35% as the outcome and the 9 individual ECG markers as predictors. Potential collinearity was evaluated in both populations by calculating the variance inflation factor (1/(1 – R2)) for each of the nine ECG markers using SAS PROC REG, where R2 was the R-squared for the model with variable Xj as the dependent variable, and all other ECG markers as the independent variables. Variance inflation factors were <1.3 for all ECG variables, indicating no multicollinearity. We ran two logistic regression models, one with ECG variables as continuous predictors if appropriate, and the second model with all ECG variables dichotomized. Variables meeting model entry criteria (p≤0.30) were retained if p≤0.10 in the final model. Continuous ECG variables were dichotomized at clinically accepted cut-points: heart rate >85bpm; QRS duration >110ms; QTc interval ≥460ms for men and ≥470ms for women; QRS-T angle >90o; PR interval >200ms; and P-wave duration >110ms. The remaining variables were dichotomous only: delayed QRS transition zone, delayed intrinsicoid deflection, and LVH.
2.4.1. Summed Expanded Panel of Abnormal ECG Markers:
Finally, in both populations we calculated a sum for the expanded abnormal ECG marker panel, in which each variable significant at p≤0.10 in either the continuous or categorical models was assigned one point (the unweighted panel). As a sensitivity analysis, a weighted panel was also constructed, with each ECG parameter weighted by its odds ratio in the categorical model rounded to the nearest integer. The sum of the number of abnormal ECG markers was modeled as a predictor of LVEF ≤35% using logistic regression. Model fit was evaluated with the Hosmer-Lemeshow goodness of fit test, and model calibration with the C-statistic. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the expanded ECG panel.
3. RESULTS
3.1. Oregon SUDS Discovery Population
Between February 1st, 2002 and January 31st, 2015, 1047 subjects (560 cardiac arrest cases, 487 controls) with both ECG and LVEF assessed by echocardiogram were identified from the Oregon SUDS. Echocardiograms were performed a median of 192 days prior to arrest / ascertainment (interquartile range 21 – 702 days), and ECGs were performed a median of 163 days from the echocardiogram (IQR 6 – 565 days). In Oregon SUDS, ECGs with paced ventricular rhythms were excluded a priori. After further excluding ECGs with acute ST elevation MI (n=33), 195 of 1014 subjects (19%) had LVEF ≤35%. Conventional ECG abnormalities (LBBB or AF) were observed in 62 (32%) of 195 subjects with LVEF ≤35% and 122 (15%) of 819 subjects with LVEF >35% (p<0.001). Presence of LBBB was significantly associated with LVEF ≤35% (odds ratio (OR) 4.4, 95% confidence interval (CI) 2.7–7.0), while AF was not (OR 1.4, 95% CI 0.9–2.2). Among the remaining 830 subjects (423 cases, 407 controls) in sinus rhythm without conventional ECG abnormalities (16% with LVEF ≤35%), all ECG parameters were significantly associated with LVEF ≤35% in univariate comparisons (p≤0.01). In multivariable models, heart rate, QRS duration, QRS-T angle, QRS transition zone, and PR-interval remained independently associated with LVEF ≤35%. In this subset, 12% of patients with LVEF ≤35% and 1% of patients with LVEF >35% had ≥4 of the abnormal ECG markers from the expanded panel (p<0.001). When these ECG parameters were combined to construct the unweighted summed expanded ECG panel, a 1-unit increase in the panel sum was associated with 2.5-fold higher odds of LVEF ≤35% (OR 2.5; 95% CI 2.0–3.1; C-statistic 0.763). The weighted panel performed similarly (C-statistic 0.747).
3.2. Cedars-Sinai Validation Population
A total of 9,742 consecutive patients (9.1% with LVEF ≤35%) had a transthoracic echocardiographic study performed between January 1st and December 31st, 2015 at the Cedars-Sinai Medical Center, with a 12-lead ECG available within 14 days of the echocardiogram (same day in 62%, within 3 days in 93%). After excluding 68 patients with acute ST-elevation MI, 1896 (20%) of the remaining 9674 patients had at least one major ECG abnormality clinically accepted to be associated with HF: LBBB, AF, or paced rhythm. After further excluding 177 subjects with other arrhythmias or missing data, 7,601 subjects remained in the final analysis. Among these patients, severely reduced LVEF ≤35% was present in 6.1%.
3.2.1. Conventional ECG abnormalities Associated with Reduced LV Function
Major ECG abnormalities (AF, paced rhythms, or LBBB) were more common in patients with lower ejection fractions (Figure 1). At least one major ECG abnormality was observed in 388 (44%) of 875 subjects with LVEF ≤35% and 1508 (17%) of 8799 subjects with LVEF >35% (p<0.001). Presence of AF was moderately associated with LVEF ≤35% (OR 1.6, 95% CI 1.3 – 1.9), while paced rhythms (OR 5.2, 95% CI 4.3 – 6.2) and LBBB (OR 4.2, 95% CI 3.3 – 5.3) had stronger associations. Presence of any one of these abnormalities for predicting LVEF ≤35% produced a sensitivity of 0.443, specificity of 0.829, PPV of 0.205, and NPV of 0.937.
Figure 1. Association of abnormal ECG markers with LVEF.
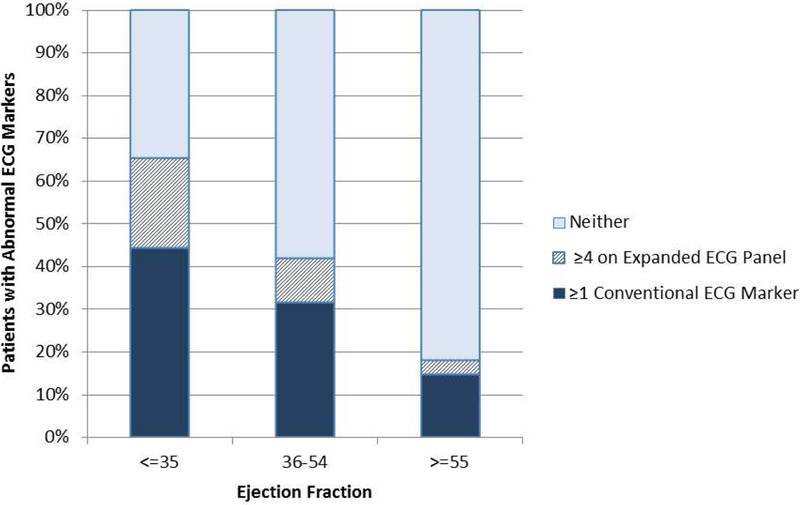
Among all patients (n=9742), ≥1 conventional abnormal ECG marker was present in 44% of patients with LVEF ≤35% (in dark). Among patients without conventional ECG abnormalities, an additional 21% of patients with LVEF ≤35% had ≥4 abnormal markers on the expanded ECG panel (in cross-hatch). Use of the expanded panel plus conventional markers increased identification of abnormal ECG findings from 44% to 65% among patients with LVEF ≤35%.
Conventional ECG abnormalities were left bundle branch, atrial fibrillation/flutter, or paced rhythms.
The expanded abnormal ECG panel included resting heart rate >85 bpm, QRS duration >110 ms, QTc interval ≥460 ms for men and ≥470 ms for women, delayed QRS transition zone, delayed intrinsicoid deflection and QRS-T angle >90o.
3.2.2. Expanded Panel of Abnormal ECG Markers Associated with Reduced Ejection Fraction
As in the discovery population, all nine of the expanded ECG parameters were associated with LVEF ≤35% in univariate comparisons in the validation cohort (p<0.001) (Table 1). In the multivariable model, heart rate, QTc interval, QRS duration, QRS-T angle, delayed QRS transition zone, and delayed intrinsicoid deflection remained independently associated with LVEF ≤35%, while LVH, prolonged PR interval, and prolonged P wave were not significant (Table 2).
Table 1.
Patient demographics and ECG parameters based on category of left ventricular ejection fraction (LVEF) in the Cedars-Sinai Health System validation population. All ECG parameters differed significantly between subjects with LVEF ≤35% and those with LVEF >35%.
| Cedars-Sinai Validation Population† (n=9742) | |||
| LVEF ≤35% (n=875) | LVEF >35% (n=8799) | p-value | |
| Demographics | |||
| Male | 648 (74%) | 4554 (52%) | <0.001 |
| Age | 68.0 ± 16.1 | 68.7 ± 17.3 | 0.23 |
| Conventional abnormal electrocardiographic findings | |||
| Left bundle branch block (LBBB) | 101 (12%) | 266 (3%) | <0.001 |
| Atrial fibrillation / flutter | 128 (15%) | 864 (9%) | <0.001 |
| Ventricular paced rhythm | 186 (21%) | 437 (5%) | <0.001 |
| ≥1 Conventional ECG abnormality | 388 (44%) | 1508 (17%) | <0.001 |
| Cedars-Sinai Validation Population without conventional abnormal ECG findings† (n=7601) | |||
| LVEF ≤35% (n=461) | LVEF >35% (n=7140) | p-value | |
| Demographics | |||
| Male | 336 (73%) | 3660 (51%) | <0.001 |
| Age | 63.8 ± 16.3 | 66.3 ± 17.4 | 0.003 |
| Expanded panel of electrocardiographic variables in subset without conventional ECG abnormalities | |||
| Heart rate >85bpm | 215 (47%) | 2193 (31%) | <0.001 |
| QRS >110ms | 170 (37%) | 1017 (14%) | <0.001 |
| Prolonged QTc‡ ≥460ms men and ≥470ms women | 311 (67%) | 1981 (28%) | <0.001 |
| QRS-T angle >90o‡ | 259 (56%) | 1300 (18%) | <0.001 |
| Delayed QRS transition‡ | 303 (66%) | 2149 (30%) | <0.001 |
| Delayed intrinsicoid deflection‡ | 156 (34%) | 412 (5.8%) | <0.001 |
| Left ventricular hypertrophy | 68 (15%) | 459 (6.4%) | <0.001 |
| PR >200ms‡ | 89 (19%) | 834 (12%) | <0.001 |
| P-wave >110ms‡ | 126 (27%) | 1303 (18%) | <0.001 |
Data are presented as n (%) or mean ±SD.
Validation population: n=9742 inpatients and outpatients from the Cedars-Sinai hospital system with LVEF assessed by echocardiogram from Jan. 1 – Dec. 31, 2015 and ECG available within 14 days of the echocardiogram. Conventional ECG abnormalities evaluated in n=9674 patients without acute MI; Expanded panel of ECG abnormalities evaluated in n=7601 patients without conventional abnormalities (ECGs in sinus rhythm and without LBBB).
Table 2.
ECG parameters associated with LVEF ≤35% in the multivariable model and included in the expanded abnormal ECG marker total, in the Cedars-Sinai validation population.
| Initial model with all ECG parameters | Final model retaining parameters if p<0.10* | |||
|---|---|---|---|---|
| ECG parameter | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| Heart rate >85bpm | 1.9 (1.5–2.3) | p<0.001 | 1.8 (1.5–2.2) | p<0.001 |
| QRS >110ms | 1.3 (1.0–1.6) | 0.06 | 1.3 (1.0–1.6) | p=0.04 |
| QTc ≥460ms men; ≥470ms women | 2.9 (2.3–3.6) | p<0.001 | 2.9 (2.3–3.6) | p<0.001 |
| QRS-T angle >90o | 2.6 (2.1–3.3) | p<0.001 | 2.6 (2.1–3.3) | p<0.001 |
| Delayed QRS transition | 3.1 (2.5–3.9) | p<0.001 | 3.1 (2.5–3.9) | p<0.001 |
| Delayed intrinsicoid deflection | 5.4 (4.2–6.9) | p<0.001 | 5.5 (4.3–7.1) | p<0.001 |
| Left ventricular hypertrophy | 1.0 (0.7–1.4) | 0.90 | -- | |
| PR >200ms‡ | 1.1 (0.8–1.4) | 0.64 | -- | |
| P-wave >110ms‡ | 1.2 (0.9–1.6) | 0.13 | -- | |
Initial multivariable model included all ECG parameters. Final multivariable model resulted from stepwise logistic regression, with p<0.30 to enter model and p<0.10 to retain in model. LVH, prolonged PR interval and P-wave duration did not remain significant, were omitted from the final model, and were not included in the abnormal ECG marker total. C-statistic of the model was 0.846, goodness of fit test p=0.11.
Based on the six statistically significant ECG markers, an unweighted expanded ECG panel sum was constructed ranging from 0 to ≥4 abnormal markers. A 1-unit increase in the panel sum was associated with 2.9-fold increased odds of LVEF ≤35% (OR 2.9; 95% CI 2.6–3.1; C-statistic 0.831). The odds ratios remained consistent in models stratified by sex and age, ranging from 2.6 to 3.5. There was no significant interaction by sex with the ECG panel sum (p=0.36). The weighted panel sum, constructed as described in the methods, ranged from 0 to 18, and had a dose-response increase in odds of LVEF ≤35%, with similar discrimination (C-statistic 0.845). Because the results were similar, we used the unweighted panel sum for further analysis.
The expanded ECG panel was significantly associated (p<0.001) with decreasing LVEF in the validation population. Starting with the overall population, use of the expanded panel in addition to the conventional markers increased identification of abnormal ECG findings from 44% to 65% among patients with LVEF ≤35% (Figure 1).
Among the subset of patients without conventional ECG abnormalities (n=7601), 461 patients had LVEF ≤35%, and 184 (40%) of these had an expanded ECG panel sum of at least 4. The majority of patients without conventional ECG abnormalities had LVEF >35% (7140 of 7601, 94%), and among these, only 5% had a panel sum of ≥4. Conversely, 61% of the 7601 patients had ≤1 abnormal markers, and among them, only 1.3% had LVEF ≤35%. Among the 564 patients (7% of the total 7601 patients) with an expanded ECG panel sum of ≥4, 184 (33%) had LVEF ≤35% (Figure 2). For identification of LVEF ≤35%, a panel sum of ≥4 had a sensitivity of 0.443, specificity of 0.947, PPV of 0.326, and NPV of 0.961.
Figure 2.
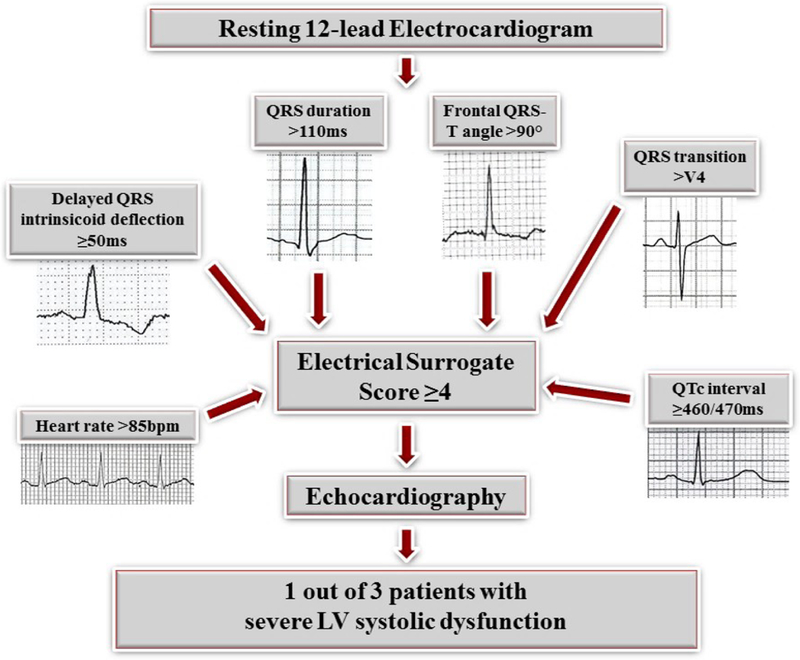
Using the expanded ECG panel, 33% of patients with an expanded panel sum of ≥4 (4 or more abnormal ECG markers) had severe LV systolic dysfunction.
4. DISCUSSION
To our knowledge, this is the first report of an expanded ECG marker panel that was consistently associated with severely reduced LVEF in two separate populations. We first identified a combination of ECG markers associated with severely reduced LV function in a discovery population and then validated our findings in a separate health-system population. Among patients with major ECG abnormalities that are conventionally associated with LVEF, such as atrial arrhythmias, LBBB, and paced rhythms, 20% had LVEF ≤35%. In the remaining patients without conventional ECG abnormalities, six specific ECG parameters (resting heart rate >85bpm, QRS duration >110ms, prolonged QTc interval, QRS-T angle >90°, delayed QRS transition, and delayed intrinsicoid deflection) remained independently associated with LVEF ≤35%. A finding of ≥4 abnormal ECG markers correlated strongly with LVEF ≤35%. On the other hand, in individuals with one or no abnormal ECG markers, severely reduced LVEF was an exceedingly rare finding.
Published studies have reported a correlation between abnormal ECG diagnoses such as atrial fibrillation, LBBB, ventricular paced rhythms and reduced LVEF; and in clinical practice, these findings generally prompt clinicians to evaluate the LVEF.21–26 Our results are also consistent with these established findings. However, a large subgroup of patients will have reduced LVEF in the absence of these conventionally accepted ECG markers.21, 24 As a consequence, there is substantial room for improvement for identification of patients with severely reduced LVEF.
Therefore, we examined an expanded panel of abnormal ECG markers that are not currently considered as indicators of LVSD in clinical practice. The association between several individual ECG markers and LVSD has been previously reported. For example, increased resting heart rate has been associated with reduced LVEF even in asymptomatic individuals in the general population.6 Several studies among heart failure patients and other populations have linked QRS prolongation with decreased LV systolic function.7–9 However, early attempts to directly estimate LV function by using measures of QRS morphology from the ECG had limited success.27 The more specific depolarization measures included in the expanded panel, i.e. delayed intrinsicoid deflection10, 28 and QRS transition zone29, were individually associated with low LVEF. In addition, prolonged QTc-interval and wide QRS-T angle have been associated with LV dysfunction.13, 14 However, to our knowledge these ECG markers have not been previously combined to examine their joint association with LVSD.
In our validation study population, after excluding patients with major ECG abnormalities conventionally associated with HF, 7.4% of patients had ≥4 abnormal ECG findings, and one-third of these patients had evidence of severe LVSD. In this heterogeneous population, positive and negative predictive values of having ≥4 abnormal ECG markers were higher than those of the traditional major ECG abnormalities.30 Furthermore, 60% of the patients had only 0–1 ECG abnormalities, and in this group the prevalence of LVEF ≤35% was under 1.5%. This observation, in accordance with previous reports23, suggests that a normal ECG virtually excludes severe LVSD. Together, these findings imply that a markedly abnormal electrical profile, even in the absence of other conventionally used major ECG abnormalities, is strongly correlated with LV systolic function.
There are several factors that could explain the relationship between increasing number of ECG abnormalities and decreased LVEF. Pathologic LV remodeling in ischemic or non-ischemic cardiomyopathy has electrical components that are reflected as abnormalities in cardiac conduction and myocardial depolarization/repolarization markers.8, 13 As highlighted recently12, structural and electrical remodeling contribute independently to risk of morbidity and mortality. Another established manifestation of the HF syndrome is abnormal autonomic remodeling, reflected by increased resting heart rate.6, 31
4.1. Study limitations
The strengths of this study lie in discovery and validation of an expanded panel of abnormal ECG markers and LVEF in two separate populations, and in the large size of the validation population. However, some potential limitations should be considered while interpreting these findings. Since the analysis was restricted to subjects undergoing echocardiographic examination, these could represent a subgroup of patients with higher morbidity. In addition, the overall prevalence of LVSD was relatively high: 16% in the Oregon SUDS population (a population enriched for sudden cardiac arrest cases and coronary disease), and 9% in our validation study population of largely hospitalized patients. Though the consistency of associations between ECG parameters and LVEF in these two very different populations is encouraging, nonetheless these results may not be generalizable to the general population or to a purely out-patient population. However, among a combination of outpatient and hospitalized patients, a set of relatively easily-obtained ECG markers was strongly correlated with severe LVSD.32
5. CONCLUSIONS
A panel of multiple, broadly available ECG markers was strongly associated with findings of severely reduced LV systolic function. These findings may have potential for improving detection of severe LVSD, with the possibility of improving early diagnosis and management of patients if prospective community-based studies corroborate the effectiveness of this expanded panel of abnormal ECG markers.
Acknowledgment:
The authors acknowledge the significant contribution of the Oregon Sudden Unexpected Death Study research subjects, American Medical Response, Portland/Gresham fire departments and the Oregon State Medical Examiner’s office.
Grant Support:
Funded by National Institutes of Health, National Heart Lung and Blood Institute (NHLBI) grants R01HL122492 and R01HL126938 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at Cedars-Sinai, Los Angeles. Dr Aro is funded by grants from the Finnish Medical Foundation, the Paavo Nurmi Foundation, the Finnish Foundation for Cardiovascular Research, the Orion Research Foundation and the Biomedicum Helsinki Foundation.
Abbreviations:
- ECG
Electrocardiogram
- HF
Heart failure
- LBBB
Left bundle branch block
- LVEF
Left ventricular ejection fraction
- LVH
Left ventricular hypertrophy
- LVSD
Left ventricular systolic dysfunction
- MI
Myocardial infarction
- Oregon SUDS
Oregon Sudden Unexpected Death Study
- SCA
Sudden cardiac arrest
- SCD
Sudden cardiac death
Footnotes
Conflicts of Interest:
All authors report no conflicts of interest.
REFERENCES
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–259 [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350 [DOI] [PubMed] [Google Scholar]
- 3.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: A follow-up study. Lancet (London, England). 2003;361:1843–1848 [DOI] [PubMed] [Google Scholar]
- 4.Investigators S, Yusuf S, Pitt B, Davis CE, Hood WB, Jr., Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The New England journal of medicine. 1992;327:685–691 [DOI] [PubMed] [Google Scholar]
- 5.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The capricorn randomised trial. Lancet (London, England). 2001;357:1385–1390 [DOI] [PubMed] [Google Scholar]
- 6.Opdahl A, Ambale Venkatesh B, Fernandes VR, Wu CO, Nasir K, Choi EY, Almeida AL, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JA. Resting heart rate as predictor for left ventricular dysfunction and heart failure: Mesa (multi-ethnic study of atherosclerosis). Journal of the American College of Cardiology. 2014;63:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murkofsky RL, Dangas G, Diamond JA, Mehta D, Schaffer A, Ambrose JA. A prolonged qrs duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction [see comment. Journal of the American College of Cardiology. 1998;32:476–482 [DOI] [PubMed] [Google Scholar]
- 8.Ilkhanoff L, Liu K, Ning H, Nazarian S, Bluemke DA, Soliman EZ, Lloyd-Jones DM. Association of qrs duration with left ventricular structure and function and risk of heart failure in middle-aged and older adults: The multi-ethnic study of atherosclerosis (mesa). European journal of heart failure. 2012;14:1285–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund LH, Jurga J, Edner M, Benson L, Dahlstrom U, Linde C, Alehagen U. Prevalence, correlates, and prognostic significance of qrs prolongation in heart failure with reduced and preserved ejection fraction. European heart journal. 2013;34:529–539 [DOI] [PubMed] [Google Scholar]
- 10.O’Neal WT, Qureshi WT, Nazarian S, Kawel-Boehm N, Bluemke DA, Lima JA, Soliman EZ. Electrocardiographic time to intrinsicoid deflection and heart failure: The multi-ethnic study of atherosclerosis. Clinical cardiology. 2016;39:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberger AL. A specific ecg triad associated with congestive heart failure. Pacing and clinical electrophysiology : PACE. 1982;5:593–599 [DOI] [PubMed] [Google Scholar]
- 12.Aro AL, Chugh SS. Clinical diagnosis of electrical versus anatomic left ventricular hypertrophy: Prognostic and therapeutic implications. Circ Arrhythm Electrophysiol. 2016;9:e003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey P Qt interval lengthening in cardiac disease relates more to left ventricular systolic dysfunction than to autonomic function. European journal of heart failure. 2000;2:265–271 [DOI] [PubMed] [Google Scholar]
- 14.Pavri BB, Hillis MB, Subacius H, Brumberg GE, Schaechter A, Levine JH, Kadish A, Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation I. Prognostic value and temporal behavior of the planar qrs-t angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117:3181–3186 [DOI] [PubMed] [Google Scholar]
- 15.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large u.S. Community. Journal of the American College of Cardiology. 2004;44:1268–1275 [DOI] [PubMed] [Google Scholar]
- 16.Narayanan K, Reinier K, Uy-Evanado A, Teodorescu C, Chugh H, Marijon E, Gunson K, Jui J, Chugh SS. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation. 2013;128:1733–1738 [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2015;28:1–39. e14 [DOI] [PubMed] [Google Scholar]
- 18.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged tpeak-to-tend interval on the resting ecg is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 2011;4:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged qrs duration on the resting ecg is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011;8:1562–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guglin ME, Thatai D. Common errors in computer electrocardiogram interpretation. International journal of cardiology. 2006;106:232–237 [DOI] [PubMed] [Google Scholar]
- 21.Baker DW, Bahler RC, Finkelhor RS, Lauer MS. Screening for left ventricular systolic dysfunction among patients with risk factors for heart failure. American Heart Journal. 2003;146:736–740 [DOI] [PubMed] [Google Scholar]
- 22.Davie AP, Francis CM, Love MP, Caruana L, Starkey IR, Shaw TR, Sutherland GR, McMurray JJ. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ (Clinical research ed.). 1996;312:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen OW, Hansen JF, Hilden J, Larsen CT, Svanegaard J. Risk assessment of left ventricular systolic dysfunction in primary care: Cross sectional study evaluating a range of diagnostic tests. BMJ (Clinical research ed.). 2000;320:220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesen LL, Andersen A. Ecg as a first step in the detection of left ventricular systolic dysfunction in the elderly. ESC Heart Failure. 2016;3:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rihal CS, Davis KB, Kennedy JW, Gersh BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. The American Journal of Cardiology. 1995;75:220–223 [DOI] [PubMed] [Google Scholar]
- 26.Talreja D, Gruver C, Sklenar J, Dent J, Kaul S. Efficient utilization of echocardiography for the assessment of left ventricular systolic function. American Heart Journal. 2000;139:394–398 [PubMed] [Google Scholar]
- 27.Young SG, Abouantoun S, Savvides M, Madsen EB, Froelicher V. Limitations of electrocardiographic scoring systems for estimation of left ventricular function. Journal of the American College of Cardiology. 1983;1:1479–1488 [DOI] [PubMed] [Google Scholar]
- 28.Darouian N, Narayanan K, Aro AL, Reinier K, Uy-Evanado A, Teodorescu C, Gunson K, Jui J, Chugh SS. Delayed intrinsicoid deflection of the qrs complex is associated with sudden cardiac arrest. Heart rhythm: the official journal of the Heart Rhythm Society. 2016;13:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aro AL, Phan D, Teodorescu C, Uy-Evanado A, Reinier K, Gunson K, Jui J, Huikuri HV, Chugh SS. Cardiac structural and functional profile of patients with delayed qrs transition zone and sudden cardiac death. Europace. 2017;19:629–635 [DOI] [PubMed] [Google Scholar]
- 30.Lieberman D Progress and challenges in colorectal cancer screening and surveillance. Gastroenterology. 2010;138:2115–2126 [DOI] [PubMed] [Google Scholar]
- 31.Floras JS. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. Journal of the American College of Cardiology. 2009;54:375–385 [DOI] [PubMed] [Google Scholar]
- 32.Galasko GI, Barnes SC, Collinson P, Lahiri A, Senior R. What is the most cost-effective strategy to screen for left ventricular systolic dysfunction: Natriuretic peptides, the electrocardiogram, hand-held echocardiography, traditional echocardiography, or their combination? European heart journal. 2006;27:193–200 [DOI] [PubMed] [Google Scholar]


